Abstract
Background
Whether additional benefit can be achieved with the use of trimetazidine (TMZ) in patients with chronic heart failure (CHF) remains controversial. We therefore performed a meta-analysis of randomized controlled trials (RCTs) to evaluate the effects of TMZ treatment in CHF patients.
Methods
We searched PubMed, EMBASE, and Cochrane databases through October 2013 and included 19 RCTs involving 994 CHF patients who underwent TMZ or placebo treatment. Risk ratio (RR) and weighted mean differences (WMD) were calculated using fixed or random effects models.
Results
TMZ therapy was associated with considerable improvement in left ventricular ejection fraction (WMD: 7.29%, 95% CI: 6.49 to 8.09, p<0.01) and New York Heart Association classification (WMD: −0.55, 95% CI: −0.81 to −0.28, p<0.01). Moreover, treatment with TMZ also resulted in significant decrease in left ventricular end-systolic volume (WMD: −17.09 ml, 95% CI: −20.15 to −14.04, p<0.01), left ventricular end-diastolic volume (WMD: −11.24 ml, 95% CI: −14.06 to −8.42, p<0.01), hospitalization for cardiac causes (RR: 0.43, 95% CI: 0.21 to 0.91, p = 0.03), B-type natriuretic peptide (BNP; WMD: −157.08 pg/ml, 95% CI: −176.55 to −137.62, p<0.01) and C-reactive protein (CRP; WMD: −1.86 mg/l, 95% CI: −2.81 to −0.90, p<0.01). However, there were no significant differences in exercise duration and all-cause mortality between patients treated with TMZ and placebo.
Conclusions
TMZ treatment in CHF patients may improve clinical symptoms and cardiac function, reduce hospitalization for cardiac causes, and decrease serum levels of BNP and CRP.
Introduction
Chronic heart failure (CHF) is a complex clinical syndrome characterized by decreased myocardial contractility, hemodynamic abnormality and neuroendocrine activation. There are multiple etiologies leading to this final common clinical pathway, which carries a 50% 5-year mortality rate and is responsible for over one third of all deaths in the United States from cardiovascular causes [1]. The past few decades have witnessed remarkable progress in the drug therapy for CHF. The clinical application of beta-adrenergic receptor blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers and aldosterone receptor antagonists has significantly reduced cardiovascular events and mortality in patients with CHF [2]. However, CHF remains a leading cause of morbidity and mortality throughout the world.
Trimetazidine (TMZ), a piperazine derivative used as an anti-anginal agent, selectively inhibits mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. By decreasing fatty acid oxidation and stimulating glucose utilization, TMZ restores coupling between glycolysis and carbohydrate oxidation, and leads to ATP production with less oxygen consumption. Previous studies have reported that TMZ exerts cardioprotective effects by reducing oxidative damage, inhibiting inflammation and apoptosis, and improving endothelial function [3]–[6].
Over the past few years, several small randomised controlled trials (RCTs) have been conducted to evaluate the effects of TMZ treatment in patients with CHF. These trials investigated clinical symptoms, cardiac function, quality of life, hospitalization, mortality and cardiovascular events, comparing TMZ with placebo. In addition, two meta-analyses of RCTs have also been performed to assess the therapeutic effects of TMZ in CHF patients [7], [8]. However, some conclusions drawn from these two meta-analyses are not consistent. We therefore performed an updated meta-analysis including a few recently published RCTs to provide more convincing evidence of TMZ therapy in patients with CHF.
Methods
Search strategy and selection criteria
We performed an electronic literature search of PubMed, EMBASE, and Cochrane databases through October 2013, using the terms “Trimetazidine”, “Vastarel”, “Idaptan”, “heart failure”, “cardiac failure”, “cardiac dysfunction”, “cardiac insufficiency”, “cardiomyopathy”, and “ventricular dysfunction”. Sensitive filters identified clinical trial or RCT in the Medline database and the EMBASE database. The search was limited to human subjects, with no restriction for language.
RCTs reporting at least one of the outcomes were considered eligible. These outcomes included cardiac function parameters (ie, left ventricular ejection fraction (LVEF), left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDV)), New York Heart Association (NYHA) classification, exercise tolerance (ie, exercise duration), all-cause mortality, hospitalization, cardiovascular events, B-type natriuretic peptide (BNP), and C-reactive protein (CRP).
Data extraction and quality assessment
Two investigators independently reviewed all potentially eligible studies using predefined eligibility criteria and collected data from the included trials. We extracted details on study characteristics, patient characteristics, inclusion criteria, ischemic etiology, intervention strategies, duration of follow-up, and clinical outcomes including LVEF, LVESV, LVEDV, NYHA classification, exercise duration, all-cause mortality, hospitalization, BNP and CRP. The quality of included RCTs was assessed by the Jadad scale [9], and a numerical score between 0 and 5 was assigned as a measure of study design.
Statistical analysis
Dichotomous data were analyzed using risk ratio (RR) with 95% confidence intervals (CI), while continuous variables were analyzed using weighted mean differences (WMD) and 95% CI. The heterogeneity of results across trials was assessed using the Chi-square based Q-test. A p value>0.10 for the Q-test indicated a lack of heterogeneity among the studies. Thus, the pooled effect was calculated using fixed effects model. Otherwise, random effects model was applied in case of significant heterogeneity across studies. Sensitivity analysis was also conducted to assess the influence of each individual study on overall estimates by sequential removal of individual studies. All statistical analyses were performed using RevMan 5.0 (Cochrane Collaboration, Oxford, UK) and STATA software 10.0 (Stata Corporation, College Station, Texas, USA). P<0.05 was considered statistically significant.
Results
Eligible studies
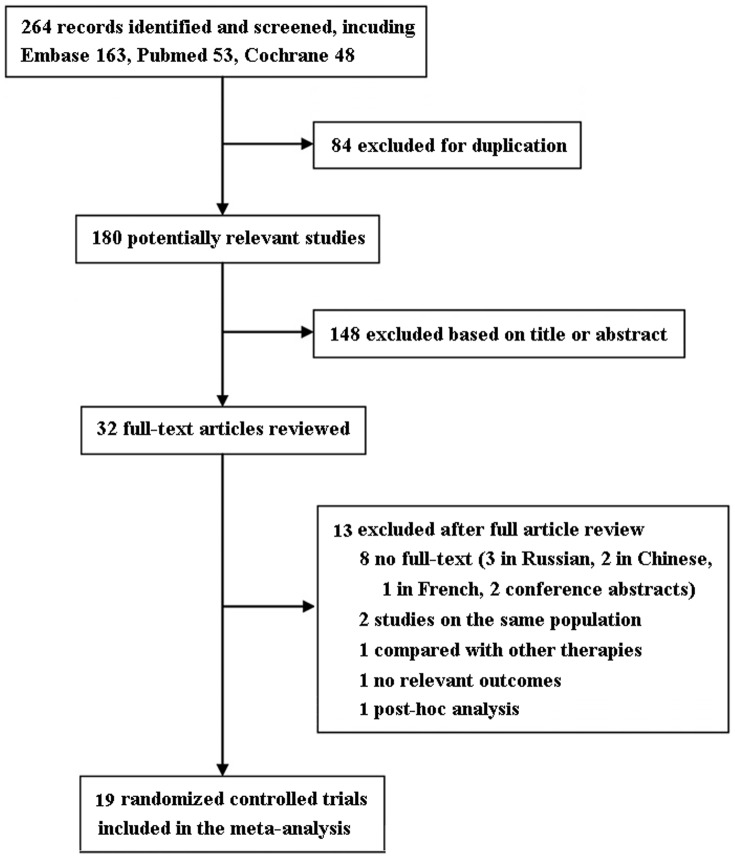
The flow of selection of studies for the meta-analysis is shown in Figure 1. Among the initial 264 RCTs, 32 trials were retrieved for detailed evaluation, and 19 studies [10]–[28] satisfying the inclusion criteria were finally analyzed. The quality assessment of included RCTs is shown in Table 1. The baseline characteristics of enrolled studies are shown in Tables 2 and 3. Among the included studies, 17 trials described LVEF [10]–[24], [26], [27], 8 NYHA classification [15], [17], [19]–[21], [24]–[26], 6 exercise duration [12], [16]–[18], [22], [27], 3 all-cause mortality [16], [17], [19], 4 hospitalization [13], [15], [17], [23], 3 BNP [17], [20], [28] and 3 CRP [19], [22], [28]. TMZ dosage ranged from 40 to 70 mg/day and follow-up periods from 1 to 24 months.
Figure 1. Flow diagram of eligible studies included in the meta-analysis.
Table 1. Quality assessment of included studies.
| Study | Reporting of randomization | Generation of random sequence | Blinding | Withdrawal description | Jadad score |
| Zhao et al., 2013 | Y | Unclear | Double-blind | Y | 4 |
| Fragasso et al., 2011 | Y | Computer generated | Single-blind | Y | 3 |
| Cera et al., 2010 | Y | Computer generated | N | Y | 3 |
| Gunes et al., 2009 | Y | Unclear | Single-blind | N | 1 |
| Marazzi et al., 2009 | Y | Unclear | Double-blind | Y | 4 |
| Tuunanen et al., 2008 | Y | Unclear | Single-blind | Y | 2 |
| Belardinelli et al., 2008 | Y | Computer generated | Double-blind | Y | 5 |
| Sisakian et al., 2007 | Y | Unclear | N | Y | 2 |
| Di Napoli et al., 2007 | Y | Sealed envelope | Double-blind | Y | 5 |
| Fragasso et al., 2006 | Y | Computer generated | N | Y | 3 |
| Fragasso et al., 2006 | Y | Computer generated | Double-blind | Y | 5 |
| Di Napoli et al., 2005 | Y | Unclear | N | Y | 2 |
| El-Kady et al., 2005 | Y | Unclear | Single-blind | Y | 2 |
| Vitale et al., 2004 | Y | Unclear | Double-blind | Y | 4 |
| Thrainsdottir et al., 2004 | Y | Unclear | Double-blind | Y | 4 |
| Rosano et al., 2003 | Y | Unclear | Double-blind | Y | 4 |
| Fragasso et al., 2003 | Y | Unclear | Double-blind | Y | 4 |
| Belardinelli et al., 2001 | Y | Unclear | Double-blind | Y | 4 |
| Brottier et al.,1990 | Y | Unclear | Double-blind | Y | 4 |
Y = yes; N = no.
Table 2. Study characteristics.
| Study | Patients (TMZ/Control) | TMZ (mg/day) | Follow-up duration | Inclusion criteria | Endpoints |
| Zhao et al., 2013 | 80 (40/40) | 60 | 6 months | Diabetes, IDCM, LVEF≤40% | Left ventricular function, exercise tolerance, CRP, BNP |
| Fragasso et al., 2011 | 44 (25/19) | 60 | 3 months | Chronic systolic HF, NYHA II–IV, LVEF <45%, | REE, LVEF, NYHA class, QOL |
| Cera et al., 2010 | 30 (17/13) | 60 | 6 months | Chronic stable HF, NYHA I–III, LVEF <45% | LVEF, NYHA class, electrophysiological indexes |
| Gunes et al., 2009 | 87 (51/36) | 60 | 3 months | Chronic stable HF, NYHA II–III, LVEF≤40% | Left and right ventricular functions |
| Marazzi et al., 2009 | 47 (23/24) | 40 | 6 months | Age ≥65 years, stable ischemic heart disease, LVEF <50% | QOL, NYHA class |
| Tuunanen et al., 2008 | 19 (12/7) | 70 | 3 months | IDCM, LVEF <47% | Echocardiographic parameters, myocardial metabolism, blood chemistry |
| Belardinelli et al., 2008 | 35 (19/16) | 60 | 3 months | Diabetes, stable ischemic heart disease | Myocardial scintigraphy parameters, blood biochemistry |
| Sisakian et al., 2007 | 82 (42/40) | 70 | 3 months | Stable ischemic heart disease, LVEF <40% | LVEF, exercise tolerance, NYHA class |
| Di Napoli et al., 2007 | 50 (25/25) | 60 | 6 months | Ischemic cardiomyopathy, LVEF <35% | Exercise tolerance, LVEF, NYHA class, BNP |
| Fragasso et al., 2006 | 65 (34/31) | 60 | 12 months | Chronic stable HF, LVEF <45% | Cardiovascular events, hospitalization, LVEF, NYHA class, QOL, BNP |
| Fragasso et al., 2006 | 12/12 | 60 | 3 months | Chronic stable HF, LVEF≤45% | Exercise tolerance, LVEF, NYHA class, cardiac PCr/ATP ratio |
| Di Napoli et al., 2005 | 61 (30/31) | 60 | 18 months | Ischemic dilated cardiomyopathy, LVEF <40% | All-cause mortality, NYHA class, LVEF, CRP |
| El-Kady et al., 2005 | 200 (100/100) | 60 | 24 months | Ischemic cardiomyopathy, LVEF <50% | SPECT parameters, exercise tolerance, LVEF |
| Vitale et al., 2004 | 47 (23/24) | 60 | 6 months | Age ≥65 years, stable ischemic heart disease, LVEF <50% | Cardiovascular events, hospitalization, LVEF, NYHA class, QOL |
| Thrainsdottir et al., 2004 | 20/20 | 60 | 4 weeks | Diabetes, stable ischemic HF, NYHA II–III, LVEF≤40% | Exercise tolerance, left ventricular function |
| Rosano et al., 2003 | 32 (16/16) | 60 | 6 months | Diabetes, stable ischemic heart disease, LVEF <50% | Left ventricular function |
| Fragasso et al., 2003 | 16/16 | 60 | 6 months | Diabetes, ischemic cardiomyopathy, LVEF≤45% | LVEF, NYHA class, exercise tolerance, blood biochemistry |
| Belardinelli et al., 2001 | 44 (22/22) | 60 | 2 months | Ischemic cardiomyopathy | Contractile response to dobutamine, left ventricular systolic function |
| Brottier et al.,1990 | 23(10/13) | 60 | 6 months | Severe ischemic cardiomyopathy, NYHA III–IV | Clinical status, LVEF, cardiac volume |
BNP = brain natriuretic peptide; CRP = C-reactive protein; HF = heart failure; IDCM = idiopathic dilated cardiomyopathy; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; QOL = quality of life; REE = resting energy expenditure; SPECT = single photon emission CT; TMZ = trimetazidine.
Table 3. Patient characteristics.
| Study | Patients (TMZ/Control) | Age (Mean, years) (TMZ/Control) | Male (N) (TMZ/Control) | Ischemic cause (%) | Diabetes (%) | NYHA class | LVEF (Mean, %) (TMZ/Control) |
| Zhao et al., 2013 | 40/40 | 59/58 | 32/30 | 0 | 100 | II–III | 34/36 |
| Fragasso et al., 2011 | 25/19 | 70 | 38 | 66 | 34 | II–IV | 35/35 |
| Cera et al., 2010 | 17/13 | 65/70 | 15/11 | 60 | 37 | I–III | 38/33 |
| Gunes et al., 2009 | 51/36 | 59/57 | 37/21 | 66 | 29 | II–III | 33/31 |
| Marazzi et al., 2009 | 23/24 | 77/78 | 18/22 | 100 | NA | I–III | <50 |
| Tuunanen et al., 2008 | 12/7 | 59/57 | 10/5 | 0 | 0 | NA | 31/38 |
| Belardinelli et al., 2008 | 19/15 | 54/54 | 16/14 | 100 | 100 | NA | 39/40 |
| Sisakian et al., 2007 | 42/40 | 64/66 | 37/33 | 100 | NA | II–III | 35/32 |
| Di Napoli et al., 2007 | 25/25 | 64/63 | 15/18 | 100 | 24 | II–IV | 28/30 |
| Fragasso et al., 2006 | 28/27 | 64/66 | 25/25 | 54 | 7 | II–IV | 34/36 |
| Fragasso et al., 2006 | 12/12 | 66 | 11 | 50 | NA | NA | 33 |
| Di Napoli et al., 2005 | 30/31 | 67/69 | 17/18 | 100 | 20 | II–IV | 30/31 |
| El-Kady et al., 2005 | 100/100 | 53/53 | 86/78 | 100 | 34 | NA | 36/37 |
| Vitale et al., 2004 | 23/24 | 77/78 | 18/22 | 100 | NA | I–III | 29/29 |
| Thrainsdottir et al.,2004 | 10/10 | 67/66 | 9/8 | 100 | 100 | II–III | 33/29 |
| Rosano et al., 2003 | 16/16 | 66/65 | 11/13 | 100 | 100 | NA | 32/33 |
| Fragasso et al., 2003 | 16/16 | 64 | 16 | 100 | 100 | I–III | 40 |
| Belardinelli et al., 2001 | 19/19 | 50/54 | 15/16 | 100 | NA | II–III | 33/33 |
| Brottier et al.,1990 | 9/11 | 57/62 | 19 | 100 | NA | III–IV | 32/29 |
LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; TMZ = trimetazidine.
Left ventricular function
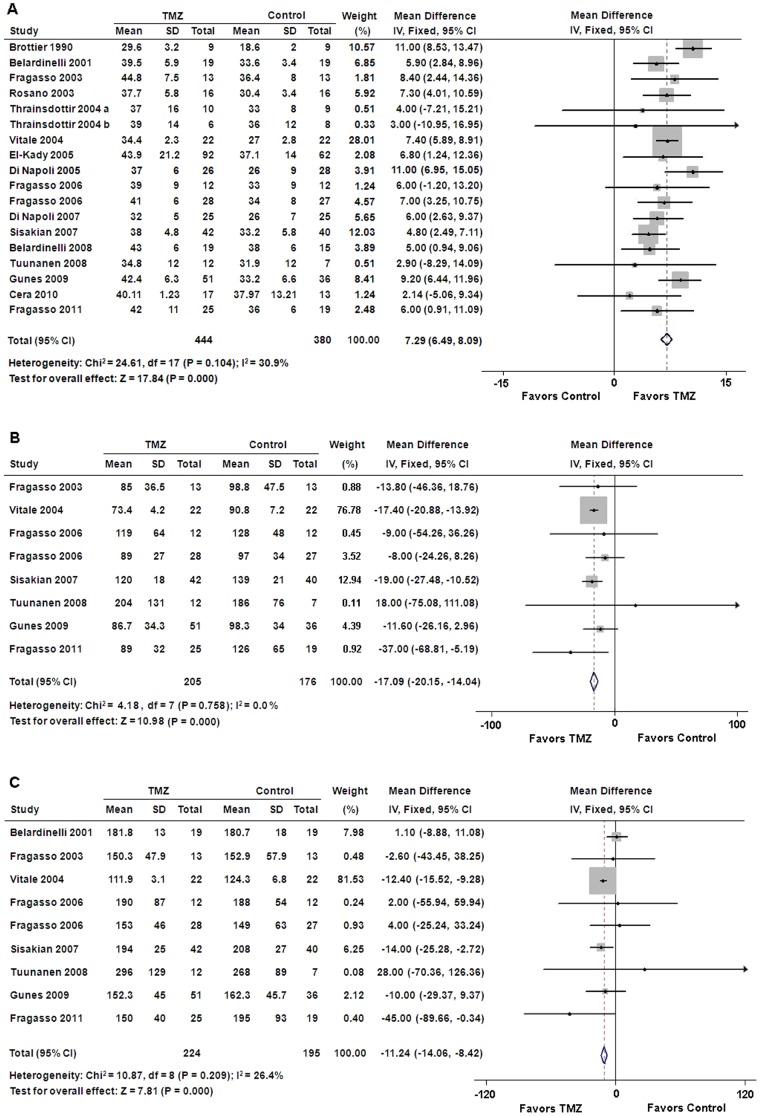
Our results indicated that additional TMZ therapy was superior to conventional treatment in terms of LVEF improvement (WMD: 7.29%, 95% CI: 6.49 to 8.09, p<0.01) (Figure 2A). In addition, LVESV and LVEDV were significantly lower in patients who received TMZ therapy than placebo treatment (WMD: −17.09 ml, 95% CI: −20.15 to −14.04, p<0.01; WMD: −11.24 ml, 95% CI: −14.06 to −8.42, p<0.01, respectively) (Figures 2B and 2C).
Figure 2. Forest plots for left ventricular function.
(A) Left ventricular ejection fraction; (B) left ventricular end-systolic volume; (C) left ventricular end-diastolic volume. CI = confidence intervals; IV = inverse variance; TMZ = trimetazidine.
NYHA classification and exercise tolerance
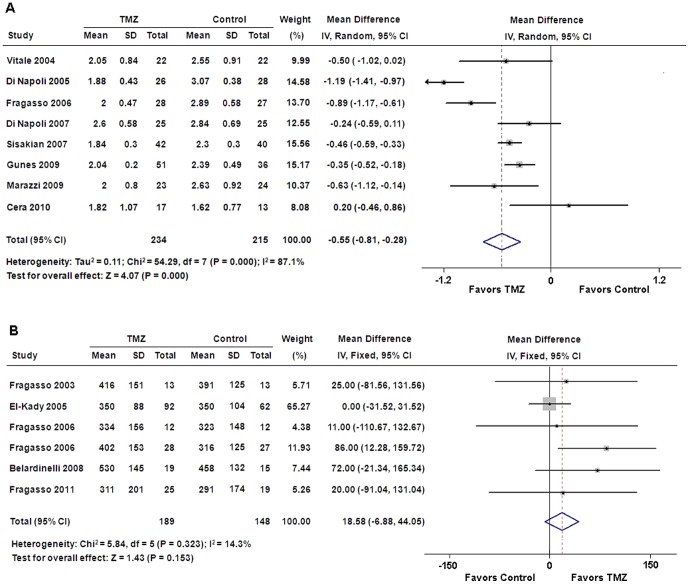
Pooled analysis showed that TMZ therapy resulted in a significant improvement in NYHA functional class compared with placebo control (WMD: −0.55, 95% CI: −0.81 to −0.28, p<0.01) (Figure 3A). However, there was no significant difference in exercise duration between patients treated with TMZ and placebo (WMD: 18.58 s; 95% CI: −6.88 to 44.05, p = 0.15) (Figure 3B).
Figure 3. Forest plots for NYHA classification and exercise duration.
(A) NYHA classification; (B) exercise duration. CI = confidence intervals; IV = inverse variance; TMZ = trimetazidine.
Sensitivity analysis
Since a significant heterogeneity across studies was observed for NYHA classification, we conducted a sensitivity analysis to assess the effect of each study on the pooled estimate under the random effects model. As shown in Table 4, removal of any individual study could not significantly reduce the heterogeneity.
Table 4. Sensitivity analysis of NYHA classification.
| Study omitted | WMD (95% CI) | P for heterogeneity | I2 |
| Vitale 2004 | −0.55 (−0.84, −0.27) | <0.001 | 88.9% |
| Di Napoli 2005 | −0.46 (−0.64, −0.27) | 0.011 | 63.7% |
| Fragasso 2006 | −0.49 (−0.78, −0.20) | <0.001 | 87.6% |
| Di Napoli 2007 | −0.59 (−0.88, −0.30) | <0.001 | 88.2% |
| Sisakian 2007 | −0.55 (−0.90, −0.21) | <0.001 | 88.1% |
| Gunes 2009 | −0.58 (−0.89, −0.26) | <0.001 | 87.1% |
| Marazzi 2009 | −0.54 (−0.83, −0.25) | <0.001 | 88.9% |
| Cera 2010 | −0.62 (−0.88, −0.35) | <0.001 | 87.8% |
NYHA = New York Heart Association; WMD = weighted mean difference; CI = confidence interval.
All-cause mortality and hospitalization for cardiac causes
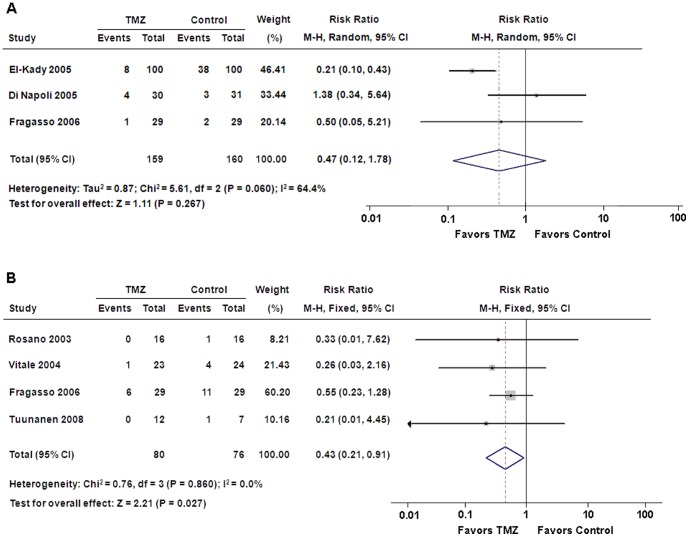
Our results suggested that there was no significant difference in all-cause mortality between patients treated with TMZ and placebo (RR: 0.47, 95% CI: 0.12 to 1.78, p = 0.27) (Figure 4A). Nevertheless, 7 of 80 patients with TMZ therapy needed hospitalization for cardiac causes, which was significantly lower than 17 of 76 patients with placebo treatment (RR: 0.43, 95% CI: 0.21 to 0.91, p = 0.03) (Figure 4B).
Figure 4. Forest plots for all-cause mortality and hospitalization for cardiac causes.
(A) All-cause mortality; (B) hospitalization for cardiac causes. CI = confidence intervals; M-H = Mantel-Haenszel; TMZ = trimetazidine.
Serum markers
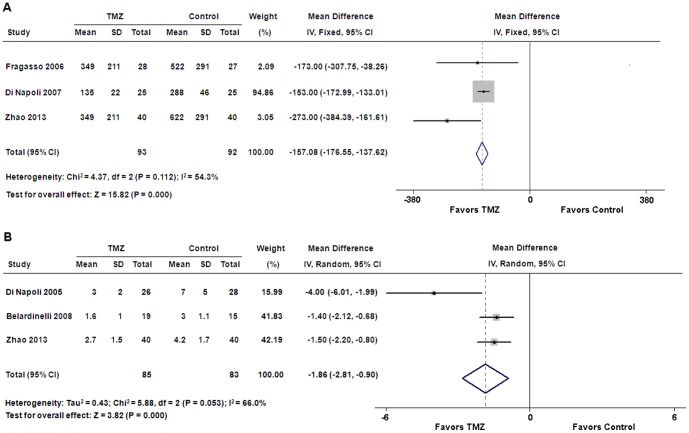
Pooled analysis showed that serum levels of BNP and CRP were significantly decreased in the TMZ group compared with those in the control group (WMD: −157.08 pg/ml, 95% CI: −176.55 to −137.62, p<0.01; WMD: −1.86 mg/l, 95% CI: −2.81 to −0.90, p<0.01, respectively) (Figures 5A and 5B).
Figure 5. Forest plots for serum markers.
(A) B-type natriuretic peptide; (B) C-reactive protein. CI = confidence intervals; IV = inverse variance; TMZ = trimetazidine.
Discussion
There is growing evidence that impaired carbohydrate oxidation and high rates of fatty acid oxidation contribute to the progression of myocardial dysfunction in CHF patients. TMZ has an inhibitory effect on the mitochondrial long-chain 3-ketoacyl coenzyme A thiolase, which plays a critical role in the fatty acid beta-oxidation pathway in the myocardium. As a result, there is a switch of cardiac metabolism from free fatty acid to glucose oxidation, which represents a more efficient metabolic pathway in terms of oxygen consumption and energy generation [29].
In the past few years, the beneficial effects of TMZ treatment in patients with CHF were confirmed in several small RCTs. Brottier et al. showed that TMZ improved clinical symptoms and LVEF in patients with severe ischemic cardiomyopathy [10]. Vitale et al. reported that TMZ improved left ventricular function and quality of life in elderly patients with coronary artery disease [15]. In addition, the other 4 reports of RCT revealed the protective effects of TMZ against left ventricular dysfunction in diabetic patients with ischemic cardiomyopathy [12]–[14], [22]. The cardioprotective effects of TMZ were also evaluated in patients with dilated cardiomyopathy. Tuunanen et al. reported that TMZ enhanced cardiac function and had both cardiac and extracardiac metabolic effects in idiopathic dilated cardiomyopathy with heart failure [23]. Zhao et al. indicated that TMZ treatment was associated with a considerable improvement of cardiac function and physical tolerance in diabetic patients with idiopathic dilated cardiomyopathy [28]. The pooled results of these studies suggested that TMZ therapy could significantly improve LVEF and NYHA classification in patients with CHF.
The effects of TMZ on all-cause mortality and hospitalization in CHF patients are still controversial. Only 3 reports of RCT with small samples described all-cause mortality [16], [17], [19], and 4 described hospitalization for cardiac causes [13], [15], [17], [23]. The pooled results of these studies demonstrated that TMZ treatment was associated with a significant decrease in hospitalization for cardiac causes. However, there was no significant difference in all-cause mortality between patients treated with TMZ and placebo. In this meta-analysis, we also evaluated the effects of TMZ on serum levels of BNP and CRP in patients with CHF. The pooled results suggested that BNP and CRP levels were significantly decreased in patients with TMZ treatment.
There are some differences in the pooled results between our meta-analysis and two previous meta-analyses performed by Gao et al. and Zhang et al. [7], [8]. The heterogeneities of LVEF, LVESV and LVEDV in our study were much smaller than those in the other two studies. In addition, our results showed no improvement in exercise duration and no decline in all-cause mortality in CHF patients treated with TMZ, which was not consistent with the other two meta-analyses. Furthermore, our pooled results also suggested that TMZ therapy could significantly decrease BNP and CRP levels in CHF patients.
Our study had several limitations. Firstly, the methodological quality of included studies was less than optimal, so we were not able to exclude the potential risk of bias in these trials. Secondly, the number of patients included in this meta-analysis was relatively small, so the conclusions drawn from this study should be interpreted with caution. Thirdly, the follow-up duration in these studies varied widely, from 4 weeks to 24 months.
In conclusion, our meta-analysis demonstrates that TMZ treatment in CHF patients may improve clinical symptoms and cardiac function, reduce hospitalization for cardiac causes, and decrease serum levels of BNP and CRP.
Supporting Information
PRISMA Checklist.
(PDF)
Funding Statement
The authors have no support or funding to report.
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, et al. (2011) Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123: e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krum H, Teerlink JR (2011) Medical therapy for chronic heart failure. Lancet 378: 713–721. [DOI] [PubMed] [Google Scholar]
- 3. Dedkova EN, Seidlmayer LK, Blatter LA (2013) Mitochondria-mediated cardioprotection by trimetazidine in rabbit heart failure. J Mol Cell Cardiol 59: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruixing Y, Wenwu L, Al-Ghazali R (2007) Trimetazidine inhibits cardiomyocyte apoptosis in a rabbit model of ischemia-reperfusion. Transl Res 149: 152–160. [DOI] [PubMed] [Google Scholar]
- 5. Zhou X, Li C, Xu W, Chen J (2012) Trimetazidine protects against smoking-induced left ventricular remodeling via attenuating oxidative stress, apoptosis, and inflammation. PLoS One 7: e40424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belardinelli R, Solenghi M, Volpe L, Purcaro A (2007) Trimetazidine improves endothelial dysfunction in chronic heart failure: an antioxidant effect. Eur Heart J 28: 1102–1108. [DOI] [PubMed] [Google Scholar]
- 7. Gao D, Ning N, Niu X, Hao G, Meng Z (2011) Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart 97: 278–286. [DOI] [PubMed] [Google Scholar]
- 8. Zhang L, Lu Y, Jiang H, Zhang L, Sun A, et al. (2012) Additional use of trimetazidine in patients with chronic heart failure: a meta-analysis. J Am Coll Cardiol 59: 913–922. [DOI] [PubMed] [Google Scholar]
- 9. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 10. Brottier L, Barat JL, Combe C, Boussens B, Bonnet J, et al. (1990) Therapeutic value of a cardioprotective agent in patients with severe ischaemic cardiomyopathy. Eur Heart J 11: 207–212. [DOI] [PubMed] [Google Scholar]
- 11. Belardinelli R, Purcaro A (2001) Effects of trimetazidine on the contractile response of chronically dysfunctional myocardium to low-dose dobutamine in ischaemic cardiomyopathy. Eur Heart J 22: 2164–2170. [DOI] [PubMed] [Google Scholar]
- 12. Fragasso G, Piatti MdPM, Monti L, Palloshi A, Setola E, et al. (2003) Short- and long-term beneficial effects of trimetazidine in patients with diabetes and ischemic cardiomyopathy. Am Heart J 146: E18. [DOI] [PubMed] [Google Scholar]
- 13. Rosano GMC, Vitale C, Sposato B, Mercuro G, Fini M (2003) Trimetazidine improves left ventricular function in diabetic patients with coronary artery disease: A double-blind placebo-controlled study. Cardiovasc Diabetol 2: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thrainsdottir IS, von Bibra H, Malmberg K, Ryden L (2004) Effects of trimetazidine on left ventricular function in patients with type 2 diabetes and heart failure. J Cardiovasc Pharmacol 44: 101–108. [DOI] [PubMed] [Google Scholar]
- 15. Vitale C, Wajngaten M, Sposato B, Gebara O, Rossini P, et al. (2004) Trimetazidine improves left ventricular function and quality of life in elderly patients with coronary artery disease. Eur Heart J 25: 1814–1821. [DOI] [PubMed] [Google Scholar]
- 16. El-Kady T, El-Sabban K, Gabaly M, Sabry A, Abdel-Hady S (2005) Effects of trimetazidine on myocardial perfusion and the contractile response of chronically dysfunctional myocardium in ischemic cardiomyopathy: a 24-month study. Am J Cardiovasc Drugs 5: 271–278. [DOI] [PubMed] [Google Scholar]
- 17. Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, et al. (2006) A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol 48: 992–998. [DOI] [PubMed] [Google Scholar]
- 18. Fragasso G, Perseghin G, Cobelli F, Esposito A, Palloshi A, et al. (2006) Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J 27: 942–948. [DOI] [PubMed] [Google Scholar]
- 19. Di Napoli P, Taccardi AA, Barsotti A (2005) Long term cardioprotective action of trimetazidine and potential effect on the inflammatory process in patients with ischaemic dilated cardiomyopathy. Heart 91: 161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Napoli P, Di Giovanni P, Gaeta MA, D'Apolito G, Barsotti A (2007) Beneficial effects of trimetazidine treatment on exercise tolerance and B-type natriuretic peptide and troponin T plasma levels in patients with stable ischemic cardiomyopathy. Am Heart J 154: 602.e1–5. [DOI] [PubMed] [Google Scholar]
- 21. Sisakian H, Torgomyan A, Barkhudaryan A (2007) The effect of trimetazidine on left ventricular systolic function and physical tolerance in patients with ischaemic cardiomyopathy. Acta Cardiol 62: 493–499. [DOI] [PubMed] [Google Scholar]
- 22. Belardinelli R, Cianci G, Gigli M, Mazzanti M, Lacalaprice F (2008) Effects of trimetazidine on myocardial perfusion and left ventricular systolic function in type 2 diabetic patients with ischemic cardiomyopathy. J Cardiovasc Pharmacol 51: 611–615. [DOI] [PubMed] [Google Scholar]
- 23. Tuunanen H, Engblom E, Naum A, Nagren K, Scheinin M, et al. (2008) Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation 118: 1250–1258. [DOI] [PubMed] [Google Scholar]
- 24. Gunes Y, Guntekin U, Tuncer M, Sahin M (2009) Improved left and right ventricular functions with trimetazidine in patients with heart failure: a tissue Doppler study. Heart Vessels 24: 277–282. [DOI] [PubMed] [Google Scholar]
- 25. Marazzi G, Gebara O, Vitale C, Caminiti G, Wajngarten M, et al. (2009) Effect of trimetazidine on quality of life in elderly patients with ischemic dilated cardiomyopathy. Adv Ther 26: 455–461. [DOI] [PubMed] [Google Scholar]
- 26. Cera M, Salerno A, Fragasso G, Montanaro C, Gardini C, et al. (2010) Beneficial electrophysiological effects of trimetazidine in patients with postischemic chronic heart failure. J Cardiovasc Pharmacol Ther 15: 24–30. [DOI] [PubMed] [Google Scholar]
- 27. Fragasso G, Salerno A, Lattuada G, Cuko A, Calori G, et al. (2011) Effect of partial inhibition of fatty acid oxidation by trimetazidine on whole body energy metabolism in patients with chronic heart failure. Heart 97: 1495–1500. [DOI] [PubMed] [Google Scholar]
- 28. Zhao P, Zhang J, Yin XG, Maharaj P, Narraindoo S, et al. (2013) The effect of trimetazidine on cardiac function in diabetic patients with idiopathic dilated cardiomyopathy. Life Sci 92: 633–638. [DOI] [PubMed] [Google Scholar]
- 29. Kantor PF, Lucien A, Kozak R, Lopaschuk GD (2000) The Antianginal Drug Trimetazidine Shifts Cardiac Energy Metabolism From Fatty Acid Oxidation to Glucose Oxidation by Inhibiting Mitochondrial Long-Chain 3-Ketoacyl Coenzyme A Thiolase. Circ Res 86: 580–588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(PDF)