Abstract
Aim:
To investigate the role of Hedgehog (Hh) signaling pathway in the invasion and metastasis of human hepatocellular carcinoma (HCC).
Methods:
Eighty six HCC tissues samples and HCC cell line Bel-7402 were examined. The protein expression of sonic hedgehog (Shh), nuclear glioma-associated oncogene-1 (Gli1), MMP-9 and p-ERK1/2 in HCC was analyzed using immunohistochemistry and Western blot analysis. Boyden chamber assay and wound-healing assay were used to quantify the invasion and metastasis of Bel-7402 cells.
Results:
In 86 HCC tissue samples, the positive ratio of Shh and nucleus Gli1 was 67.44% (58/86) and 60.47% (52/86), respectively; the expression of nucleus Gli1 was correlated with the tumor pathological grade (P=0.034), and with the ability of the tumor to invade and metastasize (P=0.001); the expression of nucleus Gli1 was also correlated with p-ERK1/2 (P=0.031) and with MMP-9 (P=0.034). Neither Shh, nor nucleus Gli1 was observed in normal liver tissue. KAAD-cyclopamine (KAAD-cyc), a specific inhibitor of the Hh pathway, at the concentrations of 1 and 4 μmol/L inhibited the invasion and migration of Bel-7402 cells and decreased the expression of Gli1 in nucleus and MMP-9, p-ERK1/2 proteins in Bel-7402 cells. On the other hand, Shh, a ligand of the Hh pathway, at the concentration of 0.5 μg/mL produced opposite effects. The MAPK pathway inhibitors U0126 and PD98059 at the concentrations of 5 and 10 μmol/L inhibited invasion and metastasis of Bel-7402 cells induced by Shh, and decreased the expression of p-ERK1/2 and MMP-9. However, U0126 and PD98059 had no effect on the expression of Gli1.
Conclusion:
Hh signaling pathway mediates invasion and metastasis of human HCC by up-regulating the protein expression of MMP-9 via ERK pathway.
Keywords: human hepatocellular carcinoma, invasion and metastasis, hedgehog signaling pathway, nuclear glioma-associated oncogene-1 (Gli1), ERK pathway, MMP-9
Introduction
Hepatocellular carcinoma (HCC) is the common primary cancer with a multifaceted molecular pathogenesis. HCC has become the fifth most prevalent malignancy worldwide and the third leading cause of cancer-related death, most importantly, the incidence of HCC is increasing1,2, and 82% of cases are in developing countries, with 55% in China alone3. Invasion and metastasis are two fundamental properties, which determine the prognosis of the HCC patients4,5. Many signaling pathways are thought to be involved in the development and invasion of HCC, including the MAPK pathway6,7, phosphatidylinositol-3 kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway8,9,10, the wnt/beta-catenin pathway9,11, hepatocyte growth factor/c-MET pathway12,13, hedgehog (Hh) signaling pathway, and so on.
Hh signaling pathway is a highly conserved system, which plays a crucial role in tissue patterning, cell differentiation and proliferation14. Hedgehog, including sonic hedgehog (Shh), Indian hedgehog (Ihh), and desert hedgehog (Dhh), appear to bind to a transmembrane receptor protein, known as Patched (Ptc), which in the absence of Shh exerts an inhibitory effect on the seven transmembrane receptor smoothened (Smo). Binding of Shh to Ptc alleviates the inhibitory effect of Ptc on Smo. Once activated, Smo induces a complex series of intracellular reactions that targets the glioma-associated oncogenes (Gli) transcription factor families, the zincfinger transcription effectors15,16. At least three members (Gli1, Gli2, and Gli3) of nuclear proteins Gli families have been identified in mammalian tissues14,17. Gli1 is a transcriptional activator18, which induces the expression of numerous target genes that regulates proliferation, differentiation, and extracellular matrix interactions19,20.
Increasing evidence has demonstrated that the Hh signaling pathway plays an important role in multiple tumor types, for example, basal cell carcinoma21,22, pancreatic cancer23, colon carcinoma24, gastric cancer25, small cell lung cancer26, prostate cancer27,28,29, breast cancer30,31, early childhood hepatoblastoma32, and esophageal cancers33.
Recent studies have revealed that the Hh signaling pathway is abnormally activated in human HCC34,35,36, and this pathway is thought to participate in the development of HCC37,38,39. Moreover, activation of the Hh pathway is correlated closely to invasion and metastasis of HCC36,40. Activated markers of the Hh signaling pathway such as Gli1 is significantly up-regulated in the HCC tumor tissues41, and Gli inhibition can also suppress HCC tumor growth and metastases in vivo and invitro42. However, the mechanism by which Hh signaling pathway is involved in HCC development is still unclear.
Hh signaling pathway can affect MAPK/ERK phosphorylation43 and lead to carcinogenesis44. For example, it also can activate ERK1/2 in the breast epithelial cell45. However, other studies have revealed that in cancer the MAPK signaling pathway regulates Hh signaling, specifically Gli activity and expression46. Therefore, what cellular mediators are involved in the crosstalk between the Hh signaling pathway and the MAPK signaling pathway in HCC? Now, there is no report about it.
Matrix metalloproteinase-9 (MMP-9 or gelatinase-B) is mostly associated with tumor migration, invasion and metastasis for various human cancers10,47,48. The Hh signaling pathway up-regulates cell migration and invasion in human gliomas and in pancreatic cancer by increasing the expressions of MMP-949,50. In addition, Smo and MMP-9 were over-expressed and associated with invasion and metastasis in HCC tissues51. Does the Hh signaling pathway mediate the migration and invasion of HCC by increasing the expressions of MMP-9? What is the correlation between MMP-9 and ERK pathway? However, they are incompletely understood in HCC.
In the present study, we investigated the mechanisms of the Hh signaling pathway in invasion and metastasis of HCC, specifically focused on the correlation between the Hh signaling pathway and the MAPK signaling pathway. Further we determined whether Hh signaling pathway involved in human HCC invasion and metastasis by up-regulating the expression of MMP-9 through ERK pathway.
Materials and methods
Patients and specimens
A total of 86 HCC patients, who had undergone liver resection without preoperative treatment at the First Affiliated Hospital of Anhui Medical University between June 2008 and December 2010, were examined. All tumor specimens were pathologically diagnosed as HCC. Prior written informed consent was obtained from all patients according to the World Medical Association Declaration of Helsinki, and the study received ethics board approval from the Affiliated Hospital of Anhui Medical University. The age of patients ranged from 33 to 75 years, with an average age of 48.1 years. There were 68 males and 18 females, 50 I–II type- and 36 III–IV type-differentiated HCC, 37 patients with invasion and/or metastasis (Table 1).
Table 1. Relationship between expression of Shh, Gli1, p-ERK1/2, and MMP-9 and clinical features of HCC (n=86).
| Clinical parameter | Cases (n) | Shh (exp-h/exp-l) | Gli1 (nuclei) (exp-h/exp-l) | p-ERK1/2(exp-h/exp-l) | MMP-9(exp-h/exp-l) | P value (Shh, Gli1, p-ERK1/2, MMP-9) |
|---|---|---|---|---|---|---|
| Age (year) | ||||||
| >50 | 40 | 31/9 | 25/15 | 23/17 | 24/16 | 0.1041, 0.8902, 0.3493, 0.6264 |
| ≤50 | 46 | 27/19 | 27/19 | 32/14 | 31/15 | |
| Tumor diameter (cm) | ||||||
| >3 | 67 | 42/25 | 36/31 | 45/22 | 42/25 | 0.1361, 0.0332, 0.3713, 0.9614 |
| ≤3 | 19 | 16/3 | 16/3 | 10/9 | 13/6 | |
| Pathological grade | ||||||
| I–II | 50 | 27/23 | 25/25 | 27/23 | 29/21 | 0.0041, 0.0342, 0.0423, 0.2604 |
| III–IV | 36 | 31/5 | 27/9 | 28/8 | 26/10 | |
| Invasion or metastasis | ||||||
| Positive | 37 | 30/7 | 30/7 | 32/5 | 30/7 | 0.0351, 0.0012, 0.0013, 0.0084 |
| Negative | 49 | 28/21 | 22/27 | 23/26 | 25/24 | |
Abbreviations: exp-h, high expression; exp-l, low expression.
Reagents
Dulbecco's modified Eagle's medium (DMEM) was obtained from Gbico Chemical Company (Gbico, USA). Shh and Gli1 antibodies were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA). p-ERK1/2 and MMP-9 antibodies were purchased from Abcam Biotechnology Inc (Abcam, UK). KAAD-cyclopamine(KAAD-cyc), Shh, U0126, and PD98059 were purchased from Toronto Research Chemicals (North York, Ontario, Canada) and Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA). All chemicals were purchased in the purest form available.
Cell line and culture conditions
Human HCC cell line Bel-7402, obtained from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences, was grown in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gbico, USA), 100 μg/mL streptomycin and 100 U/mL penicillin in a humidified atmosphere containing 5% CO2 at 37 °C. Cells during exponential growth phase were used in the experiments. Cells were treated with KAAD-cyc, the special antagonist of Smo, Shh, U0126, or PD98059, control medium contained DMSO alone. In the invasion and metastasis experiments, cells were cultured in a serum-free medium.
Immunohistochemistry staining
Briefly, immunohistochemical stains were performed on formalin-fixed and paraffin-embedded tissue sections (4 μm). The sections were prepared according to classical methods and treated with blocking solution before being sequentially incubated with primary antibodies against Shh (1:100), Gli1 (1:200), MMP-9 (1:100), and p-ERK1/2 (1:200) overnight at 4 °C. The primary antibodies were visualized by incubating in a biotinylated antibody and HRP-conjugated streptavidin. Antigen staining was performed using diaminobenzidine then counterstained with hematoxylin. Negative controls were treated with the same species normal IgG in place of primary antibody. Images of each sample were taken and the percentage of positive cancer cells was quantified as the number of positive cells over the total number of cancer cells in that image. Expression was evaluated independently by two pathologists. Staining of sections was assessed in 10 consecutive fields (200× magnification) using a validated semi-quantitative scale, which was indicated by both the percentage of positively stained tumor cells and the staining intensity. The percent positivity was scored as “0” (<5%, negative), “1” (5%–25%, sporadic), “2” (25%–50%, focal), or “3” (>50%, diffuse). The staining intensity was scored as “0” (no staining), “1” (weakly stained), “2” (moderately stained), or “3” (strongly stained). The immunostaining score was calculated as the percentage positive score × the staining intensity score. The expression levels were defined as follows: '–' (score 0–1), '+' (score 2–3), '++' (score 4–6), and '+++' (score >6). The HCC patients were divided into the low expression group (– or +) and the high expression group (++ or +++).
Boyden chamber invasion assay
The effect of KAAD-cyc, Shh, U0126, and PD98059 on HCC Bel-7402 was determined using Boyden chamber assay. Briefly, 24-well transwell units with polycarbonate membrane filters (8 μm pore size, Costar, USA) were coated with 100 μL matrigel (25 μg in 100 μL PBS, Becton Dickinson), dried in a laminar hood overnight, and reconstituted in 100 μL, washed with phosphate-buffered saline (PBS) at 37 °C for 2 h, then PBS was discharged. Bel-7402 cells were resuspended in DMEM with 0.5% BSA (5×104 cells/200 μL) in the presence or absence of KAAD-cyc, Shh, U0126, or PD98059, which was added to the upper side of the invasion chamber. DMEM (500 μL) with 2.5% FBS as chemoattractant was added to the lower chamber. After 24 h, filter inserts were removed from the wells, the cells on the upper surface of the filter were wiped off using cotton swabs. The cells that penetrated to the lower surface were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet in 20% ethanol, and counted in five randomly selected fields under phase contrast microscope. The invasion cells were monitored by photographing at 400× magnification with Olympus Microscope. The assay was performed in triplicate.
Wound-healing assay
Cell migration was examined using the wound-healing assay. Briefly, Bel-7402 cells were cultured to about 80%–90% confluence in a 6-well plate at 37 °C and 5% CO2. A wound about 1 mm width was created by scratching cells with a sterile 100 μL micropipette tip. Cells were washed with PBS (pH 6.8) three times to remove floating cells, then 1 mL serum-free DMEM was added. A computer-based microscopy imaging system was used to determine wound healing at 0 h with a microscope at 200× magnification. Then 1 mL serum-free DMEM was added with different concentrations of KAAD-cyc, Shh, U0126, or PD98059. After 24 h, photoes of the wound were taken under 200× magnification. The values of wound-healing were assessed by measuring the pixel of wound area by Photoshop 7.01 software. The experiments were performed in triplicate.
Western blots analysis
Cells were plated onto culture flask at a density of 2×105 cells/mL, cultured at 37 °C and 5% CO2. The next day, different concentrations of KAAD-cyc, Shh, U0126, or PD98059 were added. After 24 h, the levels of Shh, Gli1, MMP-9, p-ERK1/2 proteins were quantified through Western blots. The proteins were extracted through the addition of 200 μL of lysis buffer (1 mmol/L EDTA, 1.5 mmol/L MgCl2, 150 mmol/L NaCl, 50 mmol/L Hepes, 50 μmol/L DTT, 1 mmol/L phenylmethylsulfonyl fluoride and 10 mg/mL leupeptin, pH 7.4) to each well. The cell lysates were incubated on ice for 30 min vortexing every 10 min, followed by centrifugation at 12 000×g for 30 min at 4 °C, 50 μg/μL protein of cell lysate was mixed equally with 2×electrophoresis buffer [50% glycerol, 25% mercaptoethanol, 10% SDS, 0.3 mol/L Tris (pH 6.8), 0.025% bromophenol blue] and boiled for 10 min. The samples (50 μg of protein) of total cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electrophoretically transferred onto a polyvinylidene difluoride membrane (Millipore) in transfer buffer containing 25 mmol/L Tris, 150 mmol/L glycine and 20% methanol. The membranes were blocked using 5% BSA (pH 7.4, 0.5% Tween 20). The membranes were incubated with primary antibodies, anti-MMP-9 (1:1000), anti-p-ERK1/2 (1:500) and anti-Gli1 (1:500) for 16–18 h at 4 °C. The membranes were subsequently probed with anti-mouse or anti-rabbit IgG antibodies (1:5000) with the HRP for 1 h. Control blots were performed using anti-actin antibody (1:500, Santa Cruz, CA, USA). The membranes were washed in PBS for 30 min at room temperature, and detection was achieved by measuring the chemiluminescence of the blotting agent after exposure of the filters on films. At last, the densities of the bands were quantified with a computerized densitometer (Image J Launcher, Broken Symmetry Software).
Statistical analysis
Statistical analyses were performed using the SPSS 11.0 software program (SPSS Software Products, Chicago, IL, USA). All data were presented as number or mean±standard deviation (SD). Associations between protein expression and clinicopathologic variables were analyzed by ChiSquare Test. Statistical analysis among more groups was performed by one-way analysis of variance (ANOVA). The Spearman coefficient of correlation was used to examine the correlation. Statistical significance of differences were accepted at P<0.05.
Results
Overexpression of Shh, Gli1, p-ERK1/2, and MMP-9 in HCC liver tissues with invasion and metastasis compared with non-metastasis HCC liver tissue
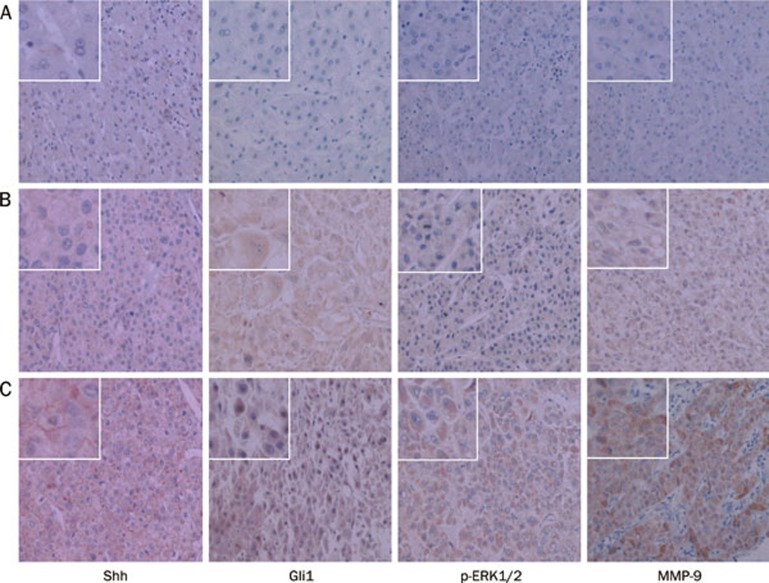
We detected Shh, Gli1 MMP-9, and p-ERK1/2 expressions in 86 cases of HCC liver tissues with or without invasion and metastasis by IHC staining. We further evaluated the relationships between Gli1 and p-ERK1/2, Gli1 and MMP-9 on invasion and metastasis of HCC. The results indicated Shh, Gli1, p-ERK1/2, and MMP-9 expressions had no notable relationship with age and tumor diameter. However, Gli1, p-ERK1/2, and MMP-9 expressions had a significant correlation with the pathological grade and metastasis of the tumor sample (Table 1). Positive expressions of Shh, p-ERK1/2, and MMP-9 were remarkably stronger in HCC liver tissues with metastasis than in non-metastasis HCC liver tissues. A significant difference was observed in expression of Gli1 in the nucleus between HCC tissues with metastasis and non-metastatic HCC liver tissue (91.89% vs 36.74%, P<0.01, Figure 1). Interestingly, expression of Gli1 was also notably correlated to expressions of MMP-9 and p-ERK1/2 (P<0.01, Table 1). Those results suggested that Hh signal pathway mediated invasion and metastasis of human HCC by up-regulating the protein expression MMP-9 and p-ERK1/2.
Figure 1.
Results of IHC staining for Shh, Gli1, p-ERK1/2, and MMP-9 from 86 HCC liver tissues including 37 cases with metastasis at 200× magnification. Representative images are displayed. Expression of Shh was defined as the cytoplasmic and plasmalemmal staining. Expression of MMP-9 was defined as cytoplasmic staining. Gli1, and p-ERK1/2 were located in cytoplasm and/or nucleus. (A) Normal liver tissues; (B) Non-metastatic HCC liver tissues; (C) Metastatic HCC liver tissues.
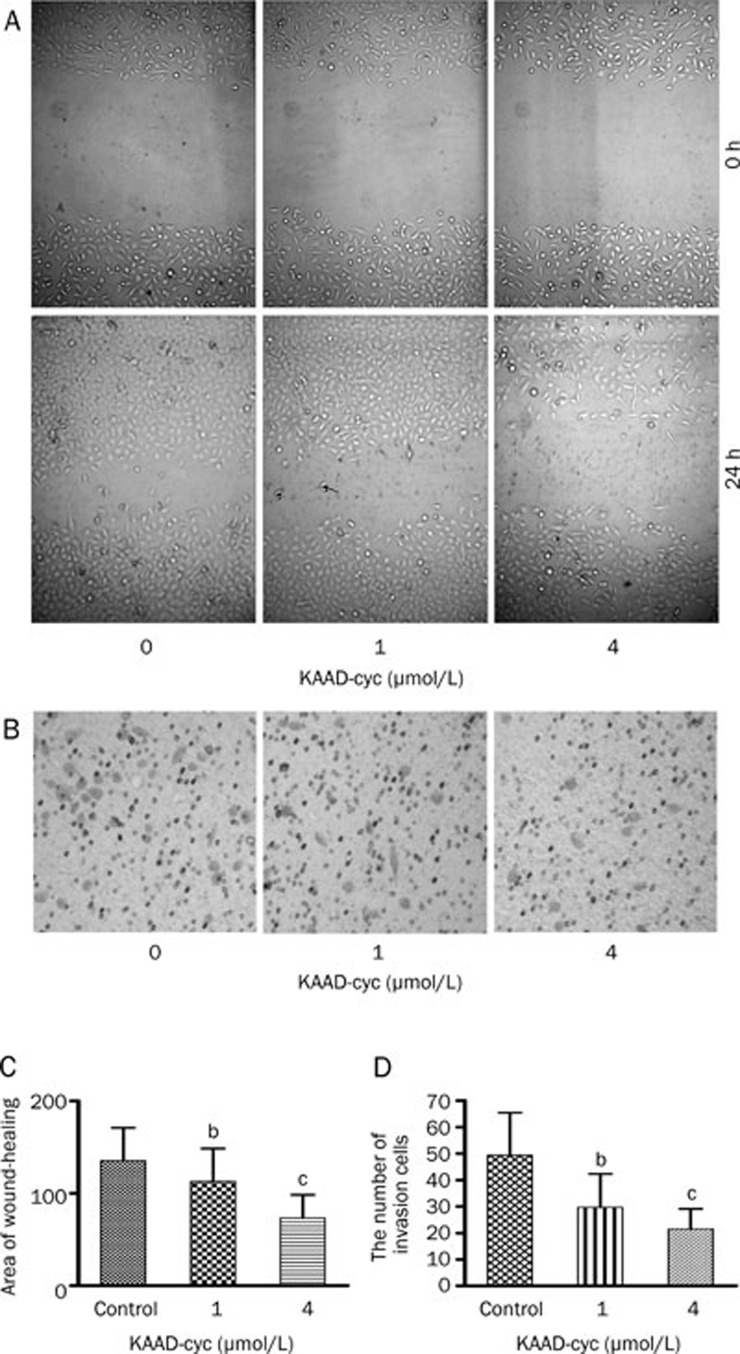
KAAD-cyc inhibited the invasion and migration of Bel-7402 cells
Bel-7402 cell invasion and motility were investigated using a Boyden chamber invasion assay and wound-healing assay, respectively. KAAD-cyc is a specific inhibitor of the Hh signaling pathway and was utilized to determine the effect of this pathway on invasion and metastasis in Bel-7402 cells. The results indicated KAAD-cyc notably inhibited migration of Bel-7402 cells on the surface of the tissue culture plate, significantly decreased area of wound-healing by 45.87% at most compared with controls in the wound-healing assay. Meanwhile, KAAD-cyc significantly decreased the numbers of cells to the lower chamber when the cells were treated with 1 μmol/L and 4 μmol/L of KAAD-cyc for 24 h in Boyden chamber invasion assay, inhibitory rates were 43.50%±15.41% and 56.36%±15.17%, respectively. Collectively, these data demonstrated that KAAD-cyc could suppress the invasion and metastasis of Bel-7402 cells (Figure 2).
Figure 2.
KAAD-cyc inhibits the invasion and migration of Bel-7402 cells. (A) Representative photographs (200×magnification) of cells treated with and without KAAD-cyc for 24 h after wounding from a representative experiment. (B) Representative photographs (400×magnification) of Bel-7402 cells treated with and without KAAD-cyc for 24 h in the invasion assay from 1 of 3 independent experiments. (C) Values of wound-healing assessed by measuring the pixel of wound-healing area. (D) The number of Bel-7402 cells in the lower chamber. Mean±SD. n=3. bP<0.05, cP<0.01 vs control.
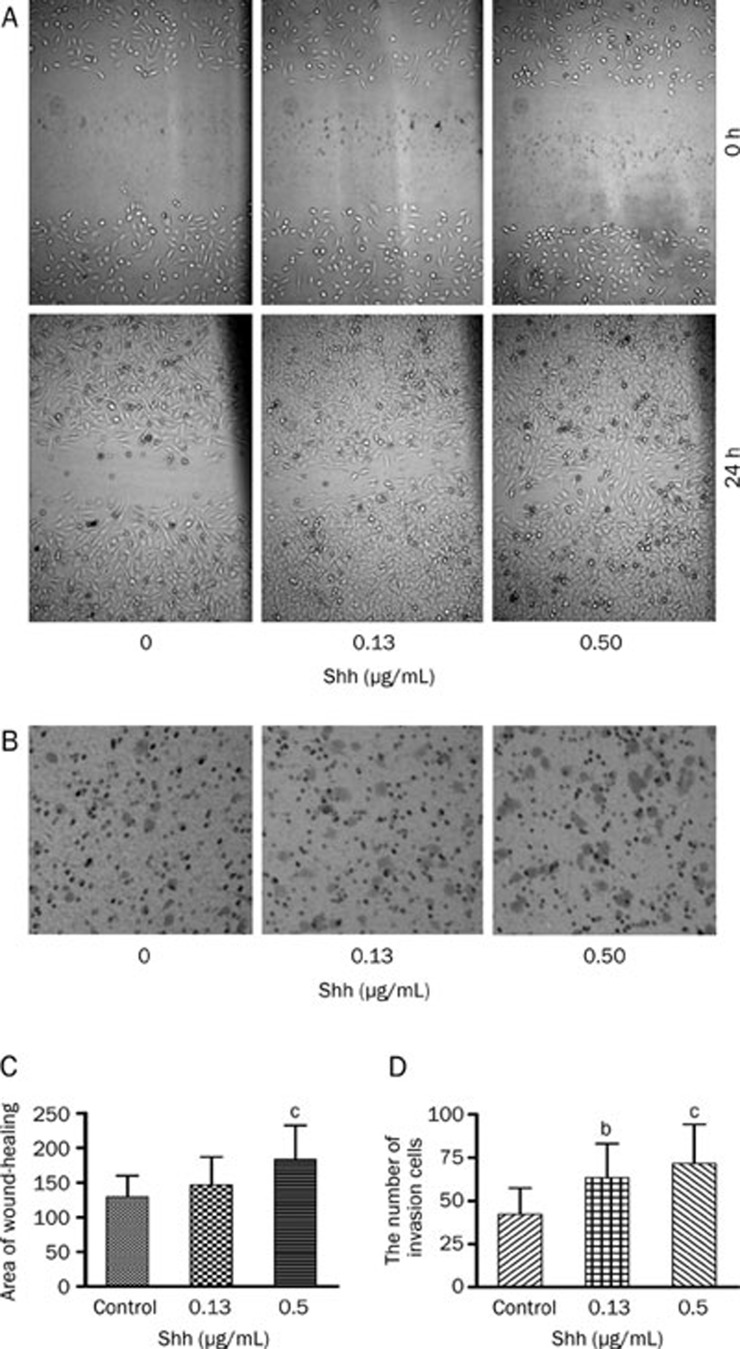
Shh increased invasion and migration of Bel-7402 cells
As illustrated in Figure 3, Shh notably improved the migration of Bel-7402 cells, significantly increased area of wound-healing by greater than 41.63%. At the same time, Shh could significantly increase the number of migrating Bel-7402 cells in the lower chamber when the cells were treated with 0.13 μg/mL and 0.5 μg/mL Shh for 24 h, incremental rates were 49.99%±14.04% with 0.13 μg/mL Shh and 69.28%±20.29% with 0.5 μg/mL Shh in invasion assays. Those data demonstrated that Shh greatly enhanced the invasive and migratory capacity of Bel-7402 cells.
Figure 3.
Shh increases invasion and migration of Bel-7402 cells. (A) Representative photographs (200×magnification) of metastatic cells treated with and without Shh 24 h after wounding from a representative experiment. (B) Representative photographs (400×magnification) of Bel-7402 cells treated with or without Shh for 24 h in invasion assay from 1 of 3 independent experiments. (C) Values of wound-healing assessed by measuring the pixel of wound area. (D) The numbers of Bel-7402 cells in the lower chamber. Mean±SD. n=3. bP<0.05, cP<0.01 vs control.
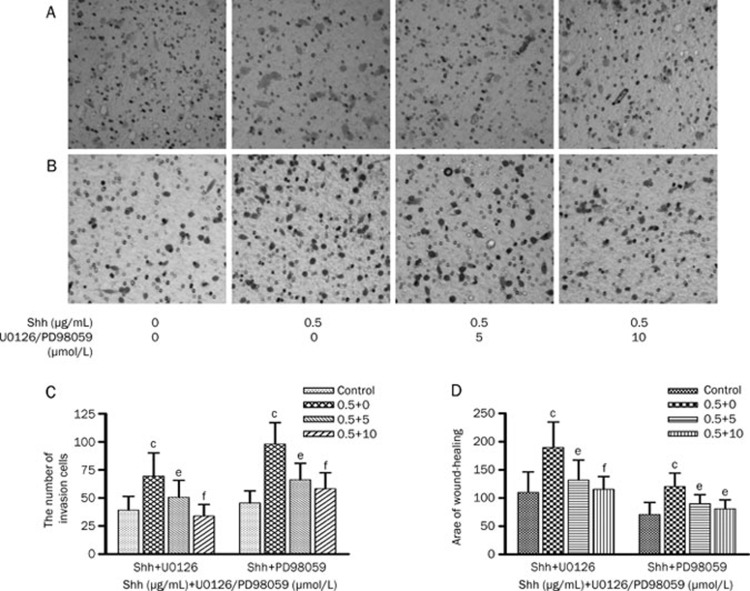
U0126 and PD98059 inhibited invasion and migration of Bel-7402 cells induced by Shh
Hh signaling pathway can lead to carcinogenesis via activation of ERK1/2. Therefore we determined whether the Hh signaling pathway promotes invasion and migration of Bel-7402 cells through p-ERK1/2. The MAPK inhibitor, U0126 and PD98059, were used in the invasion and migration assays with Bel-7402 cells in vitro. The results indicated that Shh notably increased the invasion and migration of Bel-7402 cells (P<0.01). U0126 significantly decrease the numbers of migratory Bel-7402 cells elevated by Shh. The inhibitory rates were 61.72%±18.75% with 5 μmol/L U0126 and 117.63%±28.90% with 10 μmol/L U0126. At the same time, U0126 notably inhibited migration of Bel-7402 cells induced by Shh, significantly decreased area of wound-healing by greater than 86.87% (Figure 4). PD98059 had similar effects with U0126 on the invasion and migration of Bel-7402 cells induced by Shh. These studies demonstrated that the U0126 and PD98059 could suppress the invasion and migration capacity of Bel-7402 cells induced by Shh.
Figure 4.
U0126 and PD98059 inhibit invasion and migration of Bel-7402 cells induced by Shh. (A) Representative photographs (400×magnification) of Bel-7402 cells treated with or without U0126 for 24 h after being pretreated by Shh from a representative experiment. (B) Representative photographs (400×magnification) of Bel-7402 cells treated with or without PD98059 for 24 h after being pretreated by Shh from a representative experiment. (C) The numbers of Bel-7402 cells in the lower chamber in Figure 4A and 4B, each bar represents the mean±SD of three separate experiments. (D) Values of wound-healing assessed by measuring the pixel of wound area, each bar represents the mean±SD of three separate experiments. bP<0.05, cP<0.01 vs control; eP<0.05, fP<0.01 vs Shh.
Effects of KAAD-cyc on the expression of Gli1, p-ERK1/2, and MMP-9 proteins in Bel-7402 cells
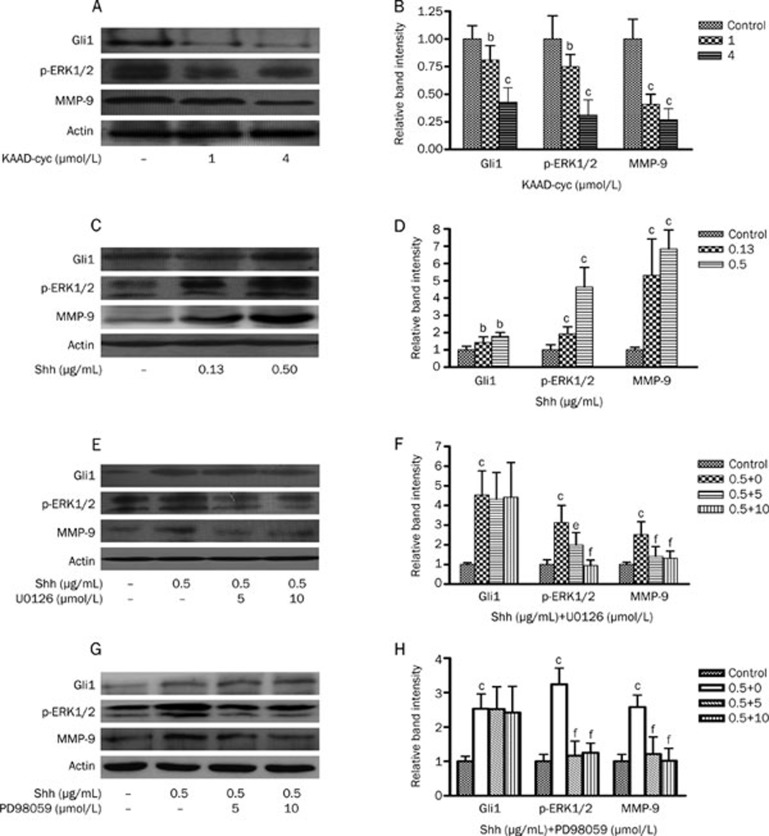
Bel-7402 cells were cultured in serum-free media containing 1 and 4 μmol/L KAAD-cyc for 24 h. The expression of Gli1, p-ERK1/2, and MMP-9 proteins were determined by Western blot analysis. The results indicated that KAAD-cyc dramatically inhibited the expression of Gli1, p-ERK1/2, and MMP-9 proteins in Bel-7402 compared with vehicle control (Figure 5A and 5B).
Figure 5.
Effects of KAAD-cyc, Shh, U0126, or PD98059 on the expressions of Gli1, p-ERK1/2, and MMP-9 proteins in Bel-7402 cells. (A) Western blot analysis of Gli1, p-ERK1/2, and MMP-9 protein levels in cell lysates from Bel-7402 cells treated with 1 or 4 μmol/L KAAD-cyc for 24 h. (B) The values under each lane indicate relative density of the band in Figure 5A normalized to β-actin, respectively. (C) Western blot analysis of Gli1, p-ERK1/2, and MMP-9 protein levels in cell lysates from Bel-7402 cells treated with 0.13 or 0.50 μg/mL Shh for 24 h. (D) The values under each lane indicate relative density of the band in Figure 5C normalized to β-actin, respectively. (E) Western blot analysis of Gli1, p-ERK1/2, and MMP-9 protein levels in cell lysates from Bel-7402 cells pretreated by 0.50 μg/mL Shh, then treated with 5 or 10 μmol/L U0126 for 24 h. (F) The values under each lane indicate relative density of the band in Figure 5E normalized to β-actin, respectively. (G) Western blot analysis of Gli1, p-ERK1/2, and MMP-9 protein levels in cell lysates from Bel-7402 cells pretreated by 0.50 μg/mL Shh, then treated with 5 or 10 μmol/L PD98059 for 24 h. (H) The values under each lane indicate relative density of the band in Figure 5G normalized to β-actin, respectively. bP<0.05, cP<0.01 vs control; eP<0.05, fP<0.01 vs Shh.
Effects of Shh on the expression of Gli1, p-ERK1/2, and MMP-9 proteins in Bel-7402 cells
Bel-7402 cells were cultured in serum-free media containing 0.13 and 0.50 μg/mL Shh for 24 h. The expression of Gli1, p-ERK1/2, and MMP-9 proteins were determined by Western blot analysis. The results indicated that Shh dramatically increased the expression of Gli1, p-ERK1/2, and MMP-9 in Bel-7402 in concentration dependent manner compared with vehicle control (Figure 5C and 5D).
Effects of U0126 and PD98059 on the expression of Gli1, p-ERK1/2, and MMP-9 in Bel-7402 cells induced by Shh
After Bel-7402 cells were treated with 0.5 μg/mL Shh, then 5 or 10 μmol/L U0126 and 5 or 10 μmol/L PD98059 were added for 24 h. Western blot analysis indicated that both U0126 and PD98059 dramatically inhibited the expression of MMP-9 and p-ERK1/2 in a concentration dependent manner. However, both U0126 and PD98059 had no effects on expression of Gli1 under the same condition compared with vehicle control (Figure 5E and 5G).
Discussion
Hepatocellular carcinoma (HCC) is one of the most malignant cancers especially in Asian countries, and its poor prognosis is mainly due to metastasis after excision52. Currently there are no available effective treatment53,54. Studies of the underlying molecular mechanism of HCC metastasis provide potential to identify new therapeutic targets. Previous evidence has demonstrated that the Hh signaling plays an important role in multiple tumor types. This signaling pathway is involved in and participates development, invasion and metastasis of HCC34,35,36,37,38,39,40. In this study, we further detected expression of Shh and Gli1 in metastasis and non-metastasis HCC liver tissues, simultaneously we used KAAD-cyc, a specific inhibitor of Hh pathway and Shh, a ligand of the Hh pathway in invasion and metastasis assays of human HCC cell line, and we confirmed the results. However, to date no report has been presented to identify a potential mechanism. Therefore we investigated the mechanisms of the Hh signaling pathway in HCC invasion and metastasis. Gli1, a transcription factor activated in the Hh pathway, significantly enhanced tumor growth and metastases of other cancers through the activation of ERK1/242,45. Overexpression of MMP-9 is also a key factor for tumor invasion and metastasis47,48,55. Moreover, the Hh signaling pathway may enhance migration and invasion of cancer cells by increasing the expression of MMP-949,50.
In the present study, we found that expressions of Shh, MMP-9, and p-ERK1/2 were remarkably stronger in HCC samples with metastasis than in non-metastasis HCC liver samples. Moreover, there was a significant difference in expression of Gli1 in nuclei of cells in HCC tissue samples with metastasis compared to and HCC samples with no metastasis. Moreover, expression of Gli1 was also positively correlated to expressions of both MMP-9 and p-ERK1/2. These data suggested that the Hh signal pathway may be involved in human HCC invasion and metastasis by up-regulating the protein expression MMP-9 and p-ERK1/2.
Since the Hh signaling pathway has been associated with MAPK/ERK pathway in different cancer43,44,45,46, furthermore our results indicate that the pathway is also notably correlated to expressions of p-ERK1/2 in HCC tissues. To confirm these results, we treated a HCC cell line, Bel-7402 cells, with KAAD-cyc, Shh, U0126, or PD98059 in vitro. Our results demonstrated that KAAD-cyc inhibited the invasion and migration of Bel-7402 cells and decreased the expression of Gli1, p-ERK1/2 proteins in Bel-7402, but Shh increased the expression of Gli1, p-ERK1/2 proteins. U0126 and PD98059 inhibited the invasion and metastasis of Bel-7402 cells induced by Shh, decreased the expression of Gli1 proteins, but they had no effect on the expression of Gli1. These indicate that the Hh signal pathway is involved in human HCC invasion and metastasis by up-regulating the expression of MAPK/ERK pathway, instead of the MAPK/ERK pathway inducing expression of Gli1.
Matrix metalloproteinases (MMPs) have long been associated with cancer cell invasion and metastasis. MMPs are proteolytic enzymes, their basic mechanism of action is to degrade proteins in extracellular matrix. Activation of MMPs has been detected in almost all type of human cancers, which is closely correlated to advanced tumor stage, increasing tumor invasion and metastasis. Onishi et al reported that the Hh signaling pathway up-regulated cell migration and invasion in human cancers by increasing expressions of MMP-950. However, the relationship among the Hh signaling pathway, MAPK/ERK pathway and MMP-9 remains unclear. Our results indicate that the Hh pathway is also correlated with expressions of p-ERK1/2 and MMP-9 in HCC tissues. In Bel-7402 cellular assays, KAAD-cyc inhibited expression of MMP-9 proteins, and Shh up-regulated expression of MMP-9 proteins. Both U0126 and PD98059 were able to inhibit the expression of MMP-9 elevated by Shh. Therefore, we concluded that the Hh signaling pathway mediated the protein expression MMP-9 through MAPK/ERK pathway.
Most importantly, we have confirmed that the Hh signal pathway is involved in human HCC invasion and metastasis. We hypothesize and also deduce that the Hh signaling pathway activates the ERK pathway, subsequently, the ERK pathway up-regulates the protein expression of MMP-9, thereby mediating human HCC invasion and metastasis. These data may assist in identifying novel diagnostic markers and therapeutic targets for the treatment of highly aggressive HCC.
Author contribution
Wei WEI and Jing-tao LU designed the research; Jing-tao LU, Wen-di ZHAO, and Wei HE performed the research; Jing-tao LU and Wei HE analyzed data; and Jing-tao LU wrote the paper.
Abbreviations
HCC, hepatocellular carcinoma; Hh, hedgehog; PI3K, phosphatidylinositol-3 kinase; Ptc, patched; Smo, smoothened; Gli, the glioma-associated oncogenes; KAAD-cyc, KAAD-cyclopamine; MMPs, matrix metalloproteinases; PBS, phosphate-buffered saline; DMEM, Dulbecco's modified Eagle's medium.
Acknowledgments
The study is supported by National Natural Science Foundation of China (No 30973543 and 81173075), and Anhui Provincial Natural Science Foundation (No 090413108 and KJ2012A160).
The authors thank senior technician Cheng-yi WU, Ai-wu ZHOU and Yun-fang ZHANG, technician Li-hua LIU for their excellent technical assistance.
References
- Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047–63. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- Walzer N, Kulik LM. Hepatocellular carcinoma: latest developments. Curr Opin Gastroenterol. 2008;24:312–9. doi: 10.1097/MOG.0b013e3282fafef3. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY, Chen YF, et al. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol. 2006;13:940–6. doi: 10.1245/ASO.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, et al. A decade's studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–96. doi: 10.1007/s00432-003-0511-1. [DOI] [PubMed] [Google Scholar]
- Min L, He B, Hui L. Mitogen-activated protein kinases in hepatocellular carcinoma development. Semin Cancer Biol. 2011;21:10–20. doi: 10.1016/j.semcancer.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Hsieh YH, Wu TT, Huang CY, Hsieh YS, Hwang JM, Liu JY. p38 mitogen-activated protein kinase pathway is involved in protein kinase Calpha-regulated invasion in human hepatocellular carcinoma cells. Cancer Res. 2007;67:4320–7. doi: 10.1158/0008-5472.CAN-06-2486. [DOI] [PubMed] [Google Scholar]
- Martínez-López N, Varela-Rey M, Fernández-Ramos D, Woodhoo A, Vázquez-Chantada M, Embade N, et al. Activation of LKB1-Akt pathway independent of phosphoinositide 3-kinase plays a critical role in the proliferation of hepatocellular carcinoma from nonalcoholic steatohepatitis. Hepatology. 2010;52:1621–31. doi: 10.1002/hep.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, et al. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: Association with MMP-9. Hepatol Res. 2009;39:177–86. doi: 10.1111/j.1872-034X.2008.00449.x. [DOI] [PubMed] [Google Scholar]
- Wei W, Chua MS, Grepper S, So SK. Blockade of Wnt-1 signaling leads to anti-tumor effects in hepatocellular carcinoma cells. Mol Cancer. 2009;8:76–81. doi: 10.1186/1476-4598-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaposi-Novak P, Lee JS, Gòmez-Quiroz L, Coulouarn C, Factor VM, Thorgeirsson SS. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116:1582–95. doi: 10.1172/JCI27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi N, Takayama H, Toyoda M, Otsuka T, Fukusato T, Merlino G, et al. Hepatocyte growth factor promotes hepatocarcinogenesis through c-Met autocrine activation and enhanced angiogenesis in transgenic mice treated with diethylnitrosamine. Oncogene. 2002;21:1791–9. doi: 10.1038/sj.onc.1205248. [DOI] [PubMed] [Google Scholar]
- Ruizi AA, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–72. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- Goetz JA, Suber LM, Zeng X, Robbins DJ. Sonic hedgehog as a mediator of long-range signaling. Bioessays. 2002;24:157–65. doi: 10.1002/bies.10056. [DOI] [PubMed] [Google Scholar]
- Nybakken K, Perrimon N. Hedgehog signal transduction: recent findings. Curr Opin Genet Dev. 2002;12:503–11. doi: 10.1016/s0959-437x(02)00333-7. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–24. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem. 2005;280:15700–8. doi: 10.1074/jbc.M413037200. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr The Hedgehog signaling network. Am J Med Genet. 2003;123A:5–28. doi: 10.1002/ajmg.a.20495. [DOI] [PubMed] [Google Scholar]
- Kump E, Ji J, Wernli M, Häusermann P, Erb P. Gli2 upregulates cFlip and renders basal cell carcinoma cells resistant to death ligand-mediated apoptosis. Oncogene. 2008;27:3856–64. doi: 10.1038/onc.2008.5. [DOI] [PubMed] [Google Scholar]
- Caro I, Low JA. The role of the hedgehog signaling pathway in the development of basal cell carcinoma and opportunities for treatment. Clin Cancer Res. 2010;16:3335–9. doi: 10.1158/1078-0432.CCR-09-2570. [DOI] [PubMed] [Google Scholar]
- Bisht S, Brossart P, Maitra A, Feldmann G. Agents targeting the Hedgehog pathway for pancreatic cancer treatment. Curr Opin Investig Drugs. 2010;11:1387–98. [PubMed] [Google Scholar]
- Yoshikawa K, Shimada M, Miyamoto H, Higashijima J, Miyatani T, Nishioka M, et al. Sonic hedgehog relates to colorectal carcinogenesis. J Gastroenterol. 2009;44:1113–7. doi: 10.1007/s00535-009-0110-2. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Katoh M. Hedgehog signaling pathway and gastric cancer. Cancer Biol Ther. 2005;4:1050–4. doi: 10.4161/cbt.4.10.2184. [DOI] [PubMed] [Google Scholar]
- Velcheti V, Govindan R. Hedgehog signaling pathway and lung cancer. J Thorac Oncol. 2007;2:7–10. doi: 10.1097/JTO.0b013e31802c0276. [DOI] [PubMed] [Google Scholar]
- Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–6. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Cozma D, Thomas-Tikhonenko A, Ahmad N, Spiegelman VS. Role of GLI2 transcription factor in growth and tumorigenicity of prostate cells. Cancer Res. 2007;67:10642–6. doi: 10.1158/0008-5472.CAN-07-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu FG, Ma QY, Wang Z. Blockade of hedgehog signaling pathway as a therapeutic strategy for pancreatic cancer. Cancer Lett. 2009;283:119–24. doi: 10.1016/j.canlet.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Haaf AT, Bektas N, von Serenyi S, Losen I, Arweiler EC, Hartmann A, et al. Expression of the glioma-associated oncogene homolog (GLI)1 in human breast cancer is associated with unfavourable overall survival. BMC Cancer. 2009;25:298–301. doi: 10.1186/1471-2407-9-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Frolova N, Sadlonova A, Novak Z, Steg A, Page GP, et al. Hedgehog signaling and response to cyclopamine differ in epithelial and stromal cells in benign breast and breast cancer. Cancer Biol Ther. 2006;5:674–83. doi: 10.4161/cbt.5.6.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenmüller M, Gruner I, Hagl B, Häberle B, Müller-Höcker J, von Schweinitz D, et al. Blocking the hedgehog pathway inhibits hepatoblastoma growth. Hepatology. 2009;49:482–90. doi: 10.1002/hep.22649. [DOI] [PubMed] [Google Scholar]
- Ma X, Sheng T, Zhang Y, Zhang X, He J, Huang S, et al. Hedgehog signaling is activated in subsets of esophageal cancers. Int J Cancer. 2006;118:139–48. doi: 10.1002/ijc.21295. [DOI] [PubMed] [Google Scholar]
- Sicklick JK, Li YX, Jayaraman A, Kannangai R, Qi Y, Vivekanandan P, et al. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis. 2006;27:748–57. doi: 10.1093/carcin/bgi292. [DOI] [PubMed] [Google Scholar]
- Huang S, He J, Zhang X, Bian Y, Yang L, Xie G, et al. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis. 2006;27:1334–40. doi: 10.1093/carcin/bgi378. [DOI] [PubMed] [Google Scholar]
- Cheng WT, Xu K, Tian DY, Zhang ZG, Liu LJ, Chen Y. Role of hedgehog signaling pathway in proliferation and invasiveness of hepatocellular carcinoma cells. Int J Oncol. 2009;34:829–36. doi: 10.3892/ijo_00000209. [DOI] [PubMed] [Google Scholar]
- Tada M, Kanai F, Tanaka Y, Tateishi K, Ohta M, Asaoka Y, et al. Down-regulation of hedgehog-interacting protein through genetic and epigenetic alterations in human hepatocellular carcinoma. Clin Cancer Res. 2008;14:3768–76. doi: 10.1158/1078-0432.CCR-07-1181. [DOI] [PubMed] [Google Scholar]
- Kim Y, Yoon JW, Xiao X, Dean NM, Monia BP, Marcusson EG. Selective down-regulation of glioma-associated oncogene 2 inhibits the proliferation of hepatocellular carcinoma cells. Cancer Res. 2007;67:3583–93. doi: 10.1158/0008-5472.CAN-06-3040. [DOI] [PubMed] [Google Scholar]
- Kim HY, Cho HK, Hong SP, Cheong J. Hepatitis B virus X protein stimulates the Hedgehog-Gli activation through protein stabilization and nuclear localization of Gli1 in liver cancer cells. Cancer Lett. 2011;309:176–84. doi: 10.1016/j.canlet.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Chen X, Lingala S, Khoobyari S, Nolta J, Zern MA, Wu J. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J Hepatol. 2010;55:838–45. doi: 10.1016/j.jhep.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Yao Y, Xu Q, Tu K, Liu Q. Evaluation of glioma-associated oncogene 1 expression and its correlation with the expression of sonic hedgehog, E-cadherin and S100a4 in human hepatocellular carcinoma. Mol Med Report. 2010;3:965–70. doi: 10.3892/mmr.2010.375. [DOI] [PubMed] [Google Scholar]
- Philips GM, Chan IS, Swiderska M, Schroder VT, Guy C, Karaca GF, et al. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS One. 2011;6:e23943. doi: 10.1371/journal.pone.0023943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia D, Madhala D, Ardon E, Reshef R, Halevy O. Sonic hedgehog promotes proliferation and differentiation of adult muscle cells: Involvement of MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta. 2007;1773:1438–46. doi: 10.1016/j.bbamcr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Schnidar H, Eberl M, Klingler S, Mangelberger D, Kasper M, Hauser-Kronberger C, et al. Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res. 2009;69:1284–92. doi: 10.1158/0008-5472.CAN-08-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Li Q, Moraes RC, Lewis MT, Hamel PA. Activation of ERK by sonic hedgehog is independent of canonical hedgehog signaling. Int J Biochem Cell Biol. 2010;42:1462–71. doi: 10.1016/j.biocel.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto M, Ohta M, Asaoka Y, Ikenoue T, Tada M, Miyabayashi K, et al. Regulation of the hedgehog signaling by the mitogen-activated protein kinase cascade in gastric cancer. Mol Carcinog. 2009;48:703–12. doi: 10.1002/mc.20516. [DOI] [PubMed] [Google Scholar]
- Donadio AC, Remedi MM, Susperreguy S, Frede S, Gilardoni MB, Tang Y, et al. Extracellular matrix metalloproteinase inducer and matrix metalloproteinases (MMPs) as regulators of tumor-host interaction in a spontaneous metastasis model in rats. Histochem Cell Biol. 2008;130:1155–64. doi: 10.1007/s00418-008-0496-6. [DOI] [PubMed] [Google Scholar]
- Cortes-Reynosa P, Robledo T, Macias-Silva M, Wu SV, Salazar EP. Src kinase regulates metalloproteinase-9 secretion induced by type IV collagen in MCF-7 human breast cancer cells. Matrix Biol. 2008;27:220–31. doi: 10.1016/j.matbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Wang K, Pan L, Che X, Cui D, Li C. Sonic Hedgehog/GLI signaling pathway inhibition restricts cell migration and invasion in human gliomas. Neurol Res. 2010;32:975–80. doi: 10.1179/016164110X12681290831360. [DOI] [PubMed] [Google Scholar]
- Onishi H, Kai M, Odate S, Iwasaki H, Morifuji Y, Ogino T, et al. Hypoxia activates the hedgehog signaling pathway in a ligand-independent manner by upregulation of Smo transcription in pancreatic cancer. Cancer Sci. 2011;102:1144–50. doi: 10.1111/j.1349-7006.2011.01912.x. [DOI] [PubMed] [Google Scholar]
- Huang XH, Chen JS, Wang Q, Chen XL, Wen L, Chen LZ, et al. miR-338-3p suppresses invasion of liver cancer cell by targeting smoothened. J Pathol. 2011;225:463–72. doi: 10.1002/path.2877. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–46. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892–9. doi: 10.1200/JCO.2005.03.196. [DOI] [PubMed] [Google Scholar]
- Grieco A, Pompili M, Caminiti G, Miele L, Covino M, Alfei B, et al. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut. 2005;54:411–8. doi: 10.1136/gut.2004.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]