Abstract
Purpose
To propose a nonisocentric treatment strategy as a special form of station parameter optimized radiation therapy, to improve sparing of critical structures while preserving target coverage in breast radiation therapy.
Methods and Materials
To minimize the volume of exposed lung and heart in breast irradiation, we propose a novel nonisocentric treatment scheme by strategically placing nonconverging beams with multiple isocenters. As its name suggests, the central axes of these beams do not intersect at a single isocenter as in conventional breast treatment planning. Rather, the isocenter locations and beam directions are carefully selected, in that each beam is only responsible for a certain subvolume of the target, so as to minimize the volume of irradiated normal tissue. When put together, the beams will provide an adequate coverage of the target and expose only a minimal amount of normal tissue to radiation. We apply the nonisocentric planning technique to 2 previously treated clinical cases (breast and chest wall).
Results
The proposed nonisocentric technique substantially improved sparing of the ipsilateral lung. Compared with conventional isocentric plans using 2 tangential beams, the mean lung dose was reduced by 38% and 50% using the proposed technique, and the volume of the ipsilateral lung receiving ≥20 Gy was reduced by a factor of approximately 2 and 3 for the breast and chest wall cases, respectively. The improvement in lung sparing is even greater compared with volumetric modulated arc therapy.
Conclusions
A nonisocentric implementation of station parameter optimized radiation therapy has been proposed for breast radiation therapy. The new treatment scheme overcomes the limitations of existing approaches and affords a useful tool for conformal breast radiation therapy, especially in cases with extreme chest wall curvature.
Introduction
The emergence of digital linear accelerators (linacs), such as TrueBeam (Varian Medical Systems, Palo Alto, CA) and Versa (Elekta, Stockholm, Sweden), has provided new opportunities for improved dose distributions and delivery efficiency. A distinct feature of the newly available digital linacs is that parameters characterizing radiation delivery, such as the motion of gantry, collimator, and couch, are discretized and can be controlled easily in an automated and programmable fashion. These capabilities of the digitally controlled delivery system call for innovations in treatment planning strategy, to better optimize radiation treatment. Current treatment plan optimization methods are designed for traditional linacs and cannot accommodate these emerging features of digital linacs (eg, the simultaneous and automated motion of gantry, collimator, and couch). This limitation of existing methods can potentially lead to compromised dose conformality and/or delivery efficiency.
Station parameter optimized radiation therapy (SPORT) has recently been introduced to harness the unique features of digital linacs (1–4). In SPORT, the concept of “station” generalizes the traditional view of a beam and gives a complete description of the state of a delivery system. The SPORT approach utilizes the optimization of station parameters, which include linac configurations such as the beam energy, aperture shape and weight, gantry angle (3), collimator angle (5), and auxiliaries such as the couch rotation (6, 7) and translation. This is compared with the approach of conventional treatments such as intensity modulated radiation therapy (IMRT) (8) and volumetric modulated arc therapy (VMAT) (9–11), which aim to optimize only a subset of the station parameters (generally aperture shape and weight) and thus are special cases of SPORT. By allowing simultaneous motion of gantry, collimator, and couch and thus adding more degrees of freedom to the treatment planning process, SPORT expands the search space of an optimal treatment plan and may lead to improved dose distributions.
In this work, we introduce the use of couch translation in breast radiation therapy planning and investigate a nonisocentric treatment strategy as a special form of SPORT to reduce lung and heart doses. Conventional breast radiation therapy planning uses 2 tangential opposed photon beams with a single isocenter. The need to adequately cover the target, given the conflict between the curved chest wall anatomy and linear edge of the radiation beam, dictates that an appreciable volume of lung will be inevitably exposed. For cases with highly concave chest wall, the lung/heart volume exposed to high radiation dose is significant, which could lead to severe late complications (12).
Methods and Materials
To minimize the volume of irradiated lung and heart in breast radiation therapy, we propose a novel nonisocentric treatment planning scheme, with the isocenters of each beam determined by the need for normal tissue sparing, while ensuring overall target coverage. Unlike conventional breast treatment planning, the central axes of these beams do not intersect at a single isocenter. Rather, the isocenter trajectory and beam directions are carefully chosen (manually), such that each beam is only responsible for a certain subvolume of the target, minimizing the volume of irradiated normal tissue.
Treatment planning
We use the clinical Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA) to implement the proposed planning scheme. To mimic a dynamic isocenter trajectory, a finite number of tangential nonopposed beams are placed in the patient, each with its own isocenter location. The isocenters are distributed roughly uniformly along the target in the axial view, approximately a few millimeters into the inner chest wall. The beam direction is chosen independently, to minimize exposure to normal tissue, resulting in each beam covering approximately one-half to two-thirds of the target volume. The collimator rotation for each beam is also adjusted in the beam’s-eye view to achieve the same goal while attempting to maximize the coverage of the target. Finally, we obtain the optimal treatment plan consisting of static IMRT beams each with its own isocenter, by using the dose–volume optimizer (version 10.0.28) in Eclipse, with lung and heart as the critical structures. We calculate the 3-dimensional dose distribution using the anisotropic analytical algorithm (version 10.0.28) in Eclipse.
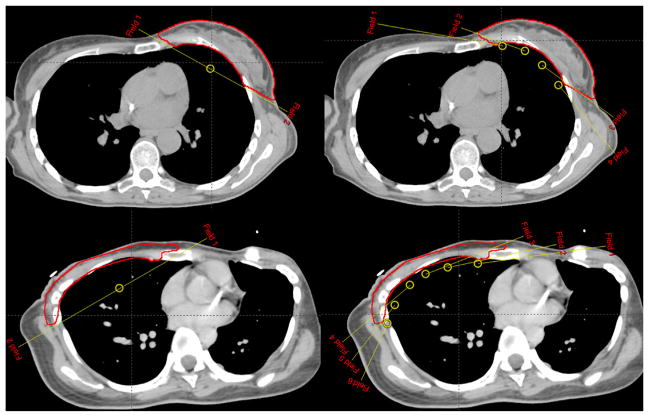
As a test, the proposed nonisocentric planning technique was applied to 2 previously treated clinical cases: a breast case and a chest wall case, under an institutional review board-approved retrospective study. Because of differences in the curvature of the target, a total of 4 tangential nonopposed beams (2 medial and 2 lateral) are used for the breast case, whereas 6 beams (3 medial and 3 lateral) are used for the chest wall case, as shown in Figure 1. For comparison purposes, an isocentric plan with 2 tangential opposed beams is generated, using the same inverse planning technique and same objective. In addition, we also optimized a RapidArc or VMAT plan, with the beam configuration similar to that in reference 13. The VMAT plan consists of two 190° coplanar arcs, with a collimator angle of 0°. Both arcs are simultaneously optimized for a total of 228 multileaf collimator segments (114 segments per arc), using the progressive resolution optimizer (version 10.0.28) in Eclipse. For efficient delivery, the first arc is rotated from 300° to 130° clockwise for left-sided target (or from 230° to 60° clockwise for right-sided target), and the second arc is rotated counter clockwise back to the starting angle. All treatment plans used the same dose volume objectives (ie, V95% >95%, V107% <5% for planning target volume [PTV], V20 Gy <15% for ipsilateral lung, V20 Gy <5% for heart, contralateral lung, and breast). A total dose of 50 Gy was prescribed to the PTV in 25 fractions. All plans are normalized such that the mean dose to the PTV equals 100% of the prescription dose.
Fig. 1.
Beam configurations for conventional breast radiation therapy planning using 2 tangential opposed beams (left) and for the proposed nonisocentric technique using multiple nonconverging beams (right). Top: breast case; bottom: chest wall case. The targets are contoured.
Plan evaluation
To evaluate the quality of the resultant treatment plans, we show the dose–volume histogram and isodose distributions. In addition, we calculate the generalized equivalent uniform dose (14–16) (gEUD) for both target and organs at risk, which is defined as , where di is the dose received by voxel i, and n is the total number of voxels in the region of interest. For tumors, negative values of a are appropriate because the gEUD is close to the minimal dose and thus is consistent with the dose response of inhomogeneously irradiated tumors. On the other hand, for normal tissues that exhibit a large volume effect and follow the parallel architecture model (eg, liver, parotids, and lungs), the mean dose (a = 1) is more consistent with the dose response (15). Here, we choose the value for parameter a to be −1 for the tumor target, and 1 for lung and heart. We further evaluate the target dose homogeneity by calculating the uniformity index, defined by D5/D95, where D5 and D95 are the minimum doses delivered to 5% and 95% of the PTV, respectively (17). The closer this number is to unity, the more homogeneous or uniform the dose distribution is.
Results
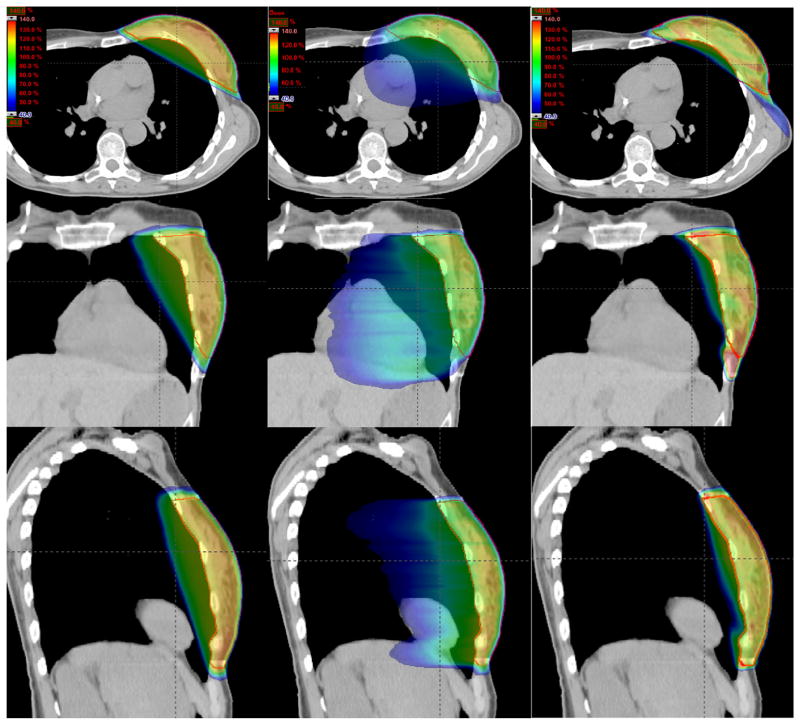
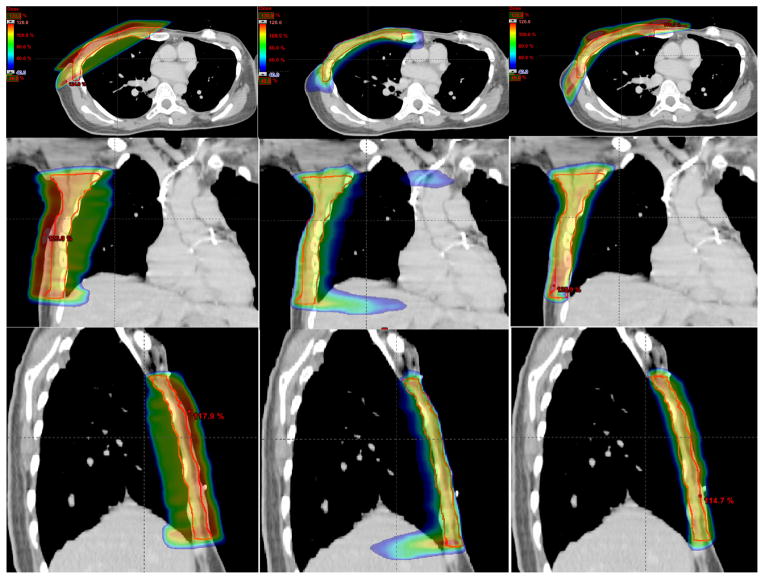
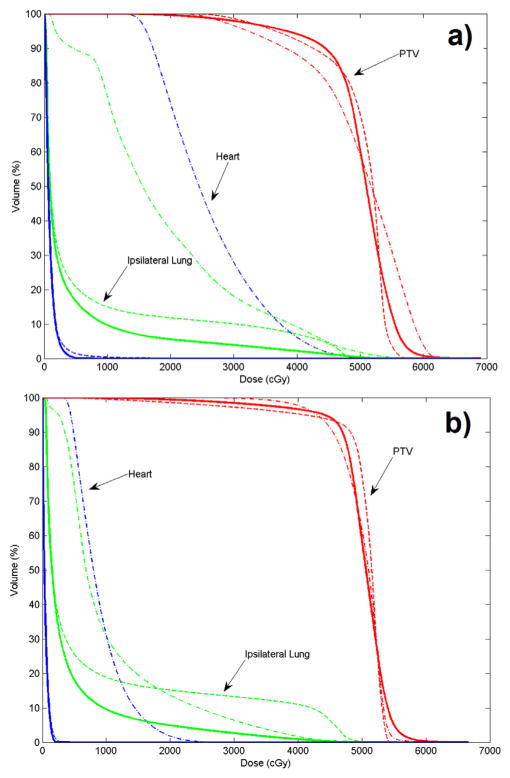
Figures 2 and 3 show the isodose distributions of the 3 treatment plans in the axial, coronal, and sagittal views for the 2 cases. Dose sparing of the lung is clearly observed in all 3 views with the nonisocentric plans. Compared with the conventional isocentric 2-tangential plans, the volume of the ipsilateral lung receiving ≥20 Gy is greatly reduced by a factor of approximately 2 and 3, for the 2 cases, respectively. Figure 4 shows the dose–volume histograms of the conventional isocentric plans and proposed nonisocentric plan for the 2 cases. It is evident that the proposed nonisocentric plan significantly reduces the lung dose while maintaining target coverage.
Fig. 2.
Isodose distributions for the breast case in the axial (top row), coronal (middle row), and sagittal (bottom row) view for the conventional isocentric 2-tangential plan (left column), volumetric modulated arc therapy plan (center column), and proposed nonisocentric plan (right column).
Fig. 3.
Same as Figure 2, except for the chest wall case. The bolus on top of the chest wall is not shown.
Fig. 4.
Dose–volume histograms for the conventional isocentric 2-tangential plan (dotted lines), volumetric modulated arc therapy plan (dashed/dotted lines), and proposed nonisocentric plan (solid lines): (a) breast case; (b) chest wall case.
As shown in Table 1, the gEUD or mean lung dose using the proposed technique is reduced by 38% and 50% for the 2 cases compared with that for the conventional isocentric opposed tangential plan. Both V5 and V20 for the ipsilateral lung are significantly reduced, by 20% and 53% for the breast case, and by 29% and 68% for the chest wall case, respectively. The dose sparing to lung is even more dramatic compared with the VMAT plans. Compared with the conventional isocentric plans, the proposed nonisocentric plan also improves the target coverage and homogeneity (eg, with a better uniformity index in both cases). Because heart is relatively far from the field in both cases, the 2-tangential and nonisocentric plans have very similar dose to heart. However, both are superior to the VMAT plans in terms of heart sparing.
Table 1.
Comparison of dose statistics between the treatment plans
| Organs | Dose statistic | Breast case
|
Chest wall case
|
||||
|---|---|---|---|---|---|---|---|
| Isocentric plan
|
Nonisocentric plan | Isocentric plan
|
Nonisocentric plan | ||||
| Opposed tangential | VMAT (2 arcs) | Opposed tangential | VMAT (2 arcs) | ||||
| PTV prescription or mean dose: 50 Gy | gEUD (Gy) | 49.1 | 48.3 | 48.8 | 47.3 | 49.7 | 48.9 |
| D95 (Gy) | 36.6 | 32.2 | 38.2 | 40.9 | 42.9 | 44.1 | |
| D5 (Gy) | 54.8 | 59.7 | 56.6 | 53.5 | 53.9 | 55.0 | |
| Uniformity index | 1.50 | 1.85 | 1.48 | 1.31 | 1.26 | 1.25 | |
| Ipsilateral lung | Mean dose or gEUD (Gy) | 6.4 | 18.9 | 4.0 | 8.2 | 10.4 | 4.1 |
| V5 (%) | 20.8 | 89.5 | 16.6 | 25.5 | 74.1 | 18.1 | |
| V20 (%) | 11.8 | 37.2 | 5.6 | 15.1 | 13.6 | 4.9 | |
| Heart | Mean dose or gEUD (Gy) | 0.9 | 26.0 | 0.8 | 0.4 | 8.9 | 0.4 |
Abbreviations: gEUD = generalized equivalent uniform dose; VMAT = volumetric modulated arc therapy.
Discussion
Radiation therapy plays an important role in breast cancer management but also carries risks of morbidity and mortality. In a prospective cohort study of approximately 300,000 women in US SEER cancer registries, a significantly increased mortality from lung cancer was observed 10–20 years after radiation therapy for early breast cancer (12). For instance, among women irradiated for breast cancer who subsequently developed lung cancer, the lung cancer mortality ratio (ipsilateral vs contralateral) was 2.0 and 2.7, respectively, 10–14 years and 15 or more years afterward. A major cause for the increased lung cancer is the extra radiation dose to lung in breast irradiation (12).
Here we have proposed a nonisocentric SPORT treatment scheme for breast radiation therapy and demonstrated substantially improved dose distributions compared with conventional isocentric treatment plans. By strategically placing multiple isocenters at the interface region of the chest wall and the irradiated breast, the resultant dose distributions can be shaped to highly curved anatomy such as the chest wall. On the other hand, with a single isocenter as in the conventional approach, the radiation beam edge is always linear, which will inevitably expose lung to extra dose due to the curved chest wall. Although attempts have been made to partially alleviate the problem using asymmetric isocentric fields (18), the conflict between curved anatomy and linear shape of radiation beam edge never disappears in the single-isocenter approach. Greater curvature in the chest wall will result in more normal tissue irradiated with conventional treatment planning, and a greater improvement using the proposed nonisocentric treatment planning method.
Some researchers have investigated the use of VMAT for improved breast dose distributions (13, 19, 20). Although the single-isocenter VMAT may provide additional angles by which dose can be delivered, the final dose distribution is limited by the beam trajectories that pass through the lungs, heart, thyroid, and contralateral breast. Low dose to the lungs using these angles can be significant, with V5 often reaching 80%–100% of the ipsilateral lung. Long-term toxicities from such low-dose irradiations are not well understood at present. The nonisocentric SPORT achieves better sparing of organs at risk through selective and individualized placement of isocenters of the incident beams. The isodose distributions shown in Figures 2 and 3 clearly demonstrate superior lung and heart sparing in both cases compared with VMAT plans.
As a possible alternative to opposed tangential photon beams for chest wall irradiation, electron beam arc therapy has been proposed and implemented for patient treatment. This technique uses an individually fitted, foam-lined cerrobend cast on the patient’s thorax as a tertiary collimator and adopts multiple electron energies and varying dose rate over the treated arc to improve dose uniformity (21, 22). Although useful for the chest wall with a relatively uniform depth, this technique may not be as effective for a complex-shaped breast target with a high curvature. Furthermore, a significant overhead is required for the manufacture of the cerrobend shields in the treatment preparation time.
In this proof of concept study, we have manually designed the isocenter locations and beam directions largely on the basis of our experience and intuition. In future studies more systematic and precise methods to determine these parameters should be investigated. Although in principle it is possible to incorporate this into the inverse planning process and optimize them together with the aperture shape and intensity, an alternative approach would be to separate the 2 distinct processes and come up with a metric to find these parameters. For example, the beam’s-eye-view–guided dosimetrics previously used for IMRT beam angle selection (23–25) could be adapted to determine the isocenter locations and beam directions here.
The optimal number of isocenters will likely depend on the need of each clinical case, in particular, the curvature of the chest wall. In this study, we tried 2–6 tangential nonopposed beams and found that using 6 beams gave the best result in the chest wall case (4 in the breast case). Intuitively, using more beams might produce even better dose distributions because the isocenters can then be placed closer to the target. In the extreme case, the beam edge can be made tangential to the target with infinite number of beams, thus sparing the largest amount of normal tissue possible. Practical challenges in planning and delivery of such treatments currently limit such an implementation.
The delivery of the nonisocentric treatment involves couch translation during treatment, whereas the delivery of the more commonly seen noncoplanar treatment involves couch rotation. The efficient delivery of the 2 can be achieved by concatenating all the beams so that they are delivered sequentially in an automated or preprogrammed fashion, without human intervention. The Developer Mode available on TrueBeam linacs allows users to define and deliver dynamic beams with arbitrary motion trajectories in monitor unit and position space for precisely controlled and predetermined variable dose rate, and simultaneous motion of all accelerator axes. The proposed nonisocentric treatment plans have been successfully delivered on a Varian True-Beam linac using the Developer Mode under experimental conditions (see Supplementary Material, available online). Table 2 lists the relevant parameters for delivery of both isocentric and nonisocentric treatment plans. The total dose delivery time (from first treatment beam on to last treatment beam off) for nonisocentric treatments is approximately twice that of isocentric opposed tangential treatments, but within a relatively short 3-minute time slot. With effective means of removing redundant segments while maintaining plan quality currently available for IMRT planning (26–28), the dose delivery time for a nonisocentric treatment that involves multiple beams is expected to be reduced to a level similar to that in conventional treatment.
Table 2.
Comparison of delivery between the treatment plans
| Delivery in TrueBeam Developer mode | Breast case
|
Chest wall case
|
||||
|---|---|---|---|---|---|---|
| Isocentric plan
|
Nonisocentric plan | Isocentric plan
|
Nonisocentric plan | |||
| Opposed tangential | VMAT (2 arcs) | Opposed tangential | VMAT (2 arcs) | |||
| No. of segments or control points | 51 | 228 | 46 | 47 | 228 | 45 |
| Total delivery time* | 1 min 23 s | 1 min 6 s | 2 min 54 s | 1 min 22 s | 1 min 10 s | 2 min 48 s |
From first treatment beam on to last treatment beam off.
A few practical issues should be pointed out here when planning and delivering nonisocentric treatments. First, because breast treatment delivery can often be tight with regard to clearance, care must be taken to avoid collision of the patient and different mechanical components, such as the couch and gantry, when designing the beam configurations during the planning stage. Second, because of the couch/patient motion required by nonisocentric treatment, the target position should be carefully monitored and maintained during the entire delivery process, and especially between the transitions of different isocenters. Onboard and in-room imaging (29–33) has provided a useful tool for intrafraction verification of target positioning based on fiducial markers or soft-tissue anatomy in conventional treatment, and is even more important for nonisocentric treatment.
Conclusion
A novel nonisocentric treatment planning scheme has been presented for breast radiation therapy. By designing beams with multiple isocenters so that they closely follow the shape of a curved breast or chest wall target, the resultant dose distributions can be optimized to substantially spare the ispilateral lung while maintaining target coverage. The new treatment scheme overcomes the limitations of the existing treatment planning approaches in current clinical practice and affords the radiation oncology discipline with a useful tool for conformal breast radiation therapy. Finally, we point out that the nonisocentric SPORT is not restricted to breast radiation therapy but applicable for treatment of any case with concave-shaped target volume.
Supplementary Material
Summary.
The authors developed a nonisocentric treatment strategy for breast cancer radiation therapy. By strategically placing multiple nonconverging beams each with its isocenter within the patient, the proposed nonisocentric technique substantially improves sparing of the ipsilateral lung. In a breast and chest wall case, the mean lung dose is reduced by 38% and 50% compared with conventional 2 opposed tangential techniques. The nonisocentric technique affords a useful tool for conformal breast radiation therapy.
Acknowledgments
This project was supported in part by grants from the National Institutes of Health (1K99 CA166186, 1R01 CA133474, and 1R21 CA153587) and the Foundation of Friends for an Earlier Breast Cancer Test.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Li R, Xing L. Bridging the gap between IMRT and VMAT: Dense angularly sampled and sparse intensity modulated radiation therapy. Med Phys. 2011;38:4912–4919. doi: 10.1118/1.3618736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim H, Li R, Lee R, et al. Dose optimization with first-order total-variation minimization for dense angularly sampled and sparse intensity modulated radiation therapy (DASSIM-RT) Med Phys. 2012;39:4316–4327. doi: 10.1118/1.4729717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li R, Xing L. An adaptive planning strategy for station parameter optimized radiation therapy (SPORT): Segmentally boosted VMAT. Med Phys. 2013;40:050701. doi: 10.1118/1.4802748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing L, Phillips MH, Orton CG. Point/counterpoint. DASSIM-RT is likely to become the method of choice over conventional IMRT and VMAT for delivery of highly conformal radiotherapy. Med Phys. 2013;40:020601. doi: 10.1118/1.4773025. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Happersett L, Yang Y, et al. Optimization of collimator trajectory in volumetric modulated arc therapy: Development and evaluation for paraspinal SBRT. Int J Radiat Oncol Biol Phys. 2010;77:591–599. doi: 10.1016/j.ijrobp.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 6.Dong P, Lee P, Ruan D, et al. 4pi noncoplanar stereotactic body radiation therapy for centrally located or larger lung tumors. Int J Radiat Oncol Biol Phys. 2013;86:407–413. doi: 10.1016/j.ijrobp.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Dong P, Lee P, Ruan D, et al. 4pi non-coplanar liver SBRT: A novel delivery technique. Int J Radiat Oncol Biol Phys. 2013;85:1360–1366. doi: 10.1016/j.ijrobp.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Ezzell GA, Galvin JM, Low D, et al. Guidance document on delivery, treatment planning, and clinical implementation of IMRT: Report of the IMRT Subcommittee of the AAPM Radiation Therapy Committee. Med Phys. 2003;30:2089–2115. doi: 10.1118/1.1591194. [DOI] [PubMed] [Google Scholar]
- 9.Yu CX. Intensity-modulated arc therapy with dynamic multileaf collimation: An alternative to tomotherapy. Phys Med Biol. 1995;40:1435–1449. doi: 10.1088/0031-9155/40/9/004. [DOI] [PubMed] [Google Scholar]
- 10.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Popple R, Suh TS, et al. Beam’s-eye-view dosimetrics-guided inverse planning for aperture-modulated arc therapy. Int J Radiat Oncol Biol Phys. 2009;75:1587–1595. doi: 10.1016/j.ijrobp.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 13.Popescu CC, Olivotto IA, Beckham WA, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys. 2010;76:287–295. doi: 10.1016/j.ijrobp.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys. 1997;24:103–110. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q, Mohan R, Niemierko A, et al. Optimization of intensity-modulated radiotherapy plans based on the equivalent uniform dose. Int J Radiat Oncol Biol Phys. 2002;52:224–235. doi: 10.1016/s0360-3016(01)02585-8. [DOI] [PubMed] [Google Scholar]
- 16.Schreibmann E, Xing L. Dose-volume based ranking of incident beam direction and its utility in facilitating IMRT beam placement. Int J Radiat Oncol Biol Phys. 2005;63:584–593. doi: 10.1016/j.ijrobp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Zhang X, Dong L, et al. Effectiveness of noncoplanar IMRT planning using a parallelized multiresolution beam angle optimization method for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2005;63:594–601. doi: 10.1016/j.ijrobp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Fogliata A, Bolsi A, Cozzi L. Critical appraisal of treatment techniques based on conventional photon beams, intensity modulated photon beams and proton beams for therapy of intact breast. Radiother Oncol. 2002;62:137–145. doi: 10.1016/s0167-8140(01)00476-5. [DOI] [PubMed] [Google Scholar]
- 19.Giorgia N, Antonella F, Alessandro C, et al. Planning strategies in volumetric modulated are therapy for breast. Med Phys. 2011;38:4025–4031. doi: 10.1118/1.3598442. [DOI] [PubMed] [Google Scholar]
- 20.Popescu CC, Beckham WA, Patenaude VV, et al. Simultaneous couch and gantry dynamic arc rotation (CG-Darc) in the treatment of breast cancer with accelerated partial breast irradiation (APBI): A feasibility study. J Appl Clin Med Phys. 2013;14:4035. doi: 10.1120/jacmp.v14i1.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peacock LM, Leavitt DD, Gibbs FA, Jr, et al. Electron arc therapy: Clinical experience with chest wall irradiation. Int J Radiat Oncol Biol Phys. 1984;10:2149–2153. doi: 10.1016/0360-3016(84)90216-5. [DOI] [PubMed] [Google Scholar]
- 22.McNeely LK, Jacobson GM, Leavitt DD, et al. Electron arc therapy: Chest wall irradiation of breast cancer patients. Int J Radiat Oncol Biol Phys. 1988;14:1287–1294. doi: 10.1016/0360-3016(88)90408-7. [DOI] [PubMed] [Google Scholar]
- 23.Pugachev A, Xing L. Pseudo beam’s-eye-view as applied to beam orientation selection in intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2001;51:1361–1370. doi: 10.1016/s0360-3016(01)01736-9. [DOI] [PubMed] [Google Scholar]
- 24.Pugachev A, Xing L. Incorporating prior knowledge into beam orientation optimization in IMRT. Int J Radiat Oncol Biol Phys. 2002;54:1565–1574. doi: 10.1016/s0360-3016(02)03917-2. [DOI] [PubMed] [Google Scholar]
- 25.Rocha H, Dias JM, Ferreira BC, et al. Beam angle optimization for intensity-modulated radiation therapy using a guided pattern search method. Phys Med Biol. 2013;58:2939–2953. doi: 10.1088/0031-9155/58/9/2939. [DOI] [PubMed] [Google Scholar]
- 26.Zhu L, Lee L, Ma Y, et al. Using total-variation regularization for intensity modulated radiation therapy inverse planning with field-specific numbers of segments. Phys Med Biol. 2008;53:6653–6672. doi: 10.1088/0031-9155/53/23/002. [DOI] [PubMed] [Google Scholar]
- 27.Kim H, Suh TS, Lee R, et al. Efficient IMRT inverse planning with a new L1-solver: Template for first-order conic solver. Phys Med Biol. 2012;57:4139–4153. doi: 10.1088/0031-9155/57/13/4139. [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Becker S, Lee R, et al. Improving IMRT delivery efficiency with reweighted L1-minimization for inverse planning. Med Phys. 2013;40:071719. doi: 10.1118/1.4811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bert C, Metheany KG, Doppke KP, et al. Clinical experience with a 3D surface patient setup system for alignment of partial-breast irradiation patients. Int J Radiat Oncol Biol Phys. 2006;64:1265–1274. doi: 10.1016/j.ijrobp.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita R, Shimizu S, Taguchi H, et al. Three-dimensional intrafractional motion of breast during tangential breast irradiation monitored with high-sampling frequency using a real-time tumor-tracking radiotherapy system. Int J Radiat Oncol Biol Phys. 2008;70:931–934. doi: 10.1016/j.ijrobp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Li R, Lewis JH, Cervino LI, et al. A feasibility study of markerless fluoroscopic gating for lung cancer radiotherapy using 4DCT templates. Phys Med Biol. 2009;54:N489–N500. doi: 10.1088/0031-9155/54/20/N03. [DOI] [PubMed] [Google Scholar]
- 32.Li R, Lewis JH, Jia X, et al. 3D tumor localization through real-time volumetric x-ray imaging for lung cancer radiotherapy. Med Phys. 2011;38:2783–2794. doi: 10.1118/1.3582693. [DOI] [PubMed] [Google Scholar]
- 33.Li R, Mok E, Chang DT, et al. Intrafraction verification of gated RapidArc by using beam-level kilovoltage X-ray images. Int J Radiat Oncol Biol Phys. 2012;83:e709–e715. doi: 10.1016/j.ijrobp.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.