Abstract
Aim:
To investigate the stereoselective binding of mexiletine or ketoprofen enantiomers with different recombinant domains of human serum albumin (HSA).
Methods:
Three domains (HSA DOM I, II and III) were expressed in Pichia pastoris GS115 cells. Blue Sepharose 6 Fast Flow was employed to purify the recombinant HSA domains. The binding properties of the standard ligands, digitoxin, phenylbutazone and diazepam, and the chiral drugs to HSA domains were investigated using ultrafiltration. The concentrations of the standard ligands, ketoprofen and mexiletine were analyzed with HPLC.
Results:
The recombinant HSA domains were highly purified as shown by SDS-PAGE and Western blotting analyses. The standard HSA ligands digitoxin, phenylbutazone and diazepam selectively binds to DOM I, DOM II and DOM III, respectively. For the chiral drugs, R-ketoprofen showed a higher binding affinity toward DOM III than S-ketoprofen, whereas S-mexiletine bound to DOM II with a greater affinity than R-mexiletine.
Conclusion:
The results demonstrate that HSA DOM III possesses the chiral recognition ability for the ketoprofen enantiomers, whereas HSA DOM II possesses that for the mexiletine enantiomers.
Keywords: mexiletine, ketoprofen, human serum albumin, protein binding, stereoselectivity, ultrafiltration technique, Pichia pastoris GS115 cells
Introduction
Human serum albumin (HSA) is the most abundant protein in human blood plasma, accounting for about half of the blood serum protein. After systemic absorption, most drugs undergo some degree of reversible binding to HSA1. The two enantiomers of a chiral drug may bind to HSA with different affinities, resulting in different free fractions. Over 50% of the drugs in current clinical use are chiral, and the majority of synthetically derived chiral drugs are administered as mixtures of the constituent stereoisomers (most commonly the racemate)2. In addition to stereoselective metabolism, stereoselective protein binding might also be responsible for the differences in pharmacokinetics between enantiomers3,4,5. Studies on the mechanism of stereoselective binding to HSA may better explain the different pharmacokinetics between enantiomers.
HSA has a limited number of high-affinity binding sites for drugs6. Fragments of HSA produced by chemical or enzymatic cleavage have been used to define the exact high-affinity binding sites for several ligands7,8,9,10. However, this method is limited by the finite number of cleavage sites in HSA, and chemical cleavage may destroy the structure of the binding site. Because HSA is composed of three quasi-independent domains, DOM I, DOM II, and DOM III1, a new method based on the cloning and expression of these three independent domains was first introduced by Dockal M11. Several studies have successfully employed recombinant HSA domains to identify the specific binding sites of several drugs, including warfarin, ochratoxin A, propofol and halothane12,13,14,15. The use of recombinant HSA fragments in a binding study with warfarin enantiomers demonstrated that the recombinant domains may also be a useful tool to reveal the stereoselective binding properties of chiral drugs16. In this study, we constructed three recombinant HSA domains to investigate the stereoselective binding properties of ketoprofen and mexiletine.
Ketoprofen and mexiletine are both chiral drugs and are currently used as racemates. The stereoselective binding of ketoprofen enantiomers to human serum albumin was discovered in 198017. In 1990, Verbeeck et al determined that ketoprofen bound extensively to HSA (above 99%)18, and that this binding may be related to the enantioselective disposition of ketoprofen in vivo. In the past several decades, studies on the stereoselective binding of ketoprofen to HSA have reached a consensus that ketoprofen mainly binds to site II of HSA and does so in a stereoselective manner19,20,21. However, contradictory stereoselective binding results have been obtained under different experimental conditions17,18,22,23,24. Studies using the method based on recombinant HSA domains may complement other binding studies to better understand the stereoselective binding properties of ketoprofen to HSA. Mexiletine is 70% bound to serum protein25, and the stereoselective disposition of mexiletine in man was first studied in 198626. In vitro studies using serum protein from healthy subjects indicated that mexiletine bound to serum protein in a stereoselective manner27. Because serum protein is made up of HSA and other proteins such as α-acid glycoprotein, the mechanism of stereoselective binding between mexiletine and HSA needs to be further studied. However, little progress has been made in identifying either binding sites in HSA or the chiral binding mechanism. In this study, the stereoselective properties of the binding between chiral drugs (mexiletine and ketoprofen) and HSA were investigated using purified recombinant HSA domains.
Materials and methods
Cloning
This protocol was a modification of previously published methods11,12. In brief, the gene segments coding for the HSA domains (the domains contained the following amino acids: HSA DOM I, 1–197; HSA DOM II, 189–385; and HSA DOM III, 381–585) were amplified by polymerase chain reaction (PCR) using the pBS-HSA plasmid as the template. The forward and reverse primers (Table 1) were designed to incorporate EcoR I and Not I sites, respectively.
Table 1. Sequence of primers.
| Primers | Sequence |
|---|---|
| Domain I | |
| Forward primer | ggcggaattcgatgcacacaagag |
| Reverse primer | atttgcggccgctctctgtttggc |
| Domain II | |
| Forward primer | agcagaattcgggaaggcttcgtct |
| Reverse primer | ataatgcggccgcctgaggctcttc |
| Domain III | |
| Forward primer | agacgaattcgtggaagagcctcag |
| Reverse primer | tatagcggccgcttataagcctaa |
The PCR products were digested overnight and then ligated into the pPIC9 vector (Invitrogen), resulting in the recombinant vectors pPIC9-HSA DOM I, pPIC9-HSA DOM II, and pPIC9-HSA DOM III. The recombinant vectors were transformed into E coli DH5α for amplification and subsequent DNA sequence analysis. The identified recombinant plasmids were linearized with Sal I and transformed into competent Pichia pastorisGS115 cells (Invitrogen). The transformants were screened for viability in the absence of glucose and histidine. The positive recombinants were confirmed by PCR and DNA sequencing.
Expression and purification
The recombinants were grown on YPD medium and then transferred to BMGY medium for induction with methanol. Methanol with a final concentration of 1% was added every 24 h to maintain induction. All incubations were performed at 28 °C on an orbital shaker at 250 r/min. Supernatant samples were collected every 12 h for SDS-PAGE and Western blot analysis.
The protein was purified using the modified procedure described by Matsushita S14. All steps of the purification procedure were performed at 4 °C. The supernatants were harvested at 72 h after induction, followed by filtration through a 0.45 μm filter. Purification was performed by precipitation with 85% (NH4)2SO4. The resulting samples were passed through a preequilibrated Blue Sepharose column (Amersham). After washing with 50 volumes of buffer 1 (50 mmol/L KH2PO4, pH 7.0), elution was performed with buffer 2 (50 mmol/L KH2PO4, 1.5 mol/L KCl, pH 7.0). The isolated protein samples were extensively dialyzed against Sorensen's phosphate buffer. The protein concentrations were measured by the Bradford method.
Western blot analysis
The purified protein samples were eluted by adding an equal volume of loading buffer and then running them on 12% SDS-PAGE gels. The separated proteins were then transferred to PVDF membranes. The membranes were incubated in the presence of goat anti-human serum albumin polyclonal antibody (Beckman). Exposure to the primary antibody was followed by incubation with a horseradish peroxidase (HRP)-conjugated secondary anti-goat IgG antibody (Sanying Biotechnology). The blots were developed using an enhanced chemiluminescence detection system (ECL) (Amersham) according to the manufacturer's instructions.
Ultrafiltration
The binding properties of the three recombinant domains with the standard ligands ketoprofen and mexiletine were investigated by ultrafiltration using a Microcon centrifugation system (America, Millipore) that utilized a filter membrane with a 30-kDa cutoff at 37 °C. Phenylbutazone, diazepam and digitoxin, each of which binds to a specific site in HSA, were chosen as the standard ligands. Aliquots of 500 μL of each HSA domain with standard ligands were centrifuged at 7000xg for 5 min. For ketoprofen and mexiletine, the centrifugations were performed at 7500xg for 10 min and 10 000xg for 15 min, respectively. The ultrafiltrate (150 μL) was collected and prepared as described above.
Nonspecific filter membrane binding was evaluated in protein-free phosphate-buffered saline (PBS). The mixture was transferred to the ultrafilter without incubation, followed by ultrafiltration at 2000xg for 5 min at 37 °C. The samples in PBS buffer with or without ultrafiltration were directly injected into an HPLC system.
The percentage adsorbed by the ultrafilter is calculated using the following formula:
P%=1–Aultrafiltrate/APBS
Aultrafiltrate, drug peak area in the ultrafiltrate; APBS, drug peak area in PBS buffer.
Sample preparation
The ultrafiltrates for the standard ligands (phenylbutazone, diazepam and digitoxin) were directly injected into the HPLC system. For ketoprofen, R-flurbiprofen was used as the internal standard. Ketoprofen was activated with 1% triethylamine and 2% thionyl chloride (both in methylene chloride) and then reacted with S-(–)-1-(1-naphthyl) ethylamine (S-NEA) (Sigma) to generate diastereoisomeric amides28. For mexiletine, R-esmolol was used as the internal standard, and the chiral derivatization was performed at 35 °C for 10 min with 2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl isothiocyanate (GITC) (Sigma)29.
HPLC analysis
The concentrations of the standard ligands, ketoprofen and mexiletine, were analyzed by HPLC. HPLC was performed on an Agilent 1100 system consisting of a G1311A pump, a G1315A (DAD) UV detector, a manual injector and ChemStation software. An Agilent Zorbax C18 (250 mm×4.6 mm, 5 μm) column was used. An aliquot of 20 μL of each sample was injected and analyzed at room temperature (Table 2).
Table 2. The estabolished HPLC methods.
| Drug | Mobile phase (v/v) | Flow rate (mL/min) | Detection (nm) |
|---|---|---|---|
| Phenylbutazone | Water-methanol (25:75) | 0.7 | 238 |
| Diazepam | Water-methanol (25:75) | 0.7 | 242 |
| Digitoxin | Water-acetonitrile (55:45) | 1 | 220 |
| Ketoprofen | Phosphate buffer (0.01 mol/L, pH 4.5)-acetonitrile (40:60) | 0.8 | 250 |
| Mexiletine | Phosphate buffer (0.02 mol/L, pH 5.5)-acetonitrile (75:25) | 0.9 | 214 |
Results
Cloning expression and purification
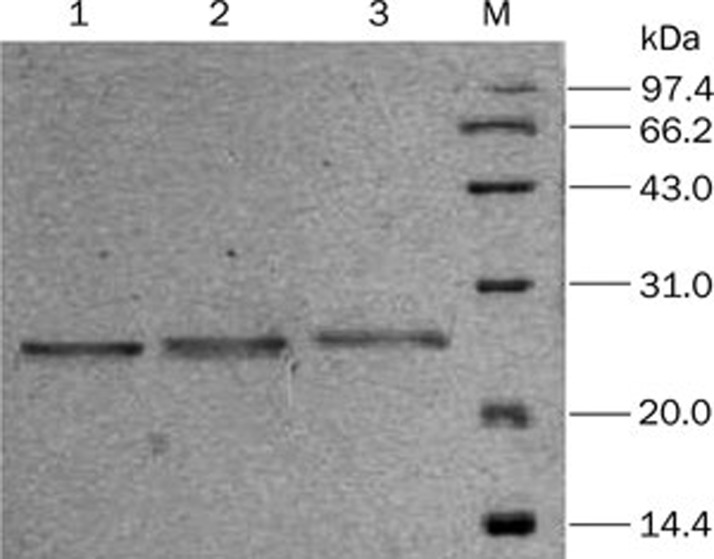
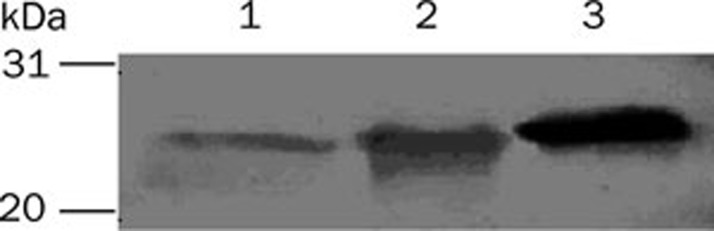
After induction with methanol, the supernatants of the pPIC9-HSA DOM I, pPIC9-HSA DOM II, and pPIC9-HSA DOM III transformants were analyzed by SDS-PAGE and Western blot. A single band was present at approximately 23 kDa, as shown in Figure 1, in accordance with the previously reported molecular masses of 22 860, 22 519, and 23 383 Da for of HSA DOM I, HSA DOM II, and HSA DOM III, respectively11. The recombinant domains were also identified by Western blot with an anti-human HSA antibody, as shown in Figure 2. The results confirmed that the recombinant HSA domains were successfully expressed and secreted into the supernatant.
Figure 1.
Analysis of the expression products by SDS-PAGE. (1) HSA DOM III; (2) HSA DOM II; (3) HSA DOM I. The positions of the molecular weight standards are indicated in the right-most lane.
Figure 2.
Analysis of the expression products by Western blot. (1) HSA DOM I; (2) HSA DOM II; (3) HSA DOM III. The positions of the molecular weight standards are indicated in the left-most lane.
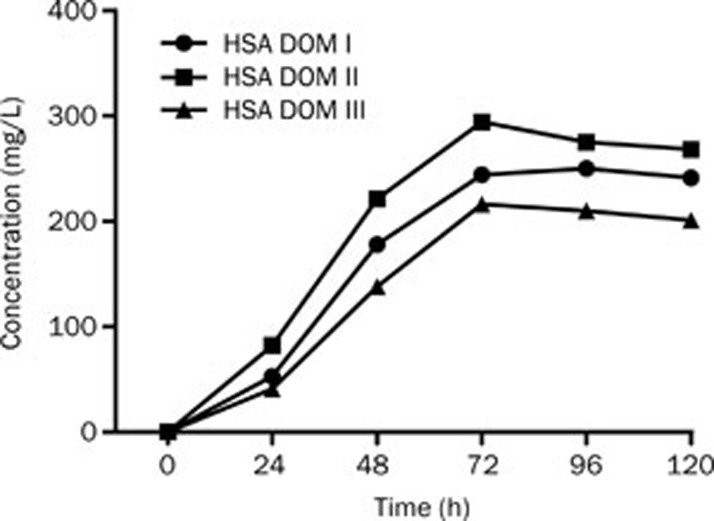
To prevent the secreted foreign proteins from being degraded by the KEX-2 proteases present on the membrane of Pichia pastoris, tryptone was added to the culture, providing excess substrate for these proteases. Because higher concentrations of methanol might inhibit the expression of the target proteins, the final concentration of methanol was less than 1%. As shown in Figure 3, the expression levels of the recombinant domains peaked at 72–96 h at a concentration of 210–275 mg/L. Consequently, the supernatants were harvested at 72 h for purification.
Figure 3.
The protein expression in recombinant P pastoris at different time points.
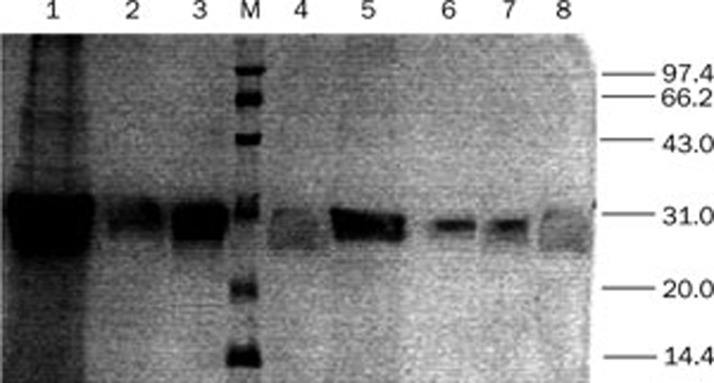
Although negligible levels of nonspecific proteins were detected by SDS-PSGE and Western blot, there were large amounts of mineral salts and metabolites in the supernatants. Impurities such as pigments, amino acids and carbohydrates may strongly inhibit the binding of the ligands with the recombinant protein fragments, resulting in a decreased protein binding rate. Blue Sepharose affinity chromatography was employed as the central step in the purification procedure. The Blue Sepharose column exhibited highly specific binding with the recombinant domains, as demonstrated by the fact that the purity of the domain preparation was greater than 98% in a previous study11. The purification efficiencies with or without precipitation were also compared. The recovery without precipitation is lower than that with precipitation (data not shown). These results indicated that the purification should be performed with precipitation. As shown in Figure 4, the secreted protein segments were highly purified and concentrated after purification.
Figure 4.
Analysis of the expression, precipitation and purification products by SDS-PAGE. (1) Precipitation of HSA DOM II; (2) Expression products of HSA DOM III; (3) Purification products of HSA DOM II; (4) Expression products of HSA DOM I; (5) Precipitation of HSA DOM I; (6) Purification products of HSA DOM I; (7) Purification products of HSA DOM III; (8) Expression products of HSA DOM III.
Confirmation of nonspecific filter binding
As shown in Table 3, the average nonspecific adsorption percentages under different concentrations were 3.53% for ketoprofen and 2.63% for mexiletine. These results indicated that the ultrafiltration system was suitable for studying the binding of ketoprofen and mexiletine to HSA.
Table 3. The nonspecific adsorption of ketoprofen and mexiletine with ultrafilter (n=3).
| Drug | Spiked amount (μg/mL) | P (%) | P (%) |
|---|---|---|---|
| Ketoprofen | 1.0 | 3.77 | 3.53±0.50 |
| 5.0 | 3.86 | ||
| 50.0 | 2.95 | ||
| Mexiletine | 0.5 | 2.25 | 2.63±1.57 |
| 5.0 | 1.28 | ||
| 50.0 | 4.36 |
Binding with standard ligands
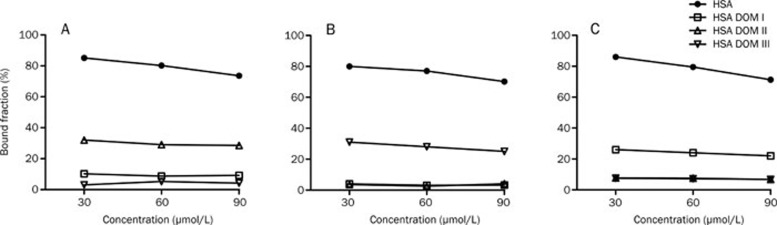
It was widely accepted that the three recombinant domains possessed the three principal binding sites of HSA: the warfarin site (site I) in DOM II, the diazepam site (site II) in DOM III and the digitoxin site (site III) in DOM I6. In the present study, the binding properties of HSA and the three HSA domains with phenylbutazone, diazepam and digitoxin, each of which represents a standard ligand for HSA, were investigated. As shown in Figure 5, phenylbutazone bound to DOM II with greater affinity than to DOM I and showed no affinity to DOM III. Diazepam bound to DOM III with high selectivity. Digitoxin mainly bound to DOM I but also slightly bound to DOM II and DOM III. The results indicated that the primary binding sites of digitoxin, phenylbutazone and diazepam were on DOM I, DOM II, and DOM III, respectively. However, there may be low-affinity sites on DOM I for phenylbutazone and on DOM II and DOM III for digitoxin.
Figure 5.
The protein binding of phenylbutazone (A), diazepam (B) and digitoxin (C) in 60 μmol/L HSA, HSA DOM I, HSA DOM II, and HSA DOM III.
The binding abilities of the HSA domains were lower than those of rHSA, in agreement with the results of a previous report14 in which the site II marker DNSS bound with greater affinity to rHSA (62.4%±5.4%) than to domain III (38.9%±7.8%). The importance of the integrated three-dimensional structure may account for this phenomenon, as the interdomain interactions may maintain the stability of the ligand binding sites.
Binding with the enantiomers of chiral drugs
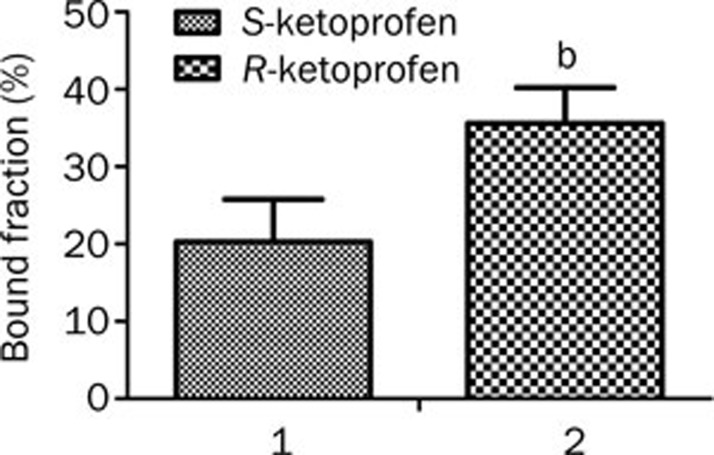
Contradictory results for the binding of ketoprofen enantiomers to HSA have been obtained in several studies17,19,24,30. Zou et al 24 reported that the S-enantiomers bind to HSA more strongly than the R-enantiomers do. According to Dubois et al19, R-ketoprofen bound more strongly than S-ketoprofen, whereas Guo et al 31 found that ketoprofen had little stereoselectivity with respect to binding to HSA. The binding of ketoprofen to DOM III showed remarkable stereoselectivity at the concentration of albumin found in plasma (5.2 μmol/mL), as R-ketoprofen exhibited a significantly higher binding affinity than S-ketoprofen (P=0.0209). The bound fractions for R-ketoprofen and S-ketoprofen were 35.5%±4.6% (n=3) and 20.5%±5.5% (n=3), respectively (Figure 6).
Figure 6.
The binding of S-ketoprofen (1) and R-ketoprofen (2) (1.25 μmol/mL) to HSA DOM III (5.2 μmol/mL) (n=3).
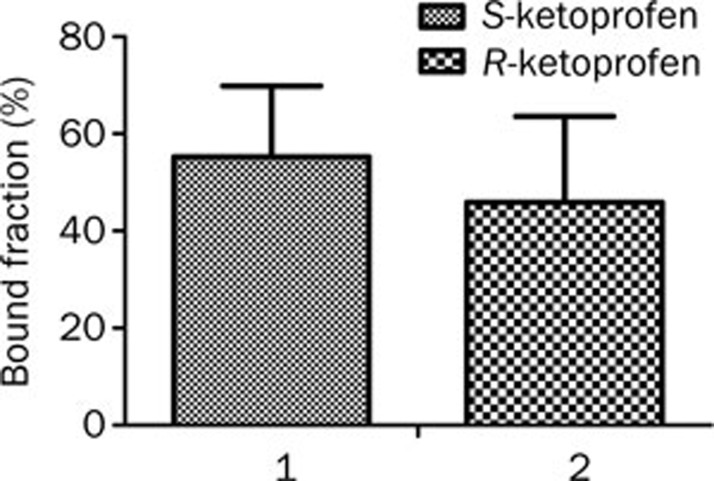
For mexiletine, the primary binding site on HSA was site I in DOM II. Enantioselectivity was also observed for mexiletine enantiomers but was the opposite of that for ketoprofen. The binding of mexiletine enantiomers to HSA was significantly stereoselective, with the bound fractions of S-mexiletine and R-mexiletine being 44.35%±1.9% (n=3) and 32.9%±2.1% (n=3), respectively (P=0.0022)31. The stereoselective trend was the same with the HSA domains. As shown in Figure 7, S-mexiletine [bound fraction of 55.3%±14.6% (n=3)] bound to DOM II with a slightly greater affinity than R-mexiletine [bound fraction of 45.9%±17.7% (n=3)]. However, the stereoselectivity of the binding of mexiletine was not statistically significant, with a P value of 0.3404.
Figure 7.
The binding of S-mexiletine (1) and R-mexiletine (2) (1.15 μmol/mL) to HSA DOM II (4.9 μmol/mL) (n=3).
Discussion
HSA binding with ketoprofen enantiomers
Ketoprofen, a chiral non-steroidal anti-inflammatory drug (NSAID) of the 2-aryl propionate family, is currently marketed and used as a racemate. S-ketoprofen possesses most of the beneficial pharmacological activity32, whereas the R-enantiomer is considered to be an impurity or a pro-drug: approximately 10% of the R-enantiomer undergoes chiral inversion upon oral administration33. Ketoprofen bound extensively to HSA (above 99%)18 in a stereoselective manner34, which may be related to the enantioselective disposition of ketoprofen in vivo35,36. Ketoprofen was reported to bind to HSA at site I and site II; the main binding site was site II (the high affinity binding site) in domain III19,20,21. In this study, the bound fraction for ketoprofen racemate was in accordance with the results of the study by Matsushita14 (bound fraction of 64.0%±5.4% for the ketoprofen racemate). These results indicated that the recombinant HSA domains produced in our study were highly purified and exhibited great activity.
The stereoselective HSA binding of ketoprofen has been identified by several studies17,22,23,24,30. However, contradictory results have been obtained under different experimental conditions. Dubois et al19 found that the enantioselective binding of ketoprofen enantiomers to HSA depended on drug and protein concentrations. Enantioselectivity was observed in HSA at 1 g/L, but the opposite enantioselectivity was observed at 40 g/L. At the concentration of HSA in plasma (40 g/L), R-ketoprofen bound more strongly than the S-isomer, and the k values of site II for S-ketoprofen were less than half of those for R-ketoprofen. Similar findings were obtained in our study, where the higher binding ability of R-ketoprofen to DOM III was detected at a similar physiological concentration. The method based on recombinant HSA domains may give a direct and thorough view of the stereoselective binding of chiral drugs.
HSA binding with mexiletine enantiomers
Mexiletine, an orally effective class 1 antiarrhythmic agent with a chiral center, is also used therapeutically as a racemate. The stereoselective disposition of mexiletine in humans was first revealed by Grech-Belanger et al26, who noted that the area under concentration-time curve (AUC) of S-mexiletine was always significantly higher (P<0.01) and that the rate of renal clearance was significantly lower (P<0.05)3 than that of the other enantiomer. It was reported that mexiletine was 70% bound to serum protein in healthy subjects25, and therefore, the differences observed between the pharmacokinetics of the enantiomers may be due largely to differences in their serum protein binding affinities.
As the stereoselective binding site and the mechanism of the binding of mexiletine enantiomers to HSA remain unknown, recombinant HSA domains were used in this study. This study represents the first attempt to identify the primary binding site of mexiletine to HSA, and it was determined that this binding site may be site I in DOM II. The bound fraction ratio of S-enantiomer to R-enantiomer on DOM II was 1.2, a value that was in accordance with stereoselective binding to recombinant HSA (ratio is 1.34). The nonsignificant difference between S-mexiletine and R-mexiletine with respect to binding to DOM II may due to the large errors. Thus, more data should be gathered in future studies to confirm the stereoselectivity.
An in vitro binding study of mexiletine enantiomers further revealed that the serum binding of mexiletine could be accounted for primarily by binding to HSA and/or AGP25. The binding of mexiletine enantiomers to AGP was examined in our previous study31, and the binding to AGP was also found to be stereoselective, with a bound fraction of 37.3%–24.1% for the R-enantiomer and a bound fraction of 31.1%–21.0% for the S-enantiomer. Although these results demonstrated opposite stereoselectivities for the binding to AGP and the binding to HSA, the stereoselective binding of the S-enantiomer to HSA may predominate in the plasma. Because the S-enantiomer possessed a slightly higher binding affinity for HSA DOM II, it had higher values for pharmacokinetic parameters, including the AUC26, the terminal elimination half-life3, and smaller values for parameters including renal clearance26 and steady-state volume of distribution3 than the R-enantiomer. These results confirmed the relationship between the HSA DOM binding of the mexiletine enantiomers and their pharmacokinetic properties.
The application of recombinant HSA domains
Recombinant HSA domains, first introduced in 199911, have been used in several studies as powerful tools for ligand binding studies. To pinpoint the essential structural elements for the formation of the warfarin binding site on HSA, Dockal et al further constructed a defined set of five recombinant proteins12. Matsushita et al14 analyzed the function of three recombinant HSA domains and considered DOM I to be a potential protein carrier for drug delivery. The same recombinant HSA domains were employed by Il'ichev et al13 to gain insight into the localization of binding sites and the nature of binding interactions between ochratoxin A and HSA. Liu et al15 utilized recombinant HSA domains to identify the main binding sites of two general anesthetics, propofol and halothane. Further analyses of the architecture of binding sites characterized the general anesthetic structure-activity relationship.
All of the above studies suggest that the recombinant HSA domains might be a suitable platform for the characterization of ligand binding. However, there has only been a single study applying the recombinant HSA domains to stereoselective binding research. Twine et al16 constructed two domain fragments of HSA corresponding to domains 1 and 2 (D12) and domains 2 and 3 (D23) and used these HSA fragments to study the binding of warfarin enantiomers to HSA. The results demonstrated that the fragments of HSA retained the ability to discriminate between pairs of warfarin enantiomers. In the present study, we performed stereoselective binding research using ketoprofen and mexiletine enantiomers and three recombinant HSA domains. This study complements other binding studies by revealing the binding properties of ketoprofen enantiomers to HSA. As the stereoselective binding of mexiletine to serum protein has not been well investigated, the nature of binding interactions between mexiletine enantiomers and HSA is further characterized in this study. The mexiletine binding sites are primarily found in DOM II, with an increased preference for the S-enantiomer.
In summary, we produced three highly purified recombinant HSA domains (HSA DOM I, HSA DOM II, and HSA DOM III), each of which had a specific ligand binding site. The recombinant domains were then employed to investigate the different chiral binding properties of the ketoprofen and mexiletine enantiomers. The results demonstrate that the method based on the recombinant HSA domains may have great potential to increase the understanding of the stereoselective binding properties of chiral drugs.
Author contribution
Su ZENG designed the study. Da SHI, Yin-xiu JIN, and Yi-hong TANG performed the experiments. Hai-hong HU and Si-yun XU contributed analytic tools. Lu-shan YU and Hui-di JIANG analyzed the data. Da SHI, Yin-xiu JIN, Yi-hong TANG, and Su ZENG wrote the paper.
Acknowledgments
This project was supported by the State Key Development Program for Basic Research of China (Grant No 2011CB710800) and National Major Projects for Science and Technology Development of the Ministry of Science and Technology of China (No 2012ZX09506001-004).
References
- He XM, Carter DC. Atomic structure and chemistry of human serum albumin. Nature. 1992;358:209–15. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- Caner H, Groner E, Levy L, Agranat I. Trends in the development of chiral drugs. Drug Discov Today. 2004;9:105–10. doi: 10.1016/s1359-6446(03)02904-0. [DOI] [PubMed] [Google Scholar]
- Igwemezie L, Kerr C, McErlane K. The pharmacokinetics of the enantiomers of mexiletine in humans. Xenobiotica. 1989;19:677–82. doi: 10.3109/00498258909042305. [DOI] [PubMed] [Google Scholar]
- Hong Y, Tang Y, Zeng S. Enantioselective plasma protein binding of propafenone: mechanism, drug interaction, and species difference. Chirality. 2009;21:692–8. doi: 10.1002/chir.20666. [DOI] [PubMed] [Google Scholar]
- Sun DL, Huang SD, Wu PS, Li J, Ye YJ, Jiang HD. Stereoselective protein binding of tetrahydropalmatine enantiomers in human plasma, HSA, and AGP, but not in rat plasma. Chirality. 2010;22:618–23. doi: 10.1002/chir.20808. [DOI] [PubMed] [Google Scholar]
- Chuang VTG, Otagiri M. Stereoselective binding of human serum albumin. Chirality. 2006;18:159–66. doi: 10.1002/chir.20237. [DOI] [PubMed] [Google Scholar]
- Reed RG, Feldhoff RC, Clute O, Peters Jr T. Fragments of bovine serum albumin produced by limited proteolysis. Conformation and ligand binding. Biochemistry. 1975;14:4578–83. doi: 10.1021/bi00692a004. [DOI] [PubMed] [Google Scholar]
- Bos OJM, Fischer MJE, Wilting J, Janssen LHM. Drug-binding and other physicochemical properties of a large tryptic and a large peptic fragment of human serum albumin. BBA-Protein Struct M. 1988;953:37–47. doi: 10.1016/0167-4838(88)90007-6. [DOI] [PubMed] [Google Scholar]
- Bos OJM, Remijn JPM, Fischer MJE, Wilting J, Janssen LHM. Location and characterization of the warfarin binding site of human serum albumin: A comparative study of two large fragments. Biochem Pharmacol. 1988;37:3905–9. doi: 10.1016/0006-2952(88)90072-x. [DOI] [PubMed] [Google Scholar]
- Bos O, Labro J, Fischer M, Wilting J, Janssen L. The molecular mechanism of the neutral-to-base transition of human serum albumin. Acid/base titration and proton nuclear magnetic resonance studies on a large peptic and a large tryptic fragment of albumin. J Biol Chem. 1989;264:953. [PubMed] [Google Scholar]
- Dockal M, Carter DC, Ruker F. The three recombinant domains of human serum albumin. Structural characterization and ligand binding properties. J Biol Chem. 1999;274:29303–10. doi: 10.1074/jbc.274.41.29303. [DOI] [PubMed] [Google Scholar]
- Dockal M, Chang M, Carter DC, Rüker F. Five recombinant fragments of human serum albumin — tools for the characterization of the warfarin binding site. Protein Sci. 2000;9:1455–65. doi: 10.1110/ps.9.8.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Il'ichev YV, Perry JL, Rüker F, Dockal M, Simon JD. Interaction of ochratoxin A with human serum albumin. Binding sites localized by competitive interactions with the native protein and its recombinant fragments. Chem-Biol Interact. 2002;141:275–93. doi: 10.1016/s0009-2797(02)00078-9. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Isima Y, Chuang VTG, Watanabe H, Tanase S, Maruyama T, et al. Functional analysis of recombinant human serum albumin domains for pharmaceutical applications. Pharm Res. 2004;21:1924–32. doi: 10.1023/b:pham.0000045248.03337.0e. [DOI] [PubMed] [Google Scholar]
- Liu R, Meng Q, Xi J, Yang J, Ha CE, Bhagavan NV, et al. Comparative binding character of two general anaesthetics for sites on human serum albumin. Biochem J. 2004;380:147. doi: 10.1042/BJ20031652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twine S, Gore M, Morton P, Fish B, Lee A, East J. Mechanism of binding of warfarin enantiomers to recombinant domains of human albumin. Arch Biochem Biophys. 2003;414:83–90. doi: 10.1016/s0003-9861(03)00173-5. [DOI] [PubMed] [Google Scholar]
- Rendic S, Albic-Kolbah T, Kajfez F, Sunjic V. Stereoselective binding of (+) and (–)-alpha-(benzoylphenylpropionic) acid (ketoprofen) to human serum albumin. Farmaco, Edizione Scientifica. 1980;35:51–9. [Google Scholar]
- Verbeeck R, Blackburn J, Loewen G. Clinical pharmacokinetics of non-steroidal anti-inflammatory drugs. Clin Pharmacokinet. 1983;8:297. doi: 10.2165/00003088-198308040-00003. [DOI] [PubMed] [Google Scholar]
- Dubois N, Lapicque F, Abiteboul M, Netter P. Stereoselective protein binding of ketoprofen: effect of albumin concentration and of the biological system. Chirality. 1993;5:126–34. doi: 10.1002/chir.530050305. [DOI] [PubMed] [Google Scholar]
- Sakai T, Maruyama T, Imamura H, Shimada H, Otagiri M. Mechanism of stereoselective serum binding of ketoprofen after hemodialysis. J Pharmacol Exp Ther. 1996;278:786. [PubMed] [Google Scholar]
- Zhivkova ZD, Russeva VN. Stereoselective binding of ketoprofen enantiomers to human serum albumin studied by high-performance liquid affinity chromatography. J Chromatogr B. 1998;714:277–83. doi: 10.1016/s0378-4347(98)00211-4. [DOI] [PubMed] [Google Scholar]
- Hayball PJ, Nation RL, Bochner F, Newton JL, Massy-Westropp RA, Hamon DPG. Plasma protein binding of ketoprofen enantiomers in man: method development and its application. Chirality. 1991;3:460–6. doi: 10.1002/chir.530030609. [DOI] [PubMed] [Google Scholar]
- Lapicque F, Muller N, Payan E, Dubois N, Netter P. Protein binding and stereoselectivity of nonsteroidal anti-inflammatory drugs. Clin Pharmacokinet. 1993;25:115. doi: 10.2165/00003088-199325020-00004. [DOI] [PubMed] [Google Scholar]
- Zou H, Wang H, Zhang Y. Stereoselective binding of warfarin and ketoprofen to human serum albumin determined by microdialysis combined with HPLC. J Liq chromatogr R T. 1998;21:2663–74. [Google Scholar]
- Kwok D, Kerr C, McErlane K. Pharmacokinetics of mexiletine enantiomers in healthy human subjects. A study of the in vivo serum protein binding, salivary excretion and red blood cell distribution of the enantiomers. Xenobiotica. 1995;25:1127–42. doi: 10.3109/00498259509061913. [DOI] [PubMed] [Google Scholar]
- Grech-Belanger O, Turgeon J, Gilbert M. Stereoselective disposition of mexiletine in man. Brit J Clin Pharmaco. 1986;21:481. doi: 10.1111/j.1365-2125.1986.tb02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McErlane K, Igwemezie L, Kerr C. Stereoselective serum protein binding of mexiletine enantiomers in man. Res Commun Chem Pathol Pharmacol. 1987;56:141. [PubMed] [Google Scholar]
- Jin YX, Tang YH, Zeng S. Analysis of flurbiprofen, ketoprofen and etodolac enantiomers by pre-column derivatization RP-HPLC and application to drug-protein binding in human plasma. J Pharmaceut Biomed. 2008;46:953–8. doi: 10.1016/j.jpba.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Jin YX, Zeng S.Determination of mexiletine enantiomers in human serum albumin after derivatization with GITC by RP-HPLC Chinese Pharmaceutical Journal 2007. Chinese.
- Guo CC, Tang YH, Hu HH, Yu LS, Jiang HD, Zeng S. Analysis of chiral non-steroidal anti-inflammatory drugs flurbiprofen, ketoprofen and etodolac binding with HSA. J Pharmaceut Anal. 1:184–90. doi: 10.1016/j.jpha.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YX.Enantioselective binding of chiral drugs, mexiletine and ketoprofen, to plasma proteinsThesis for master degree. Zhejiang University. 2006p 24–31.Chinese.
- Hutt A, Caldwell J. The importance of stereochemistry in the clinical pharmacokinetics of the 2-arylpropionic acid non-steroidal anti-inflammatory drugs. Clin Pharmacokinet. 1984;9:371–3. doi: 10.2165/00003088-198409040-00007. [DOI] [PubMed] [Google Scholar]
- Rudy AC, Liu Y, Brater C, Hall SD. Stereoselective pharmacokinetics and inversion of (R)-ketoprofen in healthy volunteers. J Clin Pharmacol. 1998;38:3S. doi: 10.1002/j.1552-4604.1998.tb04411.x. [DOI] [PubMed] [Google Scholar]
- Jamali F, Brocks D. Clinical pharmacokinetics of ketoprofen and its enantiomers. Clin Pharmacokinet. 1990;19:197. doi: 10.2165/00003088-199019030-00004. [DOI] [PubMed] [Google Scholar]
- Grubb N, Rudy D, Brater D, Hall S. Stereoselective pharmacokinetics of ketoprofen and ketoprofen glucuronide in end-stage renal disease: evidence for a 'futile cycle' of elimination. Br J Clin Pharmacol. 1999;48:494–500. doi: 10.1046/j.1365-2125.1999.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannwarth B, Lagrange F, Péhourcq F, Llanas B, Demarquez J. (S)-ketoprofen accumulation in premature neonates with renal failure who were exposed to the racemate during pregnancy. Br J Clin Pharmacol. 1999;47:459–60. doi: 10.1111/bcp.1999.47.4.459. [DOI] [PubMed] [Google Scholar]