Abstract
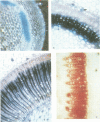
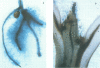
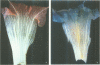
Phenylalanine ammonia-lyase (PAL) catalyses the first step in the biosynthesis of phenylpropanoids, which form a wide variety of plant secondary products. The transcription of PAL is regulated in response to various factors that induce the accumulation of flavonoids, lignin and compounds thought to be involved in plant defence reactions. The 5' upstream sequence of a PAL gene from Phaseolus vulgaris was fused to the coding region of the reporter gene encoding beta-glucuronidase (GUS), and transformed into potato and tobacco plants. Histochemical analysis of GUS expression showed that the PAL promoter was active in specific cell types that accumulated phenylpropanoid derivatives in response to mechanical wounding, and also during normal development of the xylem and flower. In xylem that had undergone secondary thickening, GUS activity occurred in rays of cells thought to be the xylem parenchyma. It was postulated that PAL activity in these cells could provide intermediates for lignin synthesis in xylem vessels that had terminally differentiated.
Full text
PDF
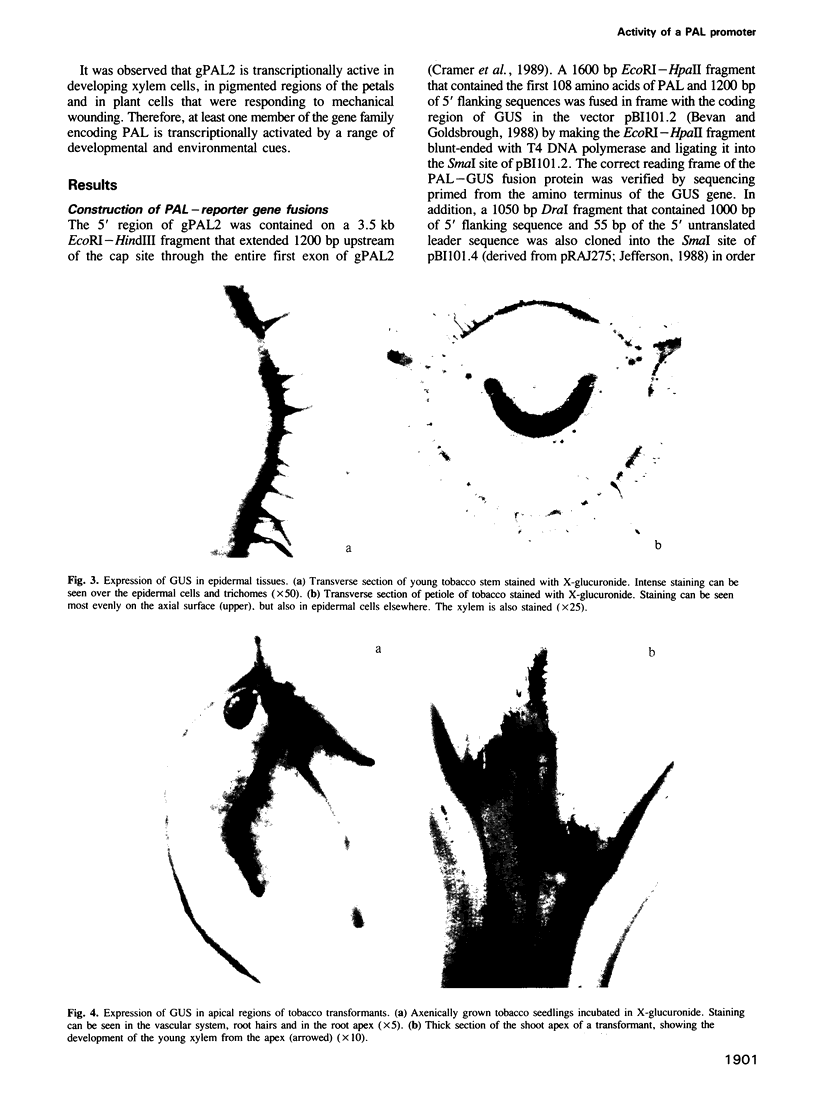

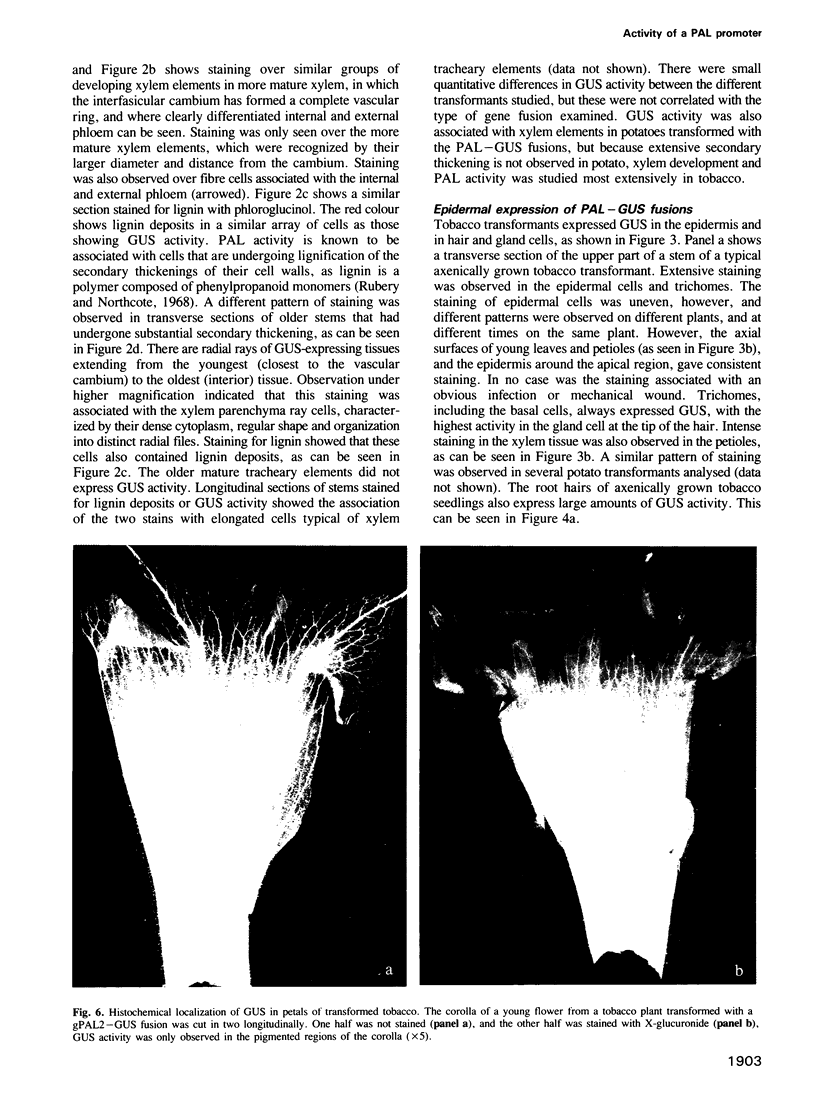



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Bell J. N., Ryder T. B., Wingate V. P., Bailey J. A., Lamb C. J. Differential accumulation of plant defense gene transcripts in a compatible and an incompatible plant-pathogen interaction. Mol Cell Biol. 1986 May;6(5):1615–1623. doi: 10.1128/mcb.6.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Bell J. N., Cramer C. L., Schuch W., Lamb C. J., Dixon R. A. L-Phenylalanine ammonia-lyase from Phaseolus vulgaris. Characterisation and differential induction of multiple forms from elicitor-treated cell suspension cultures. Eur J Biochem. 1985 Jun 3;149(2):411–419. doi: 10.1111/j.1432-1033.1985.tb08941.x. [DOI] [PubMed] [Google Scholar]
- Borchert R. Time course and spatial distribution of phenylalanine ammonia-lyase and peroxidase activity in wounded potato tuber tissue. Plant Physiol. 1978 Nov;62(5):789–793. doi: 10.1104/pp.62.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chen S. M., Coe E. H., Jr Control of anthocyanin synthesis by the C locus in maize. Biochem Genet. 1977 Apr;15(3-4):333–346. doi: 10.1007/BF00484464. [DOI] [PubMed] [Google Scholar]
- Corbin D. R., Sauer N., Lamb C. J. Differential regulation of a hydroxyproline-rich glycoprotein gene family in wounded and infected plants. Mol Cell Biol. 1987 Dec;7(12):4337–4344. doi: 10.1128/mcb.7.12.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottle W., Kolattukudy P. E. Abscisic Acid stimulation of suberization : induction of enzymes and deposition of polymeric components and associated waxes in tissue cultures of potato tuber. Plant Physiol. 1982 Sep;70(3):775–780. doi: 10.1104/pp.70.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B. B., Kolattukudy P. E. Biochemistry of Suberization: Incorporation of [1-C]Oleic Acid and [1-C]Acetate into the Aliphatic Components of Suberin in Potato Tuber Disks (Solanum tuberosum). Plant Physiol. 1977 Jan;59(1):48–54. doi: 10.1104/pp.59.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K., Cramer C. L., Bolwell G. P., Dixon R. A., Schuch W., Lamb C. J. Rapid transient induction of phenylalanine ammonia-lyase mRNA in elicitor-treated bean cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6731–6735. doi: 10.1073/pnas.82.20.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg K. Lignin: Its Constitution and Formation from p-Hydroxycinnamyl Alcohols: Lignin is duplicated by dehydrogenation of these alcohols; intermediates explain formation and structure. Science. 1965 Apr 30;148(3670):595–600. doi: 10.1126/science.148.3670.595. [DOI] [PubMed] [Google Scholar]
- Fritzemeier K. H., Cretin C., Kombrink E., Rohwer F., Taylor J., Scheel D., Hahlbrock K. Transient Induction of Phenylalanine Ammonia-Lyase and 4-Coumarate: CoA Ligase mRNAs in Potato Leaves Infected with Virulent or Avirulent Races of Phytophthora infestans. Plant Physiol. 1987 Sep;85(1):34–41. doi: 10.1104/pp.85.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. J., Merritt T. K., Butt V. S. Synthesis and removal of phenylalanine ammonia-lyase activity in illuminated discs of potato tuber parenchyme. Biochim Biophys Acta. 1979 Jan 18;582(2):196–212. doi: 10.1016/0304-4165(79)90384-2. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Hahlbrock K., Lamb C. Rapid induction of the synthesis of phenylalanine ammonia-lyase and of chalcone synthase in elicitor-treated plant cells. Eur J Biochem. 1983 Jan 1;129(3):593–601. doi: 10.1111/j.1432-1033.1983.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapid switching of plant gene expression induced by fungal elicitor. Science. 1985 Mar 8;227(4691):1240–1243. doi: 10.1126/science.227.4691.1240. [DOI] [PubMed] [Google Scholar]
- Rubery P. H., Northcote D. H. Site of phenylalanine ammonia--lyase activity and synthesis of lignin during xylem differentiation. Nature. 1968 Sep 21;219(5160):1230–1234. doi: 10.1038/2191230a0. [DOI] [PubMed] [Google Scholar]
- Ryder T. B., Cramer C. L., Bell J. N., Robbins M. P., Dixon R. A., Lamb C. J. Elicitor rapidly induces chalcone synthase mRNA in Phaseolus vulgaris cells at the onset of the phytoalexin defense response. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5724–5728. doi: 10.1073/pnas.81.18.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder T. B., Hedrick S. A., Bell J. N., Liang X. W., Clouse S. D., Lamb C. J. Organization and differential activation of a gene family encoding the plant defense enzyme chalcone synthase in Phaseolus vulgaris. Mol Gen Genet. 1987 Dec;210(2):219–233. doi: 10.1007/BF00325687. [DOI] [PubMed] [Google Scholar]
- Schmelzer E., Jahnen W., Hahlbrock K. In situ localization of light-induced chalcone synthase mRNA, chalcone synthase, and flavonoid end products in epidermal cells of parsley leaves. Proc Natl Acad Sci U S A. 1988 May;85(9):2989–2993. doi: 10.1073/pnas.85.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford A., Bevan M., Northcote D. Differential expression within a family of novel wound-induced genes in potato. Mol Gen Genet. 1989 Jan;215(2):200–208. doi: 10.1007/BF00339718. [DOI] [PubMed] [Google Scholar]
- Walter M. H., Grima-Pettenati J., Grand C., Boudet A. M., Lamb C. J. Cinnamyl-alcohol dehydrogenase, a molecular marker specific for lignin synthesis: cDNA cloning and mRNA induction by fungal elicitor. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5546–5550. doi: 10.1073/pnas.85.15.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tunen A. J., Koes R. E., Spelt C. E., van der Krol A. R., Stuitje A. R., Mol J. N. Cloning of the two chalcone flavanone isomerase genes from Petunia hybrida: coordinate, light-regulated and differential expression of flavonoid genes. EMBO J. 1988 May;7(5):1257–1263. doi: 10.1002/j.1460-2075.1988.tb02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]