Abstract
Aim:
To determine whether electrical stimulation of caudal medial prefrontal cortex (mPFC) as conditioned stimulus (CS) paired with airpuff unconditioned stimulus (US) was sufficient for establishing eyeblink conditioning in guinea pigs, and whether it was dependent on cerebellar interpositus nucleus.
Methods:
Thirty adult guinea pigs were divided into 3 conditioned groups, and trained on the delay eyeblink conditioning, short-trace eyeblink conditioning, and long-trace eyeblink conditioning paradigms, respectively, in which electrical stimulation of the right caudal mPFC was used as CS and paired with corneal airpuff US. A pseudo conditioned group of another 10 adult guinea pigs was given unpaired caudal mPFC electrical stimulation and the US. Muscimol (1 μg in 1 μL saline) and saline (1 μL) were infused into the cerebellar interpositus nucleus of the animals through the infusion cannula on d 11 and 12, respectively.
Results:
The 3 eyeblink conditioning paradigms have been successfully established in guinea pigs. The animals acquired the delay and short-trace conditioned responses more rapidly than long-trace conditioned responses. Muscimol infusion into the cerebellar interpositus nucleus markedly impaired the expression of the 3 eyeblink conditioned responses.
Conclusion:
Electrical stimulation of caudal mPFC is effective CS for establishing eyeblink conditioning in guinea pigs, and it is dependent on the cerebellar interpositus nucleus.
Keywords: associative learning, memory, eyeblink conditioning, medial prefrontal cortex, cerebellar interpositus nucleus, muscimol, guinea pig
Introduction
Classical eyeblink conditioning is one of the most widely used model systems for studying the behavioral and neurobiological mechanisms of associative learning and memory1, 2, 3. All variant of eyeblink conditioning involve paired presentations of a behaviorally neutral conditioned stimulus (CS; eg, a tone or light) and an unconditioned stimulus (US; eg, a corneal airpuff or periorbital shock). Initially, the organisms could produce only a reflexive eyeblink unconditioned response (UR) to the US. After hundreds of paired presentations of the CS and the US, the organisms could learn to close the eyes in response to the CS before the onset of the US (called the conditioned response, CR). According to the temporal relationship between the CS and the US, there are two commonly used procedures in eyeblink conditioning: trace and delay paradigms. In the trace eyeblink conditioning (TEC), a temporal gap occurs between the offset of the CS and the onset of the US, which is in contrast to the delay eyeblink conditioning (DEC), in which the CS overlaps the US and the two stimuli are terminated at the same time. It is well established that the brainstem-cerebellar circuit is the essential circuitry for the DEC1, 3, 4, 5, 6. In addition, components of the auditory CS pathway (eg, the inferior colliculus or auditory thalamus) have recently been added to the DEC circuit7, 8, 9, 10, 11, 12, 13, 14. In contrast, several forebrain structures, such as the medial prefrontal cortex (mPFC)15, 16, 17, 18, 19, 20, 21 and the hippocampus22, 23, 24, 25, 26, are required for TEC in addition to a brainstem-cerebellar circuit27, 28, 29, 30, 31.
It has been reported that electrical stimulations of the several key components in the auditory or visual CS pathway, including the cochlear nucleus32, medial auditory thalamic11, auditory cortex33, lateral geniculate, superior colliculus, visual cortex34, pontine nuclei6, 35, 36, 37, cerebellar mossy fiber38, 39, parallel fibers40, 41, interpositus nucleus42, 43, etc, can serve as effective CSs for establishing eyeblink conditioning. Moreover, eyeblink conditioning has also been successfully achieved by using stimulation of other brain areas outside of CS pathway, like the primary somatosensory cortex, the coronal-precruciate cortex44, 45, 46, etc. However, less work has been done to examine whether stimulation of the highest level in the hierarchical organization of the mammalian cortex (ie, PFC) is a sufficient CS to support associative eyeblink conditioning.
Cumulative evidence has demonstrated that PFC is implicated in many critical cognitive functions47, 48, 49 and that mPFC is closely involved in associative learning50 such as eyeblink conditioning15, 16, 17, 18, 51. Electrical stimulation of the right rostal PFC of a cat was an effective CS for eyeblink conditioning52. Given that the caudal mPFC input to the pontine nuclei is necessary for eyeblink conditioning6, it can be hypothesized that electrical stimulation of the caudal mPFC as a CS paired with an airpuff US is sufficient for establishing eyeblink conditioning, and that it is dependent on the cerebellar interpositus nucleus. The present study was designed to determine whether electrical stimulation of caudal mPFC is a sufficient CS for establishing eyeblink conditioning, and whether it is dependent on the cerebellar interpositus nucleus. Furthermore, the caudal mPFC play an important role in long TEC (eg, TEC with a 500-ms trace interval, the interval between CS offset and US onset)6, 17, 18, 53, 54, but not in short TEC (eg TEC with a 150-ms trace interval) or DEC6, 17, 18, 19, 21, 55, 56, 57, 58, 59. Thus, the current study was also designed to examine the differences in CRs among the three eyeblink conditioning paradigms when the caudal mPFC was selected as the site at which CS stimulation was applied.
Materials and methods
Subjects
A total of 40 adult female albino Dunkin-Hartley guinea pigs were included in the study. The guinea pigs weighed 500–550 g and were approximately 4–5 months old at the time of surgery. Before the experiments and between the conditioning sessions, these animals were individually housed in standard plastic cages that operated on a 12:12 light/dark cycle. The animals were granted free access to food and water ad libitum. The room temperature was maintained at 25±1 °C. All experiments were performed between 8:00 AM and 6:00 PM during the light portion of the cycle. The experimental procedures were approved by the Animal Care Committee of the Third Military Medical University and were performed in accordance with the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to optimize comfort and to minimize the use of the animals.
Surgery
The animals were allowed to remain undisturbed in their cages for 1 week prior to surgery. The guinea pigs were anesthetized with a mixture of ketamine (80 mg/kg, ip; Hengrui, Lianyungang, China) and xylazine (5 mg/kg, ip; Sigma-Aldrich, St Louis, MO, USA). The anesthetized animal's head was secured to a stereotaxic apparatus (SR-6N, Narishige, Tokyo, Japan) with lambda positioned 1.0 mm ventral to bregma. A longitudinal incision was subsequently made to reveal the skull onto which a Plexiglas headstage (1.0 cm× 1.0 cm×0.5 cm), designed to secure the animal's head, was cemented with dental cement using four stainless steel anchoring screws. One small hole (diameter: 1.0 mm) was drilled on the right side of the skull centered on the right caudal mPFC at the following stereotaxic coordinates: anteroposterior (AP) +13.0 mm, mediolateral (ML) 1.0 mm relative to the frontal zero plane, and the midline sinus, respectively. Then, a stainless steel stimulating electrode (No 792500, A-M Systems, Sequim, WA, USA; coated diameter: 33.20 μm, bare diameter: 254.00 μm) was implanted into the right caudal mPFC through the hole according to an atlas of the guinea pig brain60, and the electrode's tip was directed to the following stereotaxic coordinates: AP +13.0 mm, ML 1.0 mm, dorsoventral (DV) −2.5 mm to the skull surface (Figure 1A, 1B). Moreover, another small hole (diameter: 1.0 mm) was drilled on the left side of the skull centered on the left cerebellar interpositus nucleus at the following stereotaxic coordinates: AP −3.0 mm, ML 2.5 mm relative to the frontal zero plane, and the midline sinus, respectively. Then, a stainless steel guiding cannula (No 62001, RWD, Shenzhen, China; external diameter: 0.67 mm, internal diameter: 0.30 mm) was implanted into the left cerebellar interpositus nucleus through the hole according to an atlas of the guinea pig brain60, and its tip was directed to the following stereotaxic coordinates: (AP −3.0 mm, ML 2.5 mm, DV −5.5 mm) (Figure 1A, 1B). The infusion cannula (No 62201, RWD, Shenzhen, China; external diameter: 0.20 mm, internal diameter: 0.10 mm) extended 0.5 mm beyond the tip of the guiding cannula to the final infusion position at the following stereotaxic coordinates: (AP −3.0 mm, ML 2.5 mm, DV −6.0 mm). The reference electrode was a copper wire (0.5 mm in diameter) attached to the four stainless steel anchoring screws implanted into the skull. This wire was not in direct contact with the skull or brain tissue. To prevent occlusion, a removable stainless steel stylet (No 62101, RWD, Shenzhen, China) was inserted into the guiding cannula. The stylet provided up to 0.5 mm of extension beyond the tip of the guiding cannula. The stimulating electrode, reference electrode, and guiding cannula were fixed to the skull with dental cement. Finally, a small nylon loop was sutured into but not through the edge of the upper left eyelid. In the present study, this loop was utilized to attach the upper left eyelid to a movement-measuring device. After the surgery, the animals were allowed 1 week of recovery.
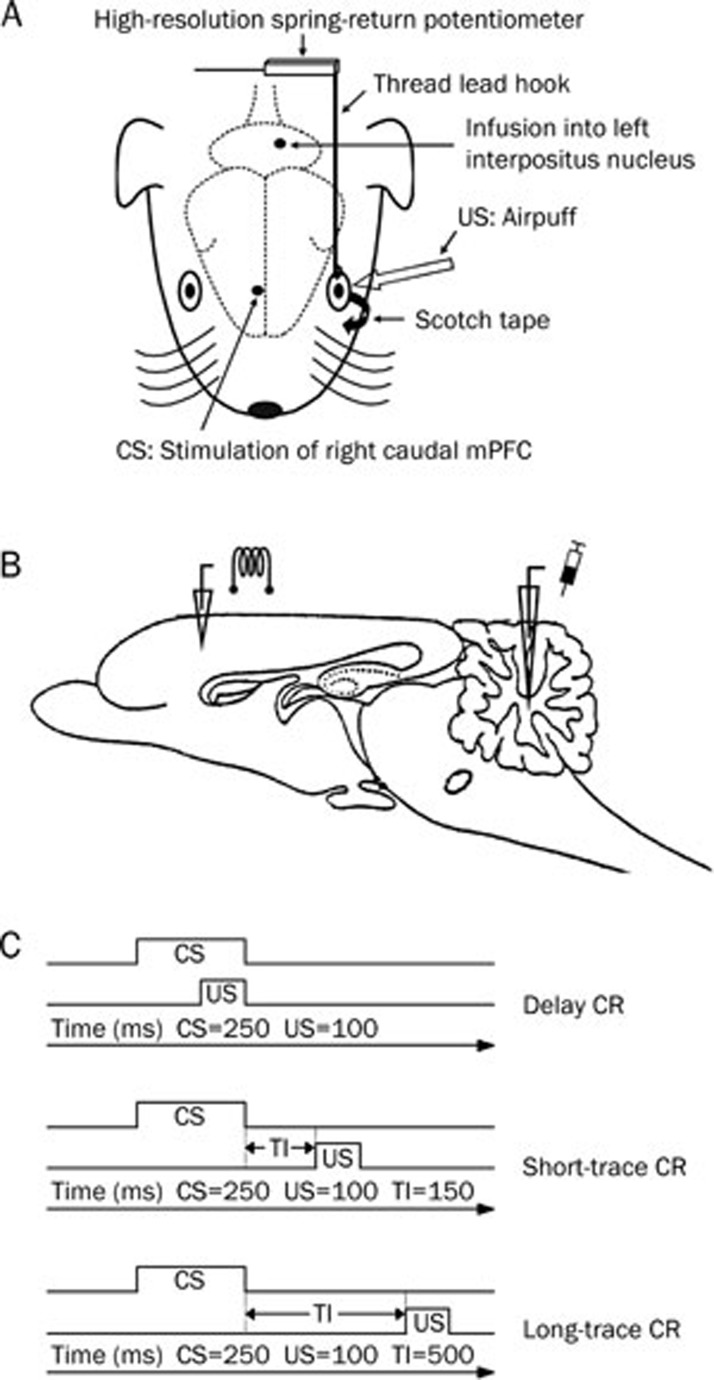
Figure 1.
Experimental design. (A) The upper left eyelid movements were measured by a high-resolution spring-return potentiometer that was attached via a thread lead that was hooked through a nylon loop, which was sutured into the left upper eyelid, and the left lower eyelid was taped open. One electrode was implanted in the right caudal medial prefrontal cortex (mPFC) and one infusion guiding cannula was implanted in left cerebellar interpositus nucleus. Electrical stimulation of right caudal mPFC was used as a conditioned stimulus (CS), and airpuff was presented to the left cornea as an unconditioned stimulus (US). (B) Diagram of the sagittal section of guinea pig brain, showing the stimulating and infusion sites. (C) Schematic diagram showing the delay, short-trace, and long-trace eyeblink conditioned response (CR) paradigms used in the present study. Note that the CS, US, and total trial lengths were equal in each conditioning. Short and long-trace CR were different in trace interval (TI) length.
Apparatus
Eyelid movements were measured by a high-resolution spring-return potentiometer (JZ101, XH, Beijing, China) that was attached via a thread lead hooked through the nylon loop sutured into the upper left eyelid. A stimulator (YC-2, Cheng Yi, Chengdu, China) was used to deliver a stimulation CS, while a plastic pipe placed 1.0 cm from the animal's left eyeball was used to deliver a corneal airpuff US (Figure 1A). A custom computerized monitoring system controlled presentations of the CS and US. Eyelid-movement mechanograms and applied-stimuli markers were digitized by a data-acquisition system (RM6280C, Cheng Yi, Chengdu, China) at a sample rate of 10 kHz and were acquired using the system's built-in software (v 4.7). A Windows PC was used to store and analyze the behavioral data.
Behavioral procedures
The 40 guinea pigs were divided into four groups: delay conditioned (n=10), short-trace conditioned (n=10), long-trace conditioned (n=10), and pseudo-conditioned (n=10). Each group represented one training paradigm. Following postoperative recovery, the animals were adapted to the experimental environment for two sessions at 60 min per session. These two sessions were followed by 10 consecutive daily sessions of acquisition training. Immediately following acquisition training, the three conditioned groups underwent 2 consecutive daily sessions of drug infusion. During the acquisition sessions and the drug infusion sessions, the animals were restrained in a Plexiglas container (25 cm×15 cm×15 cm) located in a sound- and light-attenuated chamber, and their heads were secured with blunt ear bars that pressed on the headstages. The left eye of the animal was held open in a confirmable position, with the nylon loop sutured into the left upper eyelid, which was linked to the high-resolution spring-return potentiometer. The voltage level represented the eyeblink baseline position, which was manually calibrated to a constant value. Moreover, the animal's left lower eyelid was taped open. These two measures ensured continual exposure of the left cornea.
During behavioral training, electrical stimulation of the caudal mPFC functioned as the CS, which was a 200-Hz, 250-ms train of monophasic pulses (cathodal, square, current level of 50–200 μA, a pulse duration of 0.1 ms). The electrical stimulation parameters were chosen based on the recent studies8, 11, 14, 58. The stimulation intensity for each guinea pig was set carefully before training by increasing the test current until a behavioral response was observed to avoid biasing the experimental results through an electrical startle response and a spreading of the electrical stimulation current to remote brain areas, such as hippocampus, premotor cortex, and somatosensory cortex. The current was then turned down in 5-μA increments until there was no observable behavioral response. Typical behavioral responses observed from the test stimulation included movements of the eyelid, eye, ear and/or head8, 11, 14, 58. In most of the cases (28/37) in the present experiment, the threshold stimulation was between 50–120 μA. An additional evoked field potentials recording test showed that the caudal mPFC stimulation with 200 μA or below did not evoke any field potential in the motor cortex, somatosensory cortex, or the cerebellar cortex (date not shown). The US was a 100-ms, 3.0-psi airpuff. A daily acquisition training session consisted of five 10-trial blocks. However, a daily infusion session (d 11 and d 12) consisted of three 10-trial blocks before the infusion and five 10-trial blocks after the infusion. Moreover, each block comprised nine CS-US paired trials and one CS alone trial. The trials were separated by a variable intertrial interval of 20–40 s with a mean intertribal interval of 30 s. For the delay-conditioning paradigm, the US terminated simultaneously with the offset of the CS (the interstimulus interval was 150 ms). For the short-trace and long-trace conditioning paradigms, a stimulus-free trace interval of 150 ms or 500 ms was interposed between the CS termination and the US onset, respectively (Figure 1C). For the pseudo-conditioning paradigm, the US was presented at a random interval between 1 and 10 s after the CS onset. All experiments were performed during the light phase of the light/dark cycle.
Drug infusions
Two drug infusion sessions were conducted during d 11 and d 12. Each infusion session began with three blocks of training to establish a baseline of response prior to each drug infusion. Drug infusions were performed 20 min before the subsequent beginning of conditioning training. Muscimol (Sigma-Aldrich, St Louis, MO, USA), which produces inactivation only to the soma of neurons but not to the fibers of passage, was dissolved in saline (phosphate buffer, pH 7.4), which served as the vehicle prior to use. During the first infusion session (d 11), 1.0 μg muscimol in 1.0 μL saline was infused into the guinea pigs' left cerebellar interpositus nucleus. Infusion procedures for each animal included removal of the internal stylet from the guiding cannula, insertion of a stainless steel infusion cannula that extended 0.5 mm below the tip of the guiding cannula, infusion of the drug at 0.5 μL/min via polyethylene tubing connected to a microsyringe, removal of the needle 5 min after the cessation of infusion, and finally, reinsertion of the internal stylet. During the second infusion session (d 12), 1.0 μL saline was infused into the guinea pigs' left cerebellar interpositus nucleus. The drug infusion procedures used in the second infusion session were the same as those used in the first infusion session. All of the animals were allowed 24 h to recover between the infusion sessions. Several prior studies that used similar infusion procedures have reported that muscimol spread maximally within the 10–20 min following infusion, that the effective inactivation radius was 1.5–2.0 mm, and that the blocking effect persisted for a period of up to 2.0 h in both cortical and subcortical tissues61, 62, 63. However, in present study, the exact drug diffusion into each animal is unknown because the spread of the three drugs was not measured directly.
Histology
After the completion of behavioral experiments, all of the animals were given a lethal dose of pentobarbital sodium (150 mg/kg, ip; SCRC, Shanghai, China) and were perfused transcardially with physiological saline followed by 4% paraformaldehyde, which was prepared in phosphate-bufferer (0.1 mol/L, pH 7.4). The brains were removed from the skulls and stored in 4% paraformaldehyde for several days. Four days prior to sectioning, the brains were transferred to a 30% sucrose/4% paraformaldehyde solution. Frozen coronal sections measuring 30 μm in thickness were taken from the sites of the electrode and infusion cannula implantation. The slices were stained with cresyl violet. The locations of the electrode and infusion cannula tips within the brains were carefully determined using a light microscope (SMZ1500, Nikon, Tokyo, Japan) with a digital camera (DXM1200F, Nikon, Tokyo, Japan) and were drawn onto plates using a stereotaxic atlas of the guinea pig brain60.
Behavioral data analysis
For each training trial, 2000-ms time periods were recorded during the conditioning trials and 12-s time periods were recorded during the pseudo-conditioning trials beginning 800 ms before the onset of the CS. Drug infusions were not recorded. All data presented in this paper are measurements of the left upper eyelid movements. The parameters of eyeblink responses were analyzed using custom software.
Each CS-US paired trial presented during the conditioning training was subdivided into three discontinuous analysis periods: (1) a “baseline” period, which occurred at 0–800 ms before the CS onset; (2) a “CR” period, which occurred at 140 ms before the US onset; and (3) a “UR” period, which occurred at 0–250 ms after the US onset. The “baseline” period and “CR” period of each CS-alone trial were divided in the same manner as the baseline and CR periods from the CS-US paired trials for the same training paradigm. A significant eyelid movement was defined as an increase in mechanogram amplitude that was greater than the mean baseline amplitude and had four times the standard deviation of the baseline activity. In addition, the significant eyelid movement required a minimal duration of 15 ms. Any significant eyelid movement during the latter two periods defined above was counted as a CR or a UR, respectively. The percentage of CR (CR%) was defined as the ratio of the number of trials containing the CR to the total number of valid trials. The CR peak amplitude was defined as the maximum amplitude change from baseline during the CR period. The trials containing CR were selected for analysis of CR peak amplitude. The CR relative peak latency was defined as the time interval from the CR peak to the US onset.
Only the CR% was analyzed for the animals that received pseudo-conditioning training. For each trial, a significant eyelid movement that occurred within the time period 140 ms before the US onset was defined as a CR-like eyeblink response.
Statistical analysis
All data were expressed as means±SEM. Statistical significance was determined by a least significant difference (LSD) post-hoc test following a two-way repeated measures analyses of variance (ANOVA), a separate one-way repeated measures ANOVA, or a separate one-way ANOVA using the SPSS software for Windows package (v 18.0). A value of P<0.05 was considered statistically significant.
Results
Electrode and infusion cannula tips placements
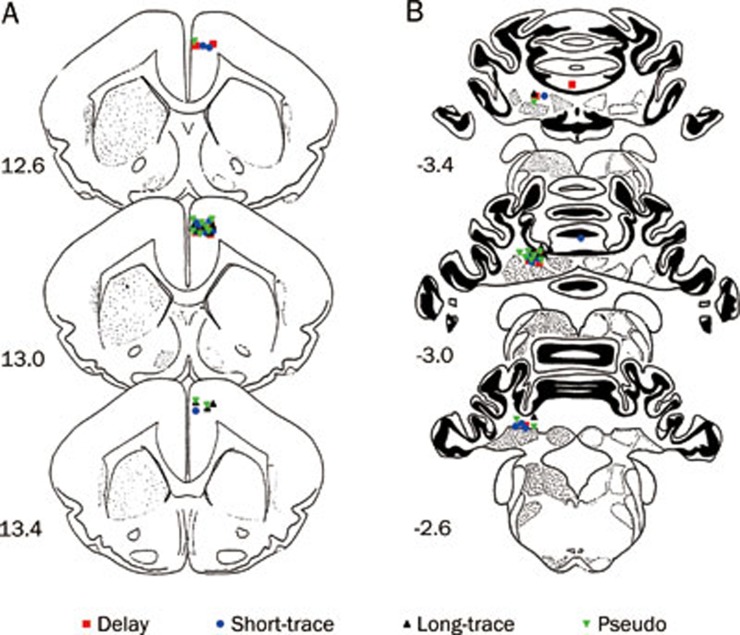
Placement of the electrode cannula and infusion tips was carefully checked before the behavioral analysis commenced. An animal's data were excluded from the analysis if the electrode tip was not in the right caudal mPFC or the infusion cannula tip was not in the left cerebellar interpositus nucleus. Electrode tip placements in the right caudal mPFC were verified by examining a series of coronal sections. All electrode tips were placed in the right caudal mPFC (n=37). The infusion cannula tip placements in the left cerebellar interpositus nucleus were also verified by examining a series of coronal sections (Figure 2A). Most of the infusion cannula tip placements were in or near the left cerebellar interpositus nucleus (n=35) with one exception each in the delay and short-trace conditioned group (Figure 2B).
Figure 2.
Histological reconstructions of the electrode and infusion cannula tips. Schematic illustration of the locations of all electrode tips (A) and all infusion cannula tips (B) for the delay (▪ n=9), short-trace (• n=10), long-trace (▴ n=8), and pseudo conditioned (▾ n=10) groups, respectively. Note that one infusion cannula tip of the delay conditioned group and one infusion cannula tip of the short-trace conditioned group were not in or near the left cerebellar interpositus nucleus. Numbers to the left represent distance (mm) from the frontal zero plane. The coronal brain plates are adapted from the atlas of Rapisarda and Bacchelli (1977).
Acquisition of eyeblink conditioning by the guinea pigs
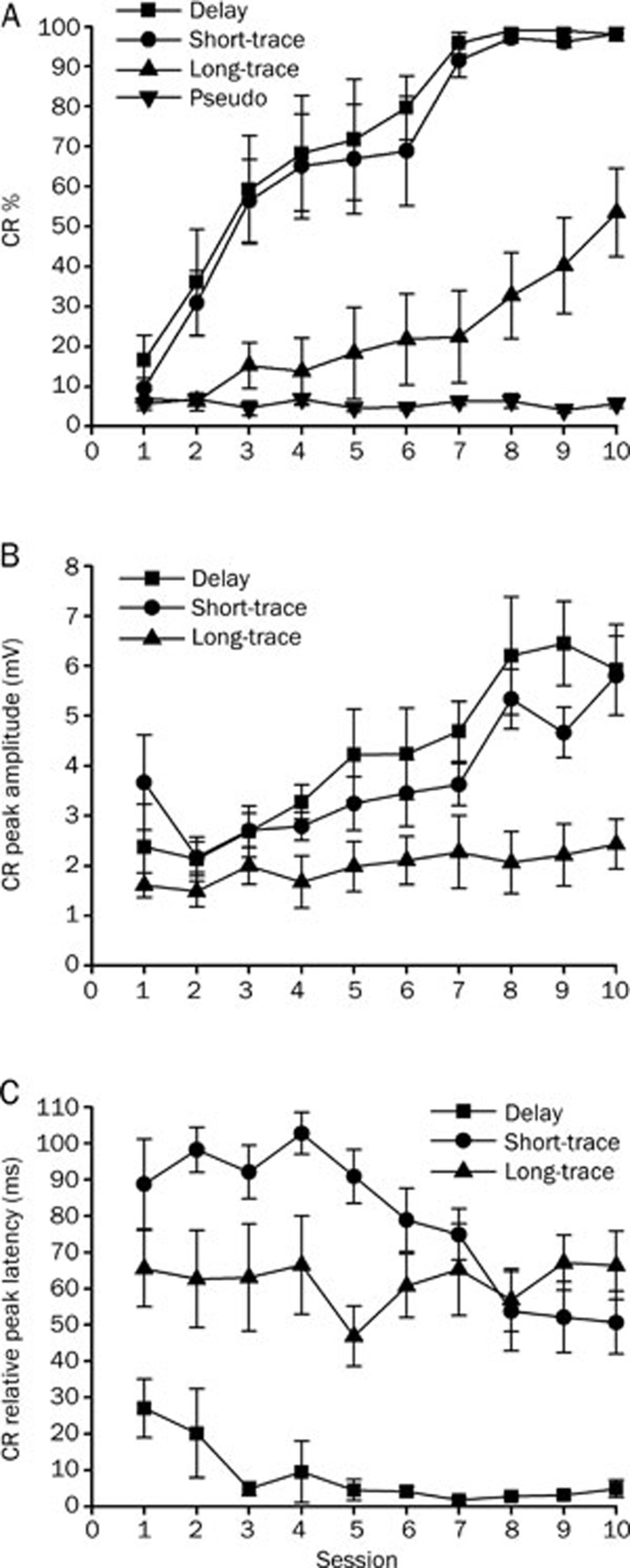
The data from two animals in the delay-conditioned group, from one animal in the short-trace conditioned group and from two animals in long-trace conditioned group were removed from the analysis, because either the infusion cannula tips placements were not in or near the left cerebellar interpositus nucleus (n=2) or the animal had died before the end of the experiment (n=3). The CR% increased as a function of sessions for the delay, short-trace and long-trace conditioned groups (Figure 3A). This increase was confirmed by a two-way repeated measures ANOVA, there was a significant interaction between groups and sessions [F(27,279)=7.289, P<0.001], and significant main effects of group [F(3,31)=39.295, P<0.001] and session [F(9,279)=38.940, P<0.001]. Furthermore, LSD post-hoc tests revealed that the CR% of the delay, short-trace, and long-trace conditioned groups was significantly higher than the CR% of the pseudo-conditioned group (P<0.001, P<0.001, and P=0.026, respectively; Figure 3A). The simple main effects of session for CR% during acquisition training were further analyzed using separate one-way repeated measures ANOVA. This analysis revealed that the simple main effects of session on CR% were significant for the delay [F(9,63)=14.517, P<0.001], short-trace [F(9,72)=2.332, P<0.001], and long-trace [F(9,63)=5.543, P<0.001] conditioned groups, but not for the pseudo-conditioned group [F(9,81)=0.626, P=0.772; Figure 3A].
Figure 3.
Acquisition of the eyeblink conditioned responses (CR) for delay (n=8), short-trace (n=9), long-trace (n=8), and pseudo (n=10) conditioned groups given training with a right caudal mPFC conditioned stimulus (CS) across 10 acquisition training sessions. (A) CR percentage, (B) CR peak amplitude, and (C) CR relative peak latency are given as mean±standard error (SEM). Error bars represent the SEM.
To investigate the effects of the different conditioning training paradigms on the CR pattern, CR peak amplitude and relative peak latency was analyzed for all of the animals. LSD post-hoc tests confirmed that the CR peak amplitude in the long-trace conditioned group was significantly lower than that in the delay (P<0.001) and short-trace (P=0.002) conditioned groups. The latter two groups did not differ significantly from each other (P=0.356; Figure 3B). In addition, a separate one-way repeated measures ANOVA revealed that the simple main effects of session on the CR peak amplitude were significant for both the delay [F(9,63)=5.143, P<0.001] and short-trace [F(9,72)=4.529, P<0.001] conditioned groups, but not for the long-trace conditioned group [F(9,81)=0.873, P=0.554; Figure 3B].
Furthermore, LSD post-hoc tests revealed that the CR relative peak latency in the delay conditioned group was significantly lower than that in either the short-trace (P<0.001) or the long-trace (P<0.001) conditioned groups. The latter two groups differed significantly from each other (P=0.019; Figure 3C). Moreover, the LSD post-hoc tests confirmed that the CR relative peak latency during session 1 was significantly higher than during session 10 for the delay (P=0.041) and short-trace (P=0.043) conditioned groups, but not for the long-trace conditioned group (P=0.994; Figure 3C).
Effects of muscimol infusion into the left cerebellar interpositus nucleus on DEC expression
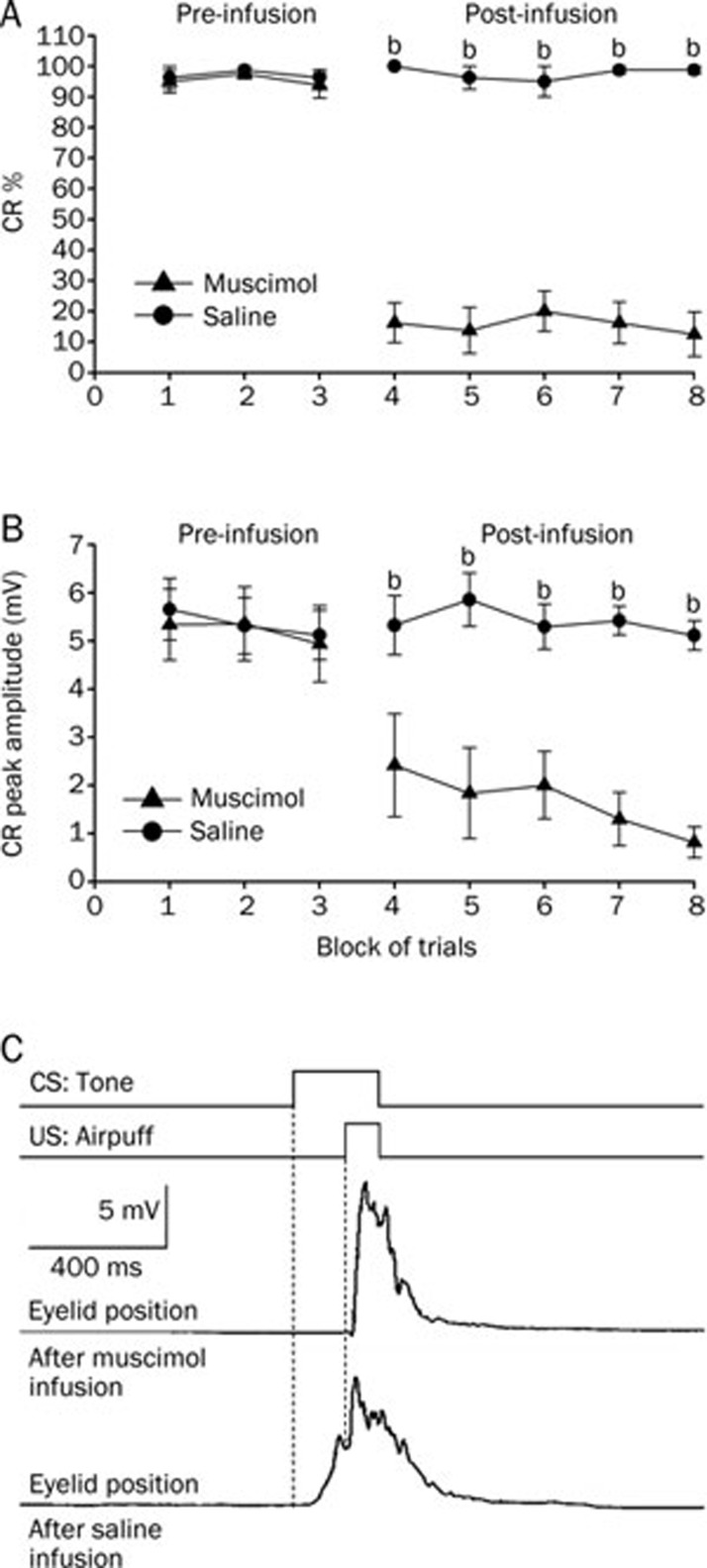
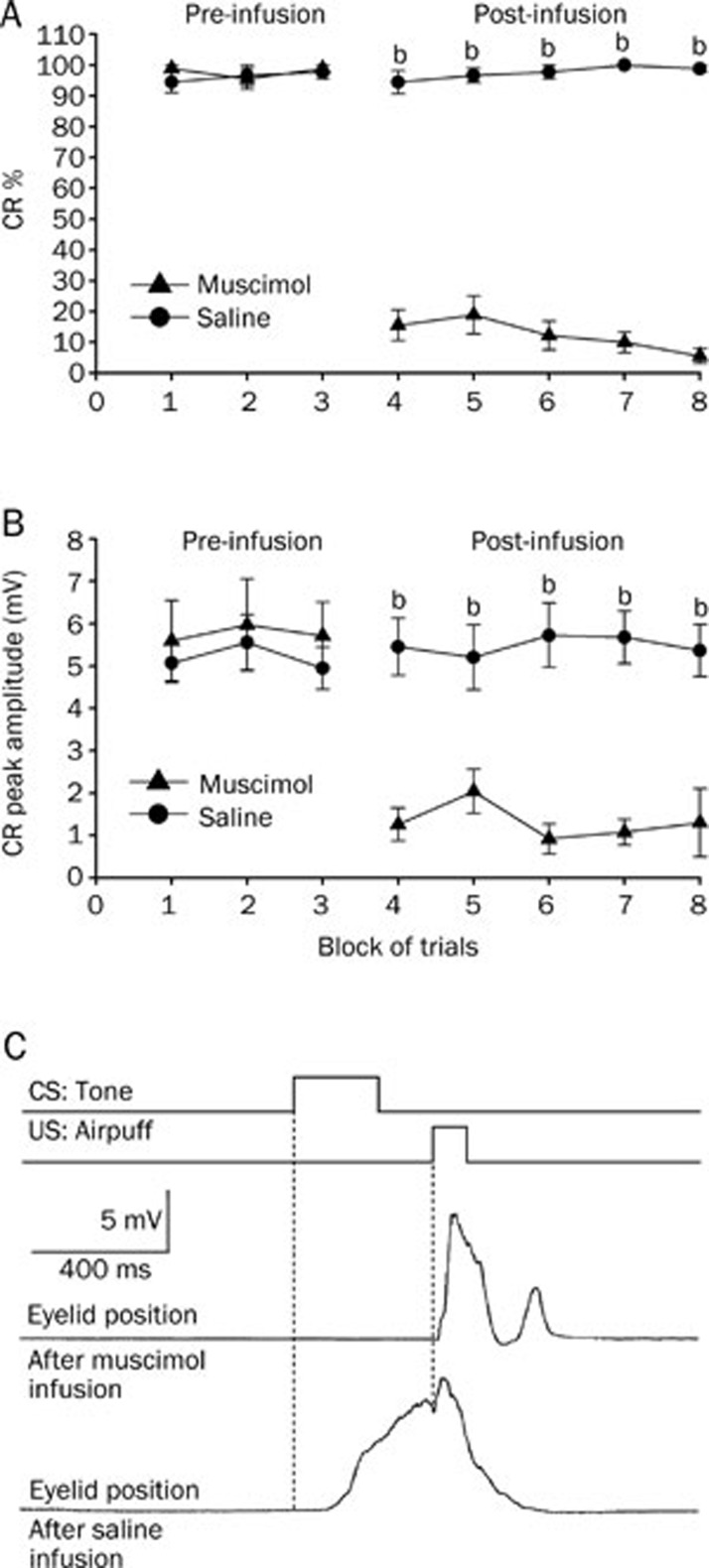
Infusion of muscimol into the left cerebellar interpositus nucleus significantly decreased the CR% (Figure 4A, right panel) and the CR peak amplitude for the group that received delay conditioning (Figure 4B, right panel). A two-way repeated measures ANOVA performed on CR% (Figure 4A, left panel) and CR peak amplitude (Figure 4B, left panel) revealed there was no significant effect of trial block [F(1,14)=0.299, P=0.593 and F(1,14)=0.028, P=0.870, respectively] during the pre-infusion block (trial blocks 1–3). However, the two-way repeated measures ANOVA confirmed that the CR% (Figure 4A, right panel) and the CR peak amplitude (Figure 4B, right panel) for the animals that received the muscimol infusion were significantly lower than those observed for the animals that received the saline infusion [F(1,14)=25.599, P<0.001 and F(1,14)=36.147, P<0.001, respectively] during the post-infusion block (trial blocks 4–8).
Figure 4.
Delay conditioned group data for the effects of muscimol (▴) and saline (•) infused into the left cerebellar interpositus nucleus. (A) Infusion of muscimol abolished the responses almost completely as illustrated by its effects on the percentage of trials in which the delay CRs were seen, whereas infusion of saline had no significant effect on the delay CRs. (B) Muscimol infusion significantly decreased the peak amplitude of the delay CRs. (C) Eyelid position of an animal after muscimol and saline infusion in the sixth trial. Upper panel: the conditioning paradigm illustrating the timing of the CS and the US. Middle panel: eyelid position after muscimol infusion. Lower panel: eyelid postion after saline infusion. All data are from the same animals. Mean±SEM. n=8. bP<0.05 vs control.
Effects of muscimol infusion into the left cerebellar interpositus nucleus on short TEC expression
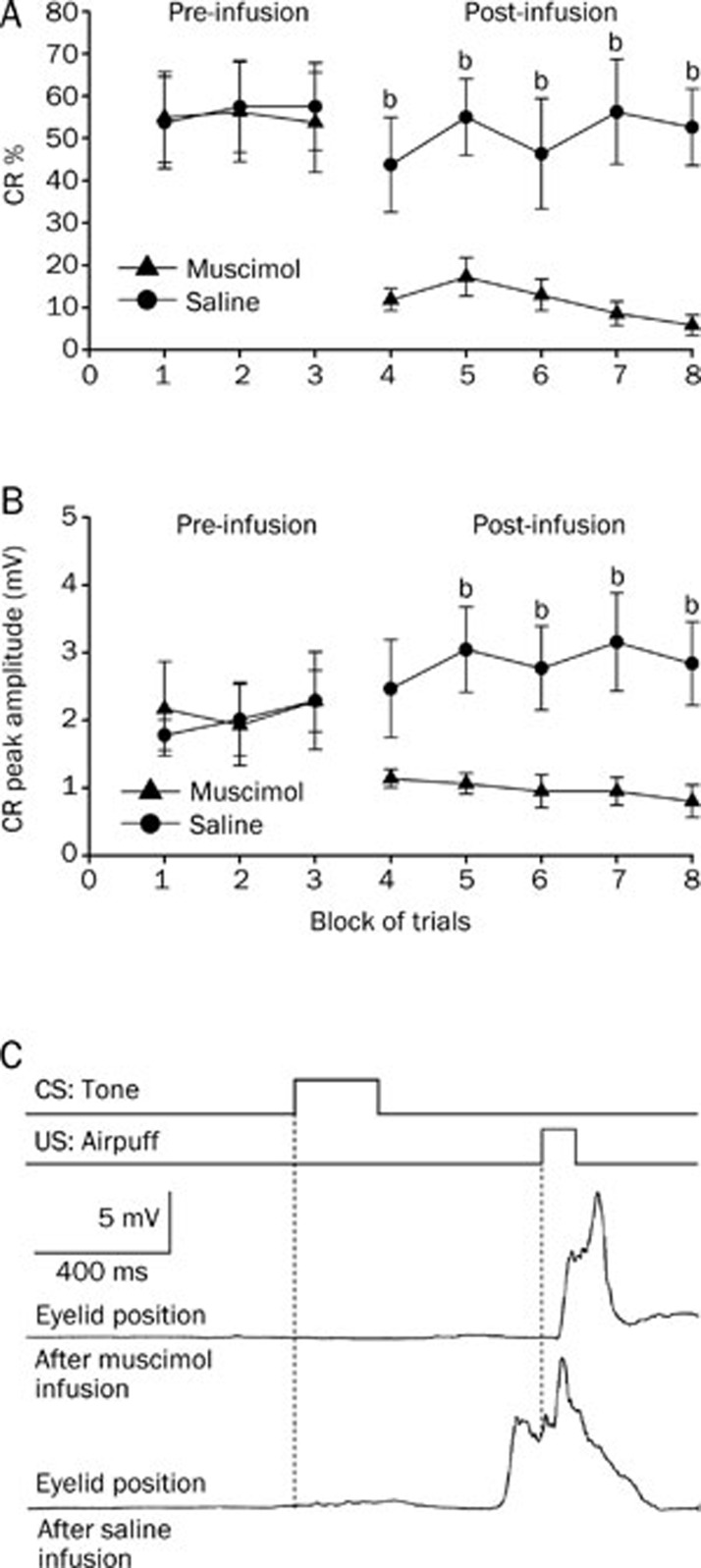
Infusion of muscimol into the left cerebellar interpositus nucleus significantly decreased the CR% (Figure 5A, right panel) and the CR peak amplitude for the group that received short-trace conditioning (Figure 5B, right panel). A two-way repeated measures ANOVA performed on CR% (Figure 5A, left panel) and CR peak amplitude (Figure 5B, left panel) revealed that there was no significant effect of trial block [F(1,16)=0.250, P=0.624 and F(1,16)=0.516, P=0.483, respectively] during the pre-infusion block (trial blocks 1–3). However, the two-way repeated measures ANOVA confirmed that the CR% (Figure 5A, right panel) and the CR peak amplitude (Figure 5B, right panel) for the animals that received the muscimol infusion were significantly lower than for the animals that received the saline infusion [F(1,16)=72.147, P<0.001 and F(1,16)=33.558, P<0.001, respectively] during the post-infusion block (trial blocks 4–8).
Figure 5.
Short-trace conditioned group data for the effects of muscimol (▴), and saline (•) infused into the left cerebellar interpositus nucleus. (A) Infusion of muscimol abolished the responses almost completely as illustrated by its effects on the percentage of trials in which the short-trace CRs are seen, whereas infusion of saline had no significant effect on the short-trace CRs. (B) Muscimol infusion significantly decreased the peak amplitude of the short-trace CRs. (C) Eyelid position of an animal after muscimol and saline infusion in the sixth trial. Upper panel: the conditioning paradigm illustrating the timing of the CS and the US. Middle panel: eyelid position after muscimol infusion. Lower panel: eyelid postion after saline infusion. All data are from the same animals. Mean±SEM. n=9. bP<0.05 vs control.
Effects of muscimol infusion into the left cerebellar interpositus nucleus on long TEC expression
Infusion of muscimol into the left cerebellar interpositus nucleus significantly decreased the CR% (Figure 6A, right panel) and the CR peak amplitude (Figure 6B, right panel) for the group that received long-trace conditioning. A two-way repeated measures ANOVA performed on CR% (Figure 6A, left panel) and CR peak amplitude (Figure 6B, left panel) revealed that there was no significant effect of trial block [F(1,14)=0.007, P=0.933 and F(1,14)=0.089, P=0.770, respectively] during the pre-infusion block (trial blocks 1–3). In contrast, the two-way repeated measures ANOVA confirmed that the CR% (Figure 6A, right panel) and the CR peak amplitude (Figure 6B, right panel) for the animals that received the muscimol infusion were significantly lower than for the animals that received the saline infusion [F(1,14)=25.801, P<0.001 and F(1,14)=8.313, P<0.001, respectively] during the post-infusion block (trial blocks 4–8).
Figure 6.
Long-trace conditioned group data for the effects of muscimol (▴) and saline (•) infused into the left cerebellar interpositus nucleus. (A) Infusion of muscimol abolished the responses almost completely as illustrated by its effects on the percentage of trials in which the long-trace CRs are seen, whereas infusion of saline had no significant effect on the long-trace CRs. (B) Muscimol infusion had significant effect on peak amplitude of the long-trace CRs. (C) Eyelid position of an animal after muscimol and saline infusion in the sixth trial. Upper panel: the conditioning paradigm illustrating the timing of the CS and the US. Middle panel: eyelid position after muscimol infusion. Lower panel: eyelid postion after saline infusion. All data are from the same animals. Mean±SEM. n=8. bP<0.05 vs control.
Discussion
In the initial portion of this study, it was demonstrated that electrical stimulation of the caudal mPFC as a CS paired with an airpuff US was sufficient for establishing eyeblink conditioning in guinea pigs. In contrast, unpaired presentations of the stimulation CS and airpuff US did not obtain CR across 10 d of training, suggesting that CRs observed in the conditioned groups were due to associative learning. Against our expectations, the guinea pigs acquired long TEC slower than both DEC and short TEC, in agreement with the previous studies using peripheral CS (eg, a tone or a light CS) in eyeblink conditioning64, 65. There is a possibility that the longer trace interval between the CS and the US makes it difficult for them to timely converge inside brain, and that additional brain regions such as the hippocampus, mPFC and amygdala are required for establishment of long TEC6, 13, 17, 18, 22, 23, 24, 53, 54, 66, 67, 68, 69. There were significant increases in CR amplitudes and decreases in CR relative peak latency in both delay and short-trace, but not in long-trace conditioned groups, attributing to that long TEC had not been well learned during successive 10 sessions. Moreover, an additional evoked field potentials recording test showed that the caudal mPFC stimulation with 200 μA or below did not evoke any field potential in the motor cortex, somatosensory cortex, or the cerebellar cortex. Thus, the current results provide direct support for our hypothesis that electrical stimulation of mPFC as a CS paired with an airpuff US is sufficient for establishing eyeblink conditioning.
In the second portion of this study, infusion of muscimol into the left interpositus nucleus impaired CRs expression in the three conditioned groups, suggesting that the interpositus nucleus is critical for expression of this special CR. It is consistent with the findings of the previous studies using peripheral CS in eyeblink conditioning that cerebellar interpositus nucleus plays an essential role in CR expression4, 27, 29, 31, 66, 70, 71. Steinmetz72 speculated that the cerebellum may only be involved in simple, discrete, aversive, and somatic associative learning that occurs with a relatively short interstimulus interval. The current results support his speculation, and show that the expression of this special CR, which is also a simple, discrete, somatic, aversive, and defensive behavior induced by stimulation of caudal mPFC as a CS, is still dependent on the cerebellum. The results from the present study further imply that it is the features of the CR rather than the CS that decide whether the cerebellum is necessary for CR operation.
Given that mPFC is necessary for long TEC6, 17, 18, 53, 54, but not for DEC and short TEC6, 17, 18, 19, 21, 55, 56, 57, 58, 59, it is expected that long TEC would be acquired more rapidly than DEC and short TEC at least in some manner when stimulation of the caudal mPFC, part of the long TEC circuit, was used as a CS. However, the present results show that the guinea pigs acquired DEC and short TEC more rapidly than long TEC. The current findings combined with the results of previous studies may indicate that one brain area at which an effective and sufficient stimulation CS for establishing CR was applied can not be interpreted as an essential area for classical eyeblink conditioning. For instance, lesions of the visual cortex did not prevent acquisition of CRs with a light CS73, suggesting that the visual cortex is not involved in the process of CR acquisition, whereas stimulation of the visual cortex can be successfully used as a CS to establish CR34. Moreover, although lesions of the pretectal nuclei73 and hippocampus22, 23, 24, 68 retarded acquisition of eyeblink conditioning, stimulation of the anterior pretectal nucleus11 and of the CA1 layer of hippocampus74 can not be served as effective CSs for establishing eyeblink conditioning. Therefore, the present results only suggest that electrical stimulation of mPFC is a very effective and sufficient CS for establishing eyeblink conditioning, and that it is dependent on the cerebellar interpositus nucleus, but can not be interpreted as providing evidence that mPFC is critically involved in DEC, short TEC, or long TEC.
It is worth noting that long TEC with a tone CS acquisition requires mPFC that persists mossy fiber activity through the stimulus-free trace interval to overlap in time with the US6, 75, 76. In the present study, however, the guinea pigs could develop long TEC to mPFC stimulation CS even when it did not co-terminate with the US. There is a possibility that the mPFC activity which begins during the stimulation CS and persists beyond the stimulation CS offset to overlap with the US may induce long TEC successful establishment. Indeed, it is reported that the activity in the neurons of mPFC began during the tone CS and persisted to overlap with the US during TEC77.
Despite careful and detailed analysis of the CRs acquisition to electrical CSs by previous studies, it is difficult to establish a minimum range of current for the electrical CS which is required for obtaining CR. The threshold stimulation of CS required to establish the CR in the present study was lower than that in most previously studies (50–200 μA in current study vs 180–250 μA for visual cortex stimulation34). This difference may in part due to differences in current spread at different sites of stimulation, but may also relate to differences in the numbers of cells which must be excited for the CS to be effective45.
One of our primary purposes here was to test the effects of stimulation of the caudal mPFC on CR establishment. Whether or not stimulation of other areas of PFC can obtain the CR is an important issue, and should be investigated in future studies. While the distributed pattern of activation induced by electrical stimulation in awake animals is currently unknown78, an additional evoked field potentials recording test showed that the caudal mPFC stimulation with 200 μA or below did not evoke any field potential in the motor cortex, somatosensory cortex, or the cerebellar cortex. Thus, the present experiment could rule out the possibility that stimulation of the caudal mPFC significantly activates other areas of cortex. In addition, the lesion and field potential data suggest that the lateral pontine nuclei conveys necessary CS signals to the cerebellum in eyeblink conditioning79, 80. Recent studies suggest that mossy fiber activity driven by input from mPFC to lateral pontine nuclei which persists through the stimulus-free trace interval to overlap in time with the US supports the auditory TEC 6, 75, 76. Therefore, the lateral pontine nuclei may also convey the electrical CS signals to the cerebellum in the three types of eyeblink conditioning induced by the electrical stimulation of caudal mPFC. However, this hypothesis needs further testing.
In conclusion, the results from this study show that electrical stimulation of caudal mPFC was a very effective CS for establishing eyeblink conditioning, and that the CR is dependent on the cerebellar interpositus nucleus. Moreover, the guinea pigs acquired delay and short-trace CRs more rapidly than long-trace CR. However, the current results should not be interpreted as providing evidence that mPFC is involved in DEC, short TEC, or long TEC.
Author contribution
Jian-feng SUI and Guang-yan WU designed research; Guang-yan WU, Juan YAO, Zheng-li FAN, and Lang-qian ZHANG performed research; Juan YAO, Xuan LI, Chuang-dong ZHAO, and Zhen-hua ZHOU analyzed data; Jian-feng SUI and Guang-yan WU wrote the paper.
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (No 30771769). We thank the assistance of De-ying CHEN in preparing the photomicrographs.
References
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–55. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Long-term storage of an associative memory trace in the cerebellum. Behav Neurosci. 2005;119:526–37. doi: 10.1037/0735-7044.119.2.526. [DOI] [PubMed] [Google Scholar]
- Thompson RF. In search of memory traces. Annu Rev Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- Weeks AC, Connor S, Hinchcliff R, LeBoutillier JC, Thompson RF, Petit TL. Eye-blink conditioning is associated with changes in synaptic ultrastructure in the rabbit interpositus nuclei. Learn Mem. 2007;14:385–9. doi: 10.1101/lm.348307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, Arenos JD. Hippocampal and cerebellar single-unit activity during delay and trace eyeblink conditioning in the rat. Neurobiol Learn Mem. 2007;87:269–84. doi: 10.1016/j.nlm.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Kreider JC, Riusech F, Mauk MD. Interactions between prefrontal cortex and cerebellum revealed by trace eyelid conditioning. Learn Mem. 2009;16:86–95. doi: 10.1101/lm.1178309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Lee I, Freeman JH. Associative plasticity in the medial auditory thalamus and cerebellar interpositus nucleus during eyeblink conditioning. J Neurosci. 2010;30:8787–96. doi: 10.1523/JNEUROSCI.0208-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Medial auditory thalamic input to the lateral pontine nuclei is necessary for auditory eyeblink conditioning. Neurobiol Learn Mem. 2010;93:92–8. doi: 10.1016/j.nlm.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Poremba A, Freeman JH. Medial auditory thalamus inactivation prevents acquisition and retention of eyeblink conditioning. Learn Mem. 2008;15:532–8. doi: 10.1101/lm.1002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Halverson HE, Hubbard EM. Inferior colliculus lesions impair eyeblink conditioning in rats. Learn Mem. 2007;14:842–6. doi: 10.1101/lm.716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Halverson HE, Freeman JH. Medial auditory thalamic stimulation as a conditioned stimulus for eyeblink conditioning in rats. Learn Mem. 2007;14:152–9. doi: 10.1101/lm.465507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Medial auditory thalamic nuclei are necessary for eyeblink conditioning. Behav Neurosci. 2006;120:880–7. doi: 10.1037/0735-7044.120.4.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald BB, Knuckley B, Maddox SA, Powell DA. Ibotenic acid lesions to ventrolateral thalamic nuclei disrupts trace and delay eyeblink conditioning in rabbits. Behav Brain Res. 2007;179:111–7. doi: 10.1016/j.bbr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Ventral lateral geniculate input to the medial pons is necessary for visual eyeblink conditioning in rats. Learn Mem. 2010;17:80–5. doi: 10.1101/lm.1572710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon B, Knuckley B, Churchwell J, Powell DA. Post–training lesions of the medial prefrontal cortex interfere with subsequent performance of trace eyeblink conditioning. J Neurosci. 2005;25:10740–6. doi: 10.1523/JNEUROSCI.3003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald BB, Maddox SA, Powell DA. Prefrontal control of trace eyeblink conditioning in rabbits: role in retrieval of the CR. Behav Neurosci. 2008;122:841–8. doi: 10.1037/0735-7044.122.4.841. [DOI] [PubMed] [Google Scholar]
- Oswald BB, Maddox SA, Tisdale N, Powell DA. Encoding and retrieval are differentially processed by the anterior cingulate and prelimbic cortices: a study based on trace eyeblink conditioning in the rabbit. Neurobiol Learn Mem. 2010;93:37–45. doi: 10.1016/j.nlm.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DA, Churchwell J, Burriss L. Medial prefrontal lesions and Pavlovian eyeblink and heart rate conditioning: effects of partial reinforcement on delay and trace conditioning in rabbits (Oryctolagus cuniculus) Behav Neurosci. 2005;119:180–9. doi: 10.1037/0735-7044.119.1.180. [DOI] [PubMed] [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav Neurosci. 2000;114:1058–67. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Activity profiles of single neurons in caudal anterior cingulate cortex during trace eyeblink conditioning in the rabbit. J Neurophysiol. 2003;90:599–612. doi: 10.1152/jn.01097.2002. [DOI] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, Kawahara S, Kirino Y. NMDA receptor-dependent processes in the medial prefrontal cortex are important for acquisition and the early stage of consolidation during trace, but not delay eyeblink conditioning. Learn Mem. 2005;12:606–14. doi: 10.1101/lm.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav Brain Res. 1999;99:123–32. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty. Neurobiol Learn Mem. 2001;76:447–61. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Tseng W, Guan R, Disterhoft JF, Weiss C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus. 2004;14:58–65. doi: 10.1002/hipo.10157. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–44. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Moyer JR Jr, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990;104:243–52. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Hu B, Yang L, Huang LS, Chen H, Zeng Y, Feng H, et al. Effect of cerebellar reversible inactivations on the acquisition of trace conditioned eyeblink responses in guinea pigs: comparison of short and long trace intervals. Neurosci Lett. 2009;459:41–5. doi: 10.1016/j.neulet.2009.04.061. [DOI] [PubMed] [Google Scholar]
- Hu B, Lin X, Huang LS, Yang L, Feng H, Sui JF. Involvement of the ipsilateral and contralateral cerebellum in the acquisition of unilateral classical eyeblink conditioning in guinea pigs. Acta Pharmacol Sin. 2009;30:141–52. doi: 10.1038/aps.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakaprot N, Kim S, Thompson RF. The role of the cerebellar interpositus nucleus in short and long term memory for trace eyeblink conditioning. Behav Neurosci. 2009;123:54–61. doi: 10.1037/a0014263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci. 2003;23:9897–905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res. 1985;348:249–60. doi: 10.1016/0006-8993(85)90443-3. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Duffel JW. Eyeblink conditioning using cochlear nucleus stimulation as a conditioned stimulus in developing rats. Dev Psychobiol. 2008;50:640–6. doi: 10.1002/dev.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Thompson RF. Conditioning using a cerebral cortical conditioned stimulus is dependent on the cerebellum and brain stem circuitry. Behav Neurosci. 1992;106:509–17. doi: 10.1037//0735-7044.106.3.509. [DOI] [PubMed] [Google Scholar]
- Halverson HE, Hubbard EM, Freeman JH. Stimulation of the lateral geniculate, superior colliculus, or visual cortex is sufficient for eyeblink conditioning in rats. Learn Mem. 2009;16:300–7. doi: 10.1101/lm.1340909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH Jr, Rabinak CA. Eyeblink conditioning in rats using pontine stimulation as a conditioned stimulus. Integr Physiol Behav Sci. 2004;39:180–91. doi: 10.1007/bf02734438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DJ, Steinmetz JE, Thompson RF. Classical discrimination conditioning of the rabbit's eyelid response using pontine stimulation as a conditioned stimulus. Behav Neural Biol. 1989;52:51–62. doi: 10.1016/s0163-1047(89)90158-1. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3:225–33. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Rosen DJ, Chapman PF, Lavond DG, Thompson RF. Classical conditioning of the rabbit eyelid response with a mossy-fiber stimulation CS: I. Pontine nuclei and middle cerebellar peduncle stimulation. Behav Neurosci. 1986;100:878–87. doi: 10.1037//0735-7044.100.6.878. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Rosen DJ, Woodruff-Pak DS, Lavond DG, Thompson RF. Rapid transfer of training occurs when direct mossy fiber stimulation is used as a conditioned stimulus for classical eyelid conditioning. Neurosci Res. 1986;3:606–16. doi: 10.1016/0168-0102(86)90057-x. [DOI] [PubMed] [Google Scholar]
- Shinkman PG, Swain RA, Thompson RF. Classical conditioning with electrical stimulation of cerebellum as both conditioned and unconditioned stimulus. Behav Neurosci. 1996;110:914–21. doi: 10.1037//0735-7044.110.5.914. [DOI] [PubMed] [Google Scholar]
- Jirenhed DA, Hesslow G. Time course of classically conditioned Purkinje cell response is determined by initial part of conditioned stimulus. J Neurosci. 2011;31:9070–4. doi: 10.1523/JNEUROSCI.1653-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Thompson RF. Timing of conditioned responses utilizing electrical stimulation in the region of the interpositus nucleus as a CS. Integr Physiol Behav Sci. 2004;39:83–94. doi: 10.1007/BF02734274. [DOI] [PubMed] [Google Scholar]
- Jimenez-Diaz L, Navarro-Lopez Jde D, Gruart A, Delgado-Garcia JM. Role of cerebellar interpositus nucleus in the genesis and control of reflex and conditioned eyelid responses. J Neurosci. 2004;24:9138–45. doi: 10.1523/JNEUROSCI.2025-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Campanario R, Delgado-Garcia JM, Gruart A. Microstimulation of the somatosensory cortex can substitute for vibrissa stimulation during Pavlovian conditioning. Proc Natl Acad Sci U S A. 2006;103:10052–7. doi: 10.1073/pnas.0603584103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody CD, Yarowsky PJ. Conditioned eye blink using electrical stimulation of coronal-precruciate cortex as conditional stimulus. J Neurophysiol. 1972;35:242–52. doi: 10.1152/jn.1972.35.2.242. [DOI] [PubMed] [Google Scholar]
- Troncoso J, Munera A, Delgado-Garcia JM. Classical conditioning of eyelid and mystacial vibrissae responses in conscious mice. Learn Mem. 2004;11:724–6. doi: 10.1101/lm.81204. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS.Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates Prog Brain Res 199085325–35.discussion 35–6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–85. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex — an update: time is of the essence. Neuron. 2001;30:319–33. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146:145–57. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Powell DA, Skaggs H, Churchwell J, McLaughlin J. Posttraining lesions of the medial prefrontal cortex impair performance of Pavlovian eyeblink conditioning but have no effect on concomitant heart rate changes in rabbits (Oryctolagus cuniculus) Behav Neurosci. 2001;115:1029–38. doi: 10.1037//0735-7044.115.5.1029. [DOI] [PubMed] [Google Scholar]
- Doty RW, Larsen RM, Ruthledge LT Jr. Conditioned reflexes established to electrical stimulation of cat cerebral cortex. J Neurophysiol. 1956;19:401–15. doi: 10.1152/jn.1956.19.5.401. [DOI] [PubMed] [Google Scholar]
- Simon B, Knuckley B, Churchwell J, Powell DA. Post-training lesions of the medial prefrontal cortex interfere with subsequent performance of trace eyeblink conditioning. J Neurosci. 2005;25:10740–6. doi: 10.1523/JNEUROSCI.3003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald BB, Maddox SA, Powell DA. Prefrontal control of trace eyeblink conditioning in rabbits: role in retrieval of the CR. Behav Neurosci. 2008;122:841–8. doi: 10.1037/0735-7044.122.4.841. [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiol Learn Mem. 1998;69:147–62. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- Chachich M, Powell DA. Both medial prefrontal and amygdala central nucleus lesions abolish heart rate classical conditioning, but only prefrontal lesions impair reversal of eyeblink differential conditioning. Neurosci Lett. 1998;257:151–4. doi: 10.1016/s0304-3940(98)00832-5. [DOI] [PubMed] [Google Scholar]
- Powell DA, Skaggs H, Churchwell J, McLaughlin J. Posttraining lesions of the medial prefrontal cortex impair performance of Pavlovian eyeblink conditioning but have no effect on concomitant heart rate changes in rabbits (Oryctolagus cuniculus) Behav Neurosci. 2001;115:1029–38. doi: 10.1037//0735-7044.115.5.1029. [DOI] [PubMed] [Google Scholar]
- Leal–Campanario R, Fairen A, Delgado-Garcia JM, Gruart A. Electrical stimulation of the rostral medial prefrontal cortex in rabbits inhibits the expression of conditioned eyelid responses but not their acquisition. Proc Natl Acad Sci U S A. 2007;104:11459–64. doi: 10.1073/pnas.0704548104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial prefrontal cortex and pavlovian conditioning: trace versus delay conditioning. Behav Neurosci. 2002;116:37–47. [PubMed] [Google Scholar]
- Rapisarda C, Bacchelli B. The brain of the guinea pig in stereotaxic coordinates. Arch Sci Biol (Bologna) 1977;61:1–37. [PubMed] [Google Scholar]
- Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127:160–4. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–59. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Sarihi A, Motamedi F, Naghdi N, Rashidy-Pour A. Lidocaine reversible inactivation of the median raphe nucleus has no effect on reference memory but enhances working memory versions of the Morris water maze task. Behav Brain Res. 2000;114:1–9. doi: 10.1016/s0166-4328(00)00176-5. [DOI] [PubMed] [Google Scholar]
- Hu B, Chen H, Yang L, Tao ZF, Yan J, Zhang YH, et al. Changes of synaptic ultrastructure in the guinea pig interpositus nuclei associate with response magnitude and timing after trace eyeblink conditioning. Behav Brain Res. 2012;226:529–37. doi: 10.1016/j.bbr.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Kotani S, Kawahara S, Kirino Y. Trace eyeblink conditioning in decerebrate guinea pigs. Eur J Neurosci. 2003;17:1445–54. doi: 10.1046/j.1460-9568.2003.02566.x. [DOI] [PubMed] [Google Scholar]
- Green JT, Arenos JD. Hippocampal and cerebellar single-unit activity during delay and trace eyeblink conditioning in the rat. Neurobiol Learn Mem. 2007;87:269–84. doi: 10.1016/j.nlm.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt LJ, Vogt BA, Sikes RW. Limbic thalamus in rabbit: architecture, projections to cingulate cortex and distribution of muscarinic acetylcholine, GABAA, and opioid receptors. J Comp Neurol. 1992;319:205–17. doi: 10.1002/cne.903190203. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–38. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Powell DA, Churchwell J. Mediodorsal thalamic lesions impair trace eyeblink conditioning in the rabbit. Learn Mem. 2002;9:10–7. doi: 10.1101/lm.45302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Chen H, Feng H, Zeng Y, Yang L, Fan ZL, et al. Disrupted topography of the acquired trace-conditioned eyeblink responses in guinea pigs after suppression of cerebellar cortical inhibition to the interpositus nucleus. Brain Res. 2010;1337:41–55. doi: 10.1016/j.brainres.2010.03.089. [DOI] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD. Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology. 1998;37:471–80. doi: 10.1016/s0028-3908(98)00055-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE. Brain substrates of classical eyeblink conditioning: a highly localized but also distributed system. Behav Brain Res. 2000;110:13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- Koutalidis O, Foster A, Weisz DJ. Parallel pathways can conduct visual CS information during classical conditioning of the NM response. J Neurosci. 1988;8:417–27. doi: 10.1523/JNEUROSCI.08-02-00417.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokasy WF, Kesner RP, Calder LD. Posttrial electrical stimulation of the dorsal hippocampus facilitates acquisition of the nictitating membrane response. Behav Neurosci. 1983;97:890–6. doi: 10.1037//0735-7044.97.6.890. [DOI] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Mauk MD. Temporal patterns of inputs to cerebellum necessary and sufficient for trace eyelid conditioning. J Neurophysiol. 2010;104:627–40. doi: 10.1152/jn.00169.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada N, Kishimoto Y, Watanabe D, Kano M, Hirano T, Funabiki K, et al. Conditioned eyeblink learning is formed and stored without cerebellar granule cell transmission. Proc Natl Acad Sci U S A. 2007;104:16690–5. doi: 10.1073/pnas.0708165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JJ, Kalmbach B, Chitwood RA, Mauk MD. Persistent activity in a cortical-to-subcortical circuit: bridging the temporal gap in trace eyelid conditioning. J Neurophysiol. 2012;107:50–64. doi: 10.1152/jn.00689.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda Y, Shikata H, Song WJ. A train of electrical pulses applied to the primary auditory cortex evokes a conditioned response in guinea pigs. Neurosci Res. 2011;71:103–6. doi: 10.1016/j.neures.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Bao S, Chen L, Thompson RF. Learning- and cerebellum-dependent neuronal activity in the lateral pontine nucleus. Behav Neurosci. 2000;114:254–61. doi: 10.1037//0735-7044.114.2.254. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proc Natl Acad Sci U S A. 1987;84:3531–5. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]