Abstract
Aim:
The sex hormones 17β-estradiol (βES) and progesterone (PRG) induce rapid non-genomic vasodilator effects which could be protective for the cardiovascular system. The purpose of this study was to analyze the mechanisms underlying their vasodilator effect in rat aortic smooth muscle preparations.
Methods:
Endothelium-denuded aorta artery rings were prepared from male Wistar rats and incubated in an organ bath. The contractions of the preparation were recorded through isometric transducers. The effects of the hormones on K+ current and L-type Ca2+ current (LTCC) were analyzed by using the whole cell voltage-clamp technique in A7r5 cells.
Results:
Both βES and PRG (1–100 μmol/L) concentration-dependently relaxed the endothelium-denuded aortic rings contracted by (–)-Bay K8644 (0.1 μmol/L) or by KCl (60 mmol/L). The IC50 values of the two hormones were not statistically different. The KV channel blocker 4-aminopyridine (2 mmol/L), BKCa channel blocker tetraethylammonium (1mmol/L) and KATP channel blocker glibenclamide (10 μmol/L) did not significantly modify the relaxant effect of the hormones. On the other hand, the blockage of the intracellular βES and PRG receptors with estradiol receptor antagonists ICI 182,780 (1 μmol/L) and PRG receptor antagonist mifepristone (30 μmol/L), respectively, did not significantly modify the relaxant action of the hormones. In A7r5 cells, both the hormones (1–100 μmol/L) rapidly and reversibly inhibited the basal and BAY-stimulated LTCC. However, these hormones had no effect on the basal K+ current.
Conclusion:
The vasorelaxant effects of βES and PRG are due to the inhibition of LTCC. The K+ channels are not involved in the effects.
Keywords: female sex hormone, 17β-estradiol, progesterone, non-genomic action, vasorelaxant effect, Ca2+ channel, K+ channel, rat aorta artery ring, A7r5 cel
Introduction
Cardiovascular diseases are one of the most common causes of death in western countries and there are different degrees of incidence of these diseases between both sexes1. Concerning the role of sex hormones in these diseases, it was reported that estrogen replacement therapy can reduce the risk of coronary and cerebrovascular diseases in postmenopausal women2, 3. Also, the results of distinct observational and clinical studies suggested that female and male sex hormones can modulate the vascular function4, 5. Nevertheless, the molecular mechanisms underlying the effects of these hormones remain undefined due to controversial data and to relatively small number of experimental studies undertaken.
According to the classic theory to explain the steroid hormones effects, these signalling molecules modulate gene transcription due to the interaction with intracellular receptors. However, these hormones can also induce rapid (seconds to minutes) non-genomic effects which are reversible and insensible to transcription and protein synthesis inhibitors1, 6, 7. Non-genomic effects of sex hormones include vasodilatation, one of the most relevant and promising action of sex steroids. Different pathways have been proposed to explain this effect. Progesterone (PRG) and 17beta-estradiol (βES) may activate endothelium-dependent vasorelaxant pathways, including pathways mediated by nitric oxide, prostacyclin and hyperpolarizing factors1, 8, 9. However, these steroids can also relax arterial smooth muscle via endothelium-independent mechanisms, mainly involving modulation of membrane ionic flux10, 11. This direct vasodilator effect of PRG and βES has been observed in different arteries from different species such as aorta12, 13, coronary arteries8, 10, 14, cerebral arteries15, omental artery16, tail artery17 and mesenteric artery9, 18, 19.
The inhibition of Ca2+ channels and the activation of K+ channels was suggested as a leading cause of the sex hormones' effect. The extracellular Ca2+ can enter into the smooth muscle cells through different types of Ca2+ channels such as store-operated Ca2+ channels, voltage-operated Ca2+ channels (VOCCs), Ca2+-permeable non-selective cation channels and the controversial receptor-operated Ca2+ channels20. Some authors showed that the inhibition of Ca2+ channels is associated to the vasodilatation mediated by estrogens21, 22, 23 and PRG24, 25. The effects of βES and PRG on VOCCs were the objective of some studies. Zhang et al have reported that in A7r5 cells estradiol inhibits two types of VOCCs: L- and T-type Ca2+ channels (LTCC and TTCC respectively)26. Nakajima et al, using tight-seal whole cell clamp technique, observed that βES inhibits LTCC, but failed to affect Ca2+-permeable non-selective cation currents evoked by endothelin or vasopressin21. In addition, these authors also observed that PRG and testosterone fail to inhibit calcium channels21. Other authors observed that environmental estrogenic pollutants and βES induced a rapid and endothelium-independent relaxation by inhibiting LTCC in vascular smooth muscle cells27. Activation of K+ channels by PRG and by βES in vascular smooth muscle may induce repolarization of plasma membrane, which will close the VOCCs and contribute to the vascular relaxation27, 28, 29, 30. This mechanism does not appear to be very important for PRG29, but the opening of Ca2+-activated K+ (BKCa) channels by βES has been observed in human coronary artery smooth muscle cells30 and rat aorta A7r5 cells27. The activation of K+ channels was suggested to be involved in the vasodilatation induced by βES in arteries from different species8, 31 and could also be important to prevent or reduce hypertension32, 33. On the contrary, K+ currents can be attenuated by 17alpha-estradiol or βES, an effect that could be mediated by estrogen-induced proteasomal degradation of these channels. These sex hormones specific bind to K+ channels and induce proteasome-dependent proteolytic degradation. This action can be elicited independently of the activation of the nuclear estradiol receptors or the accessory β1-subunit, but in the presence of β1-subunits, specific binding of estradiol to BKCa channels is significantly increased34.
In summary, PRG and βES have vascular non-genomic actions which include vasodilatation. Although the mechanistic pathways implicated in this effect are still unknown, some studies have reported, regardless of the specific site of action, the involvement of an ionic-related transduction mechanism. The purpose of this study was to analyse the mechanisms implicated in the vasodilator effect of these hormones in rat aortic smooth muscle. The relaxation induced by these hormones in endothelium-denuded rat aorta was analysed. Also, the whole cell configuration of the patch-clamp technique was used to analyse the effects of sex hormones on voltage-dependent Ca2+ current (ICa) and on the K+ current (IK) in A7r5 cells.
Materials and methods
Contractility experiments in isolated rat thoracic aorta rings
Male adult Wistar rats (Charles-River, Barcelona, Spain) weighing 400–500 g were housed and acclimatized with light cycles of 12 h light: 12 h dark and food and water ad libitum for at least one week before performing the experiments. The rats were used in accordance with the European regulations about protection of animals (Directive 86/609) and the Guide for the Care and Use of Laboratory Animals promulgated by the US National Institutes of Health (NIH Publication No 85–23, revised 1996).
The rats were sacrificed by decapitation and, after thoracotomy, the thoracic aortas were removed, placed in a thermostatized (37 °C) Krebs' modified solution and the fat and connective tissue was cleaned. Also, the vascular endothelium was mechanically removed by gentle rubbing with a cotton bud introduced through the arterial lumen. The rat aorta artery rings were placed in an organ bath (LE01.004, Letica) containing Krebs-bicarbonate solution at 37 °C continuously gassed with carbogen. The composition of the Krebs' modified solution was (mmol/L): NaCl 119, KCl 5, CaCl2·2H2O 0.5, MgSO4·7H2O 1.2, KH2PO4 1.2, NaHCO3 25, EDTA-Na2 0.03, L-(+)-ascorbic acid 0.6 and glucose 11 (pH 7.4). The rings were suspended by two parallel stainless steel wires and tension measurement was performed using isometric transducers (TRI201, Panlab SA, Spain), amplifier (ML118/D Quad Bridge, ADInstruments), interface PowerLab/4SP (ML750, ADInstruments), interface PowerLab/4SP (ML750, ADInstruments), and computerised system with Chart5 PowerLab software (ADInstruments). During the resting periods, the organ bath solution was changed every 15 min.
Initially, the rings were equilibrated for 60 minutes until a resting tension of 1.0 g. After the equilibration period, aortic rings were firstly contracted with high isosmotic KCl (60 mmol/L) and the absence of endothelium functionality was confirmed by the lack of relaxant response to acetylcholine (1 μmol/L). After that, the arteries were washed many times for at least 45 min before the next stimuli. The rings were contracted using KCl (60 mmol/L) or (–)-Bay K 8644 (BAY; 0.1 μmol/L) and vasorelaxation induced by βES and PRG (1–100 μmol/L) on these contractions was analysed.
The contraction induced by BAY (0.1 μmol/L; specific LTCC activator) was performed to observe the direct effect of these sex hormones o LTCC. The contractions induced by BAY were obtained in presence of KCl 10 mmol/L.
In some experiments, specific antagonists for the intracellular hormonal receptor were used in order to analyse the involvement of these receptors in the vasorelaxant effects of βES and PRG. In these cases, after a stable contraction with KCl (60 mmol/L), the arteries were incubated for 15 min with ICI 182,780 (1 μmol/L; a specific antagonist for the classical estradiol receptor) or with mifepristone (30 μmol/L; a specific antagonist for the classical progesterone receptor). After this incubation the vasorelaxation induced by βES or PRG in the presence of this specific antagonist was analysed.
To analyse the role of K+ channels in the effects of these sex hormones, different inhibitors of these channels were used: tetraethylammonium (TEA; 1 mmol/L), that inhibits of large-conductance Ca2+-activated potassium (BKCa) channel; glybenclamide (Gly; 10 μmol/L), that inhibits ATP-sensitive potassium (KATP) channel; and 4-aminopyridine (4-AP; 1 mmol/L), that inhibits voltage-sensitive potassium (KV) channels. In these cases, after a stable contraction with KCl (60 mmol/L), the arteries were incubated 15 min with the potassium channel inhibitors. After this incubation the effect of the sex hormones was analysed. Control experiments were performed using ethanol, the vehicle used to dissolve the channel inhibitors.
Cell culture of vascular smooth muscle cells
The A7r5 cell line, used in this study, is a commercial vascular smooth muscle cell line obtained from embryonic rat aorta (Promochem, Spain). The cells were grown in culture medium Dulbecco's modified Eagle's medium/Nutrient Mixture F-12 Hams (DMEF-F12; Sigma-Aldrich, Portugal) supplemented with NaHCO3 (1.2 μg/L), L-ascorbic acid (20 mg/L; Sigma-Aldrich), bovine serum albumin (0.5%; Sigma-Aldrich), heat-inactivated foetal bovine serum (FBS; 10%; Biochrom), and a mixture of penicillin (100 U/mL), streptomycin (100 g/mL), and amphotericin B (250 ng/mL) (Sigma-Aldrich). The cells were kept in culture at 37 °C in a humidified atmosphere with 5% CO2 in air. After confluence, the cells were placed in culture medium without FBS (FBS-free culture medium) for 24–48 h. Trypsinization was made using a solution of trypsin (0.3%) in Ca2+-Mg2+-free phosphate buffered solution with EDTA (0.025%). Subsequently, the cells were kept at 4 °C in FBS-free medium until the realisation of the electrophysiological experiments.
Electrophysiology experiments
The whole cell configuration of patch clamp technique was used to analyse the L-type calcium current (ICa,L) and the K+ current (IK). To analyse the ICa,L, the control external solution contained (mmol/L): NaCl 124.0, CaCl2 5.0, HEPES 5.0, tetraethylammonium sodium salt (TEA) 10.0, KCl 4.7 and glucose 6.0, pH 7.4 adjusted with NaOH. Patch electrodes (2–4 MΩ) were filled with internal solution (mmol/L): CsCl 119.8, CaCl2 0.06, MgCl2 4.0, Na-ATP 3.1, Na-GTP 0.4, EGTA 5.0, HEPES 10.0 and TEA 10.0, pH 7.3 adjusted with CsOH. The presence of Cs+ instead of K+ in the solutions blocked the potassium currents. The cells were maintained at a holding potential of −80 mV and routinely depolarised every 8 s to 0 mV test potential during 500 ms to measure ICa,L.
To analyse the IK, the control external solution contained (mmol/L): NaCl 134.3, CaCl2 1.0, HEPES 5.0, KCl 5.4 and glucose 6.0, pH 7.4 adjusted with NaOH. Patch electrodes (2–4 MΩ) were filled with internal solution (mmol/L): KCl 125.0, MgCl2 1.0, Na-ATP 5.0, Na-GTP 0.5, EGTA 0.1, HEPES 20.0 and glucose 10.0, pH 7.3 adjusted with KOH. For IK analysis we used the same holding potential and depolarizations to 60 mV for 300 ms were performed every 8 s.
Basal ICa,L and IK were measured 3–5 min after patch break to allow the equilibration between pipette and intracellular solutions. Currents were not compensated for capacitance and leak currents. All experiments were done at room temperature (21–25 °C) and the temperature did not vary by more than 1 °C in a given experiment. The cells were voltage clamped using the patch-clamp amplifier Axopatch 200B (Axon instruments, USA). Currents were sampled at a frequency of 10 kHz and filtered at 0.1 kHz using the analog-digital interface Digidata 1322A (Axon Instruments, USA) connected to a compatible computer with the Pclamp8 software (Axon Instruments, USA). The external solution was applied to the cell proximity by placing the cell at the opening of a 250 μm inner diameter capillary tube flowing at a rate of 20 μL/min. The basal and BAY-stimulated (10 nmol/L) ICa,L were studied in the presence of different concentrations of βES (1–100 μmol/L) and of PRG (1–100 μmol/L) dissolved in the external solution.
Drugs
All drugs were purchased from Sigma-Aldrich Química (Sintra, Portugal), except 4-aminopyridine and PRG that purchased from Biogen Cientifica (Madrid, Spain).
Mifepristone, ICI 182,780, BAY, nifedipine, βES and PRG were initially dissolved in ethanol. 4-Aminopyridine, glibenclamide, apamin and tetraethylammonium were initially dissolved in deionised water. Appropriate dilutions in Krebs' modified solution or in the corresponding electrophysiology external solution were prepared every day before the experiment. Final concentration of ethanol never exceeded 0.1% in the experiments.
Statistical analysis
Statistical treatment of data was performed using the SigmaStat Statistical Analysis System, version 1.00 (1992). Results are expressed as mean±SEM of n experiments. Comparison among multiple groups was analysed by using a one-way ANOVA followed by Dunnet's or Tukey post hoc test to determine significant differences among the means. Comparison between two groups was analysed by using Students t-test. Probability levels lower than 5% were considered significant (P<0.05).
In the contractility experiments, the relaxant responses induced by βES and PRG are expressed as a percent of the maximal contraction (Emax=100%) produced by each vasoconstrictor agent. In these experiments, sigmoidal concentration-response curves for the vasorelaxant effects were fitted and IC50 values (ie concentrations inducing 50% of relaxation) of βES and PRG were estimated for KCl- or BAY-induced contractions. The antagonist of classical progesterone receptors, mifepristone, relaxed by itself the arteries contracted by KCl, and in this case the maximal effect used to perform the concentration-response curves was the tension obtained in presence of mifepristone.
The ICa,L amplitudes were automatically calculated between the maximum current peak and the stable current plateau near the final of the every 8 s pulse. The ICa,L variations induced by the different drugs used are expressed as a percent of the basal or BAY-stimulated ICa,L. The IK variations are expressed as a percent of the basal IK obtained by depolarization in the absence of any drug.
Results
Vasorelaxant effects of female sex hormones on rat isolated aorta
The rat aortic rings without endothelium were contracted by depolarisation with isosmotic KCl (60 mmol/L) solution or by the calcium channel opener BAY (0.1 μmol/L). The maximal contractions elicited by KCl and BAY, 1134.3±39.9 mg (n=52) and 1222.8±83.1 mg (n=20) respectively, were not significantly different (P<0.05, Student's t-test). These contractile effects were reversible after washing out with Krebs' solution.
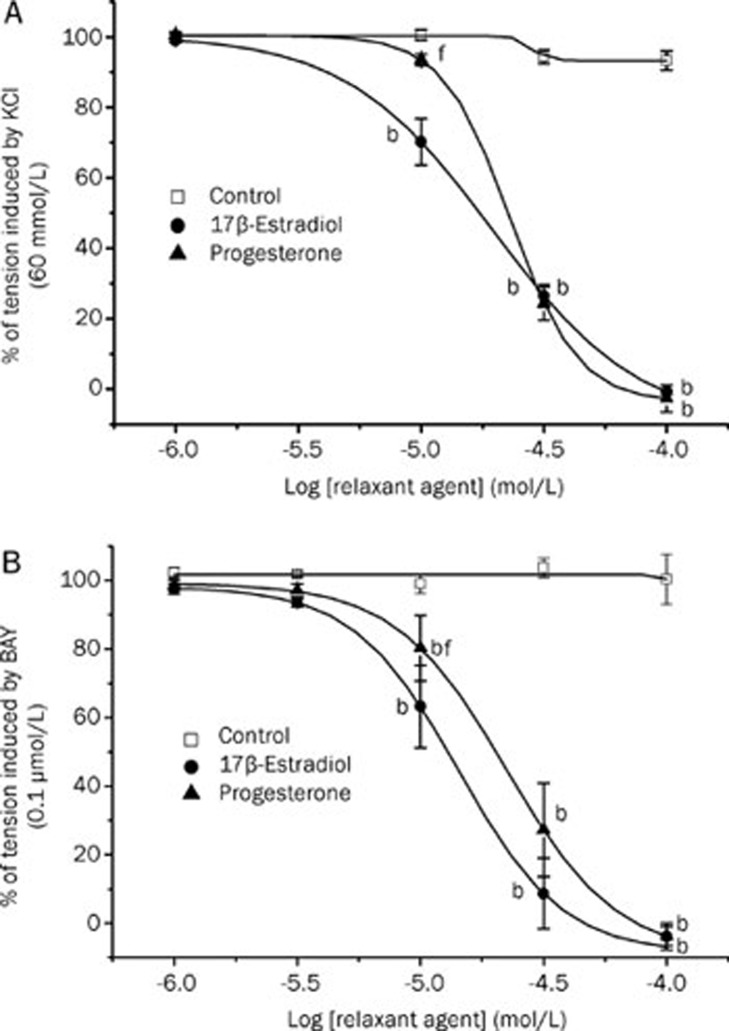
Afterwards, the effect of βES or PRG (1–100 μmol/L) on the contraction induced by KCl and Bay were analysed. Increasing concentrations of both hormones were administered and, at higher concentrations, almost completely relaxed the contractions induced by KCl and BAY (Figure 1A and 1B). The vasorelaxation induced by each dose of βES or PRG was observed 10–15 min after the application. The effect of the hormones was reversible because, after washing out, a second administration of the contractile agents elicited similar contractile effects than the previous one (P>0.05, data not shown). The maximal relaxation induced by βES and by PRG was similar in arteries contracted by KCl or BAY. However, the vasorelaxant effect induced by PRG 10 μmol/L in the arteries contracted with BAY is lower that the induced by βES at the same concentration (Figure 1B). Also, in the arteries contracted with KCl the vasorelaxant effect induced by βES 10 μmol/L is lower that the induced by PRG at the same concentration, and similar to the control arteries (Figure 1A).
Figure 1.
Relaxant effect of different concentrations of βES and PRG (1–100 μmol/L) on contractions of endothelium-denuded rat aortic rings induced by KCl (60 mmol/L) (A) or by BAY (0.1 μmol/L after KCl 10 mmol/L) (B). Each point represents the mean value and the vertical lines indicate SEM of at least 5 experiments. Control experiments were performed using with ethanol, the vehicle used to dissolve the hormones. bP<0.05 versus control. fP<0.01 βES versus PRG. One-way ANOVA followed by with Tukey post hoc test.
The IC50 values corresponding to the relaxant effects of βES or PRG on KCl-induced contraction were almost similar, being βES slightly more effective than PRG, although this difference was not significant (P>0.05; Student's t-test; Table 1). Similarly, in BAY-contracted arteries, the vasorelaxant effect of βES seems to be slightly more effective than that of PRG, as revealed the IC50 values obtained in both cases (Table 1). However, there are no significant differences between the IC50 values of βES and PRG, independently of the contractile agent (KCl or BAY) (P>0.05; Student's t-test; Table 1). Thus, in general, the vasorelaxant effect induced by βES and by PRG was similar.
Table 1. Values of IC50 (μmol/L) for the relaxant effect of 17β-estradiol and progesterone on rat aortic contractions induced by BAY (10 nmol/L) and with KCl (60 mmol/L). The influence of potassium channel inhibitors was analysed using the following drugs: the KV channel blocker 4-aminopyridine (4-AP; 2 mmol/L); the BKCa channel blocker tetraethylammonium (TEA; 1 mmol/L); and the KATP channel blocker glibenclamide (GLI; 10 μmol/L). The influence of the blockage of intracellular hormone receptors was analysed using the estradiol receptor antagonists ICI 182,780 (ICI; 1 μmol/L) and the progesterone receptor antagonists mifepristone (30 μmol/L). Each value represents the mean±SEM from the number of experiments shown in brackets.
| AGENTS | 17β-Estradiol | Progesterone |
|---|---|---|
| BAY | 14.24±0.05 (n=6) | 22.33±0.30 (n=5) |
| KCl | 19.54±1.15 (n=9) | 23.04±0.02 (n=9) |
| KCl+TEA | 22.66±2.76 (n=4) | 20.25±1.11 (n=6) |
| KCl+4-AP | 17.14±1.33 (n=7) | 22.60±1.06 (n=6) |
| KCl+GLI | 30.44±2.43 (n=3) | 18.79±2.01 (n=4) |
| KCl+ICI | 16.97±1.26 (n=6) | |
| KCl+Mifepristone | 24.49±1.35 (n=8) |
4-AP, 4-aminopyridine; BAY, (−)-Bay K8644; GLI, glibenclamide; TEA, tetraethylammonium sodium salt; ICI, ICI 182,780.
Influence of K+ channels on the vasorelaxant effects of the female sex hormones
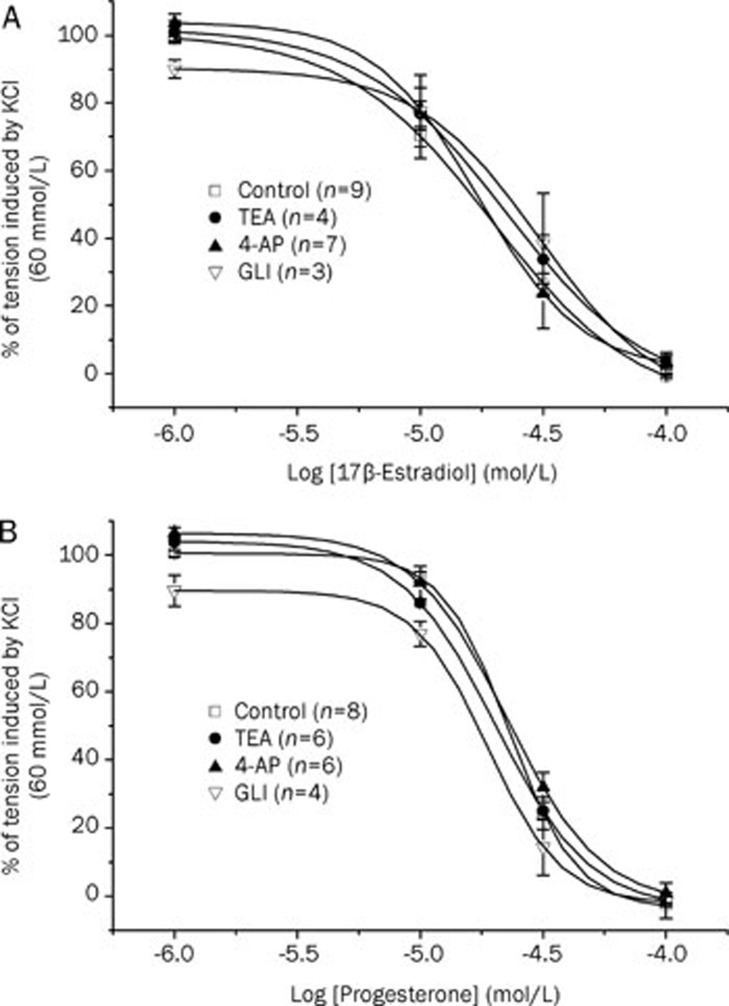
The effects of three K+ channels inhibitors (Gli, 4-AP, and TEA) were investigated to analyse the involvement of these channels in the female sex hormones-associated relaxant mechanism. Initially, after contraction with KCl 60 mmol/L, rat aorta rings were exposed for 15 min to glibenclamide (Gli; 10 μmol/L), 4-aminopyridine (4-AP; 1 mmol/L), and tetraethylammonium (TEA; 1mmol/L), either together or separately, and these inhibitors did not have a significant effect on the contraction induced by KCl 60 mmol/L (data not shown). After that, the administration of cumulative concentrations of βES or PRG induced the total relaxation of the contracted aortic rings. None of the K+ channel inhibitors tested modified significantly the relaxant effect of these hormones (Figure 2) (P>0.05, one-way ANOVA with Dunnet's post hoc test). The relaxant IC50 values for βES and PRG in the presence of any one of the K+ channels inhibitors did not differ significantly from the IC50 values calculated in the absence of them (P>0.05; Student's t-test. Table 1). Thus, the inhibition of KV, BKCa, and KATP channels did not reduce the relaxing effect of βES or PRG.
Figure 2.
Relaxant effect of different concentrations of 17β-estradiol (A) and progesterone (B) on KCl-contracted arteries in presence or absence of the following different potassium channel inhibitors: glibenclamide (GLI; 10 μmol/L); 4-aminopyridine (4-AP; 1 mmol/L); and tetraethylammonium (TEA; 1 mmol/L). Each point represents the mean value±SEM (indicated in vertical bars) from the number of experiments shown in brackets.
Implication of βES and PRG intracellular receptor in the vasorelaxant effects of these hormones
To analyse if the vasorelaxant effects of βES and PRG are mediated by the activation of the intracellular receptors, we used specific antagonists for these receptors. The arteries were contracted by KCl or BAY, after reaching a plateau of contraction the antagonists were administered and increasing concentrations of βES or PRG were added to test the relaxant effect of these hormones in these conditions. The ICI 182,780 (1 μmol/L) was used to block βES receptors and mifepristone (30 μmol/L) was employed for the receptors of PRG.
When administrated alone, mifepristone induced a significant relaxation of the contracted rat aortic rings (53.9%±3.1%). However, mifepristone and ICI 182,780 did not alter the relaxant effects of PRG or βES, respectively, because the IC50 values obtained in the absence and in the presence of these antagonist were similar (P>0.05, Student's t-test; Table 1). These results indicated that the vasorelaxant effects of βES and PRG are not mediated by classical hormonal receptor activation.
Effects of βES and PRG on ICa,L in A7r5 cells
The whole-cell patch clamp technique was used to analyse calcium current through the LTCC (ICa,L) in A7r5 cells. The mean value of basal ICa,L density was of 0.90±0.05 pA/pF (n=70). The application of BAY (10 nmol/L; specific stimulator of LTCC) significantly stimulated the calcium current by 97.7%±8.3% (n=17) above the basal level. On the contrary, nifedipine (0.1 μmol/L; LTCC inhibitor) significantly reduced the basal ICa,L until a level of 24.3%±4.5% (n=7) of the basal activity (P<0.05). The effects of BAY and/or nifedipine were completely reversible upon washout of the drug (data not shown). These results indicate that the current measured was due to the LTCC.
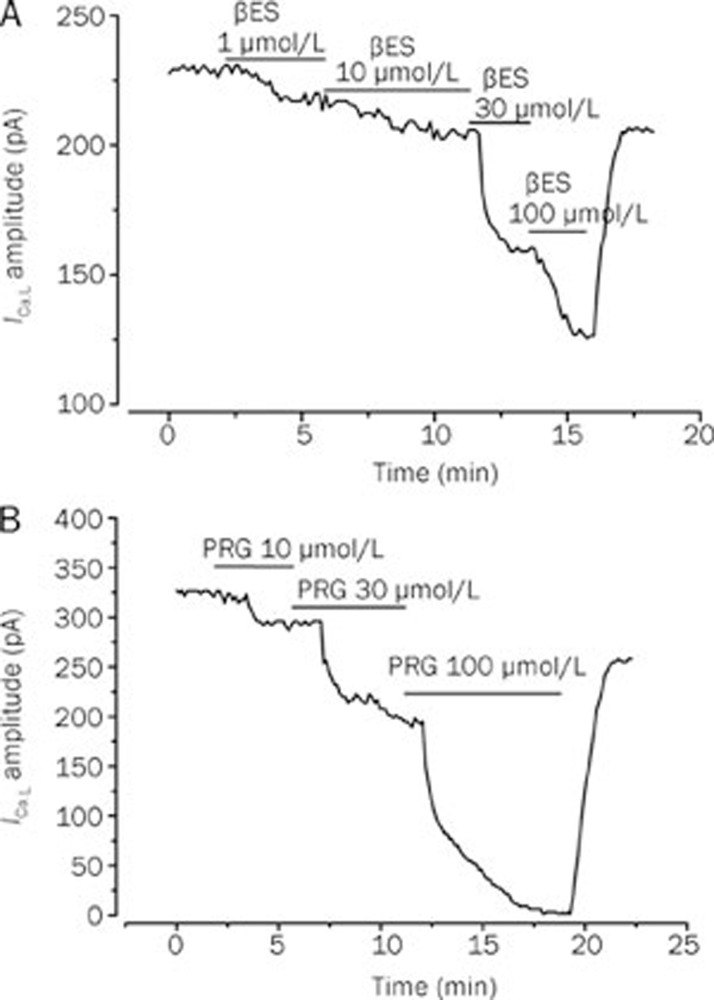
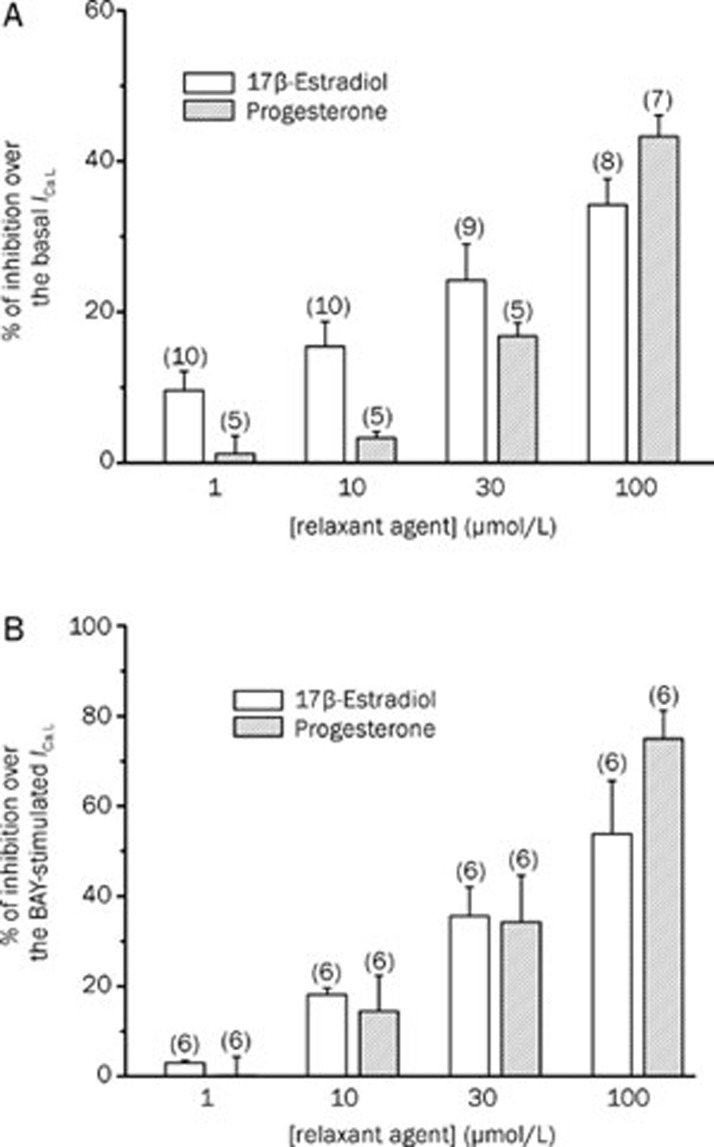
Like a proposed vasodilatation mechanism of the female sex hormones is the inhibition of LTCC, we tested the effect of βES or PRG on the ICa,L. The Figure 3A shows a typical experiment in which different concentrations of βES (1–100 μmol/L) inhibited the basal ICa,L in a reversible manner. Concerning PRG, Figure 3B shows a typical experiment in which PRG (1–100 μmol/L) almost completely inhibits the basal ICa,L. The data obtained in this type of experiments demonstrate that PRG (1–100 μmol/L) inhibits the basal ICa,L. Thus, PRG seems to have similar effects with βES on basal ICa,L (P>0.05, Student's t-test).
Figure 3.
Original records showing the effect of increasing concentrations of 17β-estradiol (A) and progesterone (B) on basal ICa,L amplitudes measured in patch-clamp experiments performed with A7r5 cells.
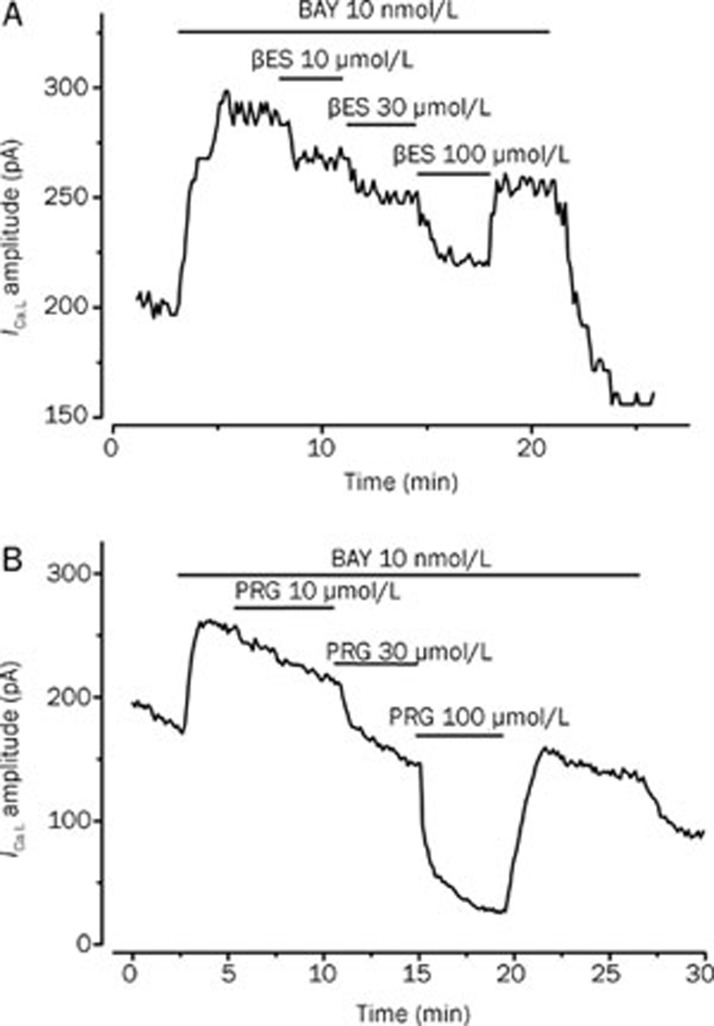
To further characterize the effect of βES on these channels, we analyse their effect on the BAY-stimulated ICa,L. Figure 4A shows a typical experiment in which βES reversibly inhibited the ICa,L stimulated by BAY (10 nmol/L) and this inhibitory effect was dependent on the concentration. The maximal effect of βES was an inhibition of 53.8%±11.8% on the BAY-stimulated ICa,L (Figure 5B). Concerning PRG effect, we also analyse its effect on the ICa,L stimulated by BAY (Figure 4B). PRG also inhibited BAY-stimulated ICa,L. At a concentration of 100 μmol/L, PRG almost completely inhibited the stimulation of BAY and reduced the ICa,L below the basal level (Figure 5B).
Figure 4.
Original records showing the effect of increasing concentrations of 17β-estradiol (A) and progesterone (B) on BAY-stimulated ICa,L amplitudes measured in patch-clamp experiments performed with A7r5 cells.
Figure 5.
Inhibitory effect (% of reduction) of 17β-estradiol and progesterone on basal ICa,L (A) and BAY-stimulated (10 nmol/L) (B) ICa,L in A7r5 cells. Each column represents the mean value±SEM (indicated in vertical bars), in percent of the basal (A) or BAY-stimulated (B) ICa,L from the number of experiments shown in brackets.
A comparison between the effects of both hormones showed that the effects of βES and PRG on BAY-stimulated ICa,L are similar (P>0.05, Student's t-test). On the other hand, ethanol (0.001%–0.1%), the vehicle used to dissolve βES and PRG did not affect basal or stimulated ICa,L (data not shown).
Effects of βES and PRG on IK in A7r5 cells
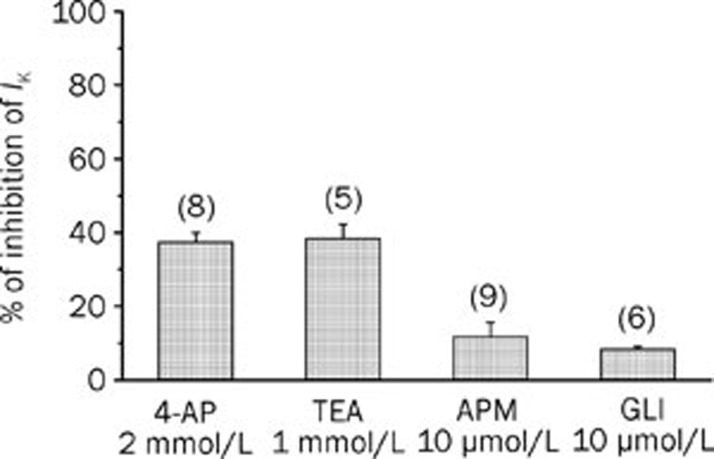
The whole-cell patch clamp technique was used to analyse potassium current (IK) in A7r5 cells. The mean value of basal IK density was of 6.1±0.7 pA/pF (n=37). In order to determine the types of K+ channels that were responsible for the total potassium current measured, we used selective blockers of different channels. The KV channel blocker 4-AP reduced basal IK by 37.4%±2.5% at +60 mV. TEA (1 mmol/L), which is used as a BKCa channel blocker, reduced net current by 38.3%±3.9% at +60 mV (Figure 6). We also tested the presence of the low conductance KCa channels using the selective blocker apamin (10 μmol/L), which induced a small reduction on the basal IK (11.7%±3.8%, n=9). Glibenclamide, usually used as a KATP channel blocker also induced a small reduction on the IK (8.3%±0.7%, n=6) (Figure 6). The effects of the K+ channels blockers used were completely reversible upon washout of the drug. Thus, our data suggest that the potassium current measured is mainly constituted by potassium exit through KV and BKCa channels.
Figure 6.
Inhibition of IK (% of reduction) induced by different potassium channel inhibitors in A7r5 cells. The bars represent the effect on IK of the following potassium channel blockers: the KV channel blocker 4-aminopyridine (4-AP; 2 mmol/L); the BKCa channel blocker tetraethylammonium (TEA; 1 mmol/L); the low conductance KCa channels blocker apamin (APM, 10 μmol/L); and the KATP channel blocker glibenclamide (GLI; 10 μmol/L). Each column represents the mean value±SEM (indicated in vertical bars), in percence of the inhibition of IK from the number of experiments shown in brackets.
In order to analyse the role of K+ channels in the relaxant mechanism of βES and PRG, the effects of these steroids on A7r5 IK were analyzed. The results show that different concentrations (1–100 μmol/L) of βES and PRG did not inhibit the IK (Table 2).
Table 2. Inhibitory effect of the basal potassium current (% of basal IK) induced by 17β-estradiol and progesterone (1–100 μmol/L) on A7r5 cells. Each value represents the mean of the % of variation of basal IK±SEM from the number of experiments shown in the brackets.
| Concentration | 17 β-estradiol | Progesterone |
|---|---|---|
| 1 μmol/L | 2.1%±2.4% (n5) | −1.4%±3.8% (n8) |
| 10 μmol/L | −1.1%±5.7% (n6) | 1.8%±3.1% (n7) |
| 30 μmol/L | −3.7%±2.8% (n5) | −0.9%±4.3% (n5) |
| 100 μmol/L | −2.1%±3.2% (n5) | −3.8%±0.8% (n5) |
Discussion
In the present study, we analyzed the vascular responses of βES and PRG in endothelium-denuded aorta from male adult rats and their effects on the ICa,L and IK measured by whole cell voltage-clamp in A7r5 cells. The female hormones (1–100 μmol/L) equally relaxed, in a rapid and concentration-dependent manner, the aortic rings contracted with KCl or BAY, suggesting an inhibitory effect on voltage-dependent Ca2+ influx currents. These voltage-dependent slow inactivated inward currents were measured in A7r5 cells and were characterised electrophysiologically and pharmacologically as ICa,L. The sex hormones studied, βES and PRG, inhibited the basal and BAY-stimulated ICa,L. On the other hand, the vasorelaxant effects of βES and PRG on rat aorta were not mediated by classic hormone receptors or by K+ channels opening. This data, obtained performing contractility experiments, were also confirmed using the whole cell configuration of the patch clamp, which show that βES and PRG failed to stimulate IK in A7r5 cells. Therefore, altogether, our results demonstrated a non-genomic inhibitory effect induced by βES and PRG on LTCC that seems to be the responsible for the endothelium-independent vasorelaxant effect of these hormones.
The vasorelaxant effects of βES and PRG on aortic rings contracted with KCl were dose-dependent (1 and 100 μmol/L) and the maximal relaxation achieved with the hormones was 100%. Because we studied the effect of these hormones at the smooth muscle level, the vascular endothelium was previously removed. Previous studies have shown the vasorelaxant effect of the female sex hormones in rat aorta, but there is no consensus about the mechanistic pathway involved in this action. Some authors defended a main role of the endothelium in the relaxant effects of these hormones1, 9. Our results demonstrate that there is a relaxant effect independent of the endothelium. These data agree with the that obtained by Perusquia et al using PRG in rat thoracic aorta35. Also, Unemoto et al described a vasorelaxant effect of βES and PRG on agonist-induced contractions in the aorta of Wistar-Kyoto and spontaneously hypertensive rats12. For these authors, the vasorelaxant effect of βES seemed to be more gifted than PRG, however they did not find statistical differences between the IC50 values calculated for the inhibitory action of the female hormones. In opposition, Rodriguez et al showed that 17α-estradiol, but not βES, relaxes calcium-dependent contractions in rat aortic strip36. On the other hand, we previously showed that testosterone and cholesterol also relax rat aorta by inhibiting LTCC37. Thus, in the sense, the vasodilator effect of cholesterol, testosterone, βES and PRG seems to be similar.
The relaxant effect induced by βES and PRG on the contractions induced by KCl or by BAY is similar, which, attending to the mode of action of both drugs, suggests that these hormones inhibit Ca2+ influx into vascular smooth muscle cells. High extracellular KCl concentrations induce plasmatic membrane depolarization, which activates the Ca2+ entry by VOCCs (mainly LTCC) and this leads to muscle contraction. BAY directly and specifically opens LTCC and induces vascular smooth muscle contraction also due to intracellular Ca2+ elevation. Thus, βES and PRG inhibited KCl and BAY-induced contractions and presumably inhibit Ca2+ influx through LTCC. This hypothesis was also supported by other investigators that studied the sex steroids effects in rat aorta12, 38 and in other arteries15, 23, 39.
Activation of K+ channels in vascular smooth muscle may induce repolarization of the plasma membrane, which leads to close VOCCs and contributes to vascular relaxation. To test the possible implication of this pathway in the vascular effects of βES and PRG we used inhibitors of these channels: TEA (BKCa channels inhibitor), glibenclamide (KATP channels inhibitor) and 4-aminopyridine (KV channel inhibitor). None of them significantly modified the rat aorta relaxant effects of βES or PRG, suggesting that potassium channel opening is not involved in the vascular action of these hormones. This conclusion may not surprise for PRG because its vascular effects were never associated to the potassium channel activation12, 29. However, in rat cerebral arteries, βES seems to enhance nitric oxide production from vascular endothelium and, by this way, activate BKCa40. According to Unemoto et al, the relaxation induced by βES in aortas from spontaneously hypertensive rats is, at least partially, mediated via KATP and KV channels stimulation in addition to Ca2+ influx blockage12. Nevertheless, these authors suggest that the K+ channels stimulation by βES does not occur in aorta of Wistar rats. Other authors demonstrate that βES can activate BKCa channels and induce artery relaxation in human coronary artery smooth muscle cell31 and in rat coronary arteries8. On the contrary, potassium currents can be attenuated in transfected HEK293 cells by 17alpha-estradiol or by βES, an effect that could be mediated by estrogen-induced proteasomal degradation of the channels34. To further investigate the effect of βES and PRG on the K+ channels, we performed patch clamp studies in A7r5 cells. The potassium current measured was significantly inhibited by the KV channel blocker (4-AP) and by the BKCa channel blocker (TEA), but the SKCa (low conductance) channel blocker (apamine) and the KATP channel blocker (glibenclamide) did not have a significant effect on the potassium current measured. In agreement with previous data37, our study show that the potassium current measured in A7r5 cells is mainly due to KV and BKCa channels. On the other hand, for the first time our study also demonstrates that βES and PRG fail to stimulate IK in A7r5 cells. These data confirm the contractility data and demonstrate that K+ channels are not implicated in the vasorelaxant effect of βES and PRG in rat aorta.
The blockage of the intracellular receptors for βES and PRG did not modify significantly the vasorelaxant action of these hormones, demonstrating that their intracellular receptors are not involved in the vascular effects induced by these hormones. This fact was previously observed by other authors23, 38. On the contrary, it has been described that the antiprogestin mifepristone reduced the PRG-induced relaxation on human placental arteries and veins, suggesting a classic receptor-activated mechanism for PRG in these vessels41. Furthermore, Han et al showed that βES activates K+ channels is due to the activation of the intracellular alpha receptor for estradiol31. On the other hand, the relaxation induced by mifepristone on KCl-contracted arteries was never referred by other authors. However, a similar effect in rat aorta was described for flutamide, a specific antagonist of the intracellular testosterone receptor42, 43. To explain this effect of flutamide, Iliescu et al suggested the involvement of the NO-cGMP pathway activation42. Also, Ba et al also observed this effect in rat arteries and a bigger relaxation in arteries from males than from females, suggesting a sex dependent mechanism for the flutamide effect43.
To further investigate the effects of the female sex hormones on calcium channels, we performed patch clamp studies in A7r5 cells. The characterisation of the ICa was made by analysing the effect of BAY, a known agonist of this type of channels, which clearly stimulate the basal ICa, and nifedipine, a selective antagonist of LTCC, that significantly blocked either basal or BAY-stimulated calcium current. These data confirm that calcium current measured is due to calcium entry through LTCC (ICa,L). Concerning the βES and PRG effects in the calcium currents, our results revealed a rapid concentration-dependent inhibitory effect on basal ICa,L, which indicates that these sex hormones have the ability to block LTCC. These results agree with previously report by Zhang et al, that showed an inhibitory effect of βES on the basal ICa,L in A7r5 cells26. Nakagima et al determined that, while βES (10 μmol/L) inhibited basal ICa,L in A7r5 cells, PRG (30 μmol/L) failed to affect these current21. In agreement with our results, these authors indicated that the inhibitory effect of βES on LTCC was already significant at a concentration of 10 μmol/L. Our results showed a more powerful effect of PRG than βES at high concentrations (100 μmol/L). The inhibition of LTCC current by PRG in A7r5 cells is here reported for the first time, although Zhang et al observed in rat tail vascular smooth muscle cells that PRG reduced the LTCC25. We already studied the effect of PRG and βES on the BAY-stimulated ICa,L. We show for the first time that PRG and βES inhibit BAY-stimulated ICa,L, confirming the inhibitory properties of these hormones on rat aorta LTCC.
The action of sex steroids on the genetic protein expression is due to diffusion of these molecules across the cell membrane that, afterwards, bind to specific intracellular receptors that regulate this expression as genetic transcription factors44. Therefore, this action needs some time to produce physiological effects. On the contrary, the inhibitory effects of βES and PRG observed in this study were rapid and reversible, because the effects disappeared after drug washing. Some author already described the existence of a non-genomic mechanism for some sex steroids through which the hormones can regulate the vascular tone45, 46. Our study also demonstrates that the effects of βES and PRG are mediated by a non-genomic pathway. Furthermore, the vasodilator effects of these female hormones are not mediated by their intracellular receptors and further investigations are needed to identify the pathway involved in this effect. In these sense, recent data have suggested that the sex steroids receptors are distributed at the membrane surface, throughout the cytosol, in the mitochondria and in the nucleus of the cells. However there is controversy regarding the exact characterization of membrane estrogen receptors. Some studies suggested the activation of the membrane surface receptors, for example, the receptor GPR30, that may activate multiple effects, including adenylate cyclase, Scr and spingosine kinases47, 48, 49. Other authors suggested a direct modulation of the ionic channels by steroids1. In this sense, further studies must be done to clarify the mechanism of non-genomic action of sex hormones on vascular tissue.
In summary, our results showed for the first time an inhibition on ICa,L induced by βES and PRG in vascular smooth muscle cells, which supports and confirms the observed vasorelaxant effects of these hormones in rat aortic rings. These sex steroids inhibit basal and BAY-stimulated ICa,L. This blockage of LTCC will reduce intracellular free Ca2+ concentration and is responsible of the rat aorta relaxation. Our data also correlate with the idea of a rapid relaxant effect of βES and PRG through a mechanism independent of the endothelium and not mediated by intracellular receptors or by potassium channel activation.
The results of this study help to further understand the non-genomic vasodilator mechanism of sex hormones. Moreover, the accumulating evidences about the cardiovascular effects of sex hormones provide promising data about their role in preventing and retaining the progression of cardiovascular diseases.
Author contribution
Ezequiel ALVAREZ: designed research, performed research, analyzed data; João Miguel CARVAS: performed research; Antonio Jose SANTOS-SILVA: contributed; Ignacio VERDE: designed research, wrote the paper.
Abbreviations
BAY, (–)-Bay K8644; βES, 17β-estradiol; BKCa, large conductance Ca2+-activated K+ channels; FBS, foetal bovine serum; ICa, voltage-dependent Ca2+ current; LTCC, L-type Ca2+ channels; PRG, progesterone; VOCCs, voltage-operated Ca2+ channels.
Acknowledgments
We thank the FCT (Fundação para a Ciência e a Tecnologia) which supported the fellowships SFRH/BPD/14458/2003 and SFRH/BDE/15532/2004.
References
- Miller VM. Sex-based differences in vascular function. Womens Health (Lond Engl) 2010;6:737–52. doi: 10.2217/whe.10.53. [DOI] [PubMed] [Google Scholar]
- Masood DE, Roach EC, Beauregard KG, Khalil RA. Impact of sex hormone metabolism on the vascular effects of menopausal hormone therapy in cardiovascular disease. Curr Drug Metab. 2010;11:693–714. doi: 10.2174/138920010794233477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab. 2011;96:1642–53. doi: 10.1210/jc.2010-2888. [DOI] [PubMed] [Google Scholar]
- Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–40. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- Kim JK, Levin ER. Estrogen signaling in the cardiovascular system. Nucl Recept Signal. 2006;4:e013. doi: 10.1621/nrs.04013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–49. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–76. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- Santos RL, Marin EB, Goncalves WL, Bissoli NS, Abreu GR, Moyses MR. Sex differences in the coronary vasodilation induced by 17 beta-oestradiol in the isolated perfused heart from spontaneously hypertensive rats. Acta Physiol (Oxf) 2010;200:203–10. doi: 10.1111/j.1748-1716.2010.02140.x. [DOI] [PubMed] [Google Scholar]
- Lekontseva O, Chakrabarti S, Jiang Y, Cheung CC, Davidge ST. Role of neuronal nitric oxide synthase in estrogen-induced relaxation in rat resistance arteries. J Pharmacol Exp Ther. 2011;339:367–75. doi: 10.1124/jpet.111.183798. [DOI] [PubMed] [Google Scholar]
- Crews JK, Khalil RA. Antagonistic effects of 17-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler Thromb Vasc Biol. 1999;19:1034–40. doi: 10.1161/01.atv.19.4.1034. [DOI] [PubMed] [Google Scholar]
- Barbagallo M, Dominguez LJ, Licata G, Shan J, Bing L, Karpinski E, et al. Vascular effects of progesterone : role of cellular calcium regulation. Hypertension. 2001;37:142–7. doi: 10.1161/01.hyp.37.1.142. [DOI] [PubMed] [Google Scholar]
- Unemoto T, Honda H, Kogo H. Differences in the mechanisms for relaxation of aorta induced by 17beta-estradiol or progesterone between normotensive and hypertensive rats. Eur J Pharmacol. 2003;472:119–26. doi: 10.1016/s0014-2999(03)01858-2. [DOI] [PubMed] [Google Scholar]
- Castillo C, Ceballos G, Rodriguez D, Villanueva C, Medina R, Lopez J, et al. Effects of estradiol on phenylephrine contractility associated with intracellular calcium release in rat aorta. Am J Physiol Cell Physiol. 2006;291:C1388–94. doi: 10.1152/ajpcell.00556.2005. [DOI] [PubMed] [Google Scholar]
- Barton M, Cremer J, Mugge A. 17Beta-estradiol acutely improves endothelium-dependent relaxation to bradykinin in isolated human coronary arteries. Eur J Pharmacol. 1998;362:73–6. doi: 10.1016/s0014-2999(98)00787-0. [DOI] [PubMed] [Google Scholar]
- Salom JB, Burguete MC, Perez-Asensio FJ, Torregrosa G, Alborch E. Relaxant effects of 17-beta-estradiol in cerebral arteries through Ca2+ entry inhibition. J Cereb Blood Flow Metab. 2001;21:422–9. doi: 10.1097/00004647-200104000-00011. [DOI] [PubMed] [Google Scholar]
- Belfort MA, Saade GR, Suresh M, Vedernikov YP. Effects of estradiol-17 beta and progesterone on isolated human omental artery from premenopausal nonpregnant women and from normotensive and preeclamptic pregnant women. Am J Obstet Gynecol. 1996;174:246–53. doi: 10.1016/s0002-9378(96)70402-7. [DOI] [PubMed] [Google Scholar]
- Kocic I, Szczepanska R, Wapniarska I. Estrogen-induced relaxation of the rat tail artery is attenuated in rats with pulmonary hypertension. Pharmacol Rep. 2010;62:95–9. doi: 10.1016/s1734-1140(10)70246-2. [DOI] [PubMed] [Google Scholar]
- Keung W, Man RY. Circulating sex hormones modulate vascular contractions and acute response to 17beta-estradiol in rat mesenteric arteries. Pharmacology. 2011;88:55–64. doi: 10.1159/000329426. [DOI] [PubMed] [Google Scholar]
- Keung W, Chan ML, Ho EY, Vanhoutte PM, Man RY. Non-genomic activation of adenylyl cyclase and protein kinase G by 17beta-estradiol in vascular smooth muscle of the rat superior mesenteric artery. Pharmacol Res. 2011;64:509–16. doi: 10.1016/j.phrs.2011.05.010. [DOI] [PubMed] [Google Scholar]
- McFadzean I, Gibson A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br J Pharmacol. 2002;135:1–13. doi: 10.1038/sj.bjp.0704468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T, Kitazawa T, Hamada E, Hazama H, Omata M, Kurachi Y. 17beta-Estradiol inhibits the voltage-dependent L-type Ca2+ currents in aortic smooth muscle cells. Eur J Pharmacol. 1995;294:625–35. doi: 10.1016/0014-2999(95)00602-8. [DOI] [PubMed] [Google Scholar]
- Okabe K, Inoue Y, Soeda H. Estradiol inhibits Ca2+ and K+ channels in smooth muscle cells from pregnant rat myometrium. Eur J Pharmacol. 1999;376:101–8. doi: 10.1016/s0014-2999(99)00353-2. [DOI] [PubMed] [Google Scholar]
- Hill BJ, Gebre S, Schlicker B, Jordan R, Necessary S. Nongenomic inhibition of coronary constriction by 17ss-estradiol, 2-hydroxyestradiol, and 2-methoxyestradiol. Can J Physiol Pharmacol. 2010;88:147–52. doi: 10.1139/y09-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HF, Zheng TZ, Li W, Qu SY, Zhang CL. Effect of progesterone on the contractile response of isolated pulmonary artery in rabbits. Can J Physiol Pharmacol. 2001;79:545–50. [PubMed] [Google Scholar]
- Zhang M, Benishin CG, Pang PK. Rapid inhibition of the contraction of rat tail artery by progesterone is mediated by inhibition of calcium currents. J Pharm Pharmacol. 2002;54:1667–74. doi: 10.1211/002235702405. [DOI] [PubMed] [Google Scholar]
- Zhang F, Ram JL, Standley PR, Sowers JR. 17beta-Estradiol attenuates voltage-dependent Ca2+ currents in A7r5 vascular smooth muscle cell line. Am J Physiol. 1994;266:C975–80. doi: 10.1152/ajpcell.1994.266.4.C975. [DOI] [PubMed] [Google Scholar]
- Ruehlmann DO, Steinert JR, Valverde MA, Jacob R, Mann GE. Environmental estrogenic pollutants induce acute vascular relaxation by inhibiting L-type Ca2+ channels in smooth muscle cells. FASEB J. 1998;12:613–9. doi: 10.1096/fasebj.12.7.613. [DOI] [PubMed] [Google Scholar]
- De Wet H, Allen M, Holmes C, Stobbart M, Lippiat JD, Callaghan R. Modulation of the BK channel by estrogens: examination at single channel level. Mol Membr Biol. 2006;23:420–9. doi: 10.1080/09687860600802803. [DOI] [PubMed] [Google Scholar]
- Tsang SY, Yao X, Chan HY, Wong CM, Chen ZY, Au CL, et al. Contribution of K+ channels to relaxation induced by 17beta-estradiol but not by progesterone in isolated rat mesenteric artery rings. J Cardiovasc Pharmacol. 2003;41:4–13. doi: 10.1097/00005344-200301000-00002. [DOI] [PubMed] [Google Scholar]
- White RE, Han G, Maunz M, Dimitropoulou C, El-Mowafy AM, Barlow RS, et al. Endothelium-independent effect of estrogen on Ca2+-activated K+ channels in human coronary artery smooth muscle cells. Cardiovasc Res. 2002;53:650–61. doi: 10.1016/s0008-6363(01)00428-x. [DOI] [PubMed] [Google Scholar]
- Han G, Yu X, Lu L, Li S, Ma H, Zhu S, et al. Estrogen receptor alpha mediates acute potassium channel stimulation in human coronary artery smooth muscle cells. J Pharmacol Exp Ther. 2006;316:1025–30. doi: 10.1124/jpet.105.093542. [DOI] [PubMed] [Google Scholar]
- Khalil RA. Sex hormones as potential modulators of vascular function in hypertension. Hypertension. 2005;46:249–54. doi: 10.1161/01.HYP.0000172945.06681.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X, McConnell KR, Khalil RA. Sex steroids and vascular responses in hypertension and aging. Gend Med. 2008;5 Suppl A:S46–S64. doi: 10.1016/j.genm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Korovkina VP, Brainard AM, Ismail P, Schmidt TJ, England SK. Estradiol binding to maxi-K channels induces their down-regulation via proteasomal degradation. J Biol Chem. 2004;279:1217–23. doi: 10.1074/jbc.M309158200. [DOI] [PubMed] [Google Scholar]
- Perusquia M, Hernandez R, Morales MA, Campos MG, Villalon CM. Role of endothelium in the vasodilating effect of progestins and androgens on the rat thoracic aorta. Gen Pharmacol. 1996;27:181–5. doi: 10.1016/0306-3623(95)00091-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Garcia de Boto MJ, Hidalgo A. Mechanisms involved in the relaxant effect of estrogens on rat aorta strips. Life Sci. 1996;58:607–15. doi: 10.1016/0024-3205(95)02330-5. [DOI] [PubMed] [Google Scholar]
- Alvarez E, Cairrao E, Morgado M, Morais C, Verde I.Testosterone and cholesterol vasodilation of rat aorta involves L-type calcium channel inhibition Adv Pharmacol Sci 2010. doi: 10.1155/2010/534184 [DOI] [PMC free article] [PubMed]
- Glusa E, Graser T, Wagner S, Oettel M. Mechanisms of relaxation of rat aorta in response to progesterone and synthetic progestins. Maturitas. 1997;28:181–91. doi: 10.1016/s0378-5122(97)00057-1. [DOI] [PubMed] [Google Scholar]
- Salom JB, Burguete MC, Perez-Asensio FJ, Centeno JM, Torregrosa G, Alborch E. Acute relaxant effects of 17-beta-estradiol through non-genomic mechanisms in rabbit carotid artery. Steroids. 2002;67:339–46. doi: 10.1016/s0039-128x(01)00185-4. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol. 1998;275:H292–300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- Omar HA, Ramirez R, Gibson M. Properties of a progesterone-induced relaxation in human placental arteries and veins. J Clin Endocrinol Metab. 1995;80:370–3. doi: 10.1210/jcem.80.2.7852492. [DOI] [PubMed] [Google Scholar]
- Iliescu R, Campos LA, Schlegel WP, Morano I, Baltatu O, Bader M. Androgen receptor independent cardiovascular action of the antiandrogen flutamide. J Mol Med. 2003;81:420–7. doi: 10.1007/s00109-003-0449-4. [DOI] [PubMed] [Google Scholar]
- Ba ZF, Wang P, Kuebler JF, Rue LW., 3rd, , Bland KI, Chaudry IH. Flutamide induces relaxation in large and small blood vessels. Arch Surg. 2002;137:1180–6. doi: 10.1001/archsurg.137.10.1180. [DOI] [PubMed] [Google Scholar]
- Truss M, Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr Rev. 1993;14:459–79. doi: 10.1210/edrv-14-4-459. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Genazzani AR. Non-genomic actions of sex steroid hormones. Eur J Endocrinol. 2003;148:281–92. doi: 10.1530/eje.0.1480281. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Mannella P, Fornari L, Caruso A, Varone G, Genazzani AR. In vitro effects of progesterone and progestins on vascular cells. Steroids. 2003;68:831–6. doi: 10.1016/j.steroids.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–90. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, et al. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–45. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–91. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]