Abstract
Purpose.
Retinal microglia become activated in diabetes and produce pro-inflammatory molecules associated with changes in retinal vasculature and increased apoptosis of retinal neurons and glial cells. We sought to determine if the action of aldose reductase (AR), an enzyme linked to the pathogenesis of diabetic retinopathy, contributes to activation of microglial cells.
Methods.
Involvement of AR in the activation process was studied using primary cultures of retinal microglia (RMG) isolated from wild-type and AR-null mice, or in mouse macrophage cultures treated with either AR inhibitors or small interfering RNA (siRNA) directed to AR. Inflammatory cytokines were measured by ELISA. Cell migration was measured using a transwell assay. Gelatin zymography was used to detect active matrix metalloproteinase (MMP)-9, while RMG-induced apoptosis of adult retinal pigment epithelium (ARPE-19) cells was studied in a cell coculture system.
Results.
Aldose reductase inhibition or genetic deficiency substantially reduced lipopolysacharide (LPS)-induced cytokine secretion from macrophages and RMG. Aldose reductase inhibition or deficiency also reduced the activation of MMP-9 and attenuated LPS-induced cell migration. Additionally, blockade of AR by sorbinil or through genetic means caused a reduction in the ability of activated RMG to induce apoptosis of ARPE-19 cells.
Conclusions.
These results demonstrate that the action of AR contributes to the activation of RMG. Inhibition of AR may be a therapeutic strategy to reduce inflammation associated with activation of RMG in disease.
Keywords: retinal microglia, aldose reductase, LPS, inflammation, migration, MMP-9, aldose reductase inhibitor, β-glucogallin
Retinal microglia contribute to the production of pro-inflammatory cytokines in the eye. We show that downregulation of aldose reductase in microglia inhibits response to endotoxin, suggesting that aldose reductase inhibitors may be useful against retinal inflammatory disease.
Introduction
Chronic inflammation is now recognized as a key factor in the pathogenesis of diabetic retinopathy (DR).1 Increased levels of pro-inflammatory signaling molecules such as TNF-α and IL-1β have been measured in patients with DR2,3 and in diabetic animal models.4 Tumor necrosis factor α–induced pathways become activated in the diabetic retina, resulting in increased levels of effector gene products such as intercellular adhesion molecule 1 (ICAM-1) in the retinal vasculature and associated alterations in interactions between circulating leukocytes and the vascular wall.5 Outside the eye, macrophages are phagocytic cells considered to be the major source of TNF-α and IL-1β,6 while microglia are thought to be the major cell type responsible for production of similar cytokines in the ocular environment.7 Microglia are considered the resident macrophages of the eye.8 Like macrophages, microglia are capable of adopting an “activated” state accompanied by elaboration of a variety of pro-inflammatory cytokines in response to metabolic stress or after encounter with foreign materials.4,9 Increased numbers of activated retinal microglia (RMG) have been detected in human eyes with DR,10 as well as in various experimental diabetic models in rats.11–14
Several potential mechanisms have been advanced to link the effects of chronic hyperglycemia on RMG activation. For example, advanced glycation endproducts (AGEs), which accumulate in the diabetic retina, have been shown to potently induce RMG activation.11,15 Gardner and colleagues16 have hypothesized that activated RMG could play a role in the pathogenesis of DR through increased release of VEGF and TNF and associated exacerbation of blood vessel permeability. Many studies have shown that treatment of diabetic rats with aldose reductase inhibitors (ARIs) leads to reduced level of VEGF in the eye.17,18 Aldose reductase inhibitors also prevent high glucose-induced TNF-α secretion.19 Taken together, these observations led us to question whether AR could play a role in regulating the activation of RMG.
Aldose reductase (AKR1B1) is an aldo-keto reductase responsible for polyol synthesis in target tissues of diabetes.20 In addition, the action of AR has been linked to the expression of pro-inflammatory molecules in a wide variety of tissues and diseases.21,22 In animal models, genetic or pharmacologic blockade of AR prevents the onset and/or progression of many defects associated with long-term diabetes, such as retinal pericyte loss23 and capillary degeneration24 as well as reduced markers of oxidative stress typically associated with DR.25 Previous studies demonstrated a potential role for AR in regulation of endotoxin-induced production of cytokines in macrophage cultures,26–29 as well as in experimental models of endotoxin-induced uveitis.22,26
Given the emergence of AR as a factor in the macrophage response to activation by endotoxin, and in light of the functional similarities between macrophages and RMG, we evaluated whether genetic or pharmacologic downregulation of AR in RMG can influence the activation response. Comparing primary cultures of RMG established from wild-type and AR-deficient mice, we examined whether absence of AR influenced the endotoxin-induced activation response. As a corollary experiment, we measured the effects of two structurally diverse AR inhibitors on various functional parameters associated with activation. Inhibitors included sorbinil, a well-characterized ARI, and β-glucogallin, a novel ARI recently identified from extracts of the Indian gooseberry plant.30 Our results point to a role for AR in RMG activation and suggest that AR inhibition may offer a strategy to downregulate this process in retinal disorders.
Materials and Methods
Materials and Cell Culture
β-glucogallin (BGG) was isolated and purified as previously reported.30 Lipopolysacharide (LPS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Sorbinil ([4S]-6-Fluoro-2,3-dihydro-spiro[4H-1-benzopyran-4,4′-imidazolidine]-2′,5′-dione) was generously provided by Pfizer Central Research (Groton, CT, USA). Control small interfering RNA (siRNA) and AKR1B3 siRNA (siAR) were purchased from Qiagen (Valencia, CA, USA). Matrix metalloproteinase (MMP)-9 inhibitor was purchase from EMD Millipore (Billerica, MA, USA). RAW264.7 macrophages were cultured in complete Dulbecco's Modified Eagle Medium (DMEM; Corning Cellgro, Manassas, VA, USA) supplemented with 4 mM L-glutamine, 10% (vol/vol) fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin. Adult retinal pigment epithelium (ARPE-19) cells were purchased from ATCC (Manassas, VA, USA) and cultured in low glucose (1 g/L) DMEM supplemented with 4 mM L-glutamine, 10% (vol/vol) fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained in a humidified incubator containing 5% carbon dioxide at 37°C.
Culture of Primary Mouse Retinal Microglia
This research was conducted in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME, USA) were handled in strict accordance with good animal practice and all animal work was approved by Institutional Animal Care and Use Committee at University of Colorado Anschutz Medical Campus (Aurora, CO, USA). Retinal microglia were isolated from retinas of 8- to 10-week-old C57BL/6 mice using a method modified from a previous study.31 Briefly, eyes were removed and retinas dissected under aseptic conditions from globes. Tissue was then incubated in Hanks' balanced salt solution (HBSS; Corning Cellgro) buffer containing papain with 180 units/mL DNase (Worthington Biochemical Corp., Lakewood, NJ, USA) at 37°C for 10 to 20 minutes. Cells were then briefly triturated, added to an equal volume of ovomucoid protease inhibitor (10 mg/mL, Worthington Biochem. Corp.), and spun at 300g for 10 minutes at 4°C. The dissociated cells were transferred to 75 cm2 flasks and cultured for 4 weeks in DMEM formulated as described above for ARPE cells. After this mixed culture had grown to confluency, the flasks were shaken at 100 rpm on an orbital shaker for 1 hour. Detached cells were then grown in 24-well plates for immunofluorescence or in 96-well plates for ELISA.
Immunofluorescence
Retinal microglia were incubated on gelatin-coated glass slides in a 24-well plate. Cells were fixed with 4% paraformaldehyde in PBS for 60 minutes at room temperature and then permeabilized with 0.1% Triton X-100 in PBS for 20 minutes. Retinal microglia were identified by incubating with rabbit anti-Iba-1antibody (1:400; Wako, Richmond, VA, USA) at 4°C overnight, followed by staining with Alexa Fluor 488 Goat Anti-Rabbit immunoglobulin G (IgG; 1:1000; Invitrogen, Carlsbad, CA, USA) and 4′,6-diamidino-2-phenylindole (DAPI; 1:5000, Sigma-Aldrich) for 60 minutes. Images were obtained using a Nikon Eclipse 80i light microscope fitted to a Nikon DS Qi1Mc camera (Nikon Instrument, Inc., Tokyo, Japan).
Western Blotting
Lysates were prepared by suspending cells in Laemmli sample buffer (Sigma-Aldrich) and heating to 100°C for 10 minutes. After resolution by SDS-PAGE (Bio-Rad, Hercules, CA, USA), materials were transferred onto nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The following primary antibodies were used for immunodetection: rabbit anti-human AR (1:1000) as reported previously,32 mouse anti-actin (1:4000; Sigma-Aldrich), or rabbit anti-MMP-9 (1:1000; Abcam, Cambridge, MA, USA). Secondary anti-mouse and anti-rabbit antibodies conjugated to horseradish peroxidase (1:5000; Millipore, Bedford, MA, USA), as well as the Western blot Substrate kit (Bio-Rad) were used to detect chemiluminescence using a BioRad ChemiDoc XRS+ imaging system.
ELISA Assay
Raw264.7 macrophages or RMG were incubated in a 6-well or 24-well plate, and media were collected after the indicated treatment. Secreted TNF-α and IL-1β in media were determined using corresponding Mouse Cytokine and Growth Factor Immunoassays (ElisaTech, Aurora, CO, USA). The optical density was detected using a BioTek Synergy 4 Hybrid Microplate Reader (BioTek, Winooski, VT, USA) and the levels of each cytokine were deduced from the absorbance value by extrapolation from a standard curve generated in parallel.
In Vitro Migration Assay
Using modified 24-well plate Boyden chambers fitted with filter inserts (pore size 8 μm; Greiner bio-one, Monroe, NC, USA), cells (2 × 104) were seeded in the upper chambers. Aldose reductase inhibitors were added to upper and lower chambers, while LPS was added to the lower chamber only. After incubating for 7 hours, cells were fixed with ice-cold methanol for 15 minutes and stained with 2% crystal violet for 30 minutes and the number of migrated cells on the side facing the lower chamber was determined. In the case of RMG, the entire filter area was counted under ×100 magnification to determine the total number of cells that migrated through the membrane. In experiments using the RAW264.7 macrophage cell line, an average number of migrated cells was determined by counting cells in at least three randomly selected fields under ×100 magnification. Results for each condition from three independent experiments was then averaged and reported as percent change relative to vehicle control.
Zymography
Matrix metalloproteinase–9 gelatinase activity was measured in conditioned medium by zymography. This procedure has been shown to estimate both proenzyme and activated MMP-9 enzyme activity. Equal amounts of conditioned medium were subjected to electrophoresis on 10% zymography gels containing 0.1% gelatin (Bio-Rad). Gels were washed with renaturing buffer (Bio-Rad) for 30 minutes, incubated in developing buffer (Bio-Rad) overnight at 37°C, and stained with Coomassie blue (Sigma-Aldrich). Gelatinase activity was detected in sample lanes by the appearance of bands of lighter gel staining due to digestion of gelatin.
Apoptosis of ARPE-19
Retinal microglia were seeded in the upper chamber of a transwell device and treated with LPS for 6 hours. After treatment, RMG were washed twice with PBS. To measure the effects of cytokines released from LPS-activated RMG, the upper chamber was removed and fitted to a lower chamber containing ARPE-19 cells. After coculture for 48 hours, the level of early apoptosis in RPE cells from the lower chamber was determined using the Annexin-V-FITC Apoptosis Detection kit (BD Biosciences, San Jose, CA, USA) as described.33 Adult retinal pigment epithelium–19 cells were released by trypsin treatment and were washed twice with cold PBS and resuspended in 1× binding buffer (10 mM HEPES [Sigma-Aldrich], pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). The cell suspension (100 μL) was transferred to 5-ml tubes, and 5 μL Annexin-V was added. After incubation with Annexin-V for 5 minutes at 4°C, 5 μL of propidium iodide (500 μg/mL) was added. The cells were incubated at 4°C in the dark for 20 minutes, and 400 μL of binding buffer was added before fluorescence-activated cell sorting (FACS) analysis.
Statistical Analysis
Results are shown as the Means ± SEM of at least three experiments. Data were analyzed by ANOVA with P value of less than 0.05 considered significant.
Results
Aldose Reductase Level in Mouse Macrophage and Retinal Microglia
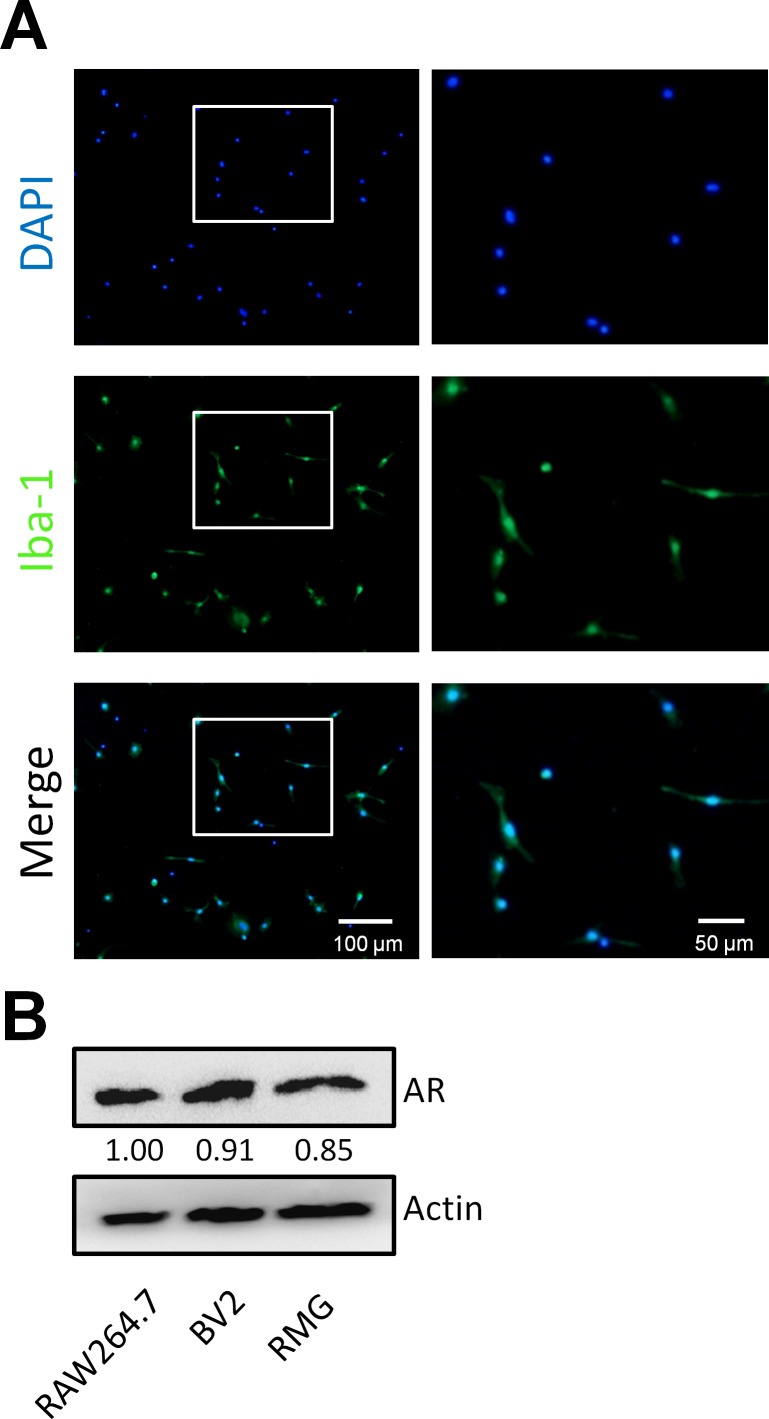
Previous studies have demonstrated high levels of AR in macrophages.27 To determine the level of AR expression in RMG we used primary cultures established from retinas dissected from the eyes of C57BL/6 mice. Using cells cultured for 4 weeks as previously described,34 we estimated purity by immunostaining for the presence of ionized calcium binding adaptor molecule 1 (Iba-1), a well-characterized marker for RMG.31 By comparison with total cell counting based on DAPI staining, we estimated that 95% (74 of 78) of cells express the Iba-1 marker (Fig. 1A). We then used immunoblotting to confirm the presence of AR in RMG, as well as in the microglial cell line BV2 and murine macrophage cell line RAW264.7 (Fig. 1B).
Figure 1.
Aldose reductase expression in RMG primary culture cells. (A) Immunofluorescence for Iba-1. Retinal microglia cell cultures established from C57BL/6 mice were immunostained for the microglial marker Iba-1 and with DAPI to visualize nuclei. (B) The AR levels were determined by Western blotting of extracts from murine macrophage RAW264.7 cells, murine microglial BV2 cells, and retinal microglia. Chemiluminescent values were used to compute the signal intensity from each band. Numerical values of band intensities BV2 and RMG samples were normalized to that observed in RAW264.7.
Aldose Reductase Inhibition or Deficiency Reduces LPS-Induced Inflammatory Cytokine Secretion
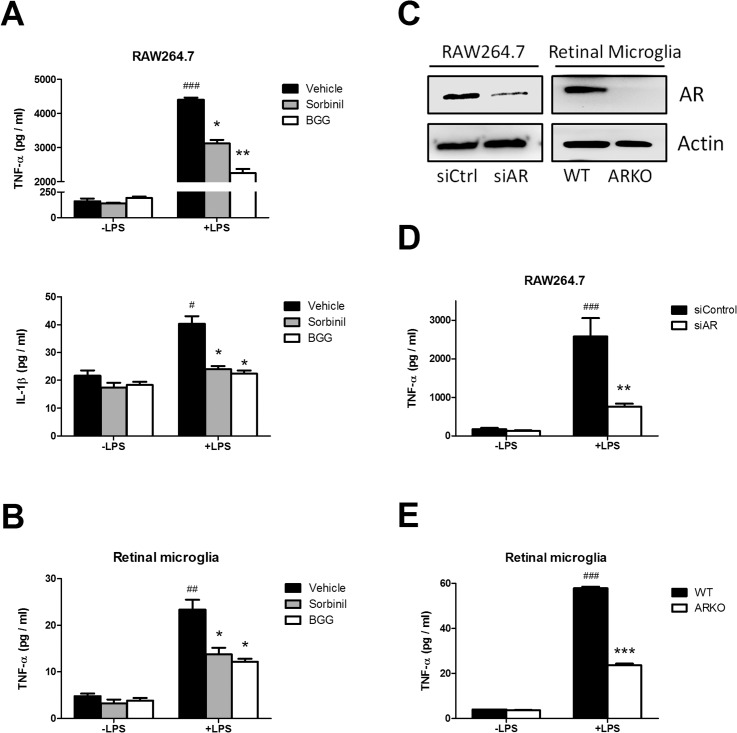
Lipopolysacharide is a potent inducer of the inflammatory response, even at very low doses, in macrophages. It has been shown that AR can mediate LPS-induced inflammatory cytokine expression.28 Therefore, we asked whether AR inhibition could attenuate cytokine secretion in RMG. To determine this, LPS-induced secretion of TNF-α and IL-1β was measured in macrophage and RMG cultures treated with the AR inhibitors sorbinil or BGG. Both ARIs attenuated the LPS-induced secretion of TNF-α and IL-1β in macrophages (Fig. 2A), as well as a significant reduction of TNF-α secretion in RMG (Fig. 2B). Next, we used a genetic approach to further confirm the role of AR in the endotoxin response. By Western blotting, we observed that AR expression was significantly reduced using a siRNA approach in RAW264.7 macrophages and was not detectable in RMG that were cultured from retinas of AR-null mice (Fig. 2C). Both AR knockdown in macrophages (Fig. 2D) and AR gene deficiency in RMG (Fig. 2E) reduced LPS-induced TNF-α secretion. These data demonstrate that endotoxin-induced secretion of TNF by RMG can be suppressed by downregulation of AR by either genetic means or through pharmacologic reduction of its enzymatic activity using ARIs.
Figure 2.
Effect of ARIs and AR deficiency on LPS-induced cytokine secretion. Macrophages and RMG were incubated with vehicle, BGG (50 μM), or Sorbinil (10 μM) for 24 hours in the absence or presence of LPS (20 ng/mL) for 4 hours. The cytokines in culture medium from macrophages (A, D) or RMG (B, E) were measured by ELISA. (C) Western blotting demonstrated that AR expression was reduced by siRNA in macrophages or gene knockout in RMG. (D) Aldose reductase expression was reduced in Raw264.7 cells by siRNA. (E) Retinal microglia were isolated from wild-type or AR-knockout mice. Both knockdown and knockout cells were treated with LPS for 4 hours before harvest. In (A) and (B), * compares vehicle versus ARI in LPS treatment. # compares LPS and no LPS between vehicle-treated groups. In D, # compares LPS versus no LPS treatment; * compares AR versus control siRNA treatment in LPS-treated group. In E, # compares LPS versus no LPS treatment; * compares wild-type and ARKO in LPS-treated group. Data are represented as the Mean ± SEM (N = 3). *, #P < 0.05; **, ##P < 0.01; ***, ###P < 0.005.
Aldose Reductase Inhibition or Deficiency Prevents Cell Migration Under LPS Exposure
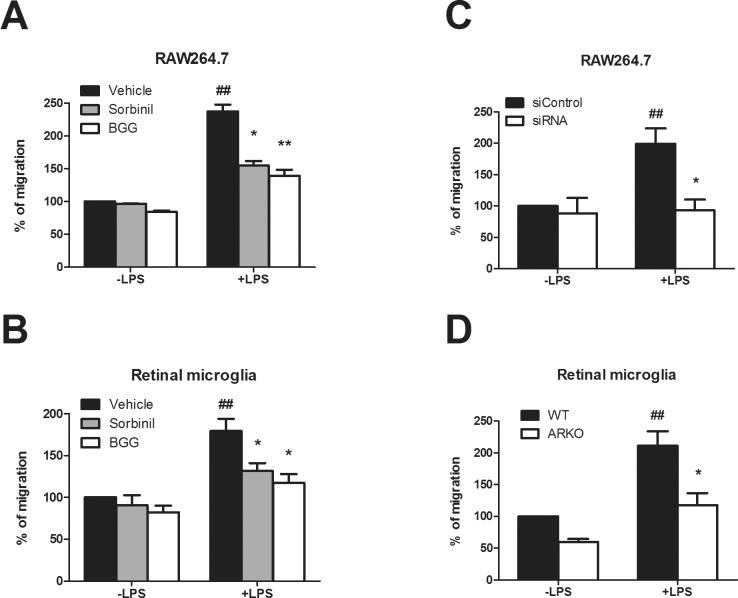
Cell motility is a key feature of macrophage and microglial cell biology, and it is known that LPS induces macrophage migration.35 However, a possible role for AR in cell migration is still unknown. To explore this possibility, we investigated the effect of AR inhibition on LPS-induced cell migration. In macrophages, LPS increased cell migration to 238% of control. However, pretreatment of cells with Sorbinil or BGG suppressed the LPS-induced increase to 155% and 140% of control, respectively (Fig. 3A). In a similar trend, LPS exposure to RMG increased cell migration to 180% of control, but migration increased only 130% and 127% by pretreatment of cells with Sorbinil or BGG, respectively (Fig. 3B). Likewise, this same effect was also achieved by downregulation of the AR gene expression using siRNA (Fig. 3C). In RMG, the LPS-induced increase in cell migration was largely absent in RMG cultured from AR-null mice (Fig. 3D). These data indicated that AR is a contributing factor important for both macrophage and RMG migration following endotoxin exposure.
Figure 3.
Importance of AR in cell migration. Macrophages (A) and retinal microglia (B) were cultured in the top chamber of a transwell insert with medium containing LPS in the bottom chamber. Cells were cultured in the absence or presence of 10 μM Sorbinil or 50 μM BGG with or without LPS (100 ng/mL), as indicated, for 7 hours. Aldose reductase in cells was reduced by siRNA (C) or gene deletion (D). Following incubation, cells were visualized by staining with 2% crystal violet and counted. In (A, B), * compares vehicle versus ARI in LPS treatment. # compares LPS and no LPS between vehicle-treated groups. In C, # compares LPS versus no LPS treatment; * compares AR versus control siRNA treatment in LPS-treated group. In (D), # compares LPS versus no LPS treatment; * compares wild-type and ARKO in LPS-treated group. Data are represented as the Mean ± SEM (N = 3). *P < 0.05; **, ##P < 0.01.
Aldose Reductase Inhibition or Knockdown Attenuates LPS-Induced MMP-9 Activation
It has been shown in lens epithelial cells and vascular smooth muscle cells that MMP-9 is activated by LPS exposure in a manner that is sensitive to AR inhibitors.36,37 To examine the effect of ARIs on MMP-9 activation, macrophages were pretreated with Sorbinil or BGG in the absence or presence of LPS. Here, we collected the culture medium and analyzed gelatinase activity by zymography. We also measured total MMP-9 protein expression in cell lysates using Western blot analysis. As shown in Figures 4A and 4B, MMP-9 was activated by LPS exposure and suppressed by ARIs, suggesting that ARIs are potentially effective in preventing cell migration by suppression of MMP-9 activation. Similarly, we found that AR knockdown reduced MMP-9 activation evidenced by zymography (Fig. 4C) and Western blotting (Fig. 4D). We further confirmed this effect using a specific MMP-9 inhibitor, which suppressed LPS-induced macrophages migration (Fig. 4E). These data are consistent with previously studies that AR inhibition suppresses MMP-9 activation.36,37
Figure 4.
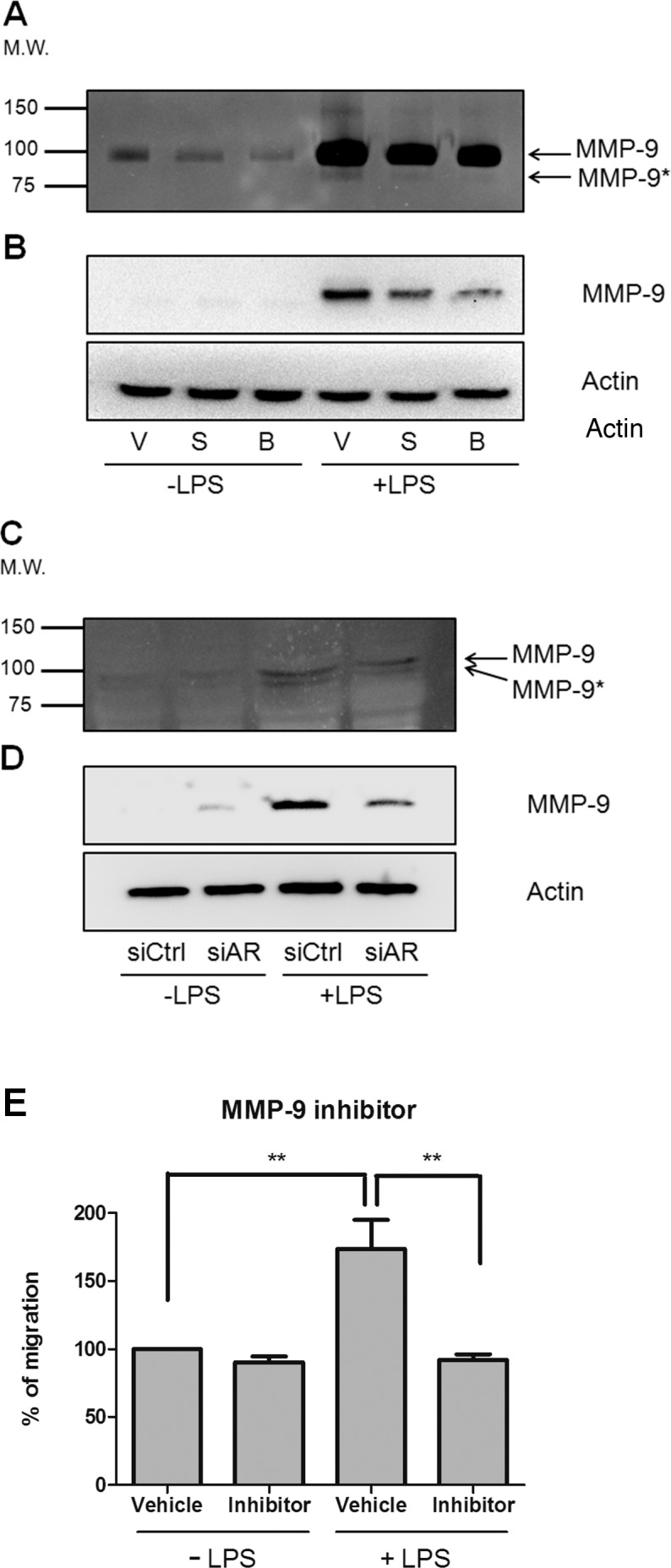
Reduction of AR expression reduces MMP-9 expression. For pharmacologic inhibition, macrophages were pretreated with vehicle (V), Sorbinil (S, 10 μM), or BGG (B, 50 μM) for 30 minutes followed by LPS (100 ng/mL) exposure for 12 hours. For genetic ablation, macrophages were transfected with control or AR siRNAs for 72 hours followed by LPS exposure for 12 hours. Pro–MMP-9 and active MMP-9 (MMP-9*) secreted into the medium were detected by gelatin zymography (A, C). Western blot detected MMP-9 in cell lysate (B, D). Macrophages migration was measured in transwell chambers using cells cotreated with MMP-9 inhibitor (1 μM) and LPS (100 ng/mL) for 7 hours before staining with crystal violet for cell counting (E). Data are represented as the Mean ± SEM (N = 3). **P < 0.01.
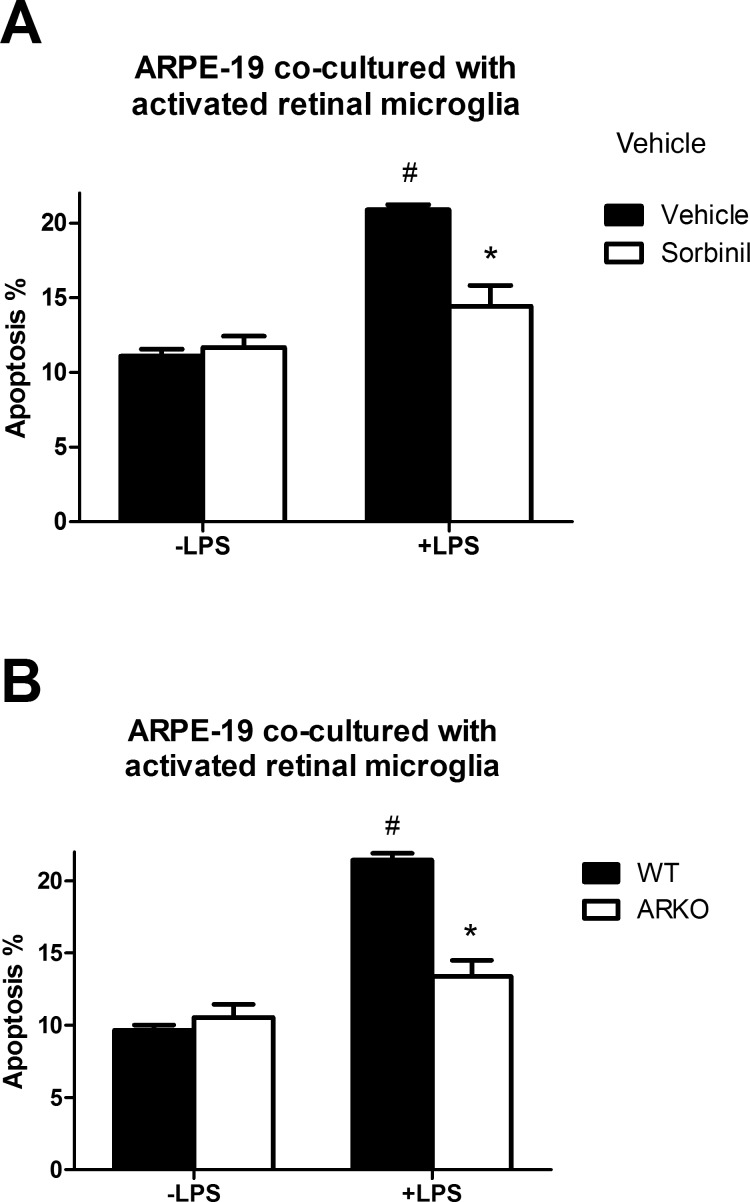
Aldose Reductase Inhibition or Deficiency Rescues Apoptosis of ARPE-19 Caused by Activated Retinal Microglia
Tumor necrosis factor α is a robust pro-inflammatory cytokine secreted under a variety of conditions. Previous studies showed that TNF-α secreted from activated microglia induces neurodegeneration.38,39 Superoxide and TNF-α have been demonstrated to cause apoptosis in ARPE-19 cells.40 We demonstrate in Figure 2 that AR inhibition or deficiency reduces TNF-α secretion, therefore, we hypothesized that reduction of AR activity in RMG may prevent RMG-induced apoptosis of ARPE-19. To test this hypothesis, we pretreated RMG with Sorbinil in the absence or presence of LPS, which significantly reduced RMG-induced apoptosis (Fig. 5A). We also examined the genetic effect of AR on apoptosis using ARPE-19 cocultured with RMG from AR-null mice, which also decreased apoptosis compared with RMG from wild-type mice (Fig. 5B). Taken together, these results indicate that AR activity is contributing to RMG-induced apoptosis in ARPE-19 cells.
Figure 5.
Effect of RMG activation on RPE apoptosis. (A) Retinal microglia were treated with vehicle or Sorbinil (10 μM) in the absence or presence of LPS (100 ng/mL) for 6 hours and then transferred for coculture with ARPE-19 cells for 48 hours as described in Materials and Methods. (B) Retinal microglia from wild-type or ARKO were treated with LPS 6 hours and then cocultured with ARPE-19 cells for 48 hours (B). In all cases, apoptosis was measured by FACS with Annexin V-PI kit. In (A) * compares vehicle versus ARI in LPS treatment. # compares LPS and no LPS between vehicle-treated groups. In (B) # compares LPS versus no LPS treatment; * compares WT and ARKO in LPS-treated group. Data are represented as the Means ± SEM (N = 3). #P < 0.05; *P < 0.05.
Discussion
Retinal microglia are phagocytic cells that are responsible for cleaning up apoptotic debris throughout the inner and outer plexiform layers of the retina.8 Resting RMG can become activated by exposure to a variety of factors, including endotoxin or glycated albumin,11,31 where they undergo a morphologic transformation to assume an ameboid shape and migrate to areas of tissue damage. As in macrophages, activated RMG upregulate extracellular signal-regulated kinase (ERK) and mitogen-activated protein kinase signaling, which leads to release of TNF-α.11 Activated RMG have been linked to a variety of ocular disorders such as AMD,31,41,42 light-induced retinal degeneration,43 DR,13 glaucoma,44 and endotoxin-induced uveitis.45
As phagocytic cells, macrophages and RMG have many functional similarities. Studies in macrophage cultures have demonstrated that AR inhibition or deficiency prevents endotoxin-induced nuclear factor–κB activation leading to production of pro-inflammatory.29 Similarly, many studies have shown that RMG secret pro-inflammatory cytokines.31,34 While AR inhibitors prevent endotoxin-induced inflammatory responses in macrophage cultures,26 the potential role for AR in regulating the inflammatory response of RMG has not been investigated, particularly the inflammatory response induced by endotoxin or other factors that cause transition of RMG to an activated state.
In agreement with studies of Ramana and coworkers,27,28 our previous studies demonstrated that reduction of AR activity, using either genetic or pharmacologic inhibitors, substantially suppressed the LPS-induced secretion of TNF-α by macrophage cultures. Reactive oxygen species (ROS) is a key factor in the induction of TNF-α through activation of p38 or ERK.11,34 Our previous study with the RAW264.7 macrophage cell line showed that AR inhibition attenuates p38 or ERK activation and suppresses ROS production following LPS exposure26 indicating that AR inhibition is capable of suppressing TNF-α secretion. In the current study, we evaluated the role of AR activity in response of RMG to activation. Using primary cultures of microglia generated from the mouse eye, we demonstrated that RMG have levels of AR that are similar to murine cell line RAW264.7 (Fig. 1). In addition, we showed that loss of AR activity through BGG or sorbinil inhibition or via AR knockout mice attenuated LPS-induced TNF-α secretion in RMG (Fig. 2). Taken together, these findings demonstrate the functional similarity between macrophages and RMG with regard to the involvement of AR in regulating cytokine production. It has been shown that TNF-α, as one of the major cytokines secreted by activated RMG,31,46 may be involved in neurodegeneration39 and uveitis.47 Therefore, inhibition of TNF-α through pharmacologic inhibition of AR in microglia may hold promise as a potential new strategy against ocular inflammation.
In the inflamed eye, activated RMG migrate into the subretinal space and cause RPE disorganization.31 Prevention of RMG migration might be another strategy to minimize collateral damage to cells during inflammation. We demonstrated that AR inhibition or genetic knock down suppresses LPS-induced cell migration in both macrophages and RMG (Fig. 3). This is the first report indicating that AR mediates endotoxin-induced migration of immune cells. This result is also consistent with our previous study that AR inhibition prevents LPS-induced infiltration of inflammatory cells into the eye.26 The mechanism linking AR activity and cell migration is not yet understood. Thus, to further characterize these mechanisms, we investigated whether AR mediates MMP-9 activation after LPS exposure. Importantly, MMP-9 is an enzyme that induces cellular morphologic changes promoting increased motility.48,49 We confirmed that AR inhibition reduces the LPS-stimulated activation of MMP-9 (Fig. 4), which further characterizes the effects of AR inhibition on mechanisms controlling cell migration.
The distribution of RMG throughout the retina changes with age. In younger animals, RMG are found primarily in the inner retina. However, with age or after adopting an “activated” state, RMG can be found in higher numbers in the subretinal space between photoreceptor outer segments and the RPE. Wong and coworkers31 have shown than activated RMG disrupt the normal organization of RPE and alter the expression of key junctional proteins such as ZO-1. To determine whether AR inhibition or ablation in RMG influences the viability of RPE cells, we carried out coculture experiments with RMG and ARPE-19. Apoptosis was significantly higher when RPE cells were cocultured with activated RMG, which was significantly diminished by pretreating with ARIs. Similarly, RMG derived from AR-null mice were substantially less effective in inducing RPE apoptosis (Fig. 5). Thus, either AR inhibition or ablation reduced the ability of activated RMG to induce apoptosis in ARPE-19 (Fig. 5). Interestingly, it appears that diabetic mice that are null for the AR gene have markedly reduced levels of defects typically associated with DR, including retinal capillary degeneration and iNOS activation.23,24 Further study will be required to determine whether the blockade of AR in RMG can translate into reduced markers of inflammation in other cells and tissues in the diabetic eye, including the retinal vasculature and neurons known to be damaged by chronic hyperglycemia such as in diabetes.5
Despite discouraging results from clinical trials of ARI against DR and neuropathy, research continues on the development of newer generations of inhibitors for clinical study.50 We recently identified β-glucogallin as a novel ARI from Indian gooseberry (Emblica officinalis).30,51 In addition to having ARI activity in aldo-keto reductase assays, we found that β-glucogallin was effective at reducing inflammatory cells in a murine uveitis model.26 Results from the current study demonstrate that β-glucogallin suppresses many of the functional responses of macrophages and RMG to LPS exposure, including cytokine production, cell migration, and induction of MMP-9. Further study will be required to determine if β-glucogallin, or structurally-related derivatives,51 are effective at downregulation of RMG activation in the diabetic retina.
Acknowledgments
The authors thank Cynthia Ju (Skaggs School of Pharmacy and Pharmaceutical Science, University of Colorado) for providing Raw264.7 murine macrophages. They also thank Joseph Brzezinski, Niklaus Mueller, Alan Palestine, Michelle Pedler, and Ko Uoon Park (Department of Ophthalmology, University of Colorado) for their many helpful suggestions and insights.
Supported by National Institutes of Health (Bethesda, MD, USA), Grants EY005856 (JMP) and EY021498 (DVL and JMP).
Disclosure: K.-C. Chang, None; J. Ponder, None; D.V. LaBarbera, P; J.M. Petrash, P
References
- 1. Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004; 18: 1450–1452 [DOI] [PubMed] [Google Scholar]
- 2. Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD. Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes–the EURODIAB Prospective Complications Study. Diabetologia. 2005; 48: 370–378 [DOI] [PubMed] [Google Scholar]
- 3. Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye. (Lond). 2006; 20: 1366–1369 [DOI] [PubMed] [Google Scholar]
- 4. Krady JK, Basu A, Allen CM, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005; 54: 1559–1565 [DOI] [PubMed] [Google Scholar]
- 5. Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011; 30: 343–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saren P, Welgus HG, Kovanen PT. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996; 157: 4159–4165 [PubMed] [Google Scholar]
- 7. Marie O, Thillaye-Goldenberg B, Naud MC, de Kozak Y. Inhibition of endotoxin-induced uveitis and potentiation of local TNF-alpha and interleukin-6 mRNA expression by interleukin-13. Invest Ophthalmol Vis Sci. 1999; 40: 2275–2282 [PubMed] [Google Scholar]
- 8. Hume DA, Perry VH, Gordon S. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol. 1983; 97: 253–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012; 366: 1227–1239 [DOI] [PubMed] [Google Scholar]
- 10. Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008; 126: 227–232 [DOI] [PubMed] [Google Scholar]
- 11. Ibrahim AS, El-Remessy AB, Matragoon S, et al. Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes. 2011; 60: 1122–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kezic JM, Chen X, Rakoczy EP, McMenamin PG. The effects of age and Cx3cr1 deficiency on retinal microglia in the Ins2(Akita) diabetic mouse. Invest Ophthalmol Vis Sci. 2013; 54: 854–863 [DOI] [PubMed] [Google Scholar]
- 13. Rungger-Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000; 41: 1971–1980 [PubMed] [Google Scholar]
- 14. Zeng XX, Ng YK, Ling EA. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci. 2000; 17: 463–471 [DOI] [PubMed] [Google Scholar]
- 15. Wang AL, Yuan M, Neufeld AH. Age-related changes in neuronal susceptibility to damage: comparison of the retinal ganglion cells of young and old mice before and after optic nerve crush. Ann N Y Acad Sci. 2007; 1097: 64–66 [DOI] [PubMed] [Google Scholar]
- 16. Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002; 47 (suppl 2): S253–S262 [DOI] [PubMed] [Google Scholar]
- 17. Guo C, Zhang Z, Zhang P, et al. Novel transgenic mouse models develop retinal changes associated with early diabetic retinopathy similar to those observed in rats with diabetes mellitus. Exp Eye Res. 2014; 119: 77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Obrosova IG, Minchenko AG, Vasupuram R, et al. Aldose reductase inhibitor fidarestat prevents retinal oxidative stress and vascular endothelial growth factor overexpression in streptozotocin-diabetic rats. Diabetes. 2003; 52: 864–871 [DOI] [PubMed] [Google Scholar]
- 19. Ramana KV, Tammali R, Reddy AB, Bhatnagar A, Srivastava SK. Aldose reductase-regulated tumor necrosis factor-alpha production is essential for high glucose-induced vascular smooth muscle cell growth. Endocrinology. 2007; 148: 4371–4384 [DOI] [PubMed] [Google Scholar]
- 20. Petrash JM. All in the family: aldose reductase and closely related aldo-keto reductases. Cell Mol Life Sci. 2004; 61: 737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhattacharya S, Manna P, Gachhui R, Sil PC. D-saccharic acid 1, 4-lactone protects diabetic rat kidney by ameliorating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via NF-kappaB and PKC signaling. Toxicol Appl Pharmacol. 2013; 267: 16–29 [DOI] [PubMed] [Google Scholar]
- 22. Yadav UC, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2007; 48: 4634–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheung AK, Fung MK, Lo AC, et al. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005; 54: 3119–3125 [DOI] [PubMed] [Google Scholar]
- 24. Tang J, Du Y, Petrash JM, Sheibani N, Kern TS. Deletion of aldose reductase from mice inhibits diabetes-induced retinal capillary degeneration and superoxide generation. PLoS One. 2013; 8: e62081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho EC, Lam KS, Chen YS, et al. Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes. 2006; 55: 1946–1953 [DOI] [PubMed] [Google Scholar]
- 26. Chang KC, Laffin B, Ponder J, et al. Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues. Chem Biol Interact. 2013; 202: 283–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006; 281: 33019–33029 [DOI] [PubMed] [Google Scholar]
- 28. Ramana KV, Srivastava SK. Mediation of aldose reductase in lipopolysaccharide-induced inflammatory signals in mouse peritoneal macrophages. Cytokine. 2006; 36: 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramana KV, Reddy AB, Tammali R, Srivastava SK. Aldose reductase mediates endotoxin-induced production of nitric oxide and cytotoxicity in murine macrophages. Free Radic Biol Med. 2007; 42: 1290–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Puppala M, Ponder J, Suryanarayana P, Reddy GB, Petrash JM, LaBarbera DV. The isolation and characterization of beta-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis. PLoS One. 2012; 7: e31399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS One. 2009; 4: e7945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang SP, Palla S, Ruzycki P, et al. Aldo-keto reductases in the eye. J Ophthalmol. 2010; 2010: 521204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang KC, Lo CW, Fan TC, et al. TNF-alpha mediates eosinophil cationic protein-induced apoptosis in BEAS-2B cells. BMC Cell Biol. 2010; 11: 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El-Remessy AB, Tang Y, Zhu G, et al. Neuroprotective effects of cannabidiol in endotoxin-induced uveitis: critical role of p38 MAPK activation. Mol Vis. 2008; 14: 2190–2203 [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. Tajima T, Murata T, Aritake K, et al. Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2). J Pharmacol Exp Ther. 2008; 326: 493–501 [DOI] [PubMed] [Google Scholar]
- 36. Pladzyk A, Reddy AB, Yadav UC, Tammali R, Ramana KV, Srivastava SK. Inhibition of aldose reductase prevents lipopolysaccharide-induced inflammatory response in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2006; 47: 5395–5403 [DOI] [PubMed] [Google Scholar]
- 37. Reddy AB, Ramana KV, Srivastava S, Bhatnagar A, Srivastava SK. Aldose reductase regulates high glucose-induced ectodomain shedding of tumor necrosis factor (TNF)-alpha via protein kinase C-delta and TNF-alpha converting enzyme in vascular smooth muscle cells. Endocrinology. 2009; 150: 63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rogove AD, Tsirka SE. Neurotoxic responses by microglia elicited by excitotoxic injury in the mouse hippocampus. Curr Biol. 1998; 8: 19–25 [DOI] [PubMed] [Google Scholar]
- 39. Medana IM, Chan-Ling T, Hunt NH. Redistribution and degeneration of retinal astrocytes in experimental murine cerebral malaria: relationship to disruption of the blood-retinal barrier. Glia. 1996; 16: 51–64 [DOI] [PubMed] [Google Scholar]
- 40. Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res. 2010; 90: 718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003; 76: 463–471 [DOI] [PubMed] [Google Scholar]
- 42. Roque RS, Rosales AA, Jingjing L, Agarwal N, Al-Ubaidi MR. Retina-derived microglial cells induce photoreceptor cell death in vitro. Brain Res. 1999; 836: 110–119 [DOI] [PubMed] [Google Scholar]
- 43. Harada T, Harada C, Kohsaka S, et al. Microglia-Müller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002; 22: 9228–9236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bosco A, Inman DM, Steele MR, et al. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2008; 49: 1437–1446 [DOI] [PubMed] [Google Scholar]
- 45. Bousquet E, Zhao M, Ly A, et al. The aldosterone-mineralocorticoid receptor pathway exerts anti-inflammatory effects in endotoxin-induced uveitis. PLoS One. 2012; 7: e49036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu YF, Fu LL, Jiang CH, Qin YW, Ni YQ, Fan JW. Naloxone inhibition of lipopolysaccharide-induced activation of retinal microglia is partly mediated via the p38 mitogen activated protein kinase signalling pathway. J Int Med Res. 2012; 40: 1438–1448 [DOI] [PubMed] [Google Scholar]
- 47. Baker D, Butler D, Scallon BJ, O'Neill JK, Turk JL, Feldmann M. Control of established experimental allergic encephalomyelitis by inhibition of tumor necrosis factor (TNF) activity within the central nervous system using monoclonal antibodies and TNF receptor-immunoglobulin fusion proteins. Eur J Immunol. 1994; 24: 2040–2048 [DOI] [PubMed] [Google Scholar]
- 48. Nelimarkka LO, Nikkari ST, Ravanti LS, Kahari VM, Jarvelainen HT. Collagenase-1, stromelysin-1 and 92 kDa gelatinase are associated with tumor necrosis factor-alpha induced morphological change of human endothelial cells in vitro. Matrix Biol. 1998; 17: 293–304 [DOI] [PubMed] [Google Scholar]
- 49. Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008; 118: 3012–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007; 2007: 61038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li L, Chang KC, Zhou Y, et al. Design of an amide N-glycoside derivative of beta-glucogallin: a stable, potent, and specific inhibitor of aldose reductase. J Med Chem. 2014; 57: 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]