Abstract
Weight loss is associated with bone loss and the risk may be greater in lean than heavier individuals, but the mechanisms involved remain unclear. We hypothesized that energy restriction (EnR) would decrease true fractional Ca absorption (TFCA) and be mediated by Ca-regulating hormones, but differently in obese and lean rats. Rats were fed a high fat (47% energy) or low fat (16% energy) diet for 4 mo. At 6 mo of age, the resulting lean [284 ± 28g (mean ± sd, n = 18)] and obese (319 ± 34g, n = 20) groups (P < 0.005) were divided into controls (CTL, ad libitum) and energy-restricted (40% restriction) groups. At baseline, bone resorption (urinary crosslinks) was higher and bone formation (serum osteocalcin) was lower in obese than in lean rats, whereas Ca balance components and Ca-regulating hormones did not differ. EnR for 10 wk reduced body weight by 25 ± 7% compared with a 6 ± 6% gain in CTL rats (P < 0.001). For both lean and obese rats, TFCA (5-d measurement, 45Ca radioisotope) decreased from 30 ± 9% to 24 ± 9% with EnR, compared with 25 ± 10% to 29 ± 11% in controls (P < 0.05). Weight loss was directly correlated with the decrease in TFCA (r = 0.34, P < 0.05). Uterine weights indicated a reduced estrogenic activity in energy-restricted rats (P < 0.0001). In lean, but not obese rats, serum estradiol (E2) correlated with weight loss (r = 0.52, P < 0.05), and tended to correlate with the decrease in TFCA (r = 0.48, P = 0.06). At the end of the study, serum 25-hydroxyvitamin-D was lower and urinary Ca was higher in lean than obese energy-restricted rats. Distinct endocrine profiles during weight loss in obese and lean rats suggest that the susceptibility of bone and Ca metabolism to EnR could differ depending on initial body weight.
Keywords: absorption, calcium, estrogen, rats, weight loss
Bone loss has been associated with weight reduction in humans (1–4) and in animal models (5–7), and may be greater in leaner subjects (3,8). However, the mechanisms regulating bone mobilization because of energy restriction (EnR)4 and the importance of initial body weight remain unclear.
Bone-regulating hormones such as 1,25 dihydroxy-vitamin D, parathyroid hormone (PTH) and estradiol (E2) may be altered during EnR (5,9,10) and thus be associated with changes in Ca absorption. For example, there is evidence that vitamin D accumulates in adipose tissue, and its release during weight loss may increase circulating levels (11). Serum PTH may rise with weight reduction (9), and the increased Ca-PTH axis activity may be driven by a decrease in Ca absorption due to EnR. In addition, intestinal Ca absorption is influenced by serum E2 (12–14), and E2 may decline with EnR (5,6), but the relationship between EnR, E2 and Ca absorption is not known. Finally, there may also be an association between weight loss, increased glucocorticoids and decreased calcium absorption (15). We hypothesized that EnR in mature lean and obese rats would decrease Ca absorption and that this could be explained by changes in bone-regulating hormones.
MATERIALS AND METHODS
Animals
Female Sprague-Dawley rats (n = 42; 2 mo old) were obtained from Taconic Farms (Taconic Farms, MD). Rats were housed in individual wire-bottomed cages and maintained on a 12-h light:dark cycle with a constant room temperature. Throughout the study, rats were weighed weekly using a balance scale. All procedures were approved by the Rutgers University Institutional Review Board for the Use and Care of Animals.
Diets
Throughout the study, rats had free access to tap water. Four purified diets (Table 1) containing 0.5% calcium and 0.3% phosphorus, levels shown to be sufficient for normal growth and bone mineralization (16), were designed specifically for these experiments (Research Diets, New Brunswick, NJ). During growth, rats consumed ad libitum either a control diet (CTL-G, 16% fat energy) based on AIN-93G (17) or a matching high fat diet (HF, 47% fat energy) to produce obese rats. At 6 mo of age, weight-matched rats were fed a control diet for mature rats (CTL-M), based on AIN-93M (17) or a 40% EnR diet. Energy restriction was accomplished by reducing the carbohydrate content and pair-feeding a reduced quantity of the EnR diets. Daily intakes of protein, fat, fiber, vitamins and minerals were the same in both diet groups
TABLE 1.
| Ingredient | CTL-G | HF | CTL-M | Energy restriction |
|---|---|---|---|---|
| g | ||||
| Casein | 200 | 200 | 140 | 140 |
| l-Cystine | 3.0 | 3.0 | 1.8 | 1.8 |
| Cornstarch | 429.5 | 139.5 | 520.7 | 214.1 |
| Maltodextrin | 100 | 125 | 100 | 75 |
| Sucrose | 100 | 50 | 100 | 46.6 |
| Cellulose | 50 | 50 | 50 | 50 |
| Soybean oil | 17.5 | 52.5 | 10 | 10 |
| Lard | 52.5 | 157.5 | 30 | 30 |
| TBHQ | 0.014 | 0.042 | 0.008 | 0.008 |
| Mineral mix S10022G3 | 35 | 35 | 0 | 0 |
| Mineral mix S10022M4 | 0 | 0 | 35 | 35 |
| Vitamin mix V100375 | 10 | 10 | 10 | 10 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 |
| Total weight, g | 1000.1 | 825.1 | 1000.1 | 615.1 |
| Calcium, mg/g diet | 5.0 | 6.1 | 5.0 | 8.1 |
| Phosphorus, mg/g diet | 3.0 | 3.6 | 3.0 | 4.9 |
| Energy, kJ/g diet (kcal/g diet) | 16.7 (4.0) | 20.1 (4.8) | 15.9 (3.8) | 15.5 (3.7) |
| % Energy | ||||
| Carbohydrate | 63.6 | 31.7 | 75.6 | 59.1 |
| Protein | 20.5 | 20.5 | 14.9 | 25.0 |
| Fat | 15.9 | 47.8 | 9.5 | 15.9 |
Prepared by Research diets, Inc., New Brunswick, NJ.
Abbreviations: CTL-G, control diet during growth period; HF, high fat diet; CTL-M, control diet for mature rats; TBHQ, tert-butylhydroxyquinone.
The mineral mix composition (AIN-93G) (17) was as follows (amount in 35 g): 5.0 g Ca, 1.56 g P, 0.5 g Mg, 3.6 g K, 0.3 g S, 1.0 g Na, 1.6 g Cl, 6.0 mg Cu, 0.2 mg I, 45.0 mg Fe, 10.5 mg Mn, 0.2 mg Se and 30.0 mg Zn.
The mineral mix composition (AIN-93M) (17) was as follows (amount in 35 g): 5.0 g Ca, 2.0 g P, 0.5 g Mg, 3.6 g K, 0.3 g S, 1.0 g Na, 1.6 g Cl, 6.0 mg Cu, 0.2 mg I, 45.0 mg Fe, 10.5 mg Mn, 0.2 mg Se and 30.0 mg Zn.
The vitamin mixture composition (AIN-93) (17) was as follows (amount in 10 g): 4000 iu vitamin A palmitate, 1000 iu cholecalciferol, 75 iu vitamin E acetate, 0.75 mg phylloquinone, 0.2 mg biotin, 25 μg cyanocobalamin, 2 mg folic acid, 30 mg nicotinic acid, 16 mg calcium pantothenate, 7 mg pyridoxine-HCl, 6 mg riboflavin, 6 mg thiamin HCl.
Protocol
Initially, 2-mo-old rats consumed the high fat diet (HF) ad libitum for 2 wk to determine those rats most responsive to the HF diet. The 24 rats with the greatest body weight gain during these 2 wk were assigned to the “obese” group, and the remaining diet-resistant rats were assigned to the “lean” group. A 12-wk period of HF or CTL-G feeding followed, until rats were 6 mo old (considered the age of skeletal maturity). After wk 12, obese rats were switched to CTL-M diet and all rats were fed the same diet for 5 d, at which time baseline measurements were taken. From this point on, all control rats (lean and obese) consumed the same diet (CTL-M) ad libitum. To control for the effect of the estrus cycle on hormones and bone markers measured in blood, the cytology of vaginal smears was evaluated according to Salas-Valdes (18) in the early morning hours, thus determining the appropriate day for blood sampling for each rat (i.e., the day of estrus, as assessed by the appearance of cornified epithelial cells and a heavy and coarse consistency of the fluid). If rats failed to present the specific estrus smear characteristics at baseline, hormones and bone markers were not evaluated. Blood was collected during the week of Ca balance in the early afternoon between 1200 and 1400 h. After baseline measurements, each body size group was divided into two weight-matched groups which were assigned to either the CTL or the 40% EnR diet. This dietary treatment continued for 10 wk, when final measurements were obtained.
Biochemical analyses
Concentrations of E2 and 25-hydroxyvitamin D [25(OH)D] were measured in acetonitrile-extracted serum using a RIA (double antibody, DPC, Los Angeles, CA and DiaSorin, Stillwater, MN, respectively). Intact bioactive serum PTH was measured using a rat-specific RIA (Immutopics, San Clemente, CA). Corticosterone was analyzed in 24-h urine samples using a rat-specific RIA kit (ICN Biomedicals, Costa Mesa, CA). Urinary creatinine excretion (No. 555 Sigma Diagnostics, St Louis, MO) was measured using a spectrophotometric method (Microplate autoreader EL311, BioTek Instruments, λ = 575). All CV were <15% as reported by the manufacturers.
Uterus weights
As an indicator of estrogenic activity during chronic EnR, uteri were dissected and weighed on a balance scale at the time of killing.
Bone turnover
Bone resorption was assessed by total urinary pyridinium crosslinks, pyridinoline (PYD) and deoxypyridinoline (DPD), which were measured by HPLC after subjecting hydrolyzed samples to a prefractionation procedure (19). Peaks were detected by fluorescence (20) and quantitated by external standards. Values are expressed as 24-h excretions (CV of 8 and 13% for PYD and DPD, respectively). Bone formation was assessed in serum using rat-specific osteocalcin (OC) with an enzyme-linked immunoassay (Biomedical Technologies, Stoughton, MA) with a CV of <8%.
Intestinal calcium absorption
Calcium absorption and intestinal Ca metabolism (intestinal Ca secretion, endogenous and total fecal Ca) were measured according to O'Loughlin and Morris (21). Briefly, rats were placed in individual metabolic cages for a 4-d adaptation period. Rats were administered 2 MBq (0.054 mCi) of 45Ca intramuscularly on d 4 to monitor the secretion of endogenous Ca into the gut. Calcium balance was determined over a 5-d period (d 6–10). Daily food consumption was recorded by weighing any food remaining at the end of each 24-h period. After the 5-d balance period, complete urine and feces were collected. The entire 5-d fecal sample was ashed at 600°C for 18 h and dissolved in 3 mol/L HCl. The pH of urine samples was adjusted to <2 with HCl to avoid Ca precipitation. Fecal and urinary 45Ca were determined by liquid scintillation counting. Fecal and urinary total Ca concentrations (Catot) were determined using atomic absorption spectrometry.
Calculations
Calcium balance, endogenous fecal Ca and Ca absorption were calculated using the following equations (21):
Data analysis
Differences between obese and lean groups at baseline (6 mo old) were analyzed by one-way ANOVA. The effects of EnR were assessed using two-way ANOVA with dietary treatment and body size as the independent factors and significant interactions or trends (P < 0.1) were further analyzed by Tukey's post-hoc comparison tests. For values measured serially over time (i.e., body weight), three-way ANOVA with repeated measures over time was performed. Pearson's correlation coefficient was used to evaluate correlations between dependent variables. Differences with P-values ≤0.05 were considered significant. Data are means ± sd unless otherwise indicated. All analyses were conducted using the SAS statistical package (SAS Institute Cary, NC; version 8.0)
RESULTS
Baseline characteristics of lean and obese rats
Body weight and food intake
Four obese and two lean rats were deleted from the analysis because of death for unknown reasons (n = 1), erratic weight fluctuations (n = 4) and low food intake during the final balance period (n = 1). Seven of the 38 remaining rats did not show vaginal smears characteristic of estrus at baseline; thus, their serum metabolites were not measured. Rats allocated to the obese group were significantly heavier than their lean counterparts, and continued to be so throughout the experiment (Fig. 1). During growth, obese compared with lean rats consumed slightly more Ca (1.7 ± 0.3 and 1.5 ± 0.3 mmol/d, respectively) and energy (234 ± 38 and 201 ± 38 kJ/d, respectively) (P < 0.05). However, energy and Ca intakes did not differ between lean and obese rats during the EnR period (Table 2).
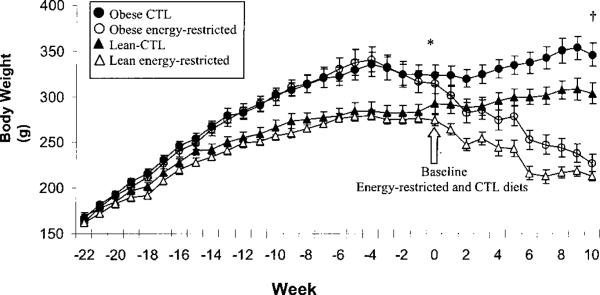
FIGURE 1.
Body weight of obese and lean rats during growth; rats consumed 40% energy-restricted (EnR) or control (CTL) diets. Each point represents mean ± sem in obese CTL (n = 10), obese EnR (n = 10), lean CTL (n = 9) and lean EnR (n = 9) groups. *Wk –22 to 0 represent the growth period [high fat (HF) or control diet]. Obese and lean rats differed significantly over time, P < 0.0001 three-way ANOVA with repeated measures over time. †EnR and CTL dietary treatment took place from wk 0 (baseline) to 10 (study conclusion). Body weight differed between obese and lean rats (P = 0.001) and between CTL and EnR groups (P < 0.0001), three-way ANOVA with repeated measures over time.
TABLE 2.
Body weight and daily food, energy and calcium intakes of obese and lean mature rats fed 40% energy-restricted (EnR) or control (CTL) diets for 10 wk1
| Obese |
Lean |
|||
|---|---|---|---|---|
| CTL | EnR | CTL | EnR | |
| n | 10 | 10 | 9 | 9 |
| Body weight | ||||
| Baseline,2g | 324 ± 34 | 315 ± 36 | 293 ± 34 | 275 ± 19 |
| Change,3% | 7.0 ± 5.8 | –27.4 ± 8.9* | 4.1 ± 5.2 | –22.2 ± 4.3* |
| Food, g/d | 15.2 ± 2.7 | 9.2 ± 1.7* | 14.9 ± 1.2 | 9.1 ± 0.6* |
| Energy, kJ/d | 244.8 ± 43.5 | 144.3 ± 26.8* | 239.3 ± 18.4 | 143.1 ± 10.0* |
| Calcium, mmol/d | 1.9 ± 0.3 | 1.9 ± 0.3 | 1.9 ± 0.1 | 1.8 ± 0.1 |
Values are means ± sd.
Differs from its control, CTL, P < 0.0001.
Significant size effect, one-way ANOVA, P < 0.01.
Significant diet effect, P < 0.0001; two-way ANOVA.
Calcium metabolism, bone turnover and biochemical analyses
None of the variables of Ca metabolism differed between the 6-mo-old obese and lean rats at baseline (Table 3). In obese rats, serum OC levels were lower, whereas 24-h urinary excretions of PYD and DPD were higher than in lean rats (Table 3). Urinary excretion of DPD remained higher in obese rats even after adjusting for body weight (P = 0.01) but this difference was not significant for PYD (P = 0.12). Body weight was directly correlated with PYD (r = 0.46, P < 0.005) and DPD (r = 0.57, P < 0.0005) excretion in all rats. Urinary corticosterone and creatinine excretions and serum PTH, E2 and 25(OH)D did not differ between groups.
TABLE 3.
| Obese | Lean | |
|---|---|---|
| Body weight, g | 319 ± 34.2 | 284 ± 28* |
| Urinary Ca, mmol/d | 0.06 ± 0.03 | 0.05 ± 0.02 |
| Fecal Ca, mmol/d | 1.32 ± 0.16 | 1.33 ± 0.14 |
| Ca balance, mmol/d | –0.04 ± 0.12 | 0.00 ± 0.16 |
| Net FCA,3% | 1.4 ± 8.0 | 3.4 ± 11.0 |
| TFCA, % | 25.4 ± 8.3 | 29.5 ± 11.3 |
| Endogenous fecal Ca, mmol/d | 0.32 ± 0.13 | 0.36 ± 0.14 |
| Intestinal Ca secretion, mmol/d | 0.44 ± 0.20 | 0.54 ± 0.28 |
| Osteocalcin, nmol/L | 10.3 ± 5.7 | 15.3 ± 4.6* |
| PYD, nmol/d | 3.1 ± 1.2 | 2.2 ± 0.7* |
| DPD, nmol/d | 1.7 ± 0.6 | 1.1 ± 0.4* |
| Creatinine, mmol/d | 0.09 ± 0.02 | 0.08 ± 0.02 |
| Corticosterone, nmol/d | 0.80 ± 0.23 | 0.69 ± 0.16 |
| PTH, pmol/L | 9.3 ± 4.2 | 7.8 ± 6.7 |
| E2, pmol/L | 46.6 ± 45.9 | 26.8 ± 16.9 |
| 25(OH) D, nmol/L | 84.9 ± 26.0 | 73.9 ± 24.0 |
Values are means ± sd, n = 20 obese and 18 lean, except for serum metabolites where n = 15 obese and 16 lean rats.
Different from obese, P < 0.05.
Blood samples: day of estrus determined by vaginal smears.
Abbreviations: net FCA, net fractional Ca absorption; TFCA, true fractional Ca absorption; urine: pyridinoline (PYD), deoxypyridinoline (DPD), creatinine and corticosterone; serum: osteocalcin, parathyroid hormone (PTH), estradiol (E2), 25-hydroxyvitamin [25(OH) D].
Energy restriction of lean and obese rats
Body weight and food intake
EnR rats consumed 40% less energy than weight-matched CTL rats (P < 0.0001) but the same amount of Ca (Table 2). EnR rats had lower body weights than CTL rats starting at 1 wk of treatment until the end of the experiment (Fig. 1, Table 2). EnR rats excreted less creatinine and this tended to be greater in lean than in obese rats (P < 0.08, Table 4).
TABLE 4.
Percentage change from baseline in serum hormones and urinary creatinine in obese and lean rats fed 40% energy restricted (EnR) or control (CTL) diets for 10 wk1
| Obese |
Lean |
P-value2 |
|||||
|---|---|---|---|---|---|---|---|
| CTL | EnR | CTL | EnR | Diet | Size | Diet × Size | |
| % Δ | |||||||
| PTH3 | –78.9 ± 21.2 | –19.0 ± 83.6 | –52.8 ± 30.4 | 29.8 ± 157.2 | 0.0415 | 0.2701 | 0.7353 |
| E2 | –9.8 ± 85.8 | 0.2 ± 92.7 | 48.9 ± 101.7 | –50.6 ± 30.1 | 0.1428 | 0.8946 | 0.0758 |
| 25(OH)D | –2.2 ± 41.9 | 13.9 ± 31.0 | –13.4 ± 24.7 | –16.4 ± 27.4 | 0.5687 | 0.0780 | 0.4095 |
| Corticosterone | 15.8 ± 34.6 | 160.0 ± 227.8 | 53.5 ± 37.1 | 140.6 ± 116.2 | 0.0111 | 0.8324 | 0.5116 |
| Creatinine | 6.5 ± 23.1ab | –2.0 ± 19.4ab | 30.0 ± 41.1a | –10.6 ± 20.4b | 0.0087 | 0.4044 | 0.0782 |
Values are means ± sd for obese CTL (n = 10 urine, n = 7 serum) and energy-restricted (n = 10 urine, n = 8 serum), and lean CTL (n = 9 urine, n = 8 serum) and energy-restricted (n = 9 urine, n = 8 serum) rats. Values in a row with different superscripts letters differ, P < 0.05.
P-value of two-way ANOVA.
Serum: parathyroid hormone (PTH), estradiol (E2), 25 hydroxyvitamin D (25(OH) D); 24-h urine: corticosterone, creatinine.
Calcium absorption
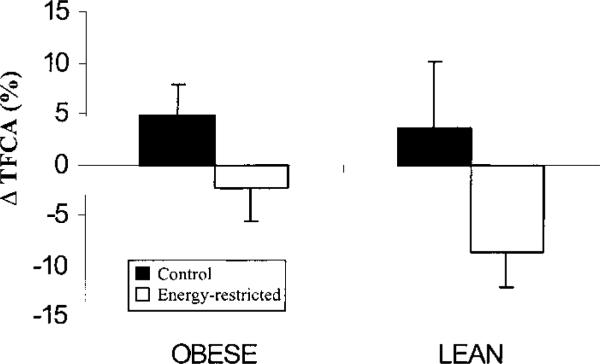
Energy restriction in lean and obese rats decreased true fractional Ca absorption (TFCA) from 29.9 ± 9.0 to 24.4 ± 8.8% compared with CTL rats who changed from 24.8 ± 10.3 to 29.0 ± 11.2% (P < 0.05, Fig. 2). Several other indices of Ca metabolism did not differ between EnR and CTL rats. However, Ca balance was lower in EnR (–0.08 ± 0.13 mmol/d) than in CTL rats (0.02 ± 0.15 mmol/d) at the end of the experiment, wk 10 (P < 0.05). In addition, urinary Ca loss was greater in EnR (0.12 ± 0.04 mmol/d) than in CTL rats (0.10 ± 0.02 mmol/d) (P < 0.05) and these losses were greater in lean (0.14 ± 0.05 mmol/d) than in obese (0.10 ± 0.03 mmol/d) EnR rats (P < 0.05).
FIGURE 2.
Difference (Δ) from baseline in true fractional Ca absorption (TFCA) in obese and lean mature rats fed 40% energy-restricted (n = 10 obese, n = 9 lean) or control (n = 10 obese, n = 9 lean) diets for 10 wk. Bars represent means ± sem of the net difference from baseline. P < 0.05 for diet effect by two-way ANOVA
Hormones
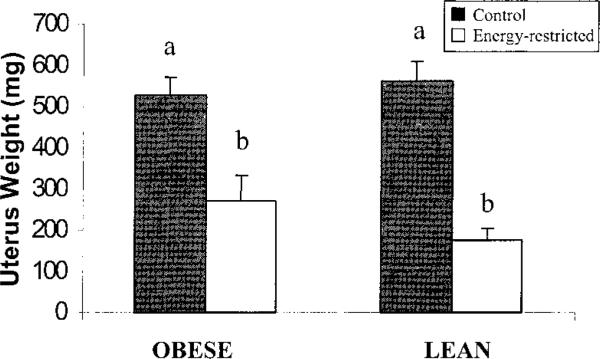
Ten weeks of EnR reduced estrogenic activity, as indicated by lower uterine weights than in controls (P < 0.0001, Fig. 3). Serum E2 tended to decrease more (P < 0.08) in lean than obese EnR rats compared with their controls (Table 4). Rats consuming food ad libitum had greater decreases in serum PTH (70%) than EnR rats (~30%) (P < 0.05, Table 4). Although there were no significant effects of diet on the changes in serum 25(OH)D (Table 4), at the end of the study there was an interaction (P < 0.05) in which absolute concentrations of 25(OH)D were higher in obese EnR than in lean EnR rats (99.1 ± 37.7 and 51.4 ± 7.5 nmol/L, respectively, P = 0.01). Excretion of corticosterone (24-h) increased more in EnR than in CTL rats (P = 0.01, Table 4).
FIGURE 3.
Uterine weights in obese and lean mature rats fed 40% energy-restricted (n = 10 obese, n = 9 lean) or control (n = 10 obese, n = 9 lean) diets for 10 wk. Bars represent means ± sem; P < 0.0001 for diet effect; bars labeled with different letters differ, P < 0.01 (two-way ANOVA and Tukey's post-hoc test).
Bone turnover
PYD, DPD and OC were not affected by 10 wk of EnR in either obese or lean rats (data not shown)
Correlation analyses
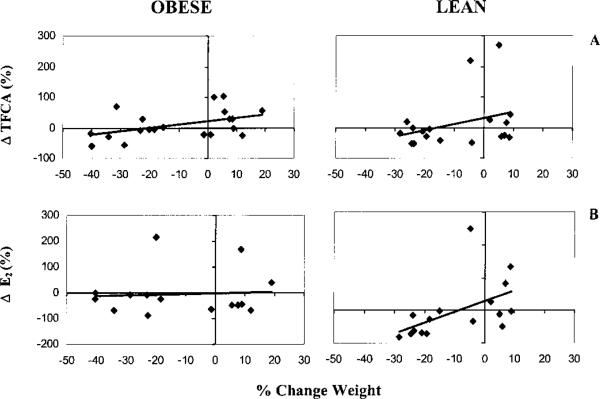
The correlations between changes in weight and changes in Ca balance, markers of bone turnover and hormones in obese and lean rats are shown in Table 5 and Figure 4. Not surprisingly, decreases in body weight correlated with decreases in creatinine excretion, but the association was stronger in lean than in obese rats. As body weight decreased, TFCA declined (Table 5, Fig. 4A), urinary corticosterone increased and PTH tended to rise (P < 0.07). Only lean rats showed a decrease in E2 with weight loss (Table 5, Fig. 4B), and importantly, this decrease tended to correlate with a decline in TFCA in lean (r = 0.48, P < 0.06) but not obese rats (r = 0.10, P < 0.73). Bone turnover markers did not correlate with weight loss (Table 5). However, decreases in urinary PYD (r = 0.53, P < 0.001) and DPD (r = 0.33, P < 0.05), but not OC, were associated with decreases in urinary creatinine excretion, a marker of muscle mass.
TABLE 5.
Pearson's correlation coefficient (r) for the relationship between the change from baseline in body weight (%) with changes in Ca absorption, Ca balance, hormones, creatinine and bone turnover in obese and lean mature rats fed 40% energy-restricted and control diets for 10 wk1
| All |
Obese2 |
Lean3 |
||||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| TFCA | 0.343 | 0.035 | 0.467 | 0.038 | 0.315 | 0.204 |
| Ca Balance | 0.282 | 0.086 | 0.335 | 0.149 | 0.117 | 0.642 |
| E2 | 0.245 | 0.184 | 0.052 | 0.854 | 0.516 | 0.041 |
| 25(OH)D | –0.200 | 0.281 | –0.265 | 0.339 | –0.042 | 0.878 |
| PTH | –0.330 | 0.070 | –0.445 | 0.096 | –0.340 | 0.198 |
| Corticosterone | –0.567 | <0.001 | –0.581 | 0.007 | –0.557 | 0.016 |
| Creatinine | 0.433 | 0.007 | 0.362 | 0.117 | 0.567 | 0.014 |
| OC | –0.048 | 0.796 | –0.093 | 0.741 | 0.444 | 0.085 |
| PYD | 0.272 | 0.098 | 0.198 | 0.403 | 0.376 | 0.124 |
| DPD | 0.242 | 0.143 | 0.070 | 0.770 | 0.405 | 0.096 |
Pearson's correlation coefficients were considered significant at P < 0.05.
Obese n = 20 (urine), n = 15 (serum).
Lean n = 18 (urine), n = 16 (serum).
4 Abbreviations: TFCA, true fractional Ca absorption; serum: estradiol (E2), 25-hydroxyvitamin D [25(OH) D], parathyroid hormone (PTH), osteocalcin (OC); 24-h urine: pyridinoline (PYD), deoxypyridinoline (DPD), corticosterone and creatinine.
FIGURE 4.
Relationships between percentage change in body weight and (A) true fractional Ca absorption (TFCA) [obese: r = 0.47, P = 0.04, n = 20; lean: r = 0.31, P = 0.20, n = 18] and (B) serum estradiol (E2) [obese: r = 0.05, P = 0.85, n = 15; lean: r = 0.52, P = 0.04, n = 16] in obese and lean mature rats fed 40% energy-restricted or control diets for 10 wk.
DISCUSSION
The results of this study indicate that a 40% restriction in energy intake for 10 wk decreases the efficiency of intestinal calcium absorption in obese and lean rats, suggesting that regardless of initial body weight, EnR will negatively affect calcium absorption in mature rats. The endocrine changes associated with weight loss, however, differed in the obese and lean groups, suggesting that the negative effect on bone may be mitigated in the obese.
It is expected that at a relatively lower initial body fat (as in our lean compared with obese rats), there will be greater loss of lean body mass with EnR (22,23). Although body composition was not measured in this study, only the lean, EnR rats showed complete absence of adipose tissue (upon visual inspection of the abdominal cavity when uteri were removed), and a trend for reduced muscle mass, as indicated by 24-h creatinine excretion. In addition, the weight of lean rats may have adapted to EnR earlier than obese rats, reaching a plateau during the last 4 wk of restriction (Fig. 1). Differences in the ratios of lean to fat tissue loss and adaptation to prolonged EnR may play an important role in the different endocrine responses and ultimate effect on Ca and bone metabolism. For example, it is possible that less fat tissue after EnR in lean compared with obese rats may have contributed to greater reduction in serum estrogen and lower final 25(OH)D concentrations.
We hypothesized that EnR would decrease estrogenic activity (5,6) and have a negative effect on intestinal calcium absorption. Ovarian hormone deficiency has been reported to impair gut calcium absorption in rats (21,24,25) and in post-menopausal women (26), whereas treatment with E2 has been shown to stimulate absorption in rats (14,21) and in humans (27). In the EnR rats, uterine weights were significantly lower than those of control rats, indicating a reduced estrogenic activity with EnR. Furthermore, the EnR-associated hypoestrogenic state at experiment conclusion was accompanied by a negative calcium balance. Hence, our results suggest that, in agreement with models of estrogen deprivation in rats and humans, the mild estrogen deficiency associated with EnR has important consequences for calcium metabolism.
The observation that weight loss was correlated with a decrease in serum E2 only in lean rats, together with the trend toward a greater decrease in serum E2 in lean compared with obese rats, suggests that the risk for estrogen deficiency due to EnR is greater in lean rats. A greater body weight may prevent the decrease in E2 due to local synthesis of estrogen in adipose tissue (28) and may protect against bone loss. In addition, the decreases in E2 during weight reduction tended to be associ- ated with decreases in calcium absorption in lean rats. There may be different mechanisms regulating the decline in calcium absorption in lean and obese rats during EnR despite reduced estrogenic activity in both lean and obese rats.
Consistent with previous findings of a trend for serum PTH to increase with weight reduction in women (9), EnR prevented the decrease in serum PTH that was observed in rats that consumed the diet ad libitum. Previously, we (5) and others (29) did not observe EnR-associated differences in serum PTH of rats. In the present study, however, we used a rat-specific bioactive PTH assay and found differences when the data were expressed as changes from baseline, whereas in previous studies (5,29) both inactive and active fragments of the PTH molecule were measured only at the conclusion of the experiments. The hypothesis that EnR decreases calcium absorption and elevates serum PTH is supported by these data.
After EnR, obese rats had higher serum 25(OH)D levels than the lean rats. This may have been because of the relatively greater fat loss that is expected in obese compared with lean rats (22,23). Brouwer et al. (11) observed that orally administered cholecalciferol in rats accumulates in adipose tissue, and food deprivation increases serum 25(OH)D. It is possible that greater degradation of fat tissue in obese than lean rats caused greater release of stored vitamin D, resulting in higher circulating 25(OH)D levels. Higher 25(OH)D levels are expected to influence calcium metabolism, and this may have been responsible for the lower urinary calcium losses in obese EnR rats compared with lean rats.
The EnR-associated hyperadrenocorticism observed in this study has been documented previously in rodents (30). Because elevated levels of glucocorticoid hormones are a risk factor for bone loss (31,32) and have recently been shown to be associated with calcium absorption (15), this may be one important mediator of the negative effects of weight reduction on calcium and bone metabolism.
Bone turnover was not affected by 10 wk of EnR in mature obese or lean rats. We expected a rise in bone turnover on the basis of our findings of decreased calcium absorption and estrogenic activity, as well as previous studies showing that EnR decreases bone mass and strength (5). However, because of the variability of bone markers, a single measurement of bone turnover may not reflect bone changes over several weeks (33). The present observations are consistent with previous findings from our laboratory (33) in which bone resorption was unchanged with EnR. However, bone formation findings differ from the previously observed EnR-associated elevation of serum OC (33). A possible explanation is that the time of blood sampling in EnR rats in relation to the last feeding differed between studies. In the Talbott study (33), blood was drawn ~24 h after the last feeding, whereas in the present study blood was drawn ~4 h postprandially for a better comparison with rats consuming food ad libitum. Our data in lean rats support the findings of Ndiaye et al. (29) that weight loss is associated with decreases in OC in male rats. Data from human studies, however, are not conclusive, with weight loss being associated with increases (2,9) or no effect (1) on serum OC.
We observed greater bone resorption and lower formation in obese compared with lean rats at 6 mo of age. In addition, body weight was directly correlated with bone resorption markers. To our knowledge, no rodent and very few clinical studies have addressed the relationship between stable body weight and markers of bone turnover in healthy subjects. The lower rates of bone formation in the obese are consistent with preliminary data from our laboratory (34) in which obese women had lower levels of OC than lean subjects. Also, others (35,36) have found inverse correlations between body mass index and OC. The intriguing findings of a less favorable bone turnover profile in the obese do not support the higher bone density and lower osteoporosis risk described in this group. Our data suggest that that there is a relationship between body weight and bone turnover, but the underlying regulating mechanisms are unknown. The implications for bone mass remain unclear and warrant further investigation.
In summary, EnR was associated with a decrease in intestinal calcium absorption in mature obese and lean rats. The effect of EnR, however, may be different depending on initial body weight due to dissimilar endocrine profiles, which are expected to have distinct effects on calcium handling in the intestine and bone metabolism. In lean, but not obese rats, there was an association between weight loss and decreases in E2 levels, and both tended to correlate with a reduction in calcium absorption. We propose that the susceptibility of calcium metabolism to EnR in mature rats depends on initial body weight and may be mediated by decreased estrogenic activity. Further studies of the mechanisms involved in the changes in calcium absorption during EnR, such as a possible downregulation of intestinal vitamin D receptors, calbindin 9k or calcium transport protein 1, are necessary to help explain the effect of EnR on bone.
Footnotes
Supported by National Institutes of Health Grant (AG-12161) to S.A.S.
Abbreviations used: 25(OH)D, 25 hydroxyvitamin D; CTL, control; DPD, deoxypyridinoline; E2, estradiol; EnR, energy restriction/energy-restricted; HF, high fat diet; OC, osteocalcin; PTH, parathyroid hormone; PYD, pyridinoline; TFCA, true fractional Ca absorption.
LITERATURE CITED
- 1.Salamone LM, Cauley JA, Black DM, Simkin-Silverman L, Lang W, Gregg E, Palermo L, Epstein RS, Kuller LH, Wing R. Effect of a lifestyle intervention on bone mineral density in premenopausal women: a randomized trial. Am. J. Clin. Nutr. 1999;70:97–103. doi: 10.1093/ajcn/70.1.97. [DOI] [PubMed] [Google Scholar]
- 2.Chao D, Espeland MA, Farmer D, Register TC, Lenchik L, Applegate WB, Ettinger WH., Jr. Effect of voluntary weight loss on bone mineral density in older overweight women. J. Am. Geriatr. Soc. 2000;48:753–759. doi: 10.1111/j.1532-5415.2000.tb04749.x. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TV, Sambrook PN, Eisman JA. Bone loss, physical activity, and weight change in elderly women: the Dubbo Osteoporosis Epidemiology Study. J. Bone Miner. Res. 1998;13:1458–1467. doi: 10.1359/jbmr.1998.13.9.1458. [DOI] [PubMed] [Google Scholar]
- 4.Shapses SA. Body weight and the Skeleton. In: Burckhardt P, Heaney R, Dawson-Hughes B, editors. Nutritional Aspects of Osteoporosis. Academic Press; New York, NY: 2001. pp. 341–354. [Google Scholar]
- 5.Talbott SM, Cifuentes M, Dunn MG, Shapses SA. Energy restriction reduces bone density and biomechanical properties in aged female rats. J. Nutr. 2001;131:2382–2387. doi: 10.1093/jn/131.9.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Zhang Y, Xiong Y, Lee CJ. Bone composition and strength of female rats subjected to different rates of weight reduction. Nutr. Res. 2000;20:1613–1622. [Google Scholar]
- 7.Colman RJ, Roecker EB, Ramsey JJ, Kemnitz JW. The effect of dietary restriction on body composition in adult male and female rhesus macaques. Aging. 1998;10:83–92. doi: 10.1007/BF03339642. [DOI] [PubMed] [Google Scholar]
- 8.Grinspoon S, Herzog D, Klibanski A. Mechanisms and treatment options for bone loss in anorexia nervosa. Psychopharmacol. Bull. 1997;33:399–404. [PubMed] [Google Scholar]
- 9.Ricci TA, Heymsfield SB, Pierson RN, Jr., Stahl T, Chowdhury HA, Shapses SA. Moderate energy restriction increases bone resorption in obese postmenopausal women. Am. J. Clin. Nutr. 2001;73:347–352. doi: 10.1093/ajcn/73.2.347. [DOI] [PubMed] [Google Scholar]
- 10.Lemann J, Jr., Gray RW, Maierhofer WJ, Adams ND. Effects of weight loss on serum 1,25-(OH)2-vitamin D concentrations in adults: a preliminary report. Calcif. Tissue Int. 1984;36:139–144. doi: 10.1007/BF02405309. [DOI] [PubMed] [Google Scholar]
- 11.Brouwer DA, van Beek J, Ferwerda H, Brugman AM, van der Klis FR, van der Heiden HJ, Muskiet FA. Rat adipose tissue rapidly accumulates and slowly releases an orally-administered high vitamin D dose. Br. J. Nutr. 1998;79:527–532. doi: 10.1079/bjn19980091. [DOI] [PubMed] [Google Scholar]
- 12.Liel Y, Shany S, Smirnoff P, Schwartz B. Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology. 1999;140:280–285. doi: 10.1210/endo.140.1.6408. [DOI] [PubMed] [Google Scholar]
- 13.Riggs BL, Khosla S, Melton LJ., 3rd A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 14.Arjmandi BH, Hollis BW, Kalu DN. In vivo effect of 17 β-estradiol on intestinal calcium absorption in rats. Bone Miner. 1994;26:181–189. doi: 10.1016/s0169-6009(08)80062-1. [DOI] [PubMed] [Google Scholar]
- 15.Arnaud SB, Navidi M, Deftos L, Thierry-Palmer M, Dotsenko R, Bigbee A, Grindeland RE. The calcium endocrine system of adolescent rhesus monkeys and controls before and after spaceflight. Am. J. Physiol. 2002;282:E514–E521. doi: 10.1152/ajpendo.00299.2001. [DOI] [PubMed] [Google Scholar]
- 16.Persson P, Gagnemo-Persson R, Hakanson R. The effect of high or low dietary calcium on bone and calcium homeostasis in young male rats. Calcif. Tissue Int. 1993;52:460–464. doi: 10.1007/BF00571337. [DOI] [PubMed] [Google Scholar]
- 17.Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J. Nutr. 1993;123:1923–1931. doi: 10.1093/jn/123.11.1923. [DOI] [PubMed] [Google Scholar]
- 18.Salas-Valdes A. A quick and inexpensive staining method for vaginal smears. Arch. Invest. Med. 1979;10:147–150. [PubMed] [Google Scholar]
- 19.Black D, Duncan A, Robins SP. Quantitative analysis of the pyridinium crosslinks of collagen in urine using ion-paired reversed-phase high-performance liquid chromatography. Anal. Biochem. 1988;169:197–203. doi: 10.1016/0003-2697(88)90274-6. [DOI] [PubMed] [Google Scholar]
- 20.Eyre DR, Koob TJ, Van Ness KP. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal. Biochem. 1984;137:380–388. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- 21.O'Loughlin PD, Morris HA. Oestrogen deficiency impairs intestinal calcium absorption in the rat. J. Physiol. 1998;511:313–322. doi: 10.1111/j.1469-7793.1998.313bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill JO, DiGirolamo M. Preferential loss of body fat during starvation in dietary obese rats. Life Sci. 1991;49:1907–1914. doi: 10.1016/0024-3205(91)90292-j. [DOI] [PubMed] [Google Scholar]
- 23.Forbes GB. Body fat content influences the body composition response to nutrition and exercise. Ann. N.Y. Acad. Sci. 2000;904:359–365. doi: 10.1111/j.1749-6632.2000.tb06482.x. [DOI] [PubMed] [Google Scholar]
- 24.Colin EM, Van Den Bemd GJ, Van Aken M, Christakos S, De Jonge HR, Deluca HF, Prahl JM, Birkenhager JC, Buurman CJ, Pols HA, Van Leeuwen JP. Evidence for involvement of 17beta-estradiol in intestinal calcium absorption independent of 1,25-dihydroxyvitamin D3 level in the rat. J. Bone Miner. Res. 1999;14:57–64. doi: 10.1359/jbmr.1999.14.1.57. [DOI] [PubMed] [Google Scholar]
- 25.Kalu DN, Orhii PB. Calcium absorption and bone loss in ovariectomized rats fed varying levels of dietary calcium. Calcif. Tissue Int. 1999;65:73–77. doi: 10.1007/s002239900660. [DOI] [PubMed] [Google Scholar]
- 26.Heaney RP, Recker RR, Saville PD. Menopausal changes in calcium balance performance. J. Lab. Clin. Med. 1978;92:953–963. [PubMed] [Google Scholar]
- 27.Gallagher JC, Riggs BL, DeLuca HF. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 1980;51:1359–1364. doi: 10.1210/jcem-51-6-1359. [DOI] [PubMed] [Google Scholar]
- 28.Simpson E, Rubin G, Clyne C, Robertson K, O'Donnell L, Davis S, Jones M. Local estrogen biosynthesis in males and females. Endocr. Relat. Cancer. 1999;6:131–137. doi: 10.1677/erc.0.0060131. [DOI] [PubMed] [Google Scholar]
- 29.Ndiaye B, Cournot G, Pelissier MA, Debray OW, Lemonnier D. Rat serum osteocalcin concentration is decreased by restriction of energy intake. J. Nutr. 1995;125:1283–1290. doi: 10.1093/jn/125.5.1283. [DOI] [PubMed] [Google Scholar]
- 30.Yu BP, Chung HY. Stress resistance by caloric restriction for longevity. Ann. N.Y. Acad. Sci. 2001;928:39–47. doi: 10.1111/j.1749-6632.2001.tb05633.x. [DOI] [PubMed] [Google Scholar]
- 31.Manelli F, Giustina A. Glucocorticoid-induced osteoporosis. Trends Endocrinol. Metab. 2000;11:79–85. doi: 10.1016/s1043-2760(00)00234-4. [DOI] [PubMed] [Google Scholar]
- 32.Dennison E, Hindmarsh P, Fall C, Kellingray S, Barker D, Phillips D, Cooper C. Profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men. J. Clin. Endocrinol. Metab. 1999;84:3058–3063. doi: 10.1210/jcem.84.9.5964. [DOI] [PubMed] [Google Scholar]
- 33.Talbott SM, Rothkopf MM, Shapses SA. Dietary restriction of energy and calcium alters bone turnover and density in younger and older female rats. J. Nutr. 1998;128:640–645. doi: 10.1093/jn/128.3.640. [DOI] [PubMed] [Google Scholar]
- 34.Cifuentes M, Johnson MA, Lewis R, Modlesky C, Shapses SA. Body weight reflects bone resorption in lean, but not overweight or obese postmenopausal women. J. Bone. Miner. Res. 2000;15:S533. [Google Scholar]
- 35.Midtby M, Magnus JH, Joakimsen RM. The Tromso Study: a population-based study on the variation in bone formation markers with age, gender, anthropometry and season in both men and women. Osteoporos. Int. 2001;12:835–843. doi: 10.1007/s001980170034. [DOI] [PubMed] [Google Scholar]
- 36.Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J. Bone Miner. Res. 1999;14:1622–1627. doi: 10.1359/jbmr.1999.14.9.1622. [DOI] [PubMed] [Google Scholar]