Abstract
Background
Greater than 200 CGG repeats in the 5′UTR of the FMR1 gene leads to epigenetic silencing and lack of the FMR1 protein, causing Fragile X Syndrome. Individuals carriers of a premutation (PM) allele with 55–200 CGG repeats are typically unmethylated and can present with clinical features defined as FMR1 associated conditions.
Methods
Blood samples from 17 male PM carriers were assessed clinically and molecularly by Southern Blot, Western Blot, PCR and QRT-PCR. Blood and brain tissue from additional 18 PM males were also similarly examined. Continuous outcomes were modeled using linear regression and binary outcomes were modeled using logistic regression.
Results
Methylated alleles were detected in different fractions of blood cells in all PM cases (n= 17). CGG repeat numbers correlated with percent of methylation and mRNA levels and, especially in the upper PM range, with greater number of clinical involvements. Inter/intra- tissue somatic instability and differences in percent methylation were observed between blood and fibroblasts (n=4) and also observed between blood and different brain regions in three of the 18 premutation cases examined. CGG repeat lengths in lymphocytes remained unchanged over a period of time ranging from 2–6 years, three cases for whom multiple samples were available.
Conclusion
In addition to CGG size instability, individuals with a PM expanded alleles can exhibit methylation and display more clinical features likely due to RNA toxicity and/or FMR1 silencing. The observed association between CGG repeat length and percent of methylation with the severity of the clinical phenotypes underscores the potential value of methylation in affected PM to further understand penetrance, inform diagnosis and to expand treatment options.
Keywords: premutation, methylation, FMR1, somatic instability
INTRODUCTION
An expanded trinucleotide mutation of >200 CGG repeats within the promoter region of FMR1 usually causes hypermethylation and epigenetic silencing of the gene. The outcome is a nearly complete depletion of FMRP, the FMR1 gene product, leading to fragile X syndrome (FXS), the number one cause for inherited intellectual disability and autism. Alleles with shorter CGG “premutation” expansions spanning 55–200 CGG repeats are generally unmethylated. The elevated expression levels of FMR1 mRNA observed in premutation carriers, [1], which is CGG dependent, creates a toxic platform for the sequestration of a number of proteins such as the heat shock protein α/β-crystallin, Lamin A/C, the RNA binding protein hnRNPA2, [2], the splicing factor Sam 68 [3] and DROSHA/DGCR8, [4] important complex with a key role in the biogenesis of miRNAs. Recently, Repeat Associated Non-ATG translation (RAN-translation) in which translation initiation begins within alternative reading frame resulting in the synthesis of polyglycine or polyalanine peptides, [5] has been proposed as a potential mechanism for toxicity in FMR1 premutations [6]. In addition expanded FMR1 transcripts are inefficiently translated resulting in reduced expression levels of FMRP, [7–9]. The association of higher CGG repeat length with higher FMR1 mRNA levels and lower FMRP levels might lead to more clinical involvement in premutation carriers or greater penetrance of FMR1 associated disorders, particularly in the upper end of the premutation range [10].
Although only some premutation carriers manifest significant clinical problems during early life certain phenotypic characteristics such as macroorchidism, [11], cognitive problems and developmental delay, including Autism Spectrum Disorders (ASD), have been described in young and adult premutation carriers, [12–19]. In addition, a significant rate of hypertension, sleep apnea, seizure, immune mediated disorders, mood, anxiety, and other psychiatric disorders are associated with the premutation and further medical problems become recognizable later in life (reviewed in [10]). Female premutation carriers frequently develop fragile X-associated primary ovarian insufficiency (FXPOI) under the age of 40, [20] and a higher frequency of immuno-mediated disorders, [21, 22]. Premutation carriers, particularly males with >50 CGG repeat alleles, have an increasing age-related risk of developing fragile X-associated tremor/ataxia syndrome (FXTAS), [23], a neurodegenerative condition with clinical presentation of intentional tremor, cerebellar gait ataxia, parkinsonism, neuropathy, autonomic dysfunction and cognitive deficits for which effective treatments are yet to be discovered, [24]. Thus, premutation carriers may develop clinical symptoms throughout their lifespan, which could be related to either RNA toxicity from elevated FMR1 mRNA or lowered FMRP expression levels.
Until recently, premutation alleles have been considered somatically stable, [25, 26]. Evidence to this effect included a postmortem study of a male premutation carrier with 94 CGG repeats showing strong similarity of the FMR1 mutation in multiple tissues, including blood, spleen, liver, kidney, testes, brain and heart, demonstrating robust somatic stability, [25]. In addition, Nolin and collaborators [26] reported that single lymphocyte cells obtained from the same premutation carrier were relatively stable in contrast to significant variations in repeat size when comparing single sperm cells originated from a single male carrier suggesting the presence of repeat instability in the germline. More recently, the presence of somatic and germline instability were demonstrated in the premutation mouse model when somatic expansions were detected in spermatogonia and spermatocyte cells, at post-natal day 18 and at 11 months of age in the same mice, [27]. The study showed somatic expansions in a human premutation lymphoblastoid cell line after one and two passages. In addition, the study detected different CGG repeat size alleles in the amygdala and frontal cortex of a 91 CGG repeat premutation human carrier, suggesting that somatic instability can occur in the premutation, as observed in these tissues, and proposing that agents that increase DNA damage could influence these expansions, [27]. One proposed hypothesis is that the occurrence of these CGG expansions could be prompted by altered levels of the mismatched repair protein MSH2, a protein important in DNA repair and that has been implicated in the mechanism of germ-line and somatic expansions in the fmr1 premutation mouse model [28].
The hypermethylation status of cells has been previously associated with somatic stability of the repeat in full expansions of individuals with FXS, [29, 30]. When identical CGG patterns were observed in multiple tissues of male fetuses affected with FXS it was proposed that CGG expansions may receive mitotic stability early in fetal life, [28], while mitotic instability was observed in unmethylated alleles, [30, 31]. Later evidence showed a correlation between DNA methylation and CGG repeat stability in primary human fibroblasts but not in human-murine somatic cell hybrids suggesting that methylation is not a sole determinant of CGG repeat stability and more complex mechanisms are likely involved, [32]. Soon after, microcell fusion transfer of either hypermethylated or unmethylated full expansion alleles on human fragile X chromosomes into pluripotent murine embryocarcinoma cells showed these became demethylated and destabilized. However, instability was reversed to stability when the destabilized expansions were reintroduced into differentiated murine cells leading to the authors’ proposal that although methylation contributes to stability of the repeat, it is not one of its primary determinants, [33].
Although premutation alleles are generally unmethylated, a small percent of cells might carry a methylated premutation allele, particularly for allele sizes in the upper premutation range. One study reported a premutation case with methylation in a small percentage of lymphocyte cells and complete unmethylation in cultured skin fibroblasts obtained from the same patient. Interestingly, the methylation status did not affect FMRP levels in the different cell lines, perhaps due to the small percent of cells with methylated alleles [34, 35].
Here, we report on a group of 17 males with both methylated and unmethylated expanded FMR1 alleles spanning mostly the premutation range. In one case, using a sensitive methylation PCR technology, [36], we identified a small percent of cells (<2%) carrying methylated alleles otherwise undetected by Southern Blot. For four of the 17 cases we demonstrated CGG allele size somatic instability in addition to differences in methylation status between tissues, including lymphocytes and primary fibroblast cell lines, derived from the same patient. Three of them also showed no major changes in lymphocytes somatic instability over time. Positive correlations between molecular and clinical measures, particularly between CGG repeat size and number of clinical involvement were also observed in this group of subjects. Somatic instability was also assessed in post-mortem brain samples, specifically cerebellum, frontal cortex and temporal lobe, and in blood derived from 18 premutation carriers who died of symptoms of FXTAS.
In conclusion, our results suggest that variability in CGG allele size and in methylation patterns within and between different tissues could have repercussions on the clinical phenotype and may contribute to the penetrance of premutation disorders.
METHODS
Molecular Measures
CGG sizing
Genomic DNA was obtained from peripheral blood, fibroblast cultures and brain regions using standard procedures (Qiagen, Valencia, CA). CGG sizing and methylation status were performed by Southern Blot analysis using Alpha View software and measured as described, [37]. Southern Blot was performed on isolated genomic DNA digested with EcoR1/Nru1, transferred on a nylon membrane and hybridized with the specified, dig-labeled, FMR1 StB12.3 probe. Details of the method have been previously described, [37]. Percent of methylation was measured using an Alpha Innotech FluorChem 8800 Image Detection System. CGG sizing was also assessed by AmplideX® PCR assay, a very sensitive and efficient amplification approach as previously described, [38] and also by PCR using the CGG primer which allows to detect the presence of AGG interruptions within the FMR1 allele as detailed in, [36, 39]. Amplified products were visualized by capillary electrophoresis (ABI 3100 Genetic Analyzer, Applied Biosystems) and separated using POP4 or POP7 polymer (Applied Biosystems) following manufacturer instructions. FAM-6 labeled PCR product visualized as a distinct band(s) amplified by the c and f primers was sized using the size standard marker (Asuragen) and amplified PCR products of known CGG repeat number by sequencing, [40]. AGG positions within the CGG element were determined using Peakscanner software (v1.0) (Applied Biosystems), [38].
Methylation PCR
DNA samples were analyzed for methylation status and confirmation of the CGG repeat length using the AmplideX FMR1 mPCR Reagents (Asuragen, Austin, TX) according to the manufacturer’s recommended protocol. Briefly, DNA samples were separately aliquoted to a control or methylation-sensitive digestion reaction. Products of the control digestion reaction were amplified using FAM-labeled primers whereas products of the methylation-sensitive reaction were amplified using HEX-labeled primers. The percent methylation for each allele was calculated as the proportion of signal in the HEX- and FAM-channels normalized to reference control signals as described, [36].
Determination of FMR1 mRNA expression levels
Total RNA was isolated prepared from 3ml of blood collected in Tempus tubes (Applied Biosystems, Foster City, CA) or from 1× 10^6 cells using Trizol (Life Technologies, Carlsbad, CA). Quantitative Real Time RT-PCR amplification was performed on total RNA using custom designed Taqman gene expression assays (Applied Biosystems, Foster City, CA) as previously described in, [1].
Protein extraction and Western Blot analysis
Lymphocyte cell pellets were lysed in SDS lysis buffer complemented with complete ULTRA protease inhibitor tablets (Roche Applied Science, Indianapolis, IN) and 1 mM AEBSF (Sigma-Aldrich, Saint Louis, MO). Total protein concentrations were measured using the RC/DC Protein Assay (BioRad, United States) and 25ug of protein per sample were separated by SDS-PAGE electrophoresis, transferred into nitrocellulose membrane (BioRad, Germany) and the membrane probed with mouse anti-FMRP (Chemicon, Temecula, CA, 1:5,000 dilution) and mouse anti-GAPDH (Chemicon, Temecula, IL, 1:80,000 dilution) primary antibodies and IRDye 800CW goat anti mouse secondary antibodies (LICOR Biosciences, Lincoln, NE; 1:50:000). Bands were imaged and at 169 μm resolution using the Odyssey infrared scanner following manufacture recommendations. Analysis was performed by densitometry using Image Studio version 2.0.
Establishment of primary fibroblast cell lines
Explants of ~ 3-mm dermal biopsies were minced and placed in a 100-mm TC-treated tissue culture dish (Corning Life Science, USA) with a small drop of Fibroblast medium [Gibco AmnioMAX-C100 Basal Medium supplemented with 15% AmnioMAX-C100 Supplement (Invitrogen, Carlsbad, CA, USA), 20 ng/ml of human basic fibroblast growth factor (bFGF; Invitrogen, Carlsbad, CA, USA) and 1X primocin (Invitrogen)]. Dishes were incubated at 37°C in a humidified 5% CO2 and 5% O2 atmosphere for 3–5 days. Fibroblast outgrowths were harvested by trypsinization, transferred into a new dish with a modified Fibroblast Medium [1:1 solution of Gibco AmnioMAX-C100 supplemented with 15% AmnioMAX-C100 Supplement (Invitrogen, Carlsbad, CA, USA) and RPMI-1640 Basal Medium supplemented with 10% fetal bovine serum (Invitrogen), 1X primocin (Invitrogen), 1% non-essential amino acids (Invitrogen), and 1:250 fungizone (J R Scientific, Woodland, CA, USA)] with media exchange every 5 days and allowed to reach 90% confluence prior to splitting. Fibroblast cultures were passaged no more than 3 times prior to collection for DNA isolation or freezing.
Cognitive and Behavioral Measures
The neuropsychological/neuropsychiatric assessments included standardized IQ tests, including different assessment methods: Stanford-Binet Intelligence Scales, Fifth Edition (SB-5), and Wechsler Adult Intelligence Scales (WAIS-III or WAIS-IV) [41, 42]. Memory function was assessed using the Wechsler Memory Scales, Third Edition (WMS-III or WMS-IV), [43, 44] and the Mullen early learning scale, [45]. Self-reported psychological problems were further assessed using the Symptom Checklist-90-R (SCL-90-R), [46]. The Autism Diagnostic Observation Schedule (ADOS), [47] and Autism Diagnostic Interview-Revised (ADI-R), [48] were used to diagnose ASD. Behavioral scales, the Vineland Adaptive Behavioral Scales, Second Edition (VABS-II), [49], and the SNAP-IV-C, [50] for ADHD were also administered.
Statistical Analysis
Multiple linear regression was used to analyze the relationship between two variables (such as CGG repeats, IQ, FMR1 mRNA expression, % methylation, or number of clinical symptoms), adjusting for a third variable. Simple linear regression was used to analyze the relationship between % methylation and CGG repeats. Simple logistic regression was used to analyze the relationship between the odds of having ASD or any other clinical feature and FMR1 mRNA levels, and multiple logistic regression was used to analyze the relationship between the odds of having ASD and CGG repeats, adjusting for percent methylation. Analyses were conducted using R, version 3.0.0, (http://www.R-project.org).
RESULTS
Human Subjects
17 males carrying FMR1 expanded alleles between 55 and 250 CGG repeats, with ages between 3 to 22 years old and one subject 69 years old, were seen at the Fragile X Research and Treatment Center at the MIND Institute and consented under different research studies with IRB approved consent forms. For four individuals, primary fibroblast cell lines were established. Post-mortem human brain tissues including cerebellum, frontal cortex and temporal lobe, were from 18 Caucasian male premutation cases (mean age 67, CGG repeats range: 59-133; Demographic information and CGG size are shown in Supplementary Table 1).
Somatic instability in premutation expanded alleles CGG repeat allele size was determined in 17 subjects by PCR analysis in peripheral blood samples. PCR amplified alleles were visualized using capillary electrophoresis. Results show the presence of somatic instability, as manifested by serial peaks on the CE electropherogram. Expanded alleles ranged from 107 CGG to the lower full mutation range (up to 250 CGG repeats) (Table 1). No AGG interruptions were detected in any of the premutation carriers except case one where 1 AGG was found.
Table 1.
CGG size, percent of methylation measured by Southern Blot analysis, and clinical measures for the 17 males with an expanded CGG repeat allele.
| Case | CGG* | Age | % methylation | IQ | Anxiety | ADHD | Perseveration | Tantrums | ASD/PD DNOS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 107 | 69 | 5 | 123 | √ | - | - | - | - |
| 2 | 121 | 19 | 9 | 91 | √ | √ | √ | √ | - |
| 3 | 138 | 3 | 9 | 97 | - | - | - | - | - |
| 4 | 155 | 14 | 6 | 51 | √ | √ | √ | √ | √ |
| 5 | 150, 180 | 8 | <2 | 116 | √ | √ | √ | √ | - |
| 6 | 157, 180 | 8 | 3 | 124 | √ | √ | √ | √ | - |
| 7 | 163 | 8 | 7 | 95 | √ | √ | √ | √ | √ |
| 8 | 162 | 21 | 19 | 68 | √ | √ | - | - | - |
| 9 | 160–190 | 22 | 2 | 97 | √ | √ | √ | √ | √ |
| 10 | 170 | 18 | 7 | 54 | √ | √ | √ | √ | √ |
| 11 | 177 | 10 | 48 | 63 | √ | √ | √ | √ | √ |
| 12 | 187 | 7 | 11 | 62 | √ | √ | √ | √ | √ |
| 13 | 188 | 9 | 21 | 60 | √ | √ | √ | √ | - |
| 14 | 192 | 7 | 25 | 111 | - | √ | √ | - | √ |
| 15 | 192 | 4 | 35 | 55 | √ | √ | √ | √ | √ |
| 16 | 220 | 21 | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
| 17 | 250 | 9 | 84 | 55 | √ | √ | √ | √ | √ |
All 17 cases show series of peaks by CE indicating the presence of multiple CGG repeat alleles. The CGG size reported in the table is correspondent to the major peak(s). Both the CGG and percent of methylation reported in the table were measured in peripheral blood.
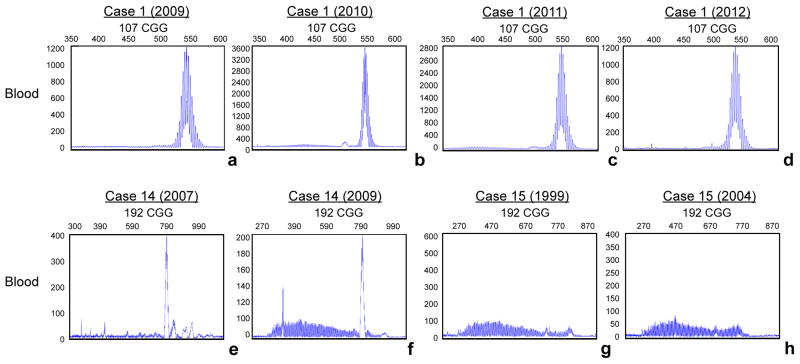
Peripheral blood leukocyte samples from three cases were collected at various visits from 2004 to 2012. Samples from case 1 were collected in 2009, 2010, 2011 and 2012. Samples from case 14 were collected in 2007 and 2009. Samples from case 15 were collected in 1999 and 2004. Size allele genotyping indicates that although somatic CGG length mosaicism is observed, the CGG repeat size showed a similar pattern between time points for the three cases analyzed (Figure 1). The PCR amplification was repeated twice for each subject to ensure consistency in the interpretation. These results demonstrate that, at least in peripheral blood from these three patients there is no indication of detectable change in CGG allele size suggesting that it is unlikely that premutation alleles may further expand with age during the lifespan of an individual (although limited to the time points analyzed, between 2 and 6 years). However, in addition, in some cases the CGG repeat profile (serial peaks) is too complex to be exactly interpreted when compared between two different time points (i.e. Figure 1g, h, case 15).
Figure 1.
CE analysis shows somatic instability in peripheral blood cells, particularly for case 14 (e and f) and case 15 (g and h). CGG repeat size was examined in three patients at the time of their first visit and at their consecutive visits. CE plots demonstrate no major detectable change in CGG repeat size after almost 6 years (case 15; g and h), after 2 years (case 14; e and f), after 1, 2, 3, and 4 years (case 1; a, b, c, d). Case numbers are as in Table 1.
FMR1 allele methylation increases proportionally with increased CGG repeat length
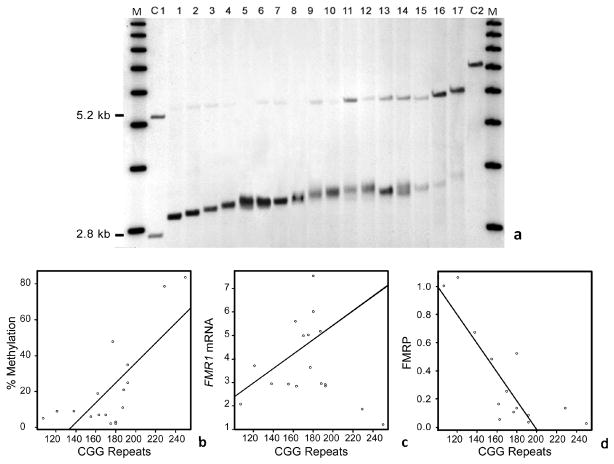
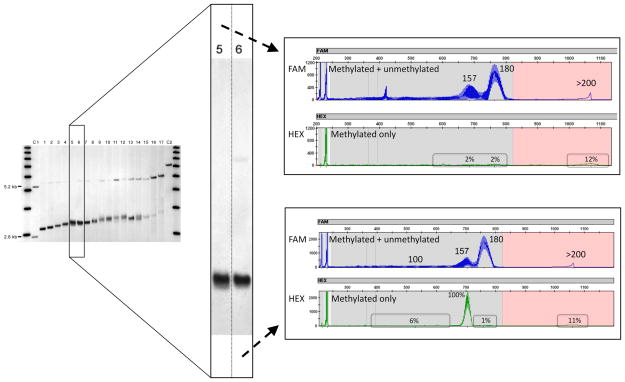
Methylation status of the FMR1 allele in each patient was assessed by both Southern Blot, which allows one to measure the fraction of cells carrying methylated alleles and is reported as a percent methylation, [37]; and by methylation PCR, [36], which quantifies allele-specific methylation at high resolution. The presence of methylated alleles was showed in the 17 individuals included in this study (Figure 2a); the percent of methylation measured as the ratio between the intensity of the methylated and unmethylated alleles on the Southern Blot for the 17 subjects listed in Table 1, was used for all statistical comparisons. Both Southern Blot and methylation PCR detected greater percent of methylation in alleles with longer CGG expansions suggesting a CGG number dependence (p= 0.001) (Figure 2b). As expected, after adjusting for % methylation a statistically significant positive correlation was observed between CGG repeat number and FMR1 mRNA (p = 0.041) (Figure 2c) while FMRP expression levels measured by western blot (Supplementary Figure 1) decreased significantly with increasing CGG repeats (P = 0.003) (Figure 2d). Methylation status determined by Southern Blot in two siblings (case 5 and 6) showed only one individual carrying a methylated allele in approximately 3% of the cells, while methylation was not detected in the other one (detection limit ~2 %) as shown in Figure 3 (lanes 5 and 6). However, mPCR detected the relative ratio of the methylated alleles in both individuals indicating a greater sensitivity of this approach for the detection of very low levels of methylated alleles, in addition to very low levels of full mutation alleles (allele with > 200 CGG repeat approximately 12% methylated) (Figure 3).
Figure 2.
(a) Southern Blot analysis shows the presence of non-methylated and methylated FMR1 alleles in DNA isolated from peripheral blood samples. 1Kb molecular weight marker is shown on the left and on the right (M). Negative control (C1) from a normal female shows a normal unmethylated (2.8 kb) and a normal methylated (5.2 kb) allele. Male carriers of expanded alleles are presented in order of increasing CGG allele size in lanes 1 through 17. Positive control corresponding to a male sample with a full mutation is shown in lane C2. A greater percent of methylation is present in the longer expanded alleles reflecting an association between methylation status and CGG repeat size. (b) Plot shows correlation between percent of methylation (measured by Southern Blot analysis) and CGG repeat length for the same 17 cases (P= 0.001; R2 = 0.56). (c) Plot shows a correlation between increasing levels of FMR1 mRNA correlates with increasing CGG repeat length for the same 17 cases after adjusting for percent methylation (P= 0.041; R2= 0.51). (d) Plot shows a negative correlation between FMRP and CGG repeat number (n= 13), after adjusting for percent methylation (P= 0.003; R2 = 0.67).
Figure 3.
Comparison of methylation patterns between Southern Blot and methylation PCR [36] for two cases (lanes 5 and 6 on Southern Blot). The Southern Blot image (left) reported approximately 3% of cells with methylated alleles in one case (lane 6), but failed to identify methylated alleles for the other case (lane 5). In both cases methylation PCR more clearly indicated the relative methylation of these premutation alleles and of a small population of newly identified full mutation alleles (right, green traces).
Methylation status can vary between peripheral blood and primary fibroblasts in individuals with high CGG repeat expansions
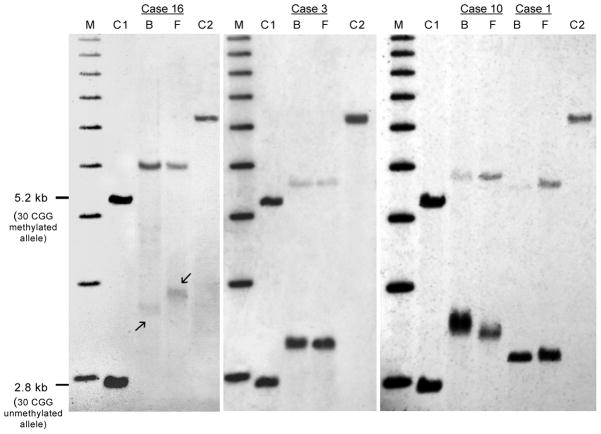
Primary fibroblast cells were obtained via skin biopsies from four patients, cultured and analyzed by PCR and Southern Blot to compare the degree of CGG instability and the FMR1 methylation status observed in peripheral blood cells. In three separate cases PCR-based allele size genotyping demonstrated that a similar pattern of CGG expansion was conserved between the two tissues, fibroblasts and blood, when obtained from a single individual (Figure 4). Although only in one case a different CGG allele size distribution was observed, methylation analysis using Southern Blot detected the presence of methylated alleles in both primary fibroblast cells and blood but in a different fraction of cells (Figure 5) with the greater percent of methylation observed in cultured fibroblast cells when compared to blood in cases 10 and 1, (Figure 5). Specifically, in case 16 the percent of methylated cells was 62% in fibroblasts and 79% in blood, in case 3 it was 11% in fibroblasts and 9% in blood, in case 10 it was 39% in fibroblasts and 7% in blood and in case 1 the percent of cells carrying methylated alleles was 23% in fibroblasts and 5% in blood cells (Figure 5).
Figure 4.
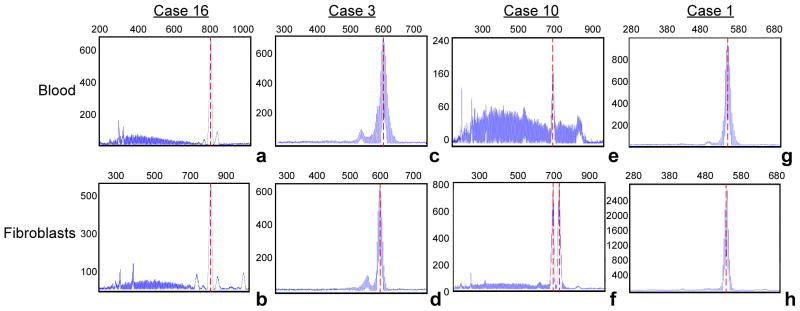
Electropherogram (CE) of PCR products amplified from DNA isolated from blood and correspondent fibroblasts from four males. The x-axis indicates the number of base pairs and the y-axis indicates relative fluorescence intensity. CE plot demonstrates the presence of allele instability in both blood and fibroblasts (a and b, case 16; c and d, case 3; e and f, case 10, and g and h, case 1; respectively from Table 1), particularly for case 10 (e and f) for which a greater difference between blood and fibroblast cells is observed (7% versus 39%, respectively, see also Figure 5).
Figure 5.
Southern Blot analysis detects differences in FMR1 percent of methylation when comparing peripheral blood to primary cultured fibroblasts derived from 4 different individuals. A 1kb molecular marker (M) and controls C1 (normal female with an unmethylated (2.8 kb) and methylated (5.2 kb) alleles) and a positive control C2 (full mutation allele) are shown. A reduced percent of methylation is observed between blood and fibroblasts, with a slightly greater percent methylation in peripheral blood cells (case 16, B) compared to the one observed in cultured fibroblast cells (case 16, F). Difference in the size of the FMR1 unmethylated alleles between blood and fibroblasts, (arrows) is also detectable (case 16). While a similar percent of methylation ratio is observed in case 3 between peripheral blood cells (case 3B) compared to cultured fibroblast cells (3F), a striking difference in percent of methylation between blood and primary cultured fibroblasts is observed for cases 10 and 1 for which fibroblasts (F) show a greater percent of methylation ratio compared to peripheral blood cells (B). Cases 3, 10 and 1 show a stable CGG repeat size. These gels illustrate an unstable pattern of percent of methylation that can exist between different tissues derived from the same individual.
Correlation of CGG repeat number and methylation status with degree of clinical features
Clinical data, including IQ, anxiety, ADHD, perseveration, tantrums and ASD or PDDNOS was available for investigating a potential association with molecular measures. After adjusting for the percent methylation, no correlation was observed between CGG length and any single clinical feature including IQ or ASD score. However, a positive correlation between a higher number of co-occurring phenotypic features (Table 1) and a larger CGG repeat length was observed (p=0.04), likely due to the effect of both RNA toxicity due to increased mRNA levels (as shown in Figure 2b) and/or reduced FMRP levels due to methylation (as shown in Figure 2d) and/or deficit in translation efficiency, particularly in the upper end of the premutation range, [1, 7, 8, 51]. Indeed, a trend was seen towards a decreased number of clinical problems in the presence of greater FMRP levels (P = 0.081; R= 0.67). Unfortunately FMRP levels were not available for 3 subjects for which a greater number of clinical involvement were observed (Table 1, cases 11, 12 and 13). We also explored the association between molecular measures obtained from blood and fibroblasts from the same individual. However, IQ was available only for three of the individuals among the group examined and for whom primary fibroblast cell lines were available and we did not have enough statistical power.
Somatic instability in premutation alleles: comparison between blood and brain tissue
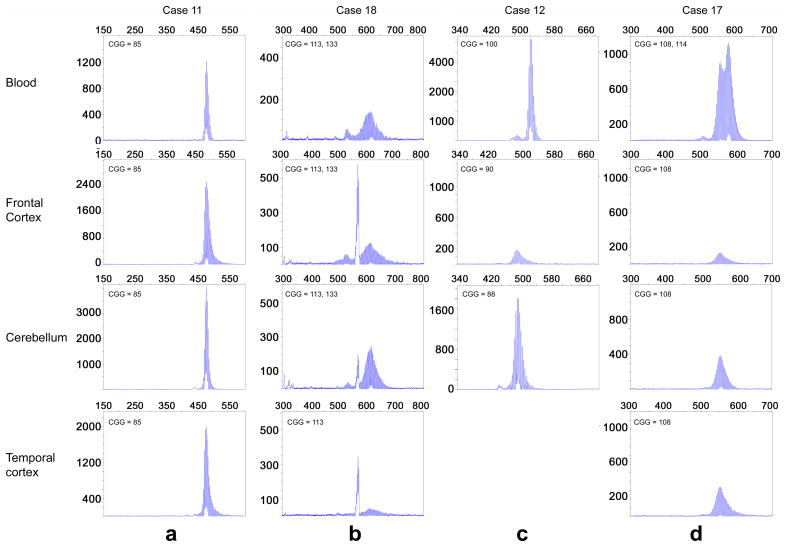
Post-mortem brain tissue and blood samples were available for 18 premutation cases with repeats ranging from 59-133 CGG, which were seen clinically at the MIND Institute prior to passing. To assess allele stability, lymphocyte blood cells and post-mortem brain tissue (cerebellum, frontal cortex and temporal lobe) derived from the same individual were examined. In the majority of cases the FMR1 allele size was similar in blood and in the three different brain regions indicating both CGG size and allele stability within the tissue and among tissues, particularly for alleles with <100 CGG repeats (Figure 6a). However, out of the 18 analyzed we identified three individuals with both inter-tissue somatic mosaicism and CGG size instability among tissues (Figure 6b, c, and d). These results suggest that CGG allele size somatic instability can occur within a tissue type and also between different tissues, as in this case, between blood and brain. Notably, methylation was not detected by Southern Blot in both blood and brain samples from these individuals (data not shown).
Figure 6.
CE plots show CGG repeat patterns observed in unmethylated FMR1 alleles from individuals with the premutation when comparing DNA isolated from blood and brain. (a) Out of the 18 cases, 11 cases with <100 CGG repeats presented inter- and intra- somatic stability. An example is illustrated by CE plot from blood and three brain regions derived from an individual carrying an 85 CGG repeat allele. Three cases presented with inter- and intra- somatic instability (b, c and d). The first case presented mostly a predominant 113 CGG repeat allele in brain tissue with a minor representation of 133 CGG repeat allele, while in blood cells the 133 CGG repeat allele was the most prominent. (c) The second case presented a 100 CGG repeat allele in blood, with a 90 CGG repeat allele in frontal cortex and an 88 CGG repeat allele in the cerebellum. Temporal lobe was not available for this individual. (d) The third case presented two major alleles (108 and 114 CGG repeats) in blood. However, only the 108 CGG repeat allele was present in all three brain regions (frontal cortex, cerebellum and temporal lobe). For four cases comparison was not possible because blood samples were not available.
DISCUSSION
The CGG expansion within the FMR1 gene predisposes it to instability, which results in size mosaicism, in which certain individuals present with alleles of different repeat expansion sizes within or in different cells or tissues. For instance, some cells may carry methylated full mutation alleles while others carry an unmethylated premutation allele, commonly referred as size mosaic full mutation. In other cases some cells may carry methylated full mutation alleles while others carry unmethylated alleles of different sizes that span from the premutation to the full mutation range (or span just the full mutation range), commonly referred as full mutation size and methylation mosaic or full mutation methylation mosaic. The latter are visualized on an agarose gel as a smear or on CE as a serial peaks. Methylation of FMR1 full mutation alleles (>200 CGG repeats) occurs early in embryonic development, [52] and is thought to play a role in stabilization of the expanded repeats, [53], while hypermethylation is found associated locally with histone deacetylation and chromatin remodeling, [54], leading to transcriptional silencing of the gene, [55].
In individuals with methylation mosaicism, some cells have fully methylated full mutation alleles while other cells carry unmethylated full mutation alleles. Such unmethylated alleles are transcriptionally active, and in many instances over expressed, [12] and, therefore, potentially translated to produce FMRP, [56]. However, FMRP production negatively correlates with the CGG repeat number, indeed FMR1 alleles, particularly in the upper premutation range, show decrease in FMRP expression levels due to a deficit in translational efficiency, [57].
Here, we present several cases with expanded methylated and unmethylated alleles as indicated by Southern Blot and by an array of different allele sizes identified by CE after PCR amplification, indicating somatic instability. In some cases the range of serial peaks (alleles) crossed over the cut off premutation range (200 CGG repeats), although peak distribution was predominantly clustered in the premutation range. These individuals exhibited allele methylation with the fraction of methylated alleles increasing with CGG repeat number; however the timing and mechanism of methylation are still unknown.
Our findings on inter- and intra- somatic instability and variability in percent methylation in different tissues, including dermal fibroblast cultured cells and peripheral blood cells, may have an impact on the clinical outcome. Indeed, we see a significant association between CGG repeat size and degree of clinical features. Although this study is limited by the small number of subjects included, it highlights the complex molecular scenario of the FMR1 gene including CGG allele size, inter- and intra- tissue somatic instability, methylation status, transcriptional levels and protein expression levels in individuals with an expanded allele and calls for the need to consider their role in the penetrance of the presented complex phenotypes. Interestingly, somatic instability was observed of a time interval of 1–7 years in peripheral blood for some cases of myotonic dystrophy, a CTG trinucleotide repeat disorder, [58]. The author suggested the expansion can occur throughout the life of an individual and that the degree of expansion correlates with the length of the initial size of the allele and can represent risk for clinical progression, [58, 59]. In this study, of the four individuals for whom fibroblast cell lines were available, of particular interest was the greater epigenetic silencing observed in the fibroblast cell lines compared to blood in three of them, while a higher percent of methylation in blood compared to fibroblasts was observed in the fourth one. Dermal fibroblasts share proliferative and molecular expression characteristics with neural precursor cells that may make them suitable for better genotype-phenotype correlations than lymphocytes [60]. The observation of a different degree of methylation in different tissues suggest that those individuals exhibiting partially methylated alleles may express lower levels of FMRP in more complex organ tissues than in blood, such may be the case in the central nervous system. Almost all genotype-phenotype studies thus far have been conducted with molecular parameters measured in blood with the suggestion that caution should be taken as mutations could be different in other tissues, including the brain. Our analysis of CGG size stability in post-mortem tissues indicates that alleles are fairly stable in the three brain regions of the majority of samples analyzed at least within the CGG repeats range available (59 –113 CGG repeats; Table S1) and in the absence of detectable methylation. However, allele size instability was observed between blood and brain regions in three cases in which the alleles were >100 CGG in size.
Finally, as the brain tissues analyzed were derived from individuals who died with symptoms of FXTAS, a question remains whether somatic instability is present in brain tissue derived from individuals with the premutation not affected with FXTAS with alleles in the same CGG repeat range.
Lastly, we note the value of emerging PCR technology, such as methylation PCR, to provide a more informed molecular view of mosaic features across different specimen types and time-points of collection. In particularly, the combination of Southern Blot and methylation PCR offers complementary data that can advance clinical research studies of FMR1 methylation in a range of study contexts.
In conclusion, our findings illustrate the need to consider multiple molecular factors including tissue variability or CGG repeat number, methylation and FMR1 and FMRP levels in modulating the penetrance of FMR1- associated disorders.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by the National Institute of Health [HD02274, HD036071]; and by the National Center for Advancing Translational Sciences [NIH UL1 TR000002]. Post mortem brain tissue was obtained from the Harvard Brain bank and from the repository of brain tissues collected at UC Davis, from fragile X and associated disorder cases [HD040661]. This work is dedicated to the memory of Matteo.
Footnotes
COMPETING INTERESTS
Dr. Randi Hagerman has received funding from Roche, Novartis, Seaside Therapeutics, Forest Curemark and the National Fragile X Foundation for clinical trials in fragile X syndrome and/or autism. She has also consulted with Novartis, Genentech and Roche regarding treatment in fragile X syndrome. Dr. Flora Tassone has consulted with Novartis and Genentech and has received funds from Roche.
References
- 1.Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66(1):6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwahashi CK, Yasui DH, An HJ, Greco CM, Tassone F, Nannen K, Babineau B, Lebrilla CB, Hagerman RJ, Hagerman PJ. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129(Pt 1):256–271. doi: 10.1093/brain/awh650. [DOI] [PubMed] [Google Scholar]
- 3.Sellier C, Rau F, Liu Y, Tassone F, Hukema RK, Gattoni R, Schneider A, Richard S, Willemsen R, Elliott DJ, Hagerman PJ, Charlet-Berguerand N. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 2010;29(7):1248–1261. doi: 10.1038/emboj.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sellier C, Freyermuth F, Tabet R, Tran T, He F, Ruffenach F, Alunni V, Moine H, Thibault C, Page A, Tassone F, Willemsen R, Disney MD, Hagerman PJ, Todd PK, Charlet-Berguerand N. Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell Rep. 2013;3(3):869–880. doi: 10.1016/j.celrep.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson CE. Repeat associated non-ATG translation initiation: one DNA, two transcripts, seven reading frames, potentially nine toxic entities! PLoS Genet. 2011;7(3):e1002018. doi: 10.1371/journal.pgen.1002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todd PK, Oh SY, Krans A, He F, Sellier C, Frazer M, Renoux AJ, Chen KC, Scaglione KM, Basrur V, Elenitoba-Johnson K, Vonsattel JP, Louis ED, Sutton MA, Taylor JP, Mills RE, Charlet-Berguerand N, Paulson HL. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron. 2013;78(3):440–455. doi: 10.1016/j.neuron.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Primerano B, Tassone F, Hagerman RJ, Hagerman P, Amaldi F, Bagni C. Reduced FMR1 mRNA translation efficiency in fragile X patients with premutations. RNA. 2002;8(12):1482–1488. [PMC free article] [PubMed] [Google Scholar]
- 8.Peprah E, He W, Allen E, Oliver T, Boyne A, Sherman SL. Examination of FMR1 transcript and protein levels among 74 premutation carriers. J Hum Genet. 2010;55(1):66–68. doi: 10.1038/jhg.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenneson A, Warren ST. The female and the fragile X reviewed. Semin Reprod Med. 2001;19(2):159–165. doi: 10.1055/s-2001-15401. [DOI] [PubMed] [Google Scholar]
- 10.Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12(8):786–798. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leehey MA, Hagerman PJ. Fragile X-associated tremor/ataxia syndrome. Handb Clin Neurol. 2012;103:373–386. doi: 10.1016/B978-0-444-51892-7.00023-1. [DOI] [PubMed] [Google Scholar]
- 12.Tassone F, Hagerman RJ, Loesch DZ, Lachiewicz A, Taylor AK, Hagerman PJ. Fragile X males with unmethylated, full mutation trinucleotide repeat expansions have elevated levels of FMR1 messenger RNA. Am J Med Genet. 2000;94(3):232–236. doi: 10.1002/1096-8628(20000918)94:3<232::aid-ajmg9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Aziz M, Stathopulu E, Callias M, Taylor C, Turk J, Oostra B, Willemsen R, Patton M. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet B Neuropsychiatr Genet. 2003;121B(1):119–127. doi: 10.1002/ajmg.b.20030. [DOI] [PubMed] [Google Scholar]
- 14.Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, Gane L, Tassone F, Hagerman P, Hagerman R. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27(2 Suppl):S137–144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- 15.Bailey DB, Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;146A(16):2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- 16.Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Tassone F, Harvey D, Rivera SM, Simon TJ. Adult Female Fragile X Premutation Carriers Exhibit Age- and CGG Repeat Length-Related Impairments on an Attentionally Based Enumeration Task. Front Hum Neurosci. 2011;5:63. doi: 10.3389/fnhum.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Tassone F, Harvey D, Rivera SM, Simon TJ. Enhanced manual and oral motor reaction time in young adult female fragile X premutation carriers. J Int Neuropsychol Soc. 2011;17(4):746–750. doi: 10.1017/S1355617711000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Srivastava S, Tassone F, Harvey D, Rivera SM, Simon TJ. Young adult female fragile X premutation carriers show age- and genetically-modulated cognitive impairments. Brain Cogn. 2011;75(3):255–260. doi: 10.1016/j.bandc.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chonchaiya W, Au J, Schneider A, Hessl D, Harris SW, Laird M, Mu Y, Tassone F, Nguyen DV, Hagerman RJ. Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet. 2012;131(4):581–589. doi: 10.1007/s00439-011-1106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20(2):402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 22.Winarni TI, Chonchaiya W, Sumekar TA, Ashwood P, Morales GM, Tassone F, Nguyen DV, Faradz SM, Van de Water J, Cook K, Hamlin A, Mu Y, Hagerman PJ, Hagerman RJ. Immune-mediated disorders among women carriers of fragile X premutation alleles. Am J Med Genet A. 2012;158A(10):2473–2481. doi: 10.1002/ajmg.a.35569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, Zhang L, Jardini T, Gane LW, Harris SW, Herman K, Grigsby J, Greco CM, Berry-Kravis E, Tassone F, Hagerman PJ. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291(4):460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 24.Tassone F, Berry-Kravis EM. The Fragile X-Associated Tremor Ataxia Syndrome (FXTAS) Springer Science+Business Media; 2010. [Google Scholar]
- 25.Tassone F, Hagerman RJ, Gane LW, Taylor AK. Strong similarities of the FMR1 mutation in multiple tissues: postmortem studies of a male with a full mutation and a male carrier of a premutation. Am J Med Genet. 1999;84(3):240–244. [PubMed] [Google Scholar]
- 26.Nolin SL, Houck GE, Jr, Gargano AD, Blumstein H, Dobkin CS, Brown WT. FMR1 CGG-repeat instability in single sperm and lymphocytes of fragile-X premutation males. Am J Hum Genet. 1999;65(3):680–688. doi: 10.1086/302543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lokanga RA, Entezam A, Kumari D, Yudkin D, Qin M, Smith CB, Usdin K. Somatic expansion in mouse and human carriers of fragile X premutation alleles. Hum Mutat. 2013;34(1):157–166. doi: 10.1002/humu.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lokanga R, Zhao XN, Usdin K. The Mismatch Repair Protein MSH2 is Rate-Limiting for Repeat Expansion in a Fragile X Premutation Mouse Model. Hum Mutat. 2013 doi: 10.1002/humu.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wohrle D, Hennig I, Vogel W, Steinbach P. Mitotic stability of fragile X mutations in differentiated cells indicates early post-conceptional trinucleotide repeat expansion. Nat Genet. 1993;4(2):140–142. doi: 10.1038/ng0693-140. [DOI] [PubMed] [Google Scholar]
- 30.Glaser D, Wohrle D, Salat U, Vogel W, Steinbach P. Mitotic behavior of expanded CGG repeats studied on cultured cells: further evidence for methylation-mediated triplet repeat stability in fragile X syndrome. Am J Med Genet. 1999;84(3):226–228. [PubMed] [Google Scholar]
- 31.Wohrle D, Salat U, Glaser D, Mucke J, Meisel-Stosiek M, Schindler D, Vogel W, Steinbach P. Unusual mutations in high functioning fragile X males: apparent instability of expanded unmethylated CGG repeats. J Med Genet. 1998;35(2):103–111. doi: 10.1136/jmg.35.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burman RW, Popovich BW, Jacky PB, Turker MS. Fully expanded FMR1 CGG repeats exhibit a length- and differentiation-dependent instability in cell hybrids that is independent of DNA methylation. Hum Mol Genet. 1999;8(12):2293–2302. doi: 10.1093/hmg/8.12.2293. [DOI] [PubMed] [Google Scholar]
- 33.Wohrle D, Salat U, Hameister H, Vogel W, Steinbach P. Demethylation, reactivation, and destabilization of human fragile X full-mutation alleles in mouse embryocarcinoma cells. Am J Hum Genet. 2001;69(3):504–515. doi: 10.1086/322739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tassone F, Longshore J, Zunich J, Steinbach P, Salat U, Taylor AK. Tissue-specific methylation differences in a fragile X premutation carrier. Clin Genet. 1999;55(5):346–351. doi: 10.1034/j.1399-0004.1999.550508.x. [DOI] [PubMed] [Google Scholar]
- 35.Allingham-Hawkins DJ, Brown CA, Babul R, Chitayat D, Krekewich K, Humphries T, Ray PN, Teshima IE. Tissue-specific methylation differences and cognitive function in fragile X premutation females. Am J Med Genet. 1996;64(2):329–333. doi: 10.1002/(SICI)1096-8628(19960809)64:2<329::AID-AJMG19>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Hadd AG, Sah S, Houghton JF, Filipovic-Sadic S, Zhang W, Hagerman PJ, Tassone F, Latham GJ. High-resolution methylation polymerase chain reaction for fragile X analysis: evidence for novel FMR1 methylation patterns undetected in Southern blot analyses. Genet Med. 2011;13(6):528–538. doi: 10.1097/GIM.0b013e31820a780f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10(1):43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filipovic-Sadic S, Sah S, Chen L, Krosting J, Sekinger E, Zhang W, Hagerman PJ, Stenzel TT, Hadd AG, Latham GJ, Tassone F. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin Chem. 2010;56(3):399–408. doi: 10.1373/clinchem.2009.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yrigollen CM, Durbin-Johnson B, Gane L, Nelson DL, Hagerman R, Hagerman PJ, Tassone F. AGG interruptions within the maternal FMR1 gene reduce the risk of offspring with fragile X syndrome. Genet Med. 2012;14(8):729–736. doi: 10.1038/gim.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yrigollen CM, Tassone F, Durbin-Johnson B. The role of AGG interruptions in the transcription of FMR1 premutation alleles. PLoS One. 2011;6(7):e21728. doi: 10.1371/journal.pone.0021728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, Tx: 1997. (WAIS-III) [Google Scholar]
- 42.Wechsler D. Wechsler Adult Intelligence Scale. 4. 2008. (WAIS-IV) [Google Scholar]
- 43.Wechsler D. Wechsler Memory Scale. 3. 2003. (WMS-III) [Google Scholar]
- 44.Wechsler D. Wechsler Memory Scale. 4. 2009. (WMS-IV) [Google Scholar]
- 45.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Services, Inc; 1995. [Google Scholar]
- 46.Derogatis LR. Symptom Checklist-90-R (SCL-90-R): Administration, Scoring, and Procedures Manual. Minneapolis: National Computer Systems; 1994. [Google Scholar]
- 47.Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19(2):185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 48.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 49.Sparrow SS, Cicchetti DV, Balla DA. Vineland Apdaptive Behaior Scales. 2. Circle Pines: AGS Publishing; 2005. [Google Scholar]
- 50.Swanson JM, Kraemer HC, Hinshaw SP. Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adloesc Psychiatry. 2001;40:168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Allen EG, Sherman S, Abramowitz A, Leslie M, Novak G, Rusin M, Scott E, Letz R. Examination of the effect of the polymorphic CGG repeat in the FMR1 gene on cognitive performance. Behav Genet. 2005;35(4):435–445. doi: 10.1007/s10519-005-2792-4. [DOI] [PubMed] [Google Scholar]
- 52.Malter HE, Iber JC, Willemsen R, de Graaff E, Tarleton JC, Leisti J, Warren ST, Oostra BA. Characterization of the full fragile X syndrome mutation in fetal gametes. Nat Genet. 1997;15(2):165–169. doi: 10.1038/ng0297-165. [DOI] [PubMed] [Google Scholar]
- 53.Devys D, Biancalana V, Rousseau F, Boue J, Mandel JL, Oberle I. Analysis of full fragile X mutations in fetal tissues and monozygotic twins indicate that abnormal methylation and somatic heterogeneity are established early in development. Am J Med Genet. 1992;43(1–2):208–216. doi: 10.1002/ajmg.1320430134. [DOI] [PubMed] [Google Scholar]
- 54.Coffee B, Zhang F, Warren ST, Reines D. Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nature Genetics. 1999;22:98–101. doi: 10.1038/8807. [DOI] [PubMed] [Google Scholar]
- 55.Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- 56.Verheij C, Bakker CE, de Graaff E, Keulemans J, Willemsen R, Verkerk AJ, Galjaard H, Reuser AJ, Hoogeveen AT, Oostra BA. Characterization and localization of the FMR-1 gene product associated with fragile X syndrome. Nature. 1993;363(6431):722–724. doi: 10.1038/363722a0. [DOI] [PubMed] [Google Scholar]
- 57.Primerano B, Tassone F, Hagerman RJ, Hagerman PJ, Amaldi F, Bagni C. Reduced FMR1 mRNA translation efficiency in Fragile X patients with premutations. RNA. 2002;8(12):1482–1488. [PMC free article] [PubMed] [Google Scholar]
- 58.Martorell L, Monckton DG, Gamez J, Johnson KJ, Gich I, Lopez de Munain A, Baiget M. Progression of somatic CTG repeat length heterogeneity in the blood cells of myotonic dystrophy patients. Hum Mol Genet. 1998;7(2):307–312. doi: 10.1093/hmg/7.2.307. [DOI] [PubMed] [Google Scholar]
- 59.Martorell L, Martinez JM, Carey N, Johnson K, Baiget M. Comparison of CTG repeat length expansion and clinical progression of myotonic dystrophy over a five year period. J Med Genet. 1995;32(8):593–596. doi: 10.1136/jmg.32.8.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilbert SF. Developmental Biology. 6. Sinauer Associats, Inc; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.