Abstract
The ability to attend to particular stimuli while ignoring others is crucial in goal-directed activities and has been linked with prefrontal cortical regions, including the dorsolateral prefrontal cortex (DLPFC). Both hyper- and hypo-activation in the DLPFC has been reported in patients with attention-deficit/hyperactivity disorder (ADHD) during many different cognitive tasks, but the network-level effects of such aberrant activity remain largely unknown. Using magnetoencephalography (MEG), we examined functional connectivity between regions of the DLPFC and the modality-specific auditory cortices during an auditory attention task in medicated and un-medicated adults with ADHD, and those without ADHD. Participants completed an attention task in two separate sessions (medicated/un-medicated), and each session consisted of two blocks (attend and no-attend). All MEG data were coregistered to structural MRI, corrected for head motion, and projected into source space. Subsequently, we computed the phase coherence (i.e., functional connectivity) between DLPFC regions and the auditory cortices. We found that un-medicated adults with ADHD exhibited greater phase coherence in the beta (14–30Hz) and gamma frequency (30–56 Hz) range in attend and no-attend conditions compared to controls. Stimulant medication attenuated these differences, but did not fully eliminate them. These results suggest that aberrant bottom-up processing may engulf executive resources in ADHD.
Keywords: Cortex, Magnetoencephalography, Stimulants, DLPFC, Beta, Gamma
1. Introduction
The capacity to focus attention on a specific stimulus or element of the environment is critical to goal directed activities, and areas of the frontal and parietal cortex have been implicated as playing a vital role in such attentional processes (Hopfinger et al., 2000; Miller and Cohen, 2001; Corbetta and Schulman, 2002). Specifically, the prefrontal cortex seems to be critical in attentional processes and is widely associated with high-level cognitive functioning, complex informational processing, and executive functioning (Posner and Peterson, 1990; Miller and Cohen, 2001; Rossi et al., 2009; Gazzaniga et al., 2009). The ability to pay attention to particular stimuli while ignoring other distracting stimuli is known as top-down or goal directed attentional processing, and has been widely studied within the context of visual attention and linked to the same cortical areas (Miller and Cohen, 2001; Corbetta and Schulman, 2002; Brazdil et al., 2007; Wang el al., 2009; Gregoriou et al., 2009; Baluch and Itti, 2011).
While the brain networks serving visual attention have been well characterized, substantially less work has been conducted in the area of auditory attention and the role of the dorsolateral prefrontal cortex (DLPFC). However, in most models of attention, it is generally assumed that the frontal cortices (including the DLPFC) are a central hub that interacts with various modality-specific regions depending on the nature of the external stimulus (Baluch and Itti, 2011; Peterson and Posner, 2012), and there is growing evidence to support this position. For example, Caclin and Fonlupt used functional magnetic resonance imaging (fMRI) to investigate the role of the DLPFC in attention to an auditory stimulus, and found that selective auditory attention resulted in increased connectivity between the DLPFC and auditory cortices in healthy adults (Caclin and Fonlupt, 2006). Such modality-specific long range neural connections are hypothesized to develop and strengthen with age (Brazdil et al., 2007; Fair et al., 2007; Wang et al., 2009), which may indicate that the fidelity of the attentional signal increases in both auditory and visual attention networks as younger participants reach adulthood or maturate to some asymptotic level (Fair et al., 2007).
Abnormalities in the prefrontal cortex, including the DLPFC, have been repeatedly associated with deficits in executive function and are known to play a crucial role in patients with attention-deficit/hyperactivity disorder (ADHD). ADHD is a common neuropsychiatric disorder characterized by symptoms of inattention, hyperactivity, and impulsivity, which generally starts in early childhood (American Psychiatric Association, 2000; Konrad and Eickhoff, 2010; Arnsten et al., 2011; Liston et al., 2011). Evidence now suggests that ADHD symptoms often persist into adulthood in about 65% of affected children, with roughly 46% displaying the full-blown disorder as adults (Kessler et al., 2010; Lara et al., 2010). Multiple brain imaging studies have shown volumetric structural deficits, functional aberrations, and abnormal structural and functional connectivity involving prefrontal regions in both children and adults with ADHD (Konrad and Eickhoff, 2010; Arnsten et al., 2011; Liston et al., 2011). In particular, it has been shown that adults with ADHD exhibit reduced activation bilaterally in the DLPFC and inferior frontal cortices during a visual oddball task, despite performing equally as well as healthy controls on the task (Cubillo et al., 2011). Additionally, psychostimulants, which have robust clinical efficacy, appear to normalize activation and functional connectivity between these prefrontal regions and posterior cortical areas about 60–75 minutes after oral administration (Konrad and Eickhoff, 2010; Peterson et al., 2009; Franzen et al., 2013). For example, one fMRI study showed that diminished functional connectivity involving prefrontal cortical regions and several other brain regions was largely normalized within 60 minutes of stimulant medication administration in children with ADHD (Rubia et al., 2009a).
Given the known role of the frontal cortices in auditory attention, as well as the substantial evidence of specific structural and functional abnormalities in the frontal cortices of patients with ADHD, it is critical to evaluate the integrity of the auditory attention network and the subsequent effects of treatment in patients with ADHD. Using magnetoencephalography (MEG), we explored neural interactions between auditory cortices and the frontal cortices during an auditory attention task in un-medicated and medicated participants with ADHD, as well as a group of their peers without ADHD. MEG is a noninvasive neurophysiological imaging technique with good spatial accuracy and millisecond temporal precision. MEG provides a direct and noninvasive measure of neuronal activity that can be easily compared to the other neuroimaging methods, while also providing important frequency-specific (i.e., neural population-level dynamics) connectivity information. We hypothesized that phase coherence (i.e. functional connectivity) between auditory cortices and frontal regions (e.g., DLPFC) would be abnormal in subjects with ADHD compared with controls, and that these abnormalities would be mitigated with the administration of psychostimulant medications.
2. Methods
2.1. Subject selection
We studied 13 adults (4 females) with ADHD, inattentive type, and 14 adults (5 females) without ADHD. Patients with ADHD were recruited from the adult psychiatry clinic at the Nebraska Medical Center (MWW). Mean ages were 42.2 (ADHD) and 44.5 years (controls) at enrollment (P=0.59). All participants were right-handed, except one participant with ADHD. The two groups were matched on age, sex, years of education, and ethnicity. All participants with ADHD had shown a satisfactory clinical response to a mixture of dextroamphetamine salts, extended release formula (MAS-XR; Adderall XR), and been prescribed the same regularly-monitored dosage for at least 6 months prior to enrollment in this study. For this study, a satisfactory clinical response was defined as mutual agreement between patient and clinician that relief of target symptoms, functional impairment, and distress related to ADHD had been consistently well-managed by the maintenance dosage of their prescription medication. ADHD diagnoses were based on a semi-structured comprehensive psychiatric assessment by a board certified psychiatrist (MWW) utilizing DSM-IV diagnostic criteria, the Adult ADHD Symptom Rating Scale (Kessler et al., 2005), and collateral history. None of the participants were currently diagnosed with anxiety, depressive, or other disorders during the study, and none of the patients were concomitantly taking other psychiatric drugs. Exclusionary criteria included any medical illness affecting CNS function, neurological disorder, Axis II disorder, history of head trauma, history of ADHD or first-degree relative with ADHD (controls only), and current substance abuse. After complete description of the study to participants, written informed consent was obtained in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the standards of the University of Nebraska Medical Center’s Institutional Review Board.
2.2. Experimental paradigm
All participants were scheduled for MEG early in the morning (e.g., 07:30–08:00h) and for the group with ADHD, about 24 hours since their last stimulant dosage (i.e., morning of the previous day). Upon arrival participants completed two blocks of binaural stimulation, each of which consisted of ~250 1 kHz tones (duration = 50 ms; inter-stimulus interval = 1.2 s). In one block, participants were instructed to monitor (“attend”) for slightly quieter tones, and to their raise their index finger each time such a tone was detected (7% of all stimuli). These quiet tones were 10 dB less than the standard tones (93%), which were presented at 90 dB. In the other block (“no-attend”), participants were instructed to remain still and not given any specific directions in regards to the stimuli. Unbeknownst to the participants, the stimulus set was identical between the two blocks, with the exception that the sequential order of stimuli differed. Half of participants in each group were presented with the “attend” block first, while the other half of participants were presented with the “no attend” block first. All stimuli were presented using TIP-300 transducers (Nicolet Biomedical, Madison, WI, USA) and foam ear inserts with 30 dB attenuation to exterior noise. Following the two blocks of auditory stimulation, all participants were moved to the patient waiting area and those with ADHD were orally administered their standard daily dosage of MAS-XR. Approximately 75 minutes later, all participants returned to the MEG room and completed the same blocks of auditory stimulation in “attend” and “no-attend” conditions.
MAS-XR medications are commonly used to treat ADHD in adolescents and adults. Upon administration, blood plasma concentration levels rise sharply then begin to asymptote toward a peak of 20–30 ng/mL at 6–8 hr post-administration. This is followed by a gradual decline in plasma concentration level over the next 16–18 hr (Clausen et al., 2005; Tulloch et al., 2002; Weisler, 2005). However, the degree to which the brain’s response curve follows the plasma concentration curve is entirely unknown. We chose 75 min because this is when the slope of the plasma concentration curve begins to flatten out, and because most prior studies of behavior and/or neuroimaging have used between 60 and 90 min from dosage to testing as the delay period (Barry et al., 2009; Chamberlain et al., 2008; Dodds et al., 2009; Peterson et al., 2009; Rubia et al., 2009a, 2009b; Weinbruch et al., 2005; Wilson et al., 2012; for an exception, see Bush et al., 2008). Thus, 75 min was near the middle of the “standard” window.
2.3. Structural magnetic resonance imaging (MRI)
High-resolution anatomic images were acquired using a Philips Achieva 3T X-series scanner. The T1-weighted sagittal images were obtained with an eight channel head coil using a 3D fast field echo sequence with the following parameters: field of view, 24 cm; slice thickness, 1 mm with no gap; in-plane resolution, 1.0 × 1.0 mm; sense factor, 1.5. Structural MRI volumes were reconstructed, aligned parallel to the anterior and posterior commissures, and transformed into the Talairach coordinate system (Talairach and Tournoux, 1988) using BrainVoyager QX (Brain Innovations, The Netherlands).
2.4. MEG data acquisition and MRI coregistration
All recordings were conducted in a one-layer magnetically shielded room with active shielding engaged. With an acquisition bandwidth of 0.1 – 330 Hz, neuromagnetic responses were sampled continuously at 1 kHz using an Elekta Neuromag system with 306 magnetic sensors (204 planar gradiometers and 102 magnetometers; Elekta, Helsinki, Finland). Using MaxFilter (v2.1.15; Elekta), MEG data from each session and subject were individually corrected for head motion, coregistered to structural MRI, and subjected to noise reduction using the signal space separation method with a temporal extension (tSSS; Taulu et al., 2005; Taulu and Simola, 2006).
Before MEG measurement, four coils were attached to the participant’s head and the locations of these coils, together with the three fiducial points (i.e., nasion, left and right periauricles) and scalp surface, were determined with a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the participant was positioned inside the magnetically-shielded room, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points), each participant’s MEG data were co-registered with their structural T1-weighted MRI data using a least-squares approach before source space analyses. Each participant’s MRI data were transformed into the Talairach coordinate system (Talairach and Tournoux, 1988), and the same transform parameters were then applied to the participant’s functional MEG data.
2.5. MEG source analysis and statistics
Artifact rejection was based on a fixed threshold method supplemented with visual inspection. Following tSSS and head-motion correction, the entire magnetic time series was transformed into a 31-node regional source model via inverse spatial filtering. Essentially, a 31-point grid with dual orthogonal orientations per point was constructed, and each orientation was used as an inverse spatial filter on the continuous sensor-level time series data of the entire recording, per condition and participant (Scherg et al., 2002; Hoechstetter et al., 2004; Wilson et al., 2013a, 2013b; Franzen et al., 2013). After transformation into source space, the current-amplitude (nAm) time series for each of the two orthogonal orientations per source was divided into epochs of 1.2-s duration, including a 0.2-s pre-stimulus baseline. For each block, epochs were transformed into the time-frequency domain using complex demodulation (Paap and Ktonas, 1977). The zero-lag phase-locking value (PLV) was then extracted, using the method described by Lachaux et al. (1999), for the dominant orientation of each source corresponding to the regions of interest (i.e., bilateral auditory and frontal cortices; see Fig. 1). Briefly, the signals were band-pass filtered at ± 0.5 Hz, and their convolution was computed using a complex Gabor wavelet centered at the target frequency. The phase of the convolution was then extracted for each time bin, trial, and source pair. These values were averaged across trials to derive the PLV, which was collapsed across the 75- to 175-ms post-stimulus time bin to estimate the average PLV per node pair during the most prominent auditory processing. The 75- to 175-ms time window was chosen because it included the largest auditory response in all participants (i.e., the M100), and because attentional effects have not been demonstrated on earlier responses in normative studies. Thus, the PLV reflects the inter-trial phase consistency between the time series of (any) two brain regions during the critical temporal period of the epoch. Values close to 1 indicate that the inter-regional phase consistency across trials (at a given frequency) varies only minutely, whereas values close to zero indicate substantial phase variation between the two regions across trials.
Fig. 1.
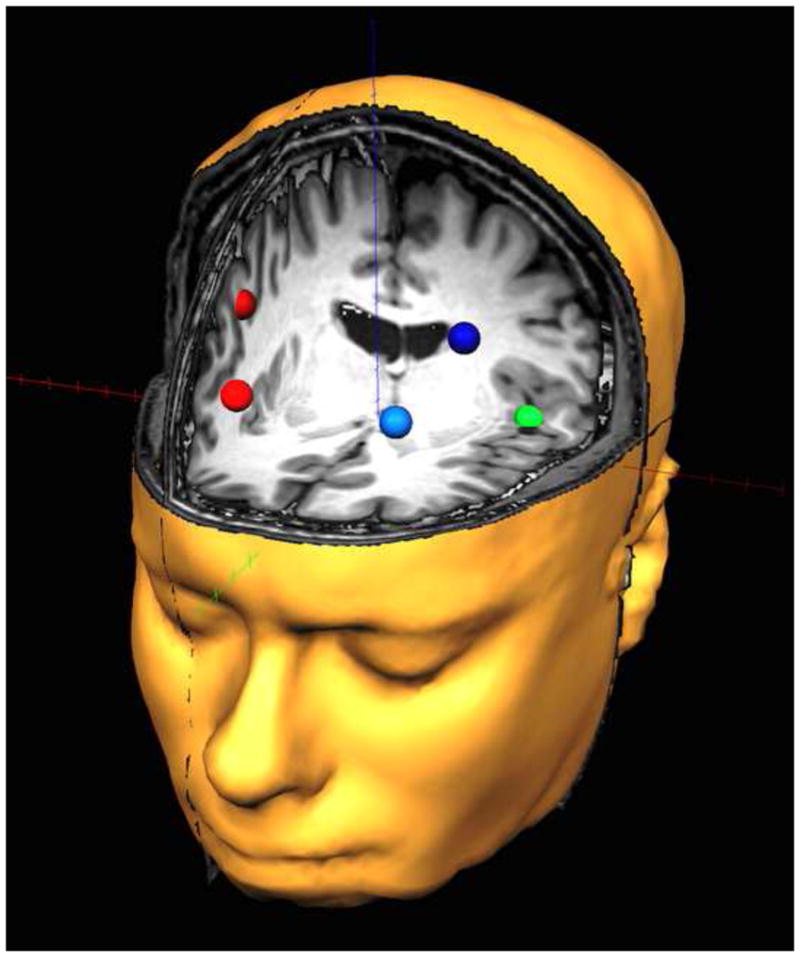
Dorsolateral prefrontal and auditory regions of interest. The red (right hemisphere) and blue (left hemisphere) nodes represent anterior and posterior regions of the prefrontal cortices corresponding to the regions that were focused upon. The green sources represent the auditory cortical regions and corresponded to the anatomical regions of heschl’s gyri (right auditory cortex is not shown). Note that the time series of each node reflects the average neuronal activity over that brain region, and not the amount of activation at a precise neuroanatomical coordinate (e.g., a voxel in Montreal Neurological Institute space).
Based on previous studies and our hypotheses, we examined phase-locking between the modality-specific auditory cortices and bilateral prefrontal regions within the theta (4–7 Hz), alpha (8–14 Hz), beta (14–30 Hz) and gamma (30–56 Hz) bands. Sources in each auditory cortex (Talairach coordinates (x, y, x); Left: −44, −16, 5; Right: 45, −16, 5) were chosen as the seed, and phase-locking values for anterior and posterior aspects of the prefrontal cortices bilaterally were computed separately (Talairach coordinates (x, y, z); left anterior frontal: −24, 44, 24; left posterior frontal: −35, 19, 40; right anterior frontal: 25, 44, 24; right posterior frontal: 36, 19, 40). The PLVs were very similar for the anterior and posterior PFC regions-of-interest and thus we averaged across these two regions to form a single PLV per auditory seed. Phase-locking values corresponding to the left and right auditory seeds were then averaged to obtain a mean phase-locking value between bilateral auditory and anterior/posterior prefrontal cortices. These values were evaluated using a repeated measures analysis of variance (ANOVA) with frequency band (4 levels) and condition (2 levels, attend and no-attend) as within-subjects factors, and group (with/without ADHD) as a between-subjects factor. Significant interaction effects were followed up using appropriate t-tests. We examined effects related to medication status separately, because three controls (~20%) did not complete the second series of attend/un-attend experimental blocks (thus, their data would have been excluded entirely in an omnibus ANOVA). Finally, it is well known that the amplitude of neural activity can bias estimations of the PLV. Thus, before conducting the PLV analysis, we examined whether the amplitude of activity (per band) differed between groups for any region of interest. This analysis was entirely negative, indicating that there were no significant differences in signal amplitude between the two groups. Statistical analyses were conducted in SPSS for Windows (Release 11.0.1). All MEG pre-processing and source modeling used the Brain Electrical Source Analysis (BESA version 5.3.2) software, and MEG-MRI coregistration and visualization used the BrainVoyager QX (Version 2.2) software.
3. Results
All participants were successful in identifying most of the quiet tones. Unmedicated patients with ADHD had a mean accuracy of 97.06%, whereas controls were over 99% accurate. This difference was not significant (P =0.4). Patients improved following medication administration (mean 97.77%), but controls did slightly worse at time 2 (mean: 98.1%); neither difference was significant. Since performance did not differ between groups and all participants performed extremely well on the task, correlations between performance and physiological measures were not assessed.
3.1. Task and group effects
The 4×2×2 mixed model ANOVA indicated significant main effects of condition, frequency band, and group, as well as a significant three-way interaction effect. None of the two-way interactions were significant. The main effect of condition indicated that phase-locking between auditory and prefrontal cortices was significantly stronger during the no-attend than during the attend condition, F(1,25) = 7.06 (P = 0.01), regardless of group status or frequency band. Follow-up testing of the frequency band main effect, F(3,75) = 13.19 (P < 0.0001), indicated that phase locking was stronger in the alpha than in the beta band (P < 0.001), and weaker in the gamma band than in all other bands (all P’s < 0.002). The group main effect, F(1,25) = 11.21 (P = 0.003), indicated that phase-locking was significantly stronger in unmedicated persons with ADHD compared with controls.
The three-way interaction effect of condition × frequency band × group, F(3,75) = 2.87 (P < 0.05), was evaluated using Bonferroni-corrected t-tests. The results indicated that unmedicated patients with ADHD had significantly greater beta-band phase-locking compared with controls in the attend, t(25) = 4.01 (P < 0.0001), and no-attend conditions, t(25) = 4.28 (P < 0.0001; see Fig. 2). When a more liberal threshold was used, unmedicated ADHD persons also exhibited greater phase-locking in the gamma band during the attend, t(25) = 2.49 (P < 0.02, uncorrected), and no-attend, t(25) = 2.05 (P = 0.05, uncorrected), conditions compared with controls (see Fig. 3).
Fig. 2.
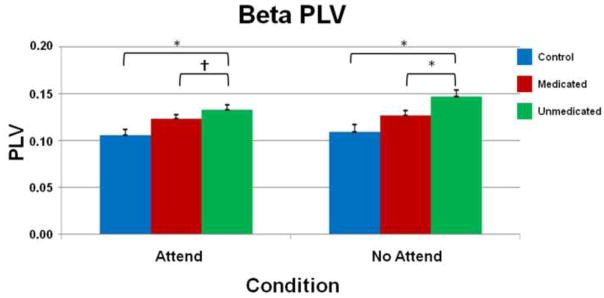
Beta band phase-locking values. Average phase locking values (PLV) between the auditory and prefrontal cortices of controls (blue), medicated (red), and unmedicated (green) adults with ADHD are grouped along the x-axis and separated by condition (attend and no-attend). The y-axis reflects mean PLV’s which vary from 0 (no phase locking) to 1 (perfect phase locking). Significant differences were noted between unmedicated ADHD and control participants in both attend and no-attend conditions (both P’s < 0.0001) and were attenuated with stimulant medications in the no-attend (P < 0.05, Bonferroni-corrected) and attend conditions (P < 0.05, uncorrected). Error bars indicate one standard error of the mean. * = P < 0.05, Bonferroni-corrected; †= P < 0.05, uncorrected.
Fig. 3.
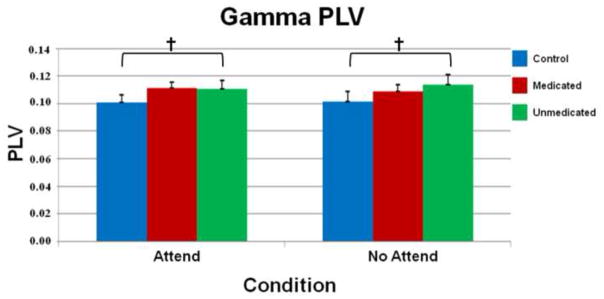
Gamma band phase-locking values. As with Fig. 2, average phase-locking values (PLV) between auditory and prefrontal cortices of controls (blue), medicated (red), and unmedicated (green) adults with ADHD are grouped along the x-axis and separated by condition (attend and no-attend). Significant differences were noted between unmedicated adults with ADHD and controls in the attend (P < 0.02, uncorrected) and no-attend conditions (P = 0.05, uncorrected). Error bars indicate one standard error of the mean. † = P < 0.05, uncorrected.
3.2. Medication effects
Since three healthy controls had data for session 1 but not session 2, we examined these data separately using within-group paired t-tests. In patients with ADHD, medication administration was associated with a significant decrease in beta-band phase-locking during the no-attend condition, t(12) = 4.94 (P < 0.05, Bonferroni-corrected), and a marginal decrease during the attend condition, t(12) = 2.36 (P < 0.05, uncorrected). These patients also showed a marginal decrease in alpha phase-locking during the no-attend condition, t(12) = 2.51 (P < 0.05, uncorrected), following medication administration. Controls did not show any significant changes from session 1 to session 2.
4. Discussion
In this study, we evaluated functional connectivity between the auditory and prefrontal cortices during an auditory attention task in subjects with ADHD and healthy controls. Our primary findings indicated that unmedicated patients with ADHD exhibited greater functional connectivity between auditory and prefrontal cortical regions relative to non-ADHD controls. Specifically, patients with ADHD had greater connectivity in the beta frequency (14–30 Hz) range in both attend and no-attend conditions, and greater functional connectivity in the gamma band (30–56 Hz) during the attend condition and (marginally) the no-attend condition. These group differences were attenuated with stimulant medication administration, as connectivity between these neural regions was reduced in ADHD subjects but not controls during the second run. Finally, we also detected stronger functional connectivity in the no-attend compared to the attend condition in both groups. Below, we discuss these findings in light of other ADHD and healthy control studies and their plausible implications.
Previous research has shown an increase in connectivity between the DLPFC and auditory cortices during an auditory attention task in healthy adults (Caclin and Funlupt, 2006). Prior work has also found increased functional connectivity between the DLPFC and visual areas during selective visual attention; thus, the general consensus is that the DLPFC serves to protect attention allocation during goal-oriented stimulus processing (Miller and Cohen, 2001; Corbetta and Schulman, 2002; Brazdil et al., 2007; Wang et al., 2009; Gregoriou et al., 2009; Baluch and Itti, 2011). However, the DLPFC is also significantly coherent with many other areas serving attention, including the anterior cingulate cortex (ACC), anterior superior parietal lobule, and cerebellum (MacDonald et al., 2000; Fan et al., 2008; Wolf et al., 2009; Prado et al., 2011). In fact, Prado and colleagues showed that diminished functional connectivity between the DLPFC and ACC were associated with increases in reaction times during a selective attention task in healthy controls (2011).
These attention networks have been shown to be dysfunctional in children and adults with ADHD during a variety of attention demanding tasks (Wolf et al., 2009; Konrad and Eickhoff, 2010; Arnsten et al., 2011; Liston et al., 2011). Using fMRI, Wolf and colleagues demonstrated reduced functional connectivity amongst nodes located in the prefrontal cortices bilaterally, anterior superior parietal lobule bilaterally, left ACC, and bilateral cerebellum in participants with ADHD during a working memory task (2009). In the current study, hyperconnectivity between prefrontal areas and auditory cortices was found, despite patients with ADHD typically exhibiting reduced DLPFC activation during attention modulation (Cubillo et al., 2011). This aberrant connectivity may indicate a bottom-up process whereby auditory stimulation is able to “engulf” executive areas, which may prevent patients with ADHD from successfully employing attentional control through the DLPFC. This inability to modulate bottom-up processing, and hence dysfunctional top-down modulation, could explain the diminished connectivity between the DLPFC and other attention areas found in previous studies (Wolf et al., 2009; Konrad and Eickhoff, 2010; Arnsten et al., 2011; Liston et al., 2011). Additionally, by perturbing the system with stimulants, we showed that both local beta and gamma functional connectivity moved toward the levels seen in controls. Although neither metric reached “normal” levels, both changed significantly pre- to post-drug and in the predicted direction, supporting the notion of abnormal hyperconnectivity in these networks. Similar gamma-band abnormalities have been observed in other neurodevelopmental disorders that are associated with attention deficits (Wilson et al., 2007, 2008, 2011).
While the results of this study are intriguing, there are some limitations that should be considered. First, we focused on modality-specific auditory regions and the bilateral frontal cortices; future studies may benefit from exploring other regions contributing to auditory attention. The fact that we did not observe differences in behavioral performance between groups or within the ADHD group (i.e., pre/post-med) could also be considered a limitation. In designing this task, our goal was to make it challenging enough to require attentional resources, but not so difficult that differences in behavior emerged. Essentially, had significant differences in behavioral performance been found, the differences in connectivity could have been attributed to the behavioral performance and not ADHD per se. This is a common argument and really a catch-22 for functional neuroimaging studies. Basically, if a neuroimaging study does not find behavioral differences than it raises the question of whether the results mean anything. On the other hand, if the same study does find behavioral differences than the results can be attributed to the performance differences and not the disorder of interest (i.e., ADHD). We propose that ceiling effects underlie the lack of behavioral differences observed in this study. Our sample size was also only moderate and future studies should utilize larger samples of patients and controls to illuminate more subtle differences, and match the two groups on additional characteristics such as socio-economic status. Our focus on the inattentive subtype could also be considered a limitation and future studies should examine different subtypes and potentially compare these groups to decipher subtype specific effects. Another potential limitation is that our medication wash-out period was only ~24 h and we cannot rule out residual medication effects in the patient group. Finally, future studies would also benefit from adding a placebo-control group and more carefully controlling for medication history, although such is difficult in adult samples who generally are decades from their initial ADHD diagnosis.
In conclusion, this study is the first to demonstrate stronger functional connectivity between frontal and auditory cortices (bilaterally) in adults with ADHD compared with controls during an auditory attention task. The results suggest neuronal hyper-connectivity in the higher frequency (beta and gamma) range between auditory and prefrontal brain regions in those with ADHD compared to controls. Additionally, the administration of psychostimulant medications in adults with ADHD attenuated these group differences, making connectivity more similar to that seen in controls. While our findings may suggest a key mechanism by which attention networks are disturbed during stimulus processing, further studies are needed to elucidate the specific alterations present in attentional networks in adults with ADHD.
Acknowledgments
The Center for Magnetoencephalography at the University of Nebraska Medical Center was founded through an endowment from an anonymous donor. This work was also supported by a grant from the Nebraska Banker’s Association and the National Institutes of Health to TWW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. APA; Washington, DC: 2000. [Google Scholar]
- Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biological Psychiatry. 2011;69:89–99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluch F, Itti L. Mechanisms of top-down attention. Trends in Neurosciences. 2011;34:210–224. doi: 10.1016/j.tins.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Hajos M, McCarthy R, Selikowitz M, Bruggemann JM. Acute atomoxetine effects on the EEG of children with attention-deficit/hyperactivity disorder. Neuropharmacology. 2009;57:702–707. doi: 10.1016/j.neuropharm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Brazdil M, Mikl M, Marecek R, Krupa P, Rektor I. Effective connectivity in target stimulus processing: A dynamic causal modeling study of visual oddball task. Neuroimage. 2007;35:827–835. doi: 10.1016/j.neuroimage.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, Makris N, Surman C, Aleardi M, Mick E, Biederman J. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Archives of General Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- Caclin A, Fonlupt P. Functional and effective connectivity in an fMRI study of an auditory-related task. European Journal of Neuroscience. 2006;23:2531–2537. doi: 10.1111/j.1460-9568.2006.04773.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Müller U, Rubia K, Del Campo N, Craig K, Regenthal R, Suckling J, Roiser JP, Grant JE, Bullmore ET, Robbins TW, Sahakian BJ. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biological Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Clausen SB, Read SC, Tulloch SJ. Single- and multiple-dose pharmacokinetics of an oral mixed amphetamine salts extended-release formulation in adults. CNS Spectrums. 2005;10(12 Suppl 20m):6–15. [PubMed] [Google Scholar]
- Corbetta M, Schulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Giampietro V, Taylor E, Rubia K. Fronto-striatal underactivation during interference inhibition and attention allocation in grown up children with attention deficit/hyperactivity disorder and persistent symptoms. Psychiatry Research: Neuroimaging. 2011;193:17–27. doi: 10.1016/j.pscychresns.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Dodds CM, Müller U, Clark L, van Loon A, Cools R, Robbins TW. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. Journal of Neuroscience. 2008;28:5976–5982. doi: 10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin F, Barch DM, Raichle ME, Peterson SE, Schlagger BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Hof PR, Guise KG, Fossella JA, Posner MI. The functional integration of the anterior cingulate cortex during conflict processing. Cerebral Cortex. 2008;18:796–805. doi: 10.1093/cercor/bhm125. [DOI] [PubMed] [Google Scholar]
- Franzen JD, Heinrichs-Graham E, White ML, Wetzel MW, Knott NL, Wilson TW. Atypical coupling between posterior regions of the default-mode network in ADHD: A pharmaco-MEG study. Journal of Psychiatry and Neuroscience. 2013;38:333–340. doi: 10.1503/jpn.120054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga MS, Ivry RB, Mangun GR. Cognitive Neuroscience: The Biology of the mind. 3. W.W. Norton & Company, Inc; New York: 2009. [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topography. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, Howes MJ, Jin R, Secnik K, Spencer T, Ustun TB, Walters EE. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychological Medicine. 2005;35:245–256. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Green JG, Adler LA, Barkley RA, Chatterji S, Faraone SV, Finkelman M, Greenhill LL, Grumer MJ, Jewell M, Russo LJ, Sampson NA, Van Brunt DL. Structure and diagnosis of adult attention-deficit/hyperactivity disorder: Analysis of expanded symptom criteria from the adult ADHD clinical diagnostic scale. Archives of General Psychiatry. 2010;67:1168–1178. doi: 10.1001/archgenpsychiatry.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human Brain Mapping. 2010;31:904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Human Brain Mapping. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara C, Fayyad J, de Graaf R, Kessler RC, Aquilar-Gaxiola S, Angermeyer M, Demytteneare K, de Girolamo G, Haro JM, Jin R, Karam EG, Lepine JP, Mora ME, Ormel J, Posada-Villa J, Sampson N. Childhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey Initiative. Biological Psychiatry. 2010;65:46–54. doi: 10.1016/j.biopsych.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Cohen MM, Teslovich T, Levenson D, Casey BJ. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biological Psychiatry. 2011;69:1168–1177. doi: 10.1016/j.biopsych.2011.03.022. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomedical Sciences Instrumentation. 1977;13:135–143. [PubMed] [Google Scholar]
- Peterson BS, Potenza MN, Wang ZS, Zhu HT, Martin A, Marsh R, Plessen KJ, Yu S. An fMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. American Journal of Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SE, Posner MI. The attention system of the human brain: 20 years after. Annual Review of Neuroscience. 2012;35:73–79. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Peterson SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Prado J, Carp J, Weissman DH. Variations of response time in a selective attention task are linked to variations of functional connectivity in the attentional network. Neuroimage. 2011;54:541–549. doi: 10.1016/j.neuroimage.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Experimental Brain Research. 2009;192:489–497. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Christakou A, Taylor E. Impulsiveness as a timing disturbance: Neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2009b;364:1919–1931. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009a;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Scherg M, Ille N, Bornfleth H, Berg P. Advanced tools for digital EEG review: virtual source montages, whole-head mapping, correlation, and phase analysis. Journal of Clinical Neurophysiology. 2002;19:91–112. doi: 10.1097/00004691-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Talairach G, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in Medicine and Biology. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M. Applications of the signal space separation method (SSS) IEEE Transactions on Signal Processing. 2005;53:3359–3372. [Google Scholar]
- Tulloch SJ, Zhang Y, McLean A, Wolf KN. SLI381 (Adderall XR), a two- component extended-release formulation of mixed amphetamine salts: bioavailability of three test formulations and comparisons of fasted, fed, and sprinkled administration. Pharmacotherapy. 2002;22:1405–1415. doi: 10.1592/phco.22.16.1405.33687. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu X, Guise GK, Knight RT, Ghajar J, Fan J. Effective connectivity of the fronto-parietal network during attentional control. Journal of Cognitive Neuroscience. 2009;22:543–553. doi: 10.1162/jocn.2009.21210. [DOI] [PubMed] [Google Scholar]
- Weisler RH. Safety, efficacy and extended duration of action of mixed amphetamine salts extended-release capsules for the treatment of ADHD. Expert Opinion on Pharmacotherapy. 2005;6:1003–1018. doi: 10.1517/14656566.6.6.1003. Review. [DOI] [PubMed] [Google Scholar]
- Wienbruch C, Paul I, Bauer S, Kivelitz H. The influence of methylphenidate on the power spectrum of ADHD children - an MEG study. BMC Psychiatry. 2005;5:29. doi: 10.1186/1471-244X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Franzen JD, Heinrichs-Graham E, White ML, Knott NL, Wetzel MW. Broadband neurophysiological abnormalities in the medial prefrontal region of the default-mode network in adults with ADHD. Human Brain Mapping. 2013a;34:566–574. doi: 10.1002/hbm.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, White ML, Knott NL, Wetzel MW. Estimating the passage of minutes: Deviant oscillatory frontal activity in medicated and unmedicated ADHD. Neuropsychology. 2013b;27:654–665. doi: 10.1037/a0034032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Hernandez OO, Asherin RM, Teale PD, Reite ML, Rojas DC. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cerebral Cortex. 2008;18:371–378. doi: 10.1093/cercor/bhm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biological Psychiatry. 2007;62:192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Developmental Neuropsychology. 2011;36:596–613. doi: 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Wetzel MW, White ML, Knott NL. Gamma-frequency neuronal activity is diminished in adults with attention-deficit/hyperactivity disorder: a pharmaco-MEG study. Journal of Psychopharmacology. 2012;26:771–777. doi: 10.1177/0269881111430731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, Plichta MM, Sambataro F, Fallgatter AJ, Jacob C, Lesch KP, Herrmann MJ, Schonfeldt-Lecuona C, Connemann BJ, Gron G, Vasic N. Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Human Brain Mapping. 2009;30:2252–2266. doi: 10.1002/hbm.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]


