Abstract
Purpose.
We described the change in visual acuity experienced by eyes successfully treated for bacterial keratitis.
Methods.
This was a prospective cohort study of a subset of study participants who had previously enrolled in the Steroids for Corneal Ulcers Trial (SCUT). All study participants had been diagnosed with culture-proven bacterial keratitis before enrollment in SCUT and subsequently were randomized to adjunctive topical corticosteroids or placebo. During SCUT, we monitored study participants at enrollment, 3 weeks, 3 months, and 12 months. We invited a subset to complete a comprehensive eye examination approximately 4 years after enrollment in SCUT. Certified refractionists assessed best spectacle-corrected visual acuity (BSCVA) using the same protocol at each study visit.
Results.
We examined 50 SCUT participants at 4 years after enrollment. Among those in this cohort, mean logMAR BSCVA at enrollment was 0.85 (Snellen equivalent, 20/160; 95% confidence interval [CI], 0.71–0.99). On average, visual acuity improved by 2.9 logMAR lines from enrollment to 3 weeks (P < 0.001), 1.2 lines from 3 weeks to 3 months (P = 0.002), and 0.8 lines from 3 to 12 months (P = 0.01). The BSCVA did not change significantly between 12 months and 4 years (0.04-line improvement, P = 0.88). After controlling for visual acuity at enrollment, BSCVA was not significantly different between the corticosteroid and placebo groups at 4 years (P = 0.53).
Conclusions.
Cases of bacterial keratitis may continue to demonstrate improvements in visual acuity up to 12 months following diagnosis, but further improvements are unlikely. These findings may guide the appropriate timing of surgical intervention in these patients. (ClinicalTrials.gov number, NCT00324168.)
Keywords: prospective, visual acuity, long-term, outcomes, keratitis, clinical trial
In a subset of study participants from the Steroids for Corneal Ulcers trial (SCUT), best spectacle corrected visual acuity improved from enrollment to the 3-month study visit, and from the 3- to 12-month study visits, but not from the 12-month to 4-year study visits.
Introduction
Microbial keratitis is a significant cause of corneal blindness and visual impairment throughout the world.1,2 Although many publications have discussed the diagnosis and management of infectious keratitis, most have focused on the resolution of epithelial defects and stromal infiltrates, and have neglected to measure visual outcomes.3–5 This is an important gap in knowledge, since ophthalmologists must counsel patients with infectious keratitis about the need and timing for penetrating keratoplasty. Such counseling currently is difficult given the lack of data regarding the natural history of this disease.
The Steroids for Corneal Ulcers Trial (SCUT) was a multi-site, double-masked, randomized clinical trial in which 500 participants with culture-positive bacterial keratitis received as adjunctive therapy either topical corticosteroids or topical placebo. The trial found no difference in 3-month visual acuity in the two treatment groups. Best spectacle-corrected visual acuity (BSCVA) was assessed in SCUT at enrollment, 3 weeks, 3 months, and 12 months, providing an opportunity to better characterize the course of visual recovery in treated bacterial keratitis. In a previous report, we showed that BSCVA improved throughout the clinical trial, including the 3- to 12-month study visit interval.6 To determine whether these improvements in visual acuity may extend beyond 12 months, we called back a subset of SCUT participants to assess their visual acuity 4 years after enrollment in SCUT.
Methods
The SCUT was a multi-center, randomized, placebo-controlled clinical trial funded by the National Eye Institute and conducted from 2006 to 2010 (ClinicalTrials.gov #NCT00324168).7 The study methods have been described previously.8 Briefly, patients with culture-positive bacterial corneal ulcers who were treated with at least 48 hours of topical moxifloxacin were randomized to adjunctive treatment with either topical prednisolone phosphate (1%) or topical placebo, tapered over 3 weeks. Topical moxifloxacin was continued until 3 weeks after enrollment, although treating ophthalmologists could alter the antibiotic therapy as necessary. The BSCVA was assessed by certified refractionists at enrollment, 3 weeks, 3 months, and 12 months.
For the current study, all SCUT participants who were enrolled at the Aravind Eye Hospital in Madurai, India between October 8, 2007 and August 18, 2008 were telephoned and invited for examination. Hospital staff made home visits to those individuals who could not be contacted to invite them to participate. Examinations were conducted in early 2012. Certified refractionists assessed BSCVA in logarithm of the minimum angle of resolution (logMAR) units using the Early Treatment of Diabetic Retinopathy Study (ETDRS) visual acuity chart with the same methodology as that described for the SCUT trial.8 Subjects with BSCVA worse than logMAR 1.6 (Snellen equivalent 20/800) were assessed for counting fingers, hand motions, light perception, or no light perception visual acuity, and were assigned a logMAR of 1.7, 1.8, 1.9, or 2.0, respectively. We performed slit-lamp biomicroscopy (900; Haag-Streit, Koeniz, Switzerland) and dilated fundus examinations for all study participants. The examiner was masked to treatment allocation for each of the follow-up visits of SCUT, including this 4-year follow-up visit. This study received institutional review board approval from the Aravind Eye Hospital Institutional Review Board and the University of California, San Francisco Committee on Human Research. The research complied with the tenets of the Declaration of Helsinki.
We calculated the change in lines of logMAR visual acuity between consecutive study visits for the cohort of study participants that had BSCVA measured for all 5 study visits. We performed descriptive statistics and tested for changes in visual acuity between consecutive study visits using a Wilcoxon signed-rank test. We compared 4-year BSCVA in the corticosteroid and placebo treatment arms with linear regression, adjusting for enrollment BSCVA. A similar linear regression was performed separately for the subgroup with ulcers caused by Nocardia species, and the subgroup with ulcers not caused by Nocardia species.
We created mixed-effects linear regression models to predict logMAR BSCVA from the study visit, with participant and the study visit associated with a participant as random effects and an unstructured covariance structure. Statistical significance was determined from the model for pairwise comparisons of adjacent time points. We tested for differential outcomes in prespecified subgroups using similar models that additionally included the interaction between the subgroup and study visit. For these subgroup analyses, statistical significance was determined from the partial interaction term for the period of time between the 12-month and 4-year study visits. To account for loss-to-follow-up at the 4-year study visit, we performed a sensitivity analysis using multiple imputation to estimate the missing BSCVA values {mi commands in Stata; missing BSCVA values at each time point imputed using multivariate normal regression with the square root of the number of days since enrollment as the explanatory variable; imputed jointly to allow within-participant interdependencies; 50 imputations, performed separately for tertiles of enrollment BSCVA [tertiles, <0.52 (Snellen equivalent 20/63); 0.52–1.44 (20/63–20/640); and ≥1.54 (20/800 and worse)]}. The 95% confidence intervals (CI) were determined for all models by bootstrapping (999 replications, resampling at the participant level). Although all visual acuity data were collected from logMAR charts and analyzed as such, we report here also the approximate Snellen equivalent using the conversion recommendations from the Diabetic Retinopathy Clinical Research Network.9
Results
Of the 500 SCUT subjects, 80 enrolled at the Madurai study site between October 2007 and August 2008, and 50 (62.5%) of these subjects were reexamined between February and March 2012 (range, 42.9–54.4 months post-enrollment; interquartile range [IQR], 45.9–52.0 months), and the remaining 30 subjects were lost to follow-up. The 50 participants with a 4-year follow-up visit had considerably better vision at enrollment than the 30 eligible study participants who were not reexamined (median Snellen equivalent, 20/100 vs. 20/500; P = 0.06), but similar vision at enrollment compared to the 420 SCUT participants not eligible for the current study (median Snellen equivalent, 20/100 vs. 20/125; P = 0.81; Table 1). Compared to this group of 420 SCUT participants ineligible for the current study, the 50 participants reexamined at 4 years were more likely to have a corneal ulcer caused by Nocardia species (28.0% vs. 9.1%, Table 1). Otherwise, the baseline characteristics of the participants included in this study were similar to participants who were eligible, but not enrolled in the current study, and to those who were not eligible for the current study.
Table 1.
Baseline Characteristics of Study Participants From the SCUT Trial, Stratified on Eligibility for and Inclusion in the Current Study
|
Baseline Characteristic |
Eligible SCUT Participants |
Ineligible SCUT Participants,N= 420 |
PValue* |
|
|
Included,N= 50 |
Excluded,N= 30 |
|||
| Female sex, N (%) | 25 (50%) | 10 (33.3%) | 192 (45.7%) | 0.33 |
| Age, y, median (IQR) | 45 (38–60) | 56 (37–65) | 53 (40–62) | 0.14 |
| LogMAR visual acuity, median (IQR) | 0.65 (0.36–1.6) | 1.36 (0.64–1.7) | 0.81 (0.36–1.7) | 0.16† |
| Infiltrate size, mm2, median (IQR) | 2.5 (1.9–3.7) | 3.2 (1.9–5.1) | 2.7 (1.9–4.0) | 0.32 |
| Steroid group, N (%) | 24 (48.0%) | 15 (50.0%) | 211 (50.2%) | 0.98 |
| Causative organism, N (%) | ||||
| S. pneumoniae | 21 (42.0%) | 15 (51.7%) | 214 (51.0%) | 0.50 |
| Nocardia species | 14 (28.0%) | 4 (13.3%) | 38 (9.1%) | 0.001‡ |
| P. aeruginosa | 12 (24.0%) | 7 (23.3%) | 92 (21.9%) | 0.88 |
| Other | 3 (6.0%) | 4 (13.3%) | 77 (18.3%) | 0.07§ |
Kruskal-Wallis test or Fisher's exact test.
Pairwise comparisons: eligible included versus eligible excluded, P = 0.06; eligible included versus ineligible, P = 0.81.
Pairwise comparisons: eligible included versus eligible excluded, P = 0.17; eligible included versus ineligible, P < 0.001.
Pairwise comparisons: eligible included versus eligible excluded, P = 0.23; eligible included versus ineligible, P < 0.02.
Of the 50 participants in the current study, three had cataract surgery and none had penetrating keratoplasty during the 4-year follow-up period. The three participants who underwent cataract surgery did so between the 12-month and 4-year study visits; all had improvement in visual acuity (final Snellen equivalent of 20/25, 20/32, and 20/50, corresponding to 2.4, 1.4, and 13.2 lines of logMAR visual acuity improvement, respectively). Because changes in visual acuity were at least partly attributable to cataract surgery for these patients, these three study participants were not included in the remaining analyses.
At the 4-year follow-up visit, 28 (59.6%) participants had visual acuity better than 20/40, 15 (31.9%) had visual acuity from 20/40 up to 20/200, 1 (2.1%) had visual acuity from 20/200 to 20/800, and 3 (6.4%) had visual acuity of counting fingers or worse. In the 15 study participants with visual acuity of 20/40 up to 20/200, the reason for vision loss was classified as corneal scar in 5 (33.3%), cataract in 2 (13.3%), a combination of corneal scar and either cataract or posterior capsular opacification in 6 (40.0%), and the combination of corneal scar and retinal problem in 2 (13.3%). In the 4 study participants with visual acuity of 20/200 or worse, the reason for vision loss was classified as corneal scar in 2 (50.0%), cataract in 1 (25.0%), and the combination of corneal scar and cataract in 1 (25.0%).
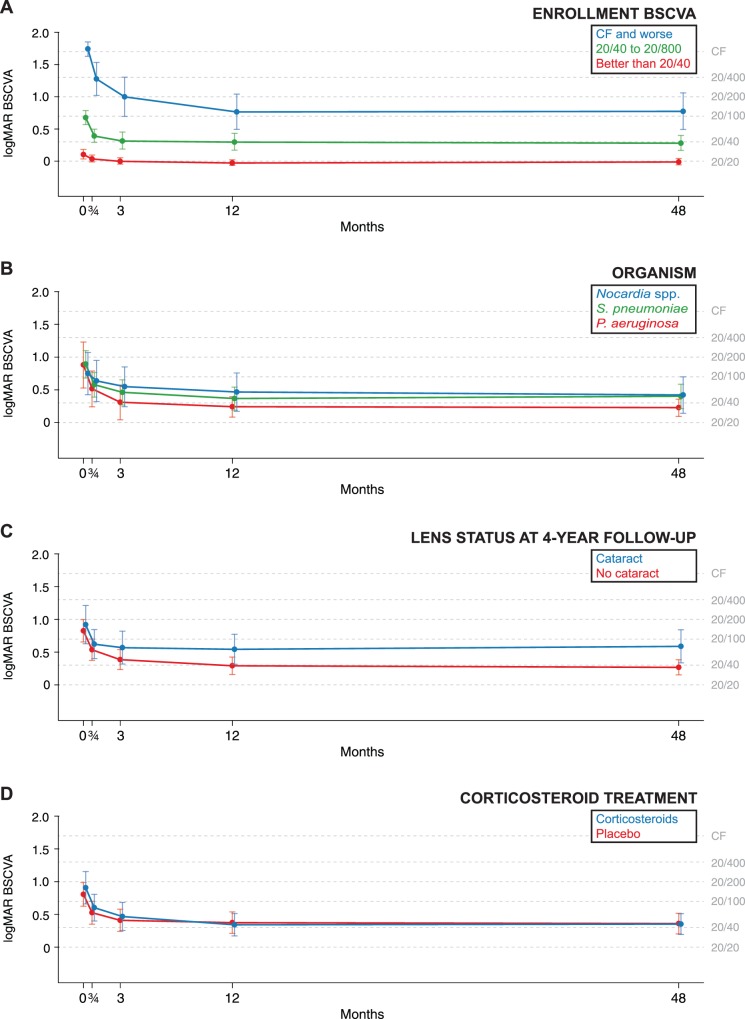
Figure 1 and Table 2 summarize the results from a repeated measures model of the 47 nonoperated participants. On average, visual acuity improved by 2.9 logMAR lines from enrollment to 3 weeks (95% CI, 1.9–3.9; P < 0.001), 1.2 lines from 3 weeks to 3 months (95% CI, 0.4–2.1; P = 0.002), and 0.8 lines from 3 to 12 months (95% CI, 0.2–1.3; P = 0.01). Thereafter, BSCVA did not change significantly, with only a 0.04-line (<1 letter) improvement from 12 months to 4 years (95% CI, −0.5–0.6; P = 0.88). In an attempt to address loss to follow-up, we performed the same analysis on the entire study population eligible for the 4-year follow-up visit who had not had cataract surgery (N = 75), using multiple imputation to account for any missing BSCVA values. Mean BSCVA was worse at each time point in this sensitivity analysis, but including the imputed values did not result in a statistically significant change in BSCVA from 12 months to 4 years (P = 0.99, Table 2). As depicted in Figure 2, the change in BSCVA from 12 months to 4 years was not statistically different for different strata of enrollment visual acuity (P = 0.95 for partial interaction term), causative organism (P = 0.82, analysis omitted three cases not due to Staphylococcus pneumoniae, Pseudomonas aeruginosa, or Nocardia species), SCUT treatment group (P = 0.83), or cataract status at the 4-year study visit (P = 0.55).
Figure 1.
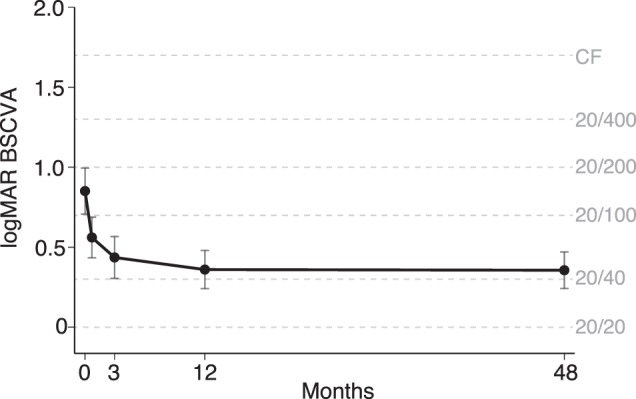
The BSCVA at five study visits in a subset of participants from the SCUT Trial. The predicted means and 95% CIs are shown for the 47 nonoperated participants who had BSCVA data at the 4-year follow-up visit.
Table 2.
Mean BSCVA at Each Study Visit and Change in Vision Over Each Time Interval, Shown Separately for the Complete Case Analysis and the Multiple Imputation Analysis
|
Study Visit |
BSCVA: Complete Case,N= 47 |
BSCVA: Multiple Imputation,N= 75 |
||
|
Mean logMAR (Snellen), 95% CI* |
PValue† |
Mean logMAR (Snellen), 95% CI* |
PValue† |
|
| Enrollment | 0.85 (20/160), 0.71–0.99 | – | 0.97 (20/200), 0.85–1.09 | – |
| 3 wks | 0.56 (20/80), 0.44–0.68 | <0.001 | 0.63 (20/100), 0.52–0.74 | <0.001 |
| 3 mo | 0.43 (20/63), 0.31–0.56 | 0.002 | 0.50 (20/63), 0.38–0.62 | 0.001 |
| 12 mo | 0.36 (20/50), 0.25–0.47 | 0.01 | 0.45 (20/63), 0.32–0.59 | 0.31 |
| 4 y | 0.36 (20/50), 0.25–0.46 | 0.88 | 0.45 (20/63), 0.20–0.70 | 0.99 |
Mean logMAR BSCVA predicted from the repeated measures model, with Snellen equivalent in parentheses, followed by 95% CIs of the logMAR BSCVA.
Wald test of the hypothesis that BSCVA is the same as that of the previous study visit, as assessed from the repeated measures model.
Figure 2.
Subgroup analysis of longitudinal BSCVA in a subset of participants from the SCUT Trial. The predicted means and 95% CIs are shown, stratified by (A) enrollment visual acuity, (B) causative organism, (C) presence of cataract at the 4-year follow-up visit, and (D) treatment allocation in SCUT (steroids versus placebo).
The proportion of study participants with cataract and glaucoma at 4 years was not significantly different when comparing those who had received corticosteroids to those who had received placebo (Table 3). After adjusting for enrollment BSCVA, 4-year BSCVA was no different in the corticosteroid or placebo treatment arms (P = 0.53). Because previous analyses of SCUT have shown that Nocardia ulcers did poorly with topical steroids, we performed a similar analysis stratified by causative organism.10 Although these analyses were consistent with a harmful effect of topical corticosteroids for Nocardia ulcers and a beneficial effect for non-Nocardia ulcers, we found no statistically significant difference between the treatment arms: in Nocardia ulcers, corticosteroids were associated with a 2.1-line reduction in 4-year BSCVA (95% CI, 8.5-line reduction to 4.2-line improvement; P = 0.48), and in non-Nocardia ulcers, corticosteroids were associated with a 1.9-line improvement in 4-year BSCVA (95% CI, 0.2-line reduction to 4.0-line improvement; P = 0.07).
Table 3.
Assessment of Cataract and Glaucoma in a Subset of Study Participants 4 Years Following Enrollment in the SCUT Trial
|
Complication at 4-y Study Visit |
Corticosteroid,N= 24 |
Placebo,N= 26 |
PValue* |
| Cataract | 4 | 9 | 0.20 |
| Cataract surgery since enrollment | 3 | 0 | 0.10 |
| Intraocular pressure ≥ 21 | 0 | 0 | NA |
| Vertical cup-to-disk ratio ≥ 0.7 | 0 | 1 | 1.0 |
NA, not applicable.
Fisher's exact test.
Discussion
The purpose of this study was to evaluate the long-term visual acuity outcomes of subjects with bacterial keratitis. To our knowledge, this is the longest reported duration of prospective follow-up for patients with this condition and, therefore, provides useful insight regarding its natural history. Although BSCVA improved during each study interval up to 12 months, there was no further improvement in visual acuity after this time. Nonetheless, visual acuity was relatively good at the 4-year follow-up visit, with almost 60% of study participants testing at better than 20/40. Poor vision at 4 years usually was due to corneal scarring, although cataract also had a role.
In a previous study, we showed that patients with treated bacterial keratitis experience an approximate 2-line improvement in visual acuity from enrollment to 3 weeks, 1-line improvement from 3 weeks to 3 months, and 1-letter improvement from 3 to 12 months.6 In the current study, we followed a subset of the larger cohort out to 4 years. Except for a higher percentage of Nocardia ulcers, the subset described in the current study had similar baseline characteristics as the larger cohort. The smaller subset experienced a similar degree of improvement in visual acuity up to the 12-month study visit as did the larger cohort, suggesting that the smaller subset is representative of the larger SCUT cohort. Between 12 months and 4 years, this subset experienced no further improvement in visual acuity. The previous study found that the improvement in vision from 3 to 12 months was attributable primarily to those patients with poor vision at enrollment. Although limited by a small sample size, the current study found no evidence for continued visual acuity improvement in the population with poor enrollment visual acuity. Therefore, the current study suggests that decisions regarding corneal transplantation in eyes with healed bacterial keratitis need not be postponed for more than 1 year following the infection, regardless of the severity at presentation.
Changes in visual acuity occur for numerous reasons, and it is possible that age-related causes of vision loss could have confounded this analysis. For this reason, we performed separate analyses stratified by cataract status. We found that even the group without cataract experienced no significant improvement in visual acuity from 12 months to 4 years, suggesting that visual acuity changes related solely to corneal remodeling may largely be complete by 12 months post-keratitis.
Although not the main objective of this study, we compared 4-year visual acuity between study participants who had received adjunctive corticosteroids during SCUT to those who had received placebo. We found no difference in BSCVA at 4 years for subjects treated with topical steroids compared to those who received placebo. Because corticosteroid therapy resulted in worse visual acuity for Nocardia ulcers in SCUT, we also performed analyses stratified on this organism. Although not statistically significant, BSCVA was better in the corticosteroid group compared to the placebo group in non-Nocardia ulcers. This observation is consistent with the 12-month SCUT results, and may indicate a potential long-term benefit of corticosteroids in non-Nocardia ulcers.11 We also observed similar rates of cataract and glaucoma in the 2 treatment groups at 4 years, suggesting that a short course of adjunctive corticosteroids for bacterial keratitis appears to have a reasonable long-term safety profile. Analyses comparing the corticosteroid and placebo groups must be interpreted with caution, since the current study population is only a small subset of the overall trial population.
These findings have practical implications. From the perspective of the ophthalmologist managing cases of bacterial keratitis that do not require immediate surgical intervention, it may be prudent to delay surgery up to 1 year to allow vision to improve. However, if vision has not reached a satisfactory level by this time, further improvement is unlikely and surgical options may be explored. From the patient's perspective, it may be comforting to know that even when the ulcer is severe, the long-term visual acuity still may be quite good. For example, among the 12 patients with counting fingers vision at enrollment, half had a BSCVA of approximately 20/70 or better at 4 years, and a quarter had vision of approximately 20/40 or better.
This study is generalizable only to patients with bacterial corneal ulcers that could be enrolled in SCUT. The population of ulcers enrolled in SCUT made up less than one-third of bacterial ulcers presenting for care during the time period of the trial, with common reasons for exclusion including an impending perforation or preexisting corneal scar.7 Therefore, the ulcers in this study are likely biased toward those individuals who had better vision, and may not be an accurate representation of the natural history of more severe bacterial corneal ulcers. Moreover, of the 80 subjects eligible for a 4-year study visit, only 50 (62.5%) were examined. Those that followed up for an examination tended to have less severe ulcers at enrollment, which may have further biased this study toward better outcomes. We addressed the loss to follow-up in a sensitivity analysis that imputed the missing values. This sensitivity analysis, while not changing the statistical significance or conclusions of the study, did estimate a mean BSCVA that was approximately one line worse at each time point. Thus, although we found the potential for relatively good vision following treatment for bacterial keratitis, it is quite possible that the majority of bacterial corneal ulcers that occur in India will experience a poorer outcome than reported here.
Strengths of this study include its prospective design, the extended duration of follow-up, and the standardized assessment of BSCVA at multiple prespecified time points. There also are several limitations. As discussed above, the participants for the current study were recruited using a convenience sample of SCUT participants. This reduced the sample size for the current study and did not allow an unbiased analysis comparing the randomization units from the clinical trial. However, the study participants included in the current study generally were similar to the overall SCUT population, suggesting that the conclusions from this small subset of patients may not be too dissimilar from the SCUT population. Nocardia ulcers were relatively over-represented in this South Indian population, which may reduce the generalizability of the study to other geographic locations. However, subgroup analyses stratified by causative organism did not suggest that the visual outcomes of Nocardia ulcers were different from that of other organisms. Finally, we relied solely on logMAR visual acuity, which does not take into account other aspects of vision that may have significant impacts on a patient's visual experience, such as contrast sensitivity, color perception, and glare.12–14 It is possible that reductions in corneal scar opacification are associated with improvements in one or more of these aspects, a finding that would have been overlooked by the current study.
In conclusion, we found that in cases of medically managed bacterial keratitis, visual acuity may improve over the first year, but further improvements are unlikely. These findings may guide the appropriate timing of surgical intervention in these patients.
Acknowledgments
The authors thank Don Everett, who was the National Eye Institute program officer for the SCUT Trial, as well as the trial's Data and Safety Monitoring Committee: Marian Fisher, PhD (chair), Anthony Aldave, MD, Donald Everett, MA, Jacqueline Glover, PhD, Kanagasabai Anandakannan, MD, Steven Kymes, PhD, Gudlavalleti Venkata Satyanarayana Murthy, MD, Ivan Schwab, MD.
Supported by National Eye Institute Grant U10 EY015114 (TML), National Eye Institute Grant K23 EY017897 and a Research to Prevent Blindness Award (NRA), National Eye Institute Grant K23 EY019071 and a Research to Prevent Blindness Award (JDK), Alcon/Novartis AG (provided moxifloxacin [Vigamox] for the trial), and Core Grant EY02162 from the National Eye Institute to the Department of Ophthalmology at the University of California, San Francisco. The authors alone are responsible for the content and writing of the paper.
Disclosure: S.M. McClintic, None; N.V. Prajna, None; M. Srinivasan, None; J. Mascarenhas, None; P. Lalitha, None; R. Rajaraman, None; C.E. Oldenburg, None; K.S. O'Brien, None; K.J. Ray, None; N.R. Acharya, None; T.M. Lietman, None; J.D. Keenan, None
References
- 1. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012; 96: 614–618 [DOI] [PubMed] [Google Scholar]
- 2. Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001; 79: 214–221 [PMC free article] [PubMed] [Google Scholar]
- 3. Morlet N, Minassian D, Butcher J. Risk factors for treatment outcome of suspected microbial keratitis. Ofloxacin Study Group. Br J Ophthalmol. 1999; 83: 1027–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol. 2001; 85: 842–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Constantinou M, Daniell M, Snibson GR, Vu HT, Taylor HR. Clinical efficacy of moxifloxacin in the treatment of bacterial keratitis: a randomized clinical trial. Ophthalmology. 2007; 114: 1622–1629 [DOI] [PubMed] [Google Scholar]
- 6. Srinivasan M, Mascarenhas J, Rajaraman R, et al. Visual recovery in treated bacterial keratitis [published online ahead of print March 5, 2014] Ophthalmology. doi: 10.1016/j.ophtha.2013.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT). Arch Ophthalmol. 2012; 130: 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Srinivasan M, Mascarenhas J, Rajaraman R, et al. The steroids for corneal ulcers trial: study design and baseline characteristics. Arch Ophthalmol. 2012; 130: 151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diabetic Retinopathy Clinical Research Network. Visual Acuity Score Conversion Chart. Available at: http://publicfiles.jaeb.org/drcrnet/Misc/VAScoreConversionChart.pdf. Accessed April 2, 2014 [Google Scholar]
- 10. Lalitha P, Srinivasan M, Rajaraman R, et al. Nocardia keratitis: clinical course and effect of corticosteroids. Am J Ophthalmol. 2012; 154: 934–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Srinivasan M, Mascarenhas J, Rajaraman R, et al. The Steroids for Corneal Ulcers Trial (SCUT): Secondary 12-month clinical outcomes of a randomized controlled trial. Am J Ophthalmol. 2013; 157: 327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mainster MA, Turner PL. Glare's causes, consequences, and clinical challenges after a century of ophthalmic study. Am J Ophthalmol. 2012; 153: 587–593 [DOI] [PubMed] [Google Scholar]
- 13. Zadnik K, Barr JT, Edrington TB, et al. Corneal scarring and vision in keratoconus: a baseline report from the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea. 2000; 19: 804–812 [DOI] [PubMed] [Google Scholar]
- 14. Owsley C, Sloane ME. Contrast sensitivity, acuity, and the perception of ‘real-world' targets. Br J Ophthalmol. 1987; 71: 791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]