Abstract
Epidemiological studies link obesity, as measured by increased body mass index (BMI) to the incidence of renal cell carcinoma (RCC) as well as to the cancer-related mortality of RCC patients. RCC is the third cancer most robustly associated with increased BMI. Understanding the role of the adipose tissue in renal carcinogenesis is therefore of major importance for the development of novel paradigms of RCC prevention and treatment. Here, we discuss the current knowledge on the impact of obesity on the development and progression of RCC as well as the role of adipose tissue-derived hormones (adipokines) in the conflict between growing tumors and the immune system.
Keywords: adipokines, immune response, renal cell carcinoma, obesity
Introduction
The incidence of obesity and obesity-associated disorders have been steadily increasing over the past few decades in developed and developing countries, making it one of the most serious health problems worldwide. Several morbidities have been etiologically associated with obesity, including Type 2 diabetes and cardiovascular diseases. Recently, an epidemiological link between obesity and the prevalence of a variety of cancers, including breast, endometrial, esophageal, gastric, colorectal, gallbladder, pancreatic, hepatic, renal, and bladder tumors, has been established.
Renal cell carcinoma (RCC) accounts for approximately 3% of all cancers in adults in several western countries, and its incidence has been rising over the last few decades.1 Although several potential risk factors have been recognized, including tobacco smoking,2 hypertension,3 and a familial history of kidney cancer, the etiology of RCC is still largely undefined. Several studies worldwide have demonstrated the influence of body mass index (BMI), i.e., weight in kg/(height m)2 on renal carcinogenesis.4 RCC is the third cancer most robustly associated with increased BMI after endometrial and esophageal tumors. One of the most rigorous meta-analysis studies performed to date revealed a strong association between overweight and RCC.5 Forty percent of RCC cases in USA and 30% in Europe are indeed associated with excessive body weight. The risk for the development of RCC has been directly correlated with increased weight in a dose-response manner, with an estimated increase of 24% for men and 34% for women for every 5 kg/m2 increase in BMI, leading to a 4% increased risk of developing RCC.5 This said, limited information is available on the variation in the risk of obese patients to develop RCC of different histological type, stage or grade, or stratified on other potential risk factors. However, it appears that clear cell RCC (ccRCC) is the histological subtype of RCC that is the most strongly associated with obesity,6,7 and for which perinephric fat invasion is a significant predictor of poor disease outcome.8 Paradoxically, obesity appears to be associated with an improved survival of cancer patients as well as with relatively poor symptomatic or local tumors, as it was documented recently by several studies. Yet, the rate of postoperative complications increases with BMI.9,10 Here, we review how a dysfunctional adipose tissue (AT) interferes with oncogenesis and may modulate anticancer immune responses.
Dysfunctional Adipose Tissue and Tumor Development
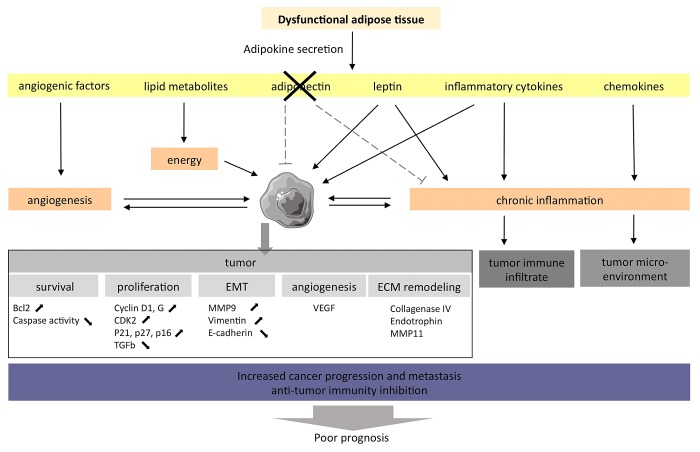
The adipose tissue (AT) is the most prevalent tissue in the human body. It is commonly found in the subcutaneous connective tissue and also surrounds various organs including the kidneys. In humans and most animal models, the development of obesity leads not only to increased fat depots in classical anatomical locations, but also to significant deposits of lipids within and around other tissues and organs, a phenomenon known as ectopic fat storage. The AT becomes increasingly dysfunctional upon weight gain. The altered physiological state of the expanding fat pads may affect carcinogenesis and impact on the progression of established renal tumors, modulating the tumor microenvironment (Fig. 1).
Figure 1. The adipose tissue of obese individuals may favor the establishment of a microenvironment that support oncogenesis and tumor progression. The adipose tissue associated with obesity displays an altered adipokine secretion pattern, hence favoring the establishment of a chronic inflammation state that promotes oncogenesis and tumor progression.
Adipokines dysregulation and RCC
The AT is not only a long-term energy storage organ, but also a major endocrine gland, being responsible for biosynthesis and secretion of a large number of hormones and cytokines, commonly referred to as adipokines. AT dysfunction results in altered circulating levels of adipokines, an alteration that may be directly involved in obesity-related tumorigenesis. Here, we discuss 2 adipokines, leptin (LEP) and adiponectin, which are among the proteins most specifically produced by adipocytes and are linked to RCC by both epidemiological and preclinical data.
LEP (also known as Ob; molecular weight = 16 kDa; 167 amino acids) is a small non glycosylated protein structurally similar to interleukin (IL)-6, IL-12, IL-15, prolactin, the growth hormone and granulocyte colony-stimulating factor (G-CSF).11 LEP exclusively binds to its receptor (LEPR, also known ObR). Several variants of the LEPR have been shown to result from the alternative splicing of the LEPR transcript (ObRA-F), with ObRB being the predominant isoform responsible for the biological actions of LEP. ObRB activates indeed the Janus kinase/signal transducer and activator of transcription (JAK/STAT3) signaling pathway, which in turn stimulates phopshoinositide-3-kinase (PI3K) to promote proliferation, migration and angiogenesis.12
Accumulating evidence supports the idea that LEP is the link between obesity and the increased incidence of various cancers.3,13 The ObR is highly expressed on multiple malignant cells, including those of mammary, pancreatic, esophageal, gastric, and colorectal origin, as compared with their normal counterparts (in which the receptor is expressed at very low levels or not at all).14 Data from animal studies reinforced the hypothesis that LEP can contribute to cancer growth. Thus, the progeny of LEP-signaling (ob/ob or db/db) mice crossed with MTTV-TGFα mice (the latter or which are prone to undergo spontaneous mammary carcinogenesis) do not develop mammary tumors.15,16 Several carcinogenic actions have been attributed to LEP, including the modulation of cell cycle-regulatory proteins as cyclin D1 and G, cyclin-dependent kinase 2 (CDK2), cyclin-dependent kinase inhibitor 1A (CDKN1A, best known as p21CIP1), CDKN1B (best known as p27KIP1), and CDKN2A (best known as p16INK4A) and transforming growth factor β1 (TGFβ1), resulting in the acceleration of cancer cell growth.17,18 LEP also sustains angiogenesis. For instance, human MCF7 and MDA-MB-231 breast carcinoma cells exposed to LEP secrete increased levels of vascular endothelial growth factor (VEGF) and are more invasive than their untreated counterparts.19 Finally, LEP signaling not only induces the expression of the anti-apoptotic proteins BCL-2 and survivin,17 but also limits the reduces the activity of caspases.20
The circulating levels of LEP in RCC patients have been the primary focus of numerous epidemiological studies highlighting the strong correlation between elevated LEP concentrations and increased risk of RCC.21 The LEPR is expressed in the renal tissue as well as in RCC cell lines, supporting a role for LEP signaling in RCC carcinogenesis. Interestingly, increased circulating levels of LEP and the overexpression of LEPRs are both associated with the invasion and progression in human RCC.22,23 Finally, Li and colleagues have recently demonstrated that LEP stimulates cell proliferation and promotes the invasion and migration capabilities of RCC Caki2 cells upon the activation of both extracellular signal-regulated kinase (ERK) and JAK/STAT3 signaling pathways.24
Adiponectin is normally produced by the AT, almost exclusively by mature adipocytes, and is an abundant circulating protein.25 Structurally, adiponectin is a polypeptide of 247 amino acids bearing an N-terminal collagen-like domain and a C-terminal globular domain. The globular domain shares significant homology with the subunits of the complement factor C1q. So far, 2 adiponectin receptors have been shown to share some homology with G protein-coupled receptors, namely, adiponectin receptor 1 (ADIPOR1) and ADIPOR2, and one to belong to the cadherin protein family, i.e., cadherin 13 (CDH13).
The study of the link between adiponectin and increased risk of oncogenesis or tumor progression led to the understanding that LEP and adiponectin always have antagonist properties.26 Several mechanisms underlying the anticarcinogenic effects of adiponectin have been described.27 Wang and colleagues showed that adiponectin significantly attenuates the proliferation of breast cancer cells by reducing the expression of cyclin D1.28 Experiments in nude mice demonstrated that both the supplementation of recombinant adiponectin and the adenovirus-mediated overexpression of this adipokine modulate the glycogen synthase kinase-3β (GSK3β)/β-catenin signaling pathway and substantially reduce the growth of MDA-MB-231 breast cancer cells in vivo.28 Adiponectin has also anti-angiogenic effects as it inhibits the proliferation and migration of endothelial cells.29,30 Additional in vitro studies revealed that adiponectin is able to limit cell growth and stimulate apoptosis by inhibiting the activation of nuclear factor-κB (NF-κB), resulting in the downregulation of BCL-2 expression.31 Finally, adiponectin can interfere with cancer progression by exerting anti-inflammatory effects through the inhibition of TNFα. Adiponectin-deficient mice manifest indeed increased levels of the TNFα-coding mRNA in the AT and higher TNFα concentrations in the plasma as compared with wild-type mice.32
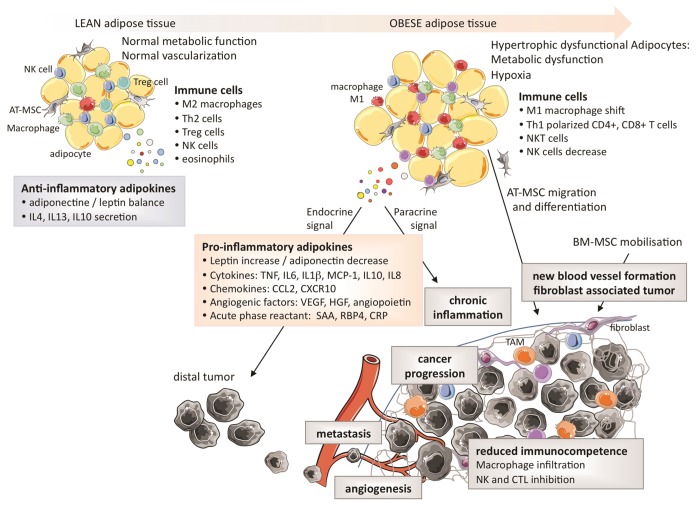
Several studies have demonstrated that adiponectin levels are reduced in RCC patients, and also that adiponectin levels inversely correlate with tumor size.21,31 Furthermore, a reduction in levels of ADIPOR2 has been associated with an increased metastatic potential.34 The protective effects of adiponectin on tumorigenesis may be mediated by the downstream effectors of ADIPOR1 and ADIPOR2, incuding the AMP-activated protein kinase (AMPK) and c-Jun N-terminal kinase 1 (JNK1), both of which can limit cell proliferation, trigger apoptosis and inhibit inflammation. Although adiponectin is downregulated in dysfunctional AT, LEP is upregulated in the very same adipocytes, suggesting that a functional crosstalk between these 2 adipokines may impact oncogenesis and tumor progression. The effects of the dysfunctional AT in obesity and their link to tumorigenesis are summarized in Figure 2.
Figure 2. Characteristics of the adipose tissue in leanness and obesity and their impact on the tumor microenvironment. The secretion of pro-inflammatory cytokines by adipocytes favor the expansion of tumor-infiltrating macrophages and limit immunocompetence.
Obesity associated inflammation
Obesity is associated with the secretion of high amounts of pro-inflammatory cytokines, which generate a low-grade chronic inflammatory state. Paradoxically, inflammation is associated with both tumor suppression and tumor progression. Inflammation is indeed required for the host immune system to kill cancer cells, yet chronic inflammation has been shown to promote cancer progression. Thus, the chronic inflammatory state sustained by adipocytes may modulate the host immunosurveillance36 and therefore exert a direct impact on both the local tumor microenvironment and on distant tumor cells (through the systemic effects of endocrine signals).37,38 This state of chronic inflammation is due (at least in part) to the accumulation of immune cells into the dysfunctional AT, macrophages constituting a large fraction of such an infiltrate.39 Moreover, the expanding AT produces high levels of pro-inflammatory cytokines, including TNFα, IL-6, IL-1β and chemokine (C-C motif) ligand 2 (CCL2, best known as MCP1),40 and high levels of acute phase reactants, i.e., proteins that are released into the circulation in response to local inflammatory processes such as serum amyloid A (SAA), retinol binding protein 4, plasma (RBP4),28 and C-reactive protein (CRP). Finally, adipocytes can support chronic inflammation by expressing or producing a multitude of additional proteins such as intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1), chemokine C-X-C motif ligand 10 (CXCL10), IL-8, IL-10, and multiple adipokines.39
Inflammatory responses play a decisive role at different stages of tumor development, including initiation, progression, malignant conversion, invasion, and metastasis. Markers of inflammation are associated with an increased risk of recurrence upon RCC surgical resection.41 Inflammatory cytokines are detected in the plasma of RCC patients and are associated with poor prognosis. In line with this notion, the treatment of RCC cells with IL-6 and IL-8 enhances their invasive potential.42 Interestingly, RCCs are not highly infiltrated by macrophages. This implies that the pro-inflammatory cytokines detected in RCC patients may be generated by the expanded AT, acting as an important initiator of oncogenesis.
Adipose tissue angiogenesis and hypoxia
The hypertrophic expansion of the AT that accompanies obesity can trigger local hypoxia, which is one of the most prominent microenvironmental stimuli for the metastatic dissemination of previously localized tumors.43,44 The master regulator of oxygen homeostasis is hypoxia-inducible factor 1α (HIF-1α). The levels of HIF-1α are increased in the AT of obese patients while its expression is reduced upon surgery-induced weight loss.45 HIF-1α stimulates inflammation and angiogenesis by deregulating the production of TNFα, VEGF, and angiopoietin. The dysfunctional AT can also enhance angiogenesis by secreting cytokines with angiogenic activities such as LEP, TNFα, IL-6, IL-8, VEGF, and hepatocyte growth factor (HGF),46,47 as well as by producing limited amounts of anti-angiogenic proteins such as adiponectin. Kleinman et al. showed that the exposure of RCC cells to adiponectin inhibits the secretion of VEGF, matrix metallopeptidase (MMP)2 and MMP9, resulting in the suppression of their invasive and migratory capacities.35 ccRCC is well known for its intense vascularity and high expression levels of angiogenic factors. HIF-1α plays an important role in ccRCC originating from alterations of von Hippel–Lindau tumor suppressor, E3 ubiquitin protein ligase (VHL), an important oncogenic event driving the development of 75% of ccRCC.48 An altered balance between pro- and anti-angiogenesis mediators may contribute to the increased risk of metastatic disease in obese subjects with RCC. This may have implications for the use of anti-angiogenic agents in RCC patients. Indeed, Ladoire et al. have recently shown that an expansion of visceral fat is associated with poor clinical outcome in RCC patients treated with anti-angiogenic agents.49
Adipose tissue and mesenchymal stromal cells
The human AT is an important source of mesenchymal stromal cells (MSCs). These cells substantially affect the tumor biology due to their ability to accumulate within the tumor stroma and to exert multiple regulatory functions in the tumor microenvironment.50,51 AT-derived MSCs share a number of key characteristics with bone marrow-derived MSCs.52 MSCs promote the proliferation of endothelial cells and the formation of new blood vessels,53 the survival and migration of malignant cells, as well as the so-called epithelial-to-mesenchymal transition (EMT).13 The accumulation of AT-derived MSCs to neoplastic lesions has been demonstrated by several studies. These cells which expand in obese individuals, may be mobilized in response to tumor-derived signals to sustain angiogenesis, hence contributing to disease progression.
Adipose tissue and the EMT transition
Over the past decade, accumulating evidence has shown that epithelial cancer cells can convert themselves into migratory mesenchymal cells. This phenotypic and behavioral shift, known as the EMT, might explain how epithelial cancer cells escape their primary anatomical location and migrate to form micrometastases in distant tissues. During the EMT, cancer cells lose epithelial characteristics, including polarization and the engagement in specialized cell-to-cell contacts, and acquire a migratory behavior. All murine and human cancer cells co-cultured with mature adipocytes exhibit increased invasive capacities in vitro and in vivo.54 In RCC, the AT may promote the EMT via multiple factors, including TNFα. The treatment of RCC cell lines with TNFα increases the expression of MMP9 and vimentin while downregulating cell-to-cell adhesion proteins such as E-cadherin, thus increasing their invasiveness and metastatic potential.55
Adipose tissue as a source of energy for cancer cells
The expanded AT constitutes not only a microenvironment that is favorable for oncogenesis as it produces and secretes pro-inflammatory cytokines,44 but also as an energy source driving the survival and proliferation of malignant cells (which generally exhibit a very intense metabolic activity). Interestingly, Nieman et al. have shown that adipocytes are able to directly transfer lipids to co-cultured ovarian cancer cells, promoting their growth in vitro and in vivo. In this setting, adipocytes manifested high levels of lipolysis while malignant cells mainly engaged in β-oxidation, suggesting that the former can act as an energy source for the latter.56 This mechanism may not be limited to the ovarian cancer setting, and may provide a rationale for the growth of malignant cells that form metastasis in the abdomen or other adipocyte-rich environments, including RCC cells.
Effects of Adipokines on Immune Cells and Antitumor Immunity
By altering the immune infiltrate and by secreting pro-inflammatory cytokines, the dysfunctional AT is known to modulate immunocompetence in humans. Several studies have found that obesity impairs natural killer (NK) cell-dependent immunity.57 NK cell activation is altered in obese rats,58 a functional defect that can be reversed by transferring them into lean animals.59 LEP differentially affects the functions of human NK cells. A short-term exposure to LEP has indeed a stimulatory effect, while long-term administration significantly impairs key NK-cell functions such as their cytotoxic potential, their ability to secrete cytokines, their proliferative potential, suggesting that LEP may increase the risk of RCC in obese people by inhibiting NK cell-dependent antitumor responses.60 The AT may also interfere with the functions of NK cells via adiponectin. Adiponectin has indeed been shown to suppress the ability of IL-2 to promote NK-cell cytotoxicity, interferon γ (IFNγ) production, as well as the IFNγ-inducible expression of TNFα-related apoptosis inducing ligand (TRAIL) and FAS ligand (FASL).28
Adipokines can also modulate adaptive immune responses. LEP favors the secretion of pro-inflammatory cytokines such as TNFα and IL-6 by macrophages,61 stimulate the accumulation of IFNγ-producing TH1 polarized cells,62 and interestingly persuades dendritic cells (DCs), major antigen-presenting cells, to drive a TH1 response.63 In addition, LEP promotes the survival of T cells64 by modulating the expression of apoptotic proteins that inhibit stress-induced apoptosis.65 Finally, LEP modulates adaptive immune response by acting on regulatory T cells (Tregs). Freshly isolated human Tregs constitutively express high amounts of LEP and LEPR. Interestingly, LEP neutralization has been shown to reverse the anergic state of Tregs in some settings.66 This is consistent with the enhanced proliferation of Tregs observed in ob/ob or db/db mice. Although LEP seems to exert positive effects on adaptive T-cell responses, its specific activity on T cells targeting tumor-associated antigens is not known and its ability to stimulate the secretion of pro-inflammatory cytokines may favor the proliferation of tumor cells and the activation of immunosuppressive myeloid-derived suppressor cells. Furthermore, CD8+ T cells are not always associated with a good prognosis in cancer patients,67,68 and the activatory effects of LEP on potentially non-relevant or immunosuppressive CD8+ T cell subsets may explain the negative impact of obesity on RCC progression.
Also adiponectin interferes with adaptive immune responses, but the precise underlying mechanisms have not been precisely elucidated. Adiponectin promotes the synthesis of anti-inflammatory cytokines such as IL-10 and IL-1 receptor antagonist (ILRA) by human monocytes, macrophages and DCs while it suppresses the production of IFNγ and TNFα by lipopolysaccharide (LPS)-stimulated human macrophages.31,69 Tsang et al. reported that adiponectin downregulates the expression of co-stimulatory molecules while increasing that inhibitory ones on DCs, resulting in the accumulation of CD4+CD25+FOXP3+ Tregs.70 Conversely, adiponectin can mediate a pro-inflammatory effects by stimulating the secretion IL-12 and IL-6. Recently, Jung et al. have reported that adiponectin activates DCs, resulting in the elicitation of TH1 and TH17 responses.71
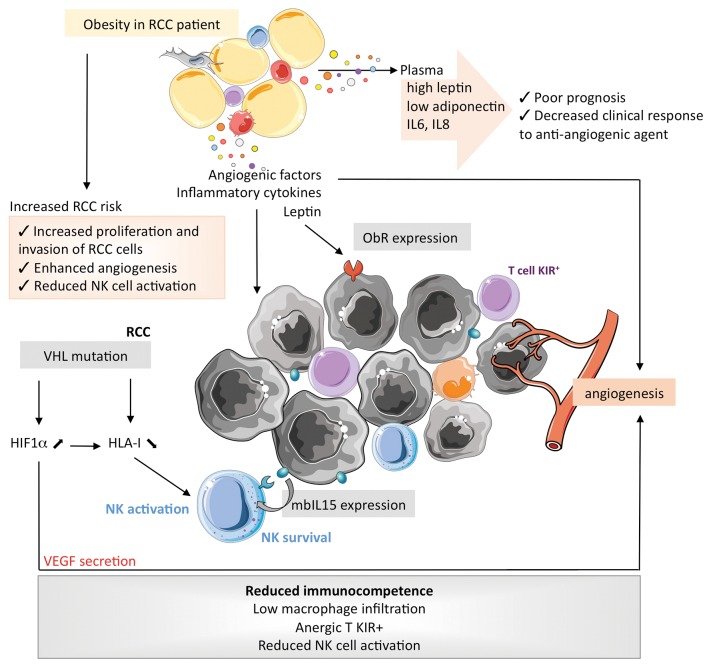
In Figure 3, we depict how the alterations of obesity-associated AT may interfere with the immune cells that infiltrate ccRCC, taking into account their particular immunological features. Renal tumors express tumor-associated antigens that induce limited T-cell activation in vivo,72,73 and the tumor-infiltrating lymphocytes that recognize self antigen are driven into anergy by the expression of inhibitory NK receptors.74,75 Conversely, NK cells participate in the immune response against RCC in a peculiar manner: (1) the percentage of NK cells infiltrating RCCs allow for subgrouping independently of the tumor-node-metastasis (TNM) classification;76 (2) cytokine-activated NK cells efficiently lyse RCC cell lines,77,78 (3) the loss-of-function of VHL results in the reduced expression of MHC class I molecules, hence favoring the activation of NK cells;79 and (4) the expression of IL-15 on the membrane of RCC cells modulates the survival of NK cells and probably maintains activated NK cells within neoplastic lesions.80 Taken together, these observations call for additional studies to understand the role of LEP in the RCC microenvironment.
Figure 3. Specific impact of obesity on the proliferation of clear cell renal cell carcinoma and the characteristics of its microenvironment. HIF1α, hypoxia-inducible factor 1α; IL, interleukin; IL15R, IL-15 receptor; KIR, killer-cell immunoglobulin-like receptor; mb, membrane-bound; NK, natural killer; ObR, obesity (leptin) receptor; RCC, renal cell carcinoma; s, soluble; VHL, von Hippel-Lindau tumor suppressor, E3 ubiquitin protein ligase.
Conclusions
Despite substantial advances in the treatment of localized RCC, disease recurrence and metastasis remain the primary cause of cancer-related mortality in this setting. It becomes now increasingly clear that metabolic changes ensuing an altered energy status and the secretion of endocrine factors facilitate oncogenesis. Malignant lesions need to escape the immune system and to rely on an abundant energy source to grow and form metastases. The hypertrophic expansion of the AT in the course of obesity can provide such an energy source and also modulate antitumor immune response. Progress in understanding the pivotal role of the AT in the tumor-immune system conflict is crucial for identifying early changes in the tumor microenvironment that contribute to malignant progression and could provide a rationale for the development of novel anticancer therapies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is supported by CMCU grant 13G0819
Glossary
Abbreviations:
- AT
adipose tissue
- BMI
body mass index
- ccRCC
clear cell RCC
- DC
dendritic cell
- EMT
epithelial-to-mesenchymal transition
- HIF-1α
hypoxia-inducible factor 1α
- IFN
interferon
- NK
natural killer
- RCC
renal cell carcinoma
- VHL
von Hippel-Lindau tumor suppressor, E3 ubiquitin protein ligase
Citation: Gati A, Kouidhi S, Marrakchi R, El Gaaied A, Kourda N, Amine D, Mohamed C, Caignard A, Perier A. Obesity and renal cancer: Role of adipokines in the tumor-immune system conflict. OncoImmunology 2014; 3:e27810; 10.4161/onci.27810
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27810
References
- 1.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Dhote R, Thiounn N, Debré B, Vidal-Trecan G. Risk factors for adult renal cell carcinoma. Urol Clin North Am. 2004;31:237–47. doi: 10.1016/j.ucl.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Duan XF, Tang P, Li Q, Yu ZT. Obesity, adipokines and hepatocellular carcinoma. Int J Cancer. 2013;133:1776–83. doi: 10.1002/ijc.28105. [DOI] [PubMed] [Google Scholar]
- 4.McGuire BB, Fitzpatrick JM. BMI and the risk of renal cell carcinoma. Curr Opin Urol. 2011;21:356–61. doi: 10.1097/MOU.0b013e32834962d5. [DOI] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Dal Maso L, Zucchetto A, Tavani A, Montella M, Ramazzotti V, Talamini R, Canzonieri V, Garbeglio A, Negri E, Tonini A, et al. Renal cell cancer and body size at different ages: an Italian multicenter case-control study. Am J Epidemiol. 2007;166:582–91. doi: 10.1093/aje/kwm108. [DOI] [PubMed] [Google Scholar]
- 7.Lowrance WT, Thompson RH, Yee DS, Kaag M, Donat SM, Russo P. Obesity is associated with a higher risk of clear-cell renal cell carcinoma than with other histologies. BJU Int. 2010;105:16–20. doi: 10.1111/j.1464-410X.2009.08706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Costa WH, Moniz RR, da Cunha IW, Fonseca FP, Guimaraes GC, de Cássio Zequi S. Impact of renal vein invasion and fat invasion in pT3a renal cell carcinoma. BJU Int. 2012;109:544–8. doi: 10.1111/j.1464-410X.2011.10366.x. [DOI] [PubMed] [Google Scholar]
- 9.Choi Y, Park B, Jeong BC, Seo SI, Jeon SS, Choi HY, Adami HO, Lee JE, Lee HM. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer. 2013;132:625–34. doi: 10.1002/ijc.27639. [DOI] [PubMed] [Google Scholar]
- 10.Rogde AJ, Gudbrandsdottir G, Hjelle KM, Sand KE, Bostad L, Beisland C. Obesity is associated with an improved cancer-specific survival, but an increased rate of postoperative complications after surgery for renal cell carcinoma. Scand J Urol Nephrol. 2012;46:348–57. doi: 10.3109/00365599.2012.678382. [DOI] [PubMed] [Google Scholar]
- 11.Imagawa K, Numata Y, Katsuura G, Sakaguchi I, Morita A, Kikuoka S, Matumoto Y, Tsuji T, Tamaki M, Sasakura K, et al. Structure-function studies of human leptin. J Biol Chem. 1998;273:35245–9. doi: 10.1074/jbc.273.52.35245. [DOI] [PubMed] [Google Scholar]
- 12.Basak S, Duttaroy AK. Leptin induces tube formation in first-trimester extravillous trophoblast cells. Eur J Obstet Gynecol Reprod Biol. 2012;164:24–9. doi: 10.1016/j.ejogrb.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Newman G, Gonzalez-Perez RR. Leptin-cytokine crosstalk in breast cancer. Mol Cell Endocrinol. 2013 doi: 10.1016/j.mce.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman G, Gonzalez-Perez RR. Leptin-cytokine crosstalk in breast cancer. Mol Cell Endocrinol. 2014;382:570–82. doi: 10.1016/j.mce.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleary MP, Phillips FC, Getzin SC, Jacobson TL, Jacobson MK, Christensen TA, Juneja SC, Grande JP, Maihle NJ. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003;77:205–15. doi: 10.1023/A:1021891825399. [DOI] [PubMed] [Google Scholar]
- 16.Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med (Maywood) 2004;229:182–93. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 17.Perera CN, Chin HG, Duru N, Camarillo IG. Leptin-regulated gene expression in MCF-7 breast cancer cells: mechanistic insights into leptin-regulated mammary tumor growth and progression. J Endocrinol. 2008;199:221–33. doi: 10.1677/JOE-08-0215. [DOI] [PubMed] [Google Scholar]
- 18.Eto I. Expression of p27Kip1, a cell cycle repressor protein, is inversely associated with potential carcinogenic risk in the genetic rodent models of obesity and long-lived Ames dwarf mice. Metabolism. 2013;62:873–87. doi: 10.1016/j.metabol.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caldefie-Chézet F, Dubois V, Delort L, Rossary A, Vasson MP. [Leptin: Involvement in the pathophysiology of breast cancer] Ann Endocrinol (Paris) 2013;74:90–101. doi: 10.1016/j.ando.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Ptak A, Kolaczkowska E, Gregoraszczuk EL. Leptin stimulation of cell cycle and inhibition of apoptosis gene and protein expression in OVCAR-3 ovarian cancer cells. Endocrine. 2013;43:394–403. doi: 10.1007/s12020-012-9788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao LM, Schwartz K, Pollak M, Graubard BI, Li Z, Ruterbusch J, Rothman N, Davis F, Wacholder S, Colt J, et al. Serum leptin and adiponectin levels and risk of renal cell carcinoma. Obesity (Silver Spring) 2013;21:1478–85. doi: 10.1002/oby.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horiguchi A, Sumitomo M, Asakuma J, Asano T, Zheng R, Asano T, Nanus DM, Hayakawa M. Leptin promotes invasiveness of murine renal cancer cells via extracellular signal-regulated kinases and rho dependent pathway. J Urol. 2006;176:1636–41. doi: 10.1016/j.juro.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 23.Horiguchi A, Sumitomo M, Asakuma J, Asano T, Zheng R, Asano T, Nanus DM, Hayakawa M. Increased serum leptin levels and over expression of leptin receptors are associated with the invasion and progression of renal cell carcinoma. J Urol. 2006;176:1631–5. doi: 10.1016/j.juro.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Gao Y, Zhang LL, He DL. Concomitant activation of the JAK/STAT3 and ERK1/2 signaling is involved in leptin-mediated proliferation of renal cell carcinoma Caki-2 cells. Cancer Biol Ther. 2008;7:1787–92. doi: 10.4161/cbt.7.11.6837. [DOI] [PubMed] [Google Scholar]
- 25.Mao X, Hong JY, Dong LQ. The adiponectin signaling pathway as a novel pharmacological target. Mini Rev Med Chem. 2006;6:1331–40. doi: 10.2174/138955706778992978. [DOI] [PubMed] [Google Scholar]
- 26.Scheid MP, Sweeney G. The role of adiponectin signaling in metabolic syndrome and cancer. Rev Endocr Metab Disord. 2013 doi: 10.1007/s11154-013-9265-5. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 27.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–5. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KY, Kim JK, Han SH, Lim JS, Kim KI, Cho DH, Lee MS, Lee JH, Yoon DY, Yoon SR, et al. Adiponectin is a negative regulator of NK cell cytotoxicity. J Immunol. 2006;176:5958–64. doi: 10.4049/jimmunol.176.10.5958. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–7. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 30.Man K, Ng KT, Xu A, Cheng Q, Lo CM, Xiao JW, Sun BS, Lim ZX, Cheung JS, Wu EX, et al. Suppression of liver tumor growth and metastasis by adiponectin in nude mice through inhibition of tumor angiogenesis and downregulation of Rho kinase/IFN-inducible protein 10/matrix metalloproteinase 9 signaling. Clin Cancer Res. 2010;16:967–77. doi: 10.1158/1078-0432.CCR-09-1487. [DOI] [PubMed] [Google Scholar]
- 31.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–32. [PubMed] [Google Scholar]
- 32.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 33.Horiguchi A, Asano T, Asano T, Ito K, Sumitomo M, Hayakawa M. Fatty acid synthase over expression is an indicator of tumor aggressiveness and poor prognosis in renal cell carcinoma. J Urol. 2008;180:1137–40. doi: 10.1016/j.juro.2008.04.135. [DOI] [PubMed] [Google Scholar]
- 34.Pinthus JH, Kleinmann N, Tisdale B, Chatterjee S, Lu JP, Gillis A, Hamlet T, Singh G, Farrokhyar F, Kapoor A. Lower plasma adiponectin levels are associated with larger tumor size and metastasis in clear-cell carcinoma of the kidney. Eur Urol. 2008;54:866–73. doi: 10.1016/j.eururo.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 35.Kleinmann N, Duivenvoorden WC, Hopmans SN, Beatty LK, Qiao S, Gallino D, Lhotak S, Daya D, Paschos A, Austin RC, et al. Underactivation of the adiponectin-adiponectin receptor 1 axis in clear cell renal cell carcinoma: implications for progression. Clin Exp Metastasis. 2013 doi: 10.1007/s10585-013-9618-1. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 36.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Meijer K, de Vries M, Al-Lahham S, Bruinenberg M, Weening D, Dijkstra M, Kloosterhuis N, van der Leij RJ, van der Want H, Kroesen BJ, et al. Human primary adipocytes exhibit immune cell function: adipocytes prime inflammation independent of macrophages. PLoS One. 2011;6:e17154. doi: 10.1371/journal.pone.0017154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci. 2011;1229:45–52. doi: 10.1111/j.1749-6632.2011.06096.x. [DOI] [PubMed] [Google Scholar]
- 41.Ohno Y, Nakashima J, Ohori M, Hatano T, Tachibana M. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol. 2010;184:873–8. doi: 10.1016/j.juro.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgerald JP, Nayak B, Shanmugasundaram K, Friedrichs W, Sudarshan S, Eid AA, DeNapoli T, Parekh DJ, Gorin Y, Block K. Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8- production. PLoS One. 2012;7:e30712. doi: 10.1371/journal.pone.0030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 44.Catalán V, Gómez-Ambrosi J, Rodríguez A, Frühbeck G. Adipose tissue immunity and cancer. Front Physiol. 2013;4:275. doi: 10.3389/fphys.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–86. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 46.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005:209–31. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 47.Gómez-Ambrosi J, Catalán V, Rodríguez A, Ramírez B, Silva C, Gil MJ, Salvador J, Frühbeck G. Involvement of serum vascular endothelial growth factor family members in the development of obesity in mice and humans. J Nutr Biochem. 2010;21:774–80. doi: 10.1016/j.jnutbio.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 48.George DJ, Kaelin WG., Jr. The von Hippel-Lindau protein, vascular endothelial growth factor, and kidney cancer. N Engl J Med. 2003;349:419–21. doi: 10.1056/NEJMp030061. [DOI] [PubMed] [Google Scholar]
- 49.Ladoire S, Bonnetain F, Gauthier M, Zanetta S, Petit JM, Guiu S, Kermarrec I, Mourey E, Michel F, Krause D, et al. Visceral fat area as a new independent predictive factor of survival in patients with metastatic renal cell carcinoma treated with antiangiogenic agents. Oncologist. 2011;16:71–81. doi: 10.1634/theoncologist.2010-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, Andreeff M, Marini FC. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One. 2012;7:e30563. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin MG. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259–66. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Bellows CF, Kolonin MG. Adipose tissue-derived progenitor cells and cancer. World J Stem Cells. 2010;2:103–13. doi: 10.4252/wjsc.v2.i5.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–9. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 54.Kushiro K, Chu RA, Verma A, Núñez NP. Adipocytes Promote B16BL6 Melanoma Cell Invasion and the Epithelial-to-Mesenchymal Transition. Cancer Microenviron. 2012;5:73–82. doi: 10.1007/s12307-011-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho MY, Tang SJ, Chuang MJ, Cha TL, Li JY, Sun GH, Sun KH. TNF-α induces epithelial-mesenchymal transition of renal cell carcinoma cells via a GSK3β-dependent mechanism. Mol Cancer Res. 2012;10:1109–19. doi: 10.1158/1541-7786.MCR-12-0160. [DOI] [PubMed] [Google Scholar]
- 56.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dovio A, Caramello V, Masera RG, Sartori ML, Saba L, Tinivella M, Prolo P, Termine A, Avagnina P, Angeli A. Natural killer cell activity and sensitivity to positive and negative modulation in uncomplicated obese subjects: relationships to leptin and diet composition. Int J Obes Relat Metab Disord. 2004;28:894–901. doi: 10.1038/sj.ijo.0802639. [DOI] [PubMed] [Google Scholar]
- 58.Nave H, Mueller G, Siegmund B, Jacobs R, Stroh T, Schueler U, Hopfe M, Behrendt P, Buchenauer T, Pabst R, et al. Resistance of Janus kinase-2 dependent leptin signaling in natural killer (NK) cells: a novel mechanism of NK cell dysfunction in diet-induced obesity. Endocrinology. 2008;149:3370–8. doi: 10.1210/en.2007-1516. [DOI] [PubMed] [Google Scholar]
- 59.Lautenbach A, Budde A, Wrann CD, Teichmann B, Vieten G, Karl T, Nave H. Obesity and the associated mediators leptin, estrogen and IGF-I enhance the cell proliferation and early tumorigenesis of breast cancer cells. Nutr Cancer. 2009;61:484–91. doi: 10.1080/01635580802610115. [DOI] [PubMed] [Google Scholar]
- 60.Wrann CD, Laue T, Hübner L, Kuhlmann S, Jacobs R, Goudeva L, Nave H. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am J Physiol Endocrinol Metab. 2012;302:E108–16. doi: 10.1152/ajpendo.00057.2011. [DOI] [PubMed] [Google Scholar]
- 61.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–46. [PubMed] [Google Scholar]
- 62.Lord GM, Matarese G, Howard JK, Bloom SR, Lechler RI. Leptin inhibits the anti-CD3-driven proliferation of peripheral blood T cells but enhances the production of proinflammatory cytokines. J Leukoc Biol. 2002;72:330–8. [PubMed] [Google Scholar]
- 63.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–8. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 64.Fernández-Riejos P, Goberna R, Sánchez-Margalet V. Leptin promotes cell survival and activates Jurkat T lymphocytes by stimulation of mitogen-activated protein kinase. Clin Exp Immunol. 2008;151:505–18. doi: 10.1111/j.1365-2249.2007.03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujita Y, Murakami M, Ogawa Y, Masuzaki H, Tanaka M, Ozaki S, Nakao K, Mimori T. Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin Exp Immunol. 2002;128:21–6. doi: 10.1046/j.1365-2249.2002.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–55. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res. 2013;19:4079–91. doi: 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]
- 68.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–38. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 69.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–5. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 70.Tsang JY, Li D, Ho D, Peng J, Xu A, Lamb J, Chen Y, Tam PK. Novel immunomodulatory effects of adiponectin on dendritic cell functions. Int Immunopharmacol. 2011;11:604–9. doi: 10.1016/j.intimp.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 71.Jung MY, Kim HS, Hong HJ, Youn BS, Kim TS. Adiponectin induces dendritic cell activation via PLCγ/JNK/NF-κB pathways, leading to Th1 and Th17 polarization. J Immunol. 2012;188:2592–601. doi: 10.4049/jimmunol.1102588. [DOI] [PubMed] [Google Scholar]
- 72.Oudard S, Rixe O, Beuselinck B, Linassier C, Banu E, Machiels JP, Baudard M, Ringeisen F, Velu T, Lefrere-Belda MA, et al. A phase II study of the cancer vaccine TG4010 alone and in combination with cytokines in patients with metastatic renal clear-cell carcinoma: clinical and immunological findings. Cancer Immunol Immunother. 2011;60:261–71. doi: 10.1007/s00262-010-0935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–61. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 74.Gati A, Guerra N, Gaudin C, Da Rocha S, Escudier B, Lécluse Y, Bettaieb A, Chouaib S, Caignard A. CD158 receptor controls cytotoxic T-lymphocyte susceptibility to tumor-mediated activation-induced cell death by interfering with Fas signaling. Cancer Res. 2003;63:7475–82. [PubMed] [Google Scholar]
- 75.Guerra N, Guillard M, Angevin E, Echchakir H, Escudier B, Moretta A, Chouaib S, Caignard A. Killer inhibitory receptor (CD158b) modulates the lytic activity of tumor-specific T lymphocytes infiltrating renal cell carcinomas. Blood. 2000;95:2883–9. [PubMed] [Google Scholar]
- 76.Eckl J, Buchner A, Prinz PU, Riesenberg R, Siegert SI, Kammerer R, Nelson PJ, Noessner E. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J Mol Med (Berl) 2012;90:55–66. doi: 10.1007/s00109-011-0806-7. [DOI] [PubMed] [Google Scholar]
- 77.Gati A, Da Rocha S, Guerra N, Escudier B, Moretta A, Chouaib S, Angevin E, Caignard A. Analysis of the natural killer mediated immune response in metastatic renal cell carcinoma patients. Int J Cancer. 2004;109:393–401. doi: 10.1002/ijc.11730. [DOI] [PubMed] [Google Scholar]
- 78.Schleypen JS, Von Geldern M, Weiss EH, Kotzias N, Rohrmann K, Schendel DJ, Falk CS, Pohla H. Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int J Cancer. 2003;106:905–12. doi: 10.1002/ijc.11321. [DOI] [PubMed] [Google Scholar]
- 79.Perier A, Fregni G, Wittnebel S, Gad S, Allard M, Gervois N, Escudier B, Azzarone B, Caignard A. Mutations of the von Hippel-Lindau gene confer increased susceptibility to natural killer cells of clear-cell renal cell carcinoma. Oncogene. 2011;30:2622–32. doi: 10.1038/onc.2010.638. [DOI] [PubMed] [Google Scholar]
- 80.Wittnebel S, Da Rocha S, Giron-Michel J, Jalil A, Opolon P, Escudier B, Validire P, Khawam K, Chouaib S, Azzarone B, et al. Membrane-bound interleukin (IL)-15 on renal tumor cells rescues natural killer cells from IL-2 starvation-induced apoptosis. Cancer Res. 2007;67:5594–9. doi: 10.1158/0008-5472.CAN-06-4406. [DOI] [PubMed] [Google Scholar]