Abstract
Aim:
To study the distribution, metabolism and excretion of S-propargyl-cysteine (SPRC), a novel hydrogen sulfide (H2S) donor, after oral administration in rats.
Methods:
Adult Sprague-Dawley rats were used. The tissue distribution of [35S] SPRC-derived radioactivity was measured using a liquid scintillation counter. The plasma protein binding of SPRC was examined using 96-well equilibrium dialysis. The excretion of SPRC in urine, bile and feces was analyzed using the LC-MS/MS method. The major metabolites in rat biomatrices were identified using MRM information-dependent, acquisition-enhanced product ion (MRM-IDA-EPI) scans on API 4000QTrap system.
Results:
After oral administration of [35S]-SPRC at a dose of 75 mg/kg, [35S] SPRC-derived radioactivity displayed broad biological distribution in various tissues of rats, including its target organs (heart and brain) with the highest in kidney. On the other hand, the binding of SPRC to human, rat and dog plasma protein was low. Only 2.18%±0.61% and 0.77%±0.61% of the total SPRC administered was excreted unchanged in the bile and urine. However, neither intact SPRC nor its metabolites were detected in rat feces. The major metabolic pathway in vivo (rat bile, urine, and plasma) was N-acetylation.
Conclusion:
The preliminary results suggest that SPRC possesses acceptable pharmacokinetic properties in rats.
Keywords: hydrogen sulfide (H2S), S-propargyl-cysteine, pharmacokinetics, distribution, metabolism, excretion, disposition
Introduction
The physiological importance of hydrogen sulfide (H2S) was first discovered in the mid-1990s. H2S is now considered to be the third novel gasotransmitter, discovered after nitric oxide and carbon monoxide. Like other gasotransmitters, H2S is a gaseous small molecule that is freely permeable across membranes. Moreover, the endogenous gas is important in the regulation of vascular tone, myocardial contractility, neurotransmission, insulin secretion, inflammation, longevity, and nociception1,2,3,4. The garlic-derived organosulfur compounds, known as H2S donors, have generated interest in the biomedical field. S-allylcysteine (SAC), one of the major compounds in aged garlic extract, has been demonstrated to have multiple biological activities, including antibacterial, antifungal, anticancer, antihepatopathic, cardioprotective, and neurotrophic properties5, 6, 7, 8.
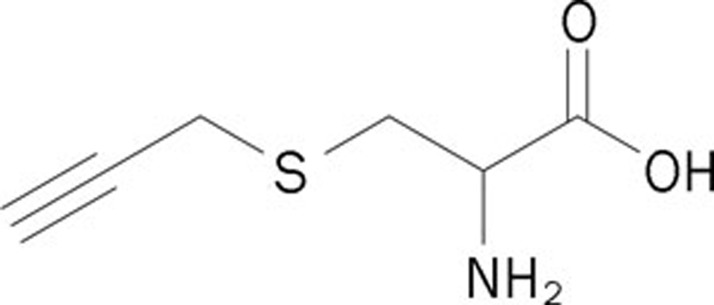
S-propargyl-cysteine (SPRC, Figure 1) is an analog of SAC and a novel H2S donor. Recently, the pharmacological activities of SPRC have been extensively studied. Wang et al demonstrated the cardioprotective effects of SPRC using an in vivo acute myocardial infarction model in rats and an in vitro hypoxic cardiomyocyte model9. Further studies revealed that the cardioprotective effect during a myocardial infarction resulted from the modulation of the endogenous levels of H2S. The release of H2S might activate signaling cascades associated with the prevention of oxidative stress10. SPRC also attenuated LPS-induced H9c2 cell activation, which would be beneficial for either the prevention or the treatment of cardiovascular inflammatory disease11. Furthermore, the protective effects of SPRC in neuroinflammation in vitro and in vivo indicate therapeutic potential for neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, ischemic stroke, multiple sclerosis, and amyotrophic lateral sclerosis12, 13. Ma et al reported that SPRC exhibited anticancer activities in SGC7901 gastric cancer cells and confirmed the in vivo antineoplastic effect of SPRC in a nude mice xenograft model14. These studies show that SPRC is emerging as a promising H2S-based therapeutic agent and candidate for future pharmaceutical development.
Figure 1.
The chemical structure of SPRC.
Preclinical pharmacokinetic characterization of a new drug candidate is an integral part of the drug discovery and development process. Such studies may also provide an in-depth understanding of the drug's mechanism of action. Unfavorable pharmacokinetic properties may lead to drug toxicity, potentially resulting in termination of the program or re-optimization of the chemical structure. Previous studies showed that SPRC was rapidly absorbed and bioavailable in rats15. Further studies characterizing the disposition of SPRC in vivo are needed to clarify its pharmacokinetic properties. The aim of this study is to investigate the distribution, metabolism, and excretion of SPRC in rats.
Materials and methods
Chemicals and reagents
SPRC was synthesized by reacting L-cysteine with propargyl bromide. The product was purified by recrystallization from an ethanol-water solution. The final product was verified by 1H nuclear magnetic resonance spectroscopy. The purity was 99.7%, as measured by high-performance liquid chromatography.
[35S] cysteine (>99% radiochemical purity), Ultima-Gold scintillation fluid, and a Soluene-350 tissue solubilizer were all purchased from Perkin-Elmer Life and Analytical Sciences (Boston, MA). [35S] SPRC (>99% purity) was synthesized by reacting [35S] cysteine with propargyl bromide as described above.
Blank Sprague-Dawley rat plasma was obtained from healthy, drug-free rats. Blank dog plasma was purchased from the Shanghai ChemPartner Co LTD (Shanghai, China). Blank human plasma was kindly provided by the Ruijin Hospital (Shanghai, China). Dialysis membranes used for the experiments had a 12 to 14 kDa molecular mass cutoff and were purchased from HTDialysis, LLC (Gales Ferry, CT, USA). HPLC-grade acetonitrile and methanol were purchased from Fisher Scientific (Pittsburgh, PA, USA). Other reagents used in this study had the highest purity commercially available.
Animal experiments
The experiments were performed in accordance with the Guidelines for Animal Experimentation of Fudan University (Shanghai, China). The protocols used in this study were approved by the Animal Ethics Committee of Fudan University (Shanghai, China). Adult Sprague-Dawley rats (body weight of 200±20 g, half males and half females) were purchased from Sino-British Sipper/BK Lab Animal Ltd (Shanghai, China; animal certificate number: SCXK hu (Shanghai) 2008-0016). The animals were maintained on a 12-h light/dark cycle under controlled conditions (temperature: 20±2 °C, relative humidity: 50%±20%). The rats had free access to food and water throughout the study unless specifically indicated.
In the biliary excretion study, rats were implanted with a cannula that was inserted into the bile duct under anesthesia with diethyl ether, and then allowed to recover before drug administration. Blank bile was collected before administration. Cumulative bile samples were collected during specific time intervals from 0–1, 1–2, 2–3, 3–4, 4–5, 5–6, 6–8, 8–12, and 12–24 h after drug administration.
Animals were housed in metabolic cages to collect urine and feces. Urine and feces were collected separately before dosing and from 0–6, 6–12, 12–24, 24–36, 36–48, and 48–72 h. The feces samples were homogenized with water. These samples were stored at -70°C until they were analyzed.
Analytical method for SPRC
The feces homogenate, bile, or urine samples (20 μL) was precipitated with acetonitrile containing 0.44 μg/mL internal standard. After centrifugation at 12 000×g for 5 min, 5 μL of the supernatant was used for LC-MS/MS analysis.
SPRC concentrations were quantitated using the LC-MS/MS method. The analytical method has been previously validated and reported for determining the concentration of SPRC in rat plasma15.
Tissue distribution study of [35S] SPRC-derived radioactivity in rats
The tissue distribution of [35S] SPRC-derived radioactivity was evaluated in Sprague-Dawley rats after an oral administration of 75 mg/kg [35S] SPRC solution (25 μCi/mL, 10 g/L). Animals were euthanized at 0.5 h, 1.5 h, and 6 h after drug administration. Following euthanasia, the rats were exsanguinated, and then plasma and tissues (heart, liver, spleen, lung, kidney, brain, stomach, intestine, and muscle) were collected and homogenized (0.25 g/mL).
Aliquots (400 μL) of the tissue samples were digested in 1 mL of Soluene-350 tissue solubilizer for 3 h at 60 °C. Ultima Gold scintillation fluid (10 mL) was then added to each sample. For plasma samples, 100 μL of plasma was directly mixed with 10 mL of scintillation fluid. All the sample preparations were stored in the dark for 24 h before analysis. All of the radioactivity measurements were made using a Tricarb 2910TR liquid scintillation analyzer (Perkin-Elmer, Wellesley, MA, USA). The tissue radioactivity levels were expressed as a percent injected dose per gram of tissue (%ID/g).
Measurement of the plasma protein binding of SPRC using 96-well equilibrium dialysis
Protein binding of SPRC to human, rat, and dog plasma was measured using a 96-well microequilibrium Teflon dialysis device (HTDialysis, LLC, Gales Ferry, CT). Dialysis membranes were soaked in distilled water for 20 min and then soaked in 30% (v/v) ethanol for 15 min. Just prior to use, the membrane was rinsed three times in deionized water and then rinsed once with isotonic sodium phosphate buffer. After assembling the dialysis plates, 110 μL of blank buffer and spiked plasma samples (2, 10, and 50 μg/mL) were added to the receiver and donor side of the equilibrium dialysis block, respectively. The dialysis block was covered with a plastic lid and placed in a shaker (37 °C, 100 r/min) for 6 h. Warfarin was used as a positive control.
SPRC samples from both sides of the chamber were measured using the LC-MS/MS method. Percent binding was calculated using the following equation:
Bound %=100×([Donor]–[Receiver])/[Donor]
Metabolite identification using LightSight software
Preliminary metabolites in rat biomatrices were identified using MRM information-dependent, acquisition-enhanced product ion (MRM-IDA-EPI) scans on the API 4000QTrap system. Pooled rat plasma (0–24 h), bile (0–24 h), urine (0–72 h), and feces (0–72 h) samples were precipitated with acetonitrile. The mobile phase consisted of a methanol/ammonium acetate buffer [(10 mmol/L, pH=4.0): 15/85 (v/v)]. Other analytical conditions were the same as those applied to the quantification of SPRC, described above.
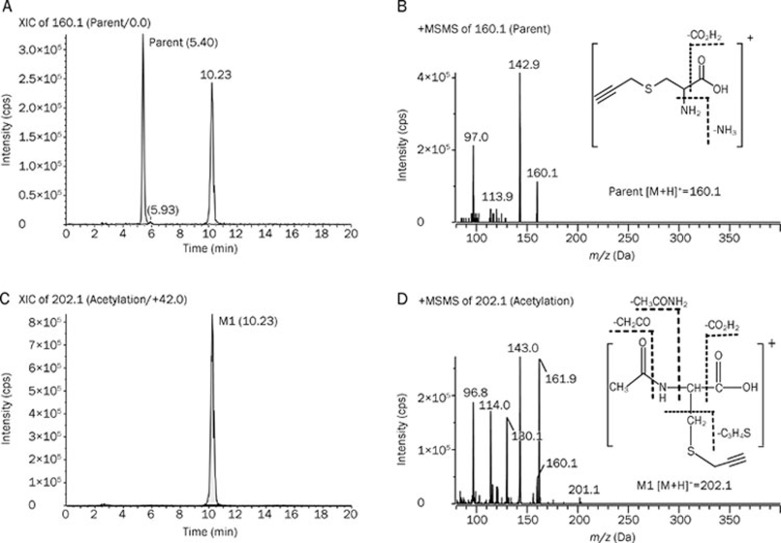
Mass spectrometer conditions were optimized by infusing 5 μg/mL SPRC solution in distilled water via a syringe pump at a 10 μL/min flow rate. The EPI analyte spectra were collected to identify the major fragments of SPRC ([M+H]+=160.1). The three most abundant fragments were m/z 143.0, 114.0, and 97.0. Therefore, three MRM survey channels at 160.1→143.0, 160.1→114.0, and 160.1→97.0 were used to identify the metabolites of SPRC. Metabolite identification was accomplished using LightSight software, by matching the product ions and neutral losses of the EPI spectra of detected metabolites to the parent compound.
The IDA threshold was set to 500 cps, above which the EPI scan was triggered to collect the fragment ion spectra. The EPI scan rate was 4000 amu/s, and the scan range was 80 to 400 amu. The CEs were set at 30 eV, with a CE spread of 15 eV. Other parameters were set as follows: ion source voltage, +4500 V; temperature, 400 °C; curtain gas, 15 psi; ion source gas 1, 20 psi; ion source gas 2, 20 psi.
Other types of mass spectrometric analyses, including enhanced mass spectrometry (EMS), precursor ion (PI), and neutral loss (NL) scans, were performed to ensure that unexpected metabolites were not missed.
Data analysis
The amount of SPRC excreted into bile and urine during each time interval was calculated by multiplying the SPRC concentration by the volume of sample. The cumulative amount of SPRC (X) over a certain time period was calculated by adding all excreted amounts within the period. The same data were also expressed (in the form of % of dose) by dividing X with the total dose administered.
A one-way ANOVA, followed by S-N-K multiple comparisons, was used to evaluate the statistical significance of interspecies differences in plasma protein binding. The differences were considered significant when P<0.05.
Results
Tissue distribution of [35S] SPRC-derived radioactivity
The distribution of [35S] SPRC-derived radioactivity in various organs at three time points was determined after an oral administration of a 75 mg/kg [35S] SPRC solution. SPRC-derived radioactivity was broadly distributed in all of the tissues examined (Table 1). At 0.5 h (the Tmax in the plasma kinetics), the concentrations observed were in the following order: kidney>plasma>stomach>liver>lung>intestine>spleen> heart>muscle>brain. When compared with the corresponding plasma concentrations, the kidney tissues showed the highest exposure. The mean concentrations in the kidney tissue were 1.1, 1.5, and 1.8 times that present in the plasma at 0.5 h, 1.5 h, and 6 h, respectively. SPRC-related radioactivity in all the other tissues was the same as or lower than the corresponding plasma concentration.
Table 1. Tissue distribution of [35S]-SPRC derived radioactivity after oral administration (25 μCi/mg, 75 mg/kg) of SPRC in rats. Data represent Mean±SD (n=5).
| Tissue |
Distribution amount of [35S]-SPRC derived radioactivity (%ID/g) |
||
|---|---|---|---|
| 0.5 h | 1.5 h | 6 h | |
| Plasma | 0.40±0.04 | 0.31±0.08 | 0.18±0.07 |
| Brain | 0.07±0.01 | 0.08±0.02 | 0.05±0.01 |
| Muscle | 0.11±0.02 | 0.12±0.03 | 0.05±0.02 |
| Heart | 0.15±0.06 | 0.10±0.02 | 0.05±0.02 |
| Spleen | 0.15±0.05 | 0.13±0.04 | 0.07±0.02 |
| Kidney | 0.44±0.19 | 0.47±0.14 | 0.32±0.12 |
| Stomach | 0.29±0.22 | 0.12±0.03 | 0.08±0.02 |
| Liver | 0.23±0.04 | 0.21±0.02 | 0.15±0.07 |
| Lung | 0.19±0.09 | 0.17±0.07 | 0.12±0.06 |
| Intestine | 0.17±0.06 | 0.09±0.01 | 0.10±0.06 |
Plasma protein binding
The extent of SPRC bound to human, rat, and dog plasma was studied at three concentration levels using a rapid equilibrium dialysis method. The protein binding to the positive control compound (warfarin) was within the normal range (98.4%±1.1%), indicating the reliability of the equilibrium dialysis study. The fraction bound ranged from -0.8% to -14.5% for human, 3.7% to 10.9% for rat, and -5.2% to 0.6% for dog. The total SPRC recovery after dialysis was >98%. No statistical differences were found at the three concentration levels tested. The statistical analysis revealed no significant difference in the plasma protein binding fractions of SPRC among the three species.
Excretion of SPRC in rats
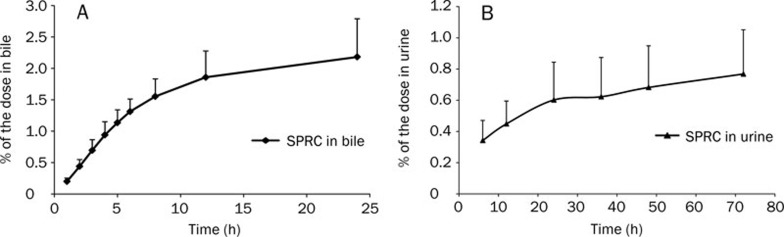
The excretion of unchanged SPRC after oral administration has been studied in rats using the LC-MS/MS method. No unchanged SPRC was found in rat feces up to 72 h post-dose using a relatively sensitive analytical method (the low limit of quantification was 50 ng/mL). After oral administration in rats, only 2.18%±0.61% and 0.77%±0.61% of the dose was excreted in the bile and urine as the parent drug up to 24 and 72 h, respectively (Figure 2). Less than 3% of the oral SPRC dose was recovered unchanged.
Figure 2.
The cumulative excretion of unchanged SPRC in rat bile (A) and urine (B) after oral administration. SPRC concentrations were determined by the LC-MS/MS method. All values are expressed as the mean±SD. (n=6).
Identification of the major metabolites of SPRC in urine, bile, feces and plasma
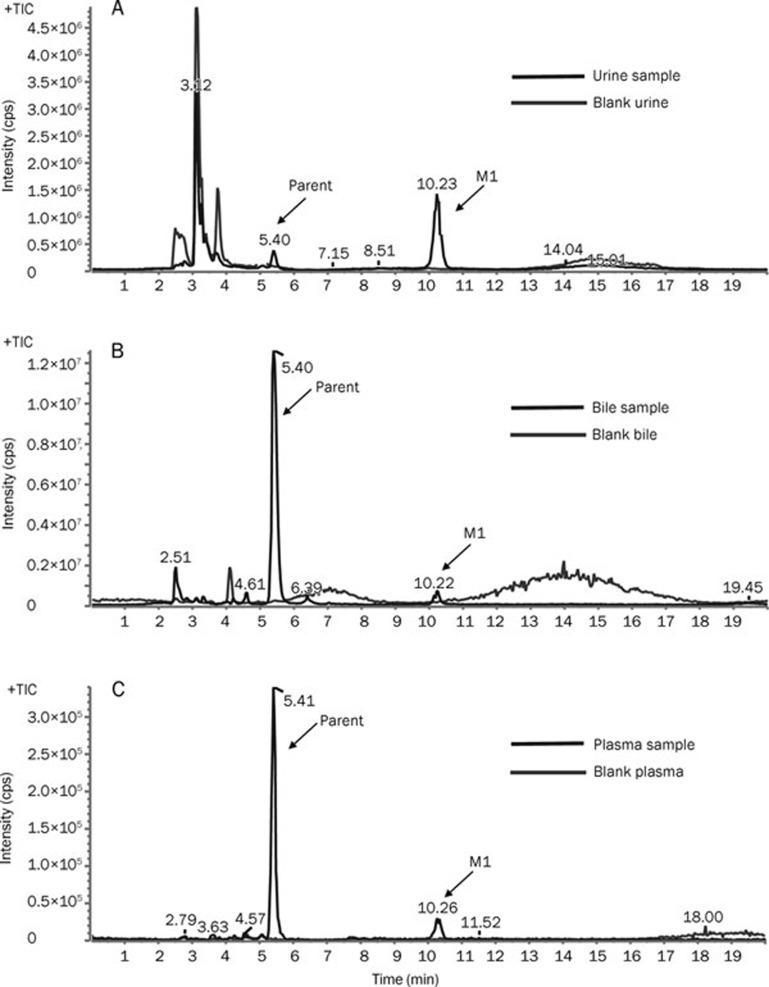
The representative total ion current (TIC) chromatograms of the drug-treated rat biological samples were compared with the corresponding blank samples. Only one metabolite (M1, RT=10.2 min) of SPRC was found in rat urine, bile, and plasma (Figure 3). However, neither the parent drug nor the N-acetyl metabolite was observed in rat feces (data not shown).
Figure 3.
The representative total ion current (TIC) chromatogram obtained from rat biological samples following oral administration of SPRC using the MRM-IDA-EPI method. (A) TIC chromatograph of a rat urine sample collected 96 h post-drug administration; (B) TIC chromatograph of a rat bile sample collected 24 h post-drug administration; (C) TIC chromatograph of a rat plasma sample collected 24 h post-drug administration.
The extracted ion chromatograms (EICs) and representative EPI spectra of M1 were also compared with the parent SPRC (Figure 4). M1 was proposed to be an acetylated metabolite because the precursor ion (m/z 202.1) was 42 m/z larger than the corresponding precursor ion (m/z 160.1) of SPRC. In addition, the fragmentation pattern of M1 is similar to that of SPRC, indicating that the same ions (m/z 143, m/z 114, and m/z 97) were found in the EPI spectra.
Figure 4.
The representative extracted ion chromatography (EIC) and its enhanced product ion (EPI) spectra obtained from the urine sample after oral dosing of the SPRC solution using the MRM-IDA method. (A) EIC of parent drug (160.1 m/z); (B) EPI spectra and structure of parent drug; (C) EIC of M1 (202.1 m/z); (D) EPI spectra and structure of M1.
To ensure that no unexpected metabolites were missed using the MRM survey scan, different types of survey scan triggering EPI method, were used to detect any other metabolites of SPRC. These methods included EMS-EPI, PI-EPI, and NL-EPI. However, no other metabolites were found using these methods (data not shown).
Discussion
SPRC, a novel sulfur-containing amino acid derivative, has been proven to be a potent H2S donor. It was selected for development, in part because of its potent in vitro and in vivo biological activities. However, the lack of comprehensive knowledge about the pharmacokinetic properties of SPRC hampers its further development as a new drug candidate. In the present study, we analyzed the pharmacokinetic properties of SPRC, including the tissue distribution, identification of major metabolites, and route of excretion.
SPRC shares pharmacokinetic properties with other cysteine derivatives, such as SAC16, 17. These cysteine derivatives were all bioavailable and were absorbed rapidly and easily in the gastrointestinal tract15. After oral administration, [35S] SPRC-derived radioactivity was extensively distributed in various tissues. An accumulation of [35S] SPRC-derived radioactivity exceeding plasma concentrations was mainly observed in the kidney at three different time points. The radioactivity in other tissues was approximate to or lower than the observed plasma concentration. Previous studies showed that SAC was retained at a fairly high concentration in the kidney, and it was speculated that SAC was reabsorbed in the kidney16. Therefore, the kidney may play a major role in the elimination of cysteine derivatives in rats and deserves further study.
In the rat excretion study, less than 3% of the oral dose of unchanged SPRC was recovered in the urine and bile. This result indicates that the drug is almost completely metabolized before elimination from the body. The major metabolic pathway of SPRC was N-acetylation, which forms the conjugate compound found in urine, bile, and plasma. The N-acetylated SAC was also the major metabolite of SAC16. Neither the parent drug nor the N-acetyl metabolite was found in rat feces. Previous studies demonstrated that S-allyl-L-cysteine sulfoxide and N-allyl-L-cysteine sulfoxide were the expected metabolites of SAC in rat urine18. However, these sulfoxide metabolites of SPRC were not observed in our experiment.
In summary, this study examined in detail the pharmacokinetic properties of SPRC in Sprague-Dawley rats. Our study examined the tissue distribution, plasma protein binding, excretion, and metabolites of SPRC. The tissue distribution of [35S] SPRC-derived radioactivity in rats was rapid and extensive. SPRC was distributed mainly in the kidney. The levels of human, rat, and dog plasma SPRC protein binding were low. Only approximately 0.77% and 2.18% of the parent SPRC could be recovered after oral administration in rat urine and bile, respectively. The major metabolite of SPRC in rat urine, bile, and plasma was the N-acetylated metabolite. No parent drug or metabolite was found in rat feces. In addition to increasing the knowledge of SPRC biological activities, this pharmacokinetic study contributes to the further development of this new drug candidate.
Author contribution
Yuan-ting ZHENG, Yi-zhun ZHU, and Wei-min CAI designed the experiments, Yuan-ting ZHENG, Jian-hua ZHU, Guo MA, Qing ZHU, Ping YANG, Bo TAN, Jin-lian ZHANG, Hai-xing SHEN, and Jia-lin XU performed the experiments, Yuan-ting ZHENG, and Wei-min CAI wrote the manuscript.
Acknowledgments
This project was supported by the National Drug Innovative Program (2009ZX09301-011) and Traditional Chinese Medicine Modernization (10DZ1972100).
References
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–71. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- Dello Russo C, Tringali G, Ragazzoni E, Maggiano N, Menini E, Vairano M, et al. Evidence that hydrogen sulphide can modulate hypothalamo-pituitary-adrenal axis function: in vitro and in vivo studies in the rats. J Neuroendoctinol. 2000;12:225–33. doi: 10.1046/j.1365-2826.2000.00441.x. [DOI] [PubMed] [Google Scholar]
- Laggner H, Hermann M, Esterbauer H, Muellner MK, Exner M, Gmeiner BM, et al. The novel gaseous vasorelaxant hydrogen sulfide inhibits angiotensin-converting enzyme activity of endothelial cells. J Hypertens. 2007;25:2100–4. doi: 10.1097/HJH.0b013e32829b8fd0. [DOI] [PubMed] [Google Scholar]
- Chu Q, Ling MT, Feng H, Cheung HW, Tsao SW, Wang X, et al. A novel anticancer effect of garlic derivatives: inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis. 2006;27:2180–9. doi: 10.1093/carcin/bgl054. [DOI] [PubMed] [Google Scholar]
- Cao Y, Adhikari S, Ang AD, Moore PK, Bhatia M. Mechanism of induction of pancreatic acinar cell apoptosis by hydrogen sulfide. Am J Physiol Cell Physiol. 2006;291:C503–10. doi: 10.1152/ajpcell.00547.2005. [DOI] [PubMed] [Google Scholar]
- Garcia E, Limon D, Perez-De La Cruz V, Giordano M, Diaz-Muñoz M, Maldonado PD, et al. Lipid peroxidation, mitochondrial dysfunction and neurochemical and behavioural deficits in different neurotoxic models: protective role of S-allylcysteine. Free Radic Res. 2008;42:892–902. doi: 10.1080/10715760802506356. [DOI] [PubMed] [Google Scholar]
- Rose P, Whiteman M, Moore PK, Zhu YZ. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: the chemistry of potential therapeutic agents. Nat Prod Rep. 2005;22:351–68. doi: 10.1039/b417639c. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu HR, Mu Q, Rose P, Zhu YZ. S-propargyl-cysteine protects both adult rat hearts and neonatal cardiomyocytes from ischemia/hypoxia injury: the contribution of the hydrogen sulfide-mediated pathway. J Cardiovasc Pharmacol. 2009;54:139–46. doi: 10.1097/FJC.0b013e3181ac8e12. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang XL, Liu HR, Rose P, Zhu YZ. Protective effects of cysteine analogues on acute myocardial ischemia: novel modulators of endogenous H2S production. Antioxid Redox Signal. 2010;12:1155–65. doi: 10.1089/ars.2009.2947. [DOI] [PubMed] [Google Scholar]
- Pan LL, Liu XH, Gong QH, Zhu YZ. S-Propargyl-cysteine (SPRC) attenuated lipopolysaccharide-induced inflammatory response in H9c2 cells involved in a hydrogen sulfide-dependent mechanism. Amino Acids. 2011;41:205–15. doi: 10.1007/s00726-011-0834-1. [DOI] [PubMed] [Google Scholar]
- Gong QH, Wang Q, Pan LL, Liu XH, Xin H, Zhu YZ. S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: involvement of TNF signaling and NF-kappaB pathway in rats. Brain Behav Immun. 2011;25:110–9. doi: 10.1016/j.bbi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Gong QH, Pan LL, Liu XH, Wang Q, Huang H, Zhu YZ. S-propargyl-cysteine (ZYZ-802), a sulphur-containing amino acid, attenuates beta-amyloid-induced cognitive deficits and pro-inflammatory response: involvement of ERK1/2 and NF-kappaB pathway in rats. Amino Acids. 2011;40:601–10. doi: 10.1007/s00726-010-0685-1. [DOI] [PubMed] [Google Scholar]
- Ma K, Liu Y, Zhu Q, Liu CH, Duan JL, Tan BK, et al. H2S donor, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: evidence for a novel anti-cancer effect of endogenous H2S. PLoS One. 2011;6:e20525. doi: 10.1371/journal.pone.0020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YT, Liu HR, Ma G, Yang P, Zhang L, Gu Y, et al. Determination of S-propargyl-cysteine in rat plasma by mixed-mode reversed-phase and cation-exchange HPLC-MS/MS method and its application to pharmacokinetic studies. J Pharm Biomed Anal. 2011;54:1187–91. doi: 10.1016/j.jpba.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Nagae S, Ushijima M, Hatono S, Imai J, Kasuga S, Matsuura H, et al. Pharmacokinetics of the garlic compound S-allylcysteine. Planta Med. 1994;60:214–7. doi: 10.1055/s-2006-959461. [DOI] [PubMed] [Google Scholar]
- Yan CK, Zeng FD. Pharmacokinetics and tissue distribution of S-allylcysteine in rats. Asian J Drug Metab Pharmacokinet. 2004;5:61–9. [Google Scholar]
- Krause RJ, Glocke SC, Elfarra AA. Sulfoxides as urinary metabolites of S-allyl-L-cysteine in rats: evidence for the involvement of flavin-containing monooxygenases. Drug Metab Dispos. 2002;30:1137–42. doi: 10.1124/dmd.30.10.1137. [DOI] [PubMed] [Google Scholar]