Abstract
Objective
Metastatic lymph nodes (LN) are an adverse prognostic factor in head and neck squamous cell carcinoma (SCC). In this study, we tested the hypothesis that nodal metastases have reduced impact on survival in tonsil cancer in the HPV-predominant era.
Methods
Incidence and mortality data of tonsil and oral cavity SCC between 1988 and 2007 was obtained from the SEER database. Based on published literature, we considered cases of tonsil cancer from 1988–1997 the pre-HPV cohort (N=752), and 1998–2007 as the HPV-predominant cohort (N=2,755).
Results
Comparing the two cohorts, Kaplan-Meier five-year overall survival (OS) for tonsil SCC improved from 54.0% to 74.3% (p<0.0001), and cancer-specific survival (CSS) improved from 66.0 to 82.9% (p<0.0001). Stratifying by LN involvement showed improved OS in the HPV-predominant cohort with one (63.6 vs. 79.7%, p<0.0001), two to three (54.2 vs. 75.9%, P<0.0001), four to eight (40.3 vs. 68.9%, p<0.0001), and greater than eight positive nodes (25.5 vs. 41.9%, p<0.0001).
Conclusion
While metastatic LNs still negatively affect prognosis, their impact on OPC survival has diminished in the HPV-predominant era. This finding provides a rationale for additional studies of the prognostic significance of LN metastases in OPC cohorts of defined HPV status, and supports the concept that HPV-related OPC is a disease distinct from “classical” OPC, with unique prognostic features.
Keywords: head and neck neoplasms, oropharyngeal neoplasms, oropharynx, papillomavirus infections, squamous cell cancer, human papillomavirus (HPV)
INTRODUCTION
The number of cancer-containing metastatic lymph nodes is a well-established prognostic factor in cancer of the aerodigestive tract, including lung cancer, [1] oral cancer, [2–4], and other head and neck mucosal sites [5]. Almost two decades ago, Leemans et al. reported that patients with head and neck squamous cell carcinoma (HNSCC) with three or more pathologically positive nodes were at a higher risk of having distant metastases than patients with two or less positive nodes, and thus recommended that patients with three or more positive nodes receive adjuvant therapy [6]. A year later, this same group showed that patients having a greater number of positive nodes experienced a higher five-year rate of recurrence, as 26.2% of patients with three or more positive nodes experienced recurrence, 10.8% of patients with one or two nodes experienced recurrence, and 9.5% of N0 patients experienced recurrence at five years [7]. A similar study showed that a greater number of positive nodes was also associated with decreased survival, with patients with one positive node experiencing a 50.3% five-year survival, two nodes experiencing a 40% five-year survival, three nodes experiencing a 29.6% five-year survival, and four or more nodes with 15% five-year survival [8].
Longstanding alcohol and tobacco use have traditionally been considered the main risk factors for HNSCC. However, in recent years, human papilloma virus (HPV) has become an increasingly prevalent cause of HNSCC [9–11], especially in the oropharynx [12]. There are striking differences between HPV-associated and environmental carcinogen-induced oropharyngeal cancer, including the fact that HPV-positive tumors are much more responsive to radiation therapy and have a more favorable prognosis, supporting the now well-accepted theory that HPV-associated oropharyngeal cancer (OPC) is a different clinical entity [13–15]. Unfortunately, large cancer registries such as the Surveillance, End Results, and Epidemiology (SEER) registry in the United States do not contain information on HPV status. There is evidence, however, to suggest that representation of HPV-associated cancer in the SEER registry parallels that in the country at large, and that the ratio of HPV-positive to HPV-negative OPC increased to a critical level in the mid-1990s [11]. Chaturvedi et al. observed that the incidence of HPV positive oropharyngeal cancers increased by 225% from 1988 to 2004, or from 0.8 to 2.6 per 100,000 in a representative subset of the United States [11,16].
With this epidemiologic shift in mind, the goal of this study was to determine in a large group of patients with OPC whether the yield of pathologically positive lymph nodes has decreased prognostic significance in patients with HPV-associated cancer. By stratifying patients by year of diagnosis into pre-1998 and post-1998 groups as a proxy for HPV status, and performing parallel analyses for patients with oral cavity SCC (a site rarely associated with HPV), we sought to determine whether the impact of positive lymph nodes on survival in OPC patients is diminished in the era of HPV-predominant disease.
METHODS
Incidence-based mortality data was obtained from the Surveillance, Epidemiology, and End Results (SEER) database, a network of cancer registries maintained by the National Cancer Institute which captures approximately 10% of all US cancer cases [17]. This database contains information such as TNM staging, lymph node involvement, and tumor type for all cancer diagnoses in defined geographic areas within the United States. Cases for this study were obtained from the SEER 9 dataset, and limited to patients diagnosed between 1988 and 2007. Based on prior epidemiologic studies, we considered OPC cases diagnosed in 1988–1997 the pre-HPV cohort, and those diagnosed in 1998–2007 the HPV-predominant cohort [16]. Only primary cancers were included in this analysis; second primary cancers were excluded.
In order to make samples as homogeneous as possible for comparison, patients were excluded from the study if they met the following criteria: if they had distant metastases at presentation, did not undergo neck dissection, had no positive lymph nodes at the time of neck dissection, or received preoperative radiation. Cases where less than five lymph nodes were removed at the time of neck dissection were also excluded from the analysis. The number of positive lymph nodes in a patient was assigned based on the number of cancer-containing lymph nodes reported by pathologic analysis. We limited our analysis to cancers of the tonsil, coded in the SEER database as sites C090–C099, to focus on the oropharyngeal site most associated with HPV. To serve as a comparison in a site where HPV association is much less common,[18,19] we identified a similar cohort of patients with SCC of the oral cavity. For this analysis, we defined oral cavity as including cancers of the gum and other mouth (C030–C039, C050–C059, C060–C069), floor of mouth (C040–C049), and tongue (C019–C029), according to the SEER site classifications.
To compare groups with varying numbers of lymph node metastases, we stratified patients into groups of one, two to three, four to eight, and greater than eight positive lymph nodes for comparison. Data were extracted in SEER*Stat v. 6.4.4, and statistical analyses were performed in SAS (Version 9.3, Cary, NC). Overall survival was determined from the number of patients alive after excluding death from any cause, and cause-specific survival was determined from the number of patients alive after excluding death due specifically to the primary cancer. Age, sex, race, and whether the patient received postoperative radiation therapy were all included as covariates in the Cox multivariate regression model used to calculate mortality, using a hazard ratio to compare survival between the two time periods. These variables were included in the multivariate model after being found to be independently significant as univariate predictors of survival.
RESULTS
From a total of 13,644 patients diagnosed with squamous cell carcinoma of the tonsil in the SEER 9 registry between 1988 and 2007, we analyzed 752 patients in the pre-HPV cohort, and 2,755 patients in the HPV-predominant cohort who had regional disease and did not receive preoperative radiation (Figure 1). Overall, patients from the HPV-predominant cohort were younger (p=0.0094), more likely to be male (p<0.0001) and white (p<0.0001), and more likely to have a smaller nodal burden (p=0.0090) (Table 1). By comparison, the oral cavity 1988–1997 cohort contained 922 patients, and the 1998–2007 cohort contained 1,968 patients with regional disease and no preoperative radiation. The oral cavity population showed no statistically significant difference in age (p=0.4640), gender (p=0.2705), or nodal burden between the two cohorts. There was a small but statistically significant increase in the percentage of white patients with oral cavity SCC in the HPV-predominant era (Table 1).
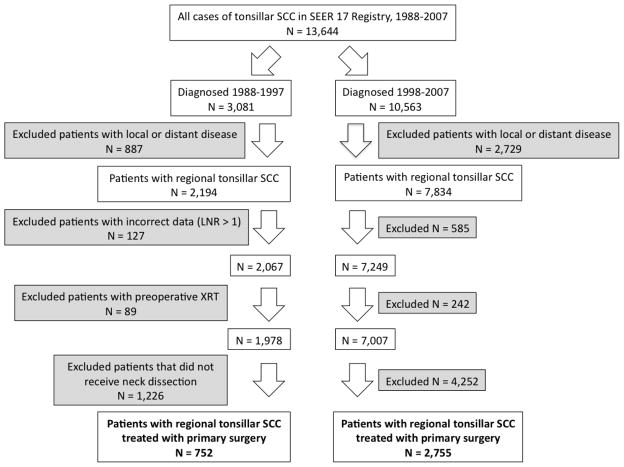
Figure 1.
Inclusion and exclusion criteria for patients with squamous cell carcinoma of the tonsil in the SEER 9 Registry, United States, 1988–2007. LNR = lymph node ratio, or the number of metastatic lymph nodes divided by the number of lymph nodes examined.
Table 1.
Characteristics of patients with regional SCC of the tonsil and oral cavity in the SEER 9 Registry, United States, 1988–2007.
| Patient Characteristics | Tonsil | p-value for difference* | Oral Cavity | p-value for difference* | ||
|---|---|---|---|---|---|---|
| 1988–1997 | 1998–2007 | 1988–1997 | 1998–2007 | |||
| Age | % (n) | % (n) | 0.0094 | % (n) | % (n) | 0.4640 |
| < 50 years | 28.9 (217) | 30.2 (833) | 12.4 (114) | 13.7 (269) | ||
| 50–64 years | 57.6 (433) | 61.5 (1,694) | 58.4 (538) | 53.9 (1,060) | ||
| > 65 years | 13.6 (102) | 8.3 (228) | 29.3 (270) | 32.5 (639) | ||
| Gender | <0.0001 | 0.2705 | ||||
| Male | 75.8 (570) | 82.7 (2,277) | 68.3 (630) | 66.3 (1,304) | ||
| Race | <0.0001 | 0.0309 | ||||
| White | 84.2 (633) | 90.7 (2,499) | 80.6 (743) | 82.8 (1,629) | ||
| Black | 11.0 (83) | 5.8 (161) | 14.5 (134) | 11.5 (226) | ||
| Asian/Pacific Islander | 4.4 (33) | 2.9 (79) | 3.6 (33) | 5.2 (103) | ||
| American Indian/Alaska Native | 0.4 (3) | 0.6 (16) | 1.3 (12) | 0.5 (10) | ||
| Postoperative RT | 0.0004 | 0.7140 | ||||
| Was not given | 26.6 (200) | 20.6 (565) | 28.9 (266) | 29.5 (580) | ||
| Given | 73.4 (552) | 79.4 (2,182) | 71.2 (656) | 70.5 (1,385) | ||
| Positive Nodes | 0.0090 | 0.9280 | ||||
| 1 node | 45.6 (343) | 51.7 (1,425) | 41.0 (378) | 41.8 (823) | ||
| 2–3 nodes | 29.9 (225) | 26.8 (738) | 34.1 (314) | 31.9 (627) | ||
| 4–8 nodes | 17.2 (129) | 15.1 (417) | 19.0 (175) | 20.6 (405) | ||
| > 8 nodes | 7.3 (55) | 6.4 (175) | 6.0 (55) | 5.7 (113) | ||
PROC TTEST was used in SAS to calculate the p-value using the Pooled method for equal variances between groups, and the Satterthwaite method for unequal variances.
RT = Radiation therapy.
We subsequently examined overall survival (OS) and cancer-specific survival across the two time periods, using a Kaplan-Meier survival curve. The five-year OS for tonsil SCC improved from 54.0% to 74.3% (p<0.0001). Similarly, CSS improved from 66.0 to 82.9% (p<0.0001).
We then compared Kaplan-Meier survival curves according to the number of pathologically positive lymph nodes found during neck dissection in the pre-HPV and HPV-predominant tonsil and oral cavity cancer cohorts. After stratifying by the number of positive lymph nodes in tonsil cancers we found highly significant improvements in five year OS for all node-positive patients. This was seen in patients with one positive node (63.6 to 79.7 percent, p<0.0001), two to three positive nodes (54.2 to 75.9 percent, p<0.0001), four to eight positive nodes (40.3 to 68.9 percent, p<0.0001), and greater than eight positive nodes (25.5 to 41.9 percent, p<0.0001) (Figure 2). The relative prognostic impact of increasing nodal burden on overall survival was also markedly attenuated in the HPV-predominant era. Whereas, in the pre-HPV era, 10 year overall survival rates among patients with 1, 2–3, or 4–8 nodes ranged from 30.2 percent to 51.4 percent (a spread of 21 percent), survival rates for patients in these categories was both remarkably improved and the range greatly compressed in the HPV-predominant era (ranging from 61.1 percent to 63.0 percent, a 2 percent difference).
Figure 2.
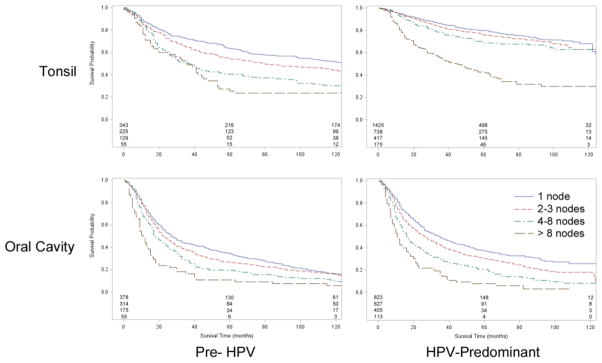
Overall survival of patients with regionally-staged tonsil and oral cavity SCC, stratified by number of pathologically positive nodes.
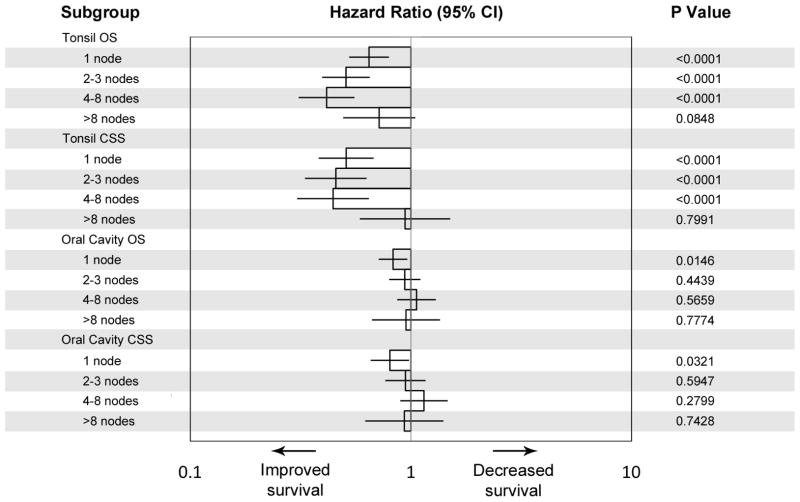
A similar narrowing of the survival difference between patients with 1, 2–3, or 4–8 positive nodes was seen for cancer-specific survival (Figure 3). We found improved five year CSS among patients with one positive node (74.1 to 88.2 percent, p<0.0001), two to three nodes (65.9 to 84.3 percent, p<0.0001), and four to eight nodes (54.4 to 76.1 percent, p<0.0001). However, OPC patients with an extremely high nodal burden (>8 positive nodes) continue to have markedly worse prognosis in the HPV-predominant era. Figure 4 summarizes these results by showing hazard ratios for survival in the HPV-predominant era with respect to the pre-HPV era.
Figure 3.
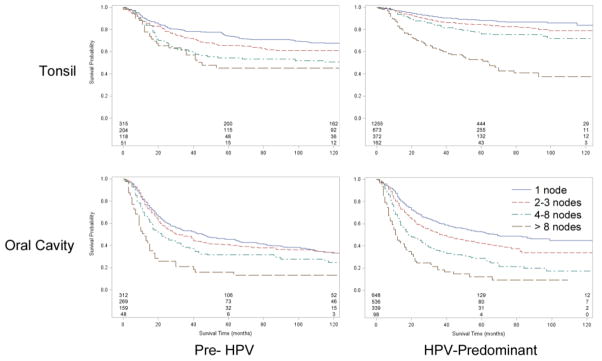
Cancer-specific survival for patients with regionally-staged tonsil and oral cavity SCC, stratified by number of positive nodes.
Figure 4.
Hazard ratios for overall and cancer-specific survival of patients with regionally-staged SCC of the tonsil and oral cavity, stratified by the number of pathologically positive nodes found on neck dissection. Survival of the HPV-predominant cohort is presented relative to that of the pre-HPV cohort. (Error bars represent the 95% confidence interval).
DISCUSSION
This analysis of over 3,000 patients diagnosed with tonsillar squamous cell carcinoma from 1988 through 2007 suggests that lymph node metastases have a greatly diminished impact on survival of OPC patients in the HPV-predominant era. Specifically, the poorer survival associated with >1 positive lymph node, previously observed in the pre-HPV era, is markedly attenuated in the era of HPV-associated OPC, with the exception of patients with >8 positive lymph nodes. In addition, both five-year overall survival (OS) and cancer-specific survival (CSS) improved in tonsil SCC patients with one to eight lymph node metastases, consistent with prior studies describing improved survival of patients with HPV-positive cancers [16,15,13]. Studying OS and CSS across all patients reveals that, in the HPV-predominant era, five-year survival has improved for both tonsil and oral cavity cancer patients; however, the improvement was far more pronounced in the tonsil cohort. The improvement in CSS was almost twice as great in the tonsil cohort, with a 23 percent increase in five-year CSS, as compared to 14 percent in the oral cavity cohort. Of note, neither OS nor CSS in tonsil SCC patients with greater than eight positive nodes improved from the pre-HPV to the HPV-predominant cohort, while for OPC patients all other categories (one, two to three, or four to eight positive nodes) improved significantly. This difference was much less pronounced in the oral cavity cohort, where the only statistically significant improvement in survival was in patients with one positive node. These findings strongly support the hypothesis that regional metastasis has a greatly decreased impact on survival of patients with HPV-related cancers, as compared to non-HPV-related cancers.
It is important to note that the difference in survival between the two cohorts could potentially be explained by increased use of concomitant chemotherapy in patients receiving postoperative radiation therapy in the HPV era [20]. However, because we did not see a comparable improvement in survival in the comparison group of patients with oral cavity cancer for whom postoperative management is anticipated to be similar, this is not likely to be a major contributing factor. Another possible confounding factor is the shift in treatment patterns over time towards increasing use of aggressive radiation and chemoradiation regimens for patients with advanced OPC, which may potentially result in skewing of the stage distribution of surgical patients towards lower stages of disease. However, the issue of whether patients received radiation therapy postoperatively was accounted for in the multivariate regression, and the percentage of patients excluded from the analysis because they did not undergo primary surgical management (and instead received definitive chemoradiotherapy) was identical (60%) in both cohorts (Figure 1).
The main limitation of this analysis is the lack of direct information about HPV status, since this information is not captured in the SEER database. We used year of diagnosis as proxy for likelihood of HPV infection based on published literature suggesting that trends in HPV incidence in the US as a whole are well-represented in the SEER population [16]. However, this is likely to result in misclassification of many patients in both cohorts, which would tend to weaken the association between HPV status and altered prognostic impact of lymphatic metastases. Thus, our analysis is likely to underestimate the magnitude of decrease in prognostic impact of lymphatic metastasis in patients with HPV-related disease. In addition to potential changes in treatment paradigms, it is also true that comparison of patients treated in sequential time periods can be complicated by changes in staging and use of diagnostic imaging. While the use of diagnostic cross sectional imaging (primarily CT scan) is anticipated to be higher in the HPV-predominant cohort, we categorize patients in this study according to pathologic and not clinical nodal staging. A final limitation is the lack of detailed information about patient therapy, since the SEER treatment categories contain relatively little detail; for example, there is no information about chemotherapy in the database. Additional cohort studies of patients with defined HPV status and detailed treatment information will be required to confirm these findings. TNM staging data, for example, could help to further provide evidence. However, because the SEER data only provides TNM stage data from 2004 forward, this information could not be included in this analysis.
In conclusion, we have demonstrated improved survival of OPC patients with multiple pathologically positive lymph nodes in the HPV era, suggesting a greatly diminished impact of nodal yield on the prognosis of patients with HPV-related OPC. Since advanced nodal disease is a common rationale for treatment intensification (addition of chemotherapy to radiotherapy, or addition of induction chemotherapy to chemoradiotherapy), these data underscore the need for further studies of the impact of lymphatic metastases on OPC patients in cohorts of defined HPV status.
Acknowledgments
Funding: This work was supported by the Doris Duke Charitable Foundation.
Footnotes
Conflicts of Interest: None.
References
- 1.Wisnivesky JP, Arciniega J, Mhango G, Mandeli J, Halm EA. Lymph node ratio as a prognostic factor in elderly patients with pathological N1 non-small cell lung cancer. Thorax. 2010 doi: 10.1136/thx.2010.148601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gil Z, Carlson DL, Boyle JO, Kraus DH, Shah JP, Shaha AR, Singh B, Wong RJ, Patel SG. Lymph node density is a significant predictor of outcome in patients with oral cancer. Cancer. 2009;115 (24):5700–5710. doi: 10.1002/cncr.24631. [DOI] [PubMed] [Google Scholar]
- 3.Shrime MG, Bachar G, Lea J, Volling C, Ma C, Gullane PJ, Gilbert RW, Irish JC, Brown DH, Goldstein DP. Nodal ratio as an independent predictor of survival in squamous cell carcinoma of the oral cavity. Head Neck. 2009;31 (11):1482–1488. doi: 10.1002/hed.21114. [DOI] [PubMed] [Google Scholar]
- 4.Shrime MG, Ma C, Gullane PJ, Gilbert RW, Irish JC, Brown DH, Goldstein DP. Impact of nodal ratio on survival in squamous cell carcinoma of the oral cavity. Head Neck. 2009;31 (9):1129–1136. doi: 10.1002/hed.21073. [DOI] [PubMed] [Google Scholar]
- 5.Olsen KD, Caruso M, Foote RL, Stanley RJ, Lewis JE, Buskirk SJ, Frassica DA, DeSanto LW, O’Fallon WM, Hoverman VR. Primary head and neck cancer. Histopathologic predictors of recurrence after neck dissection in patients with lymph node involvement. Arch Otolaryngol Head Neck Surg. 1994;120 (12):1370–1374. doi: 10.1001/archotol.1994.01880360066012. [DOI] [PubMed] [Google Scholar]
- 6.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993;71 (2):452–456. doi: 10.1002/1097-0142(19930115)71:2<452::aid-cncr2820710228>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Leemans CR, Tiwari R, Nauta JJP, Vanderwaal I, Snow GB. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer. 1994;73 (1):187–190. doi: 10.1002/1097-0142(19940101)73:1<187::aid-cncr2820730132>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Mamelle G, Pampurik J, Luboinski B, Lancar R, Lusinchi A, Bosq J. Lymph node prognostic factors in head and neck squamous cell carcinomas. Am J Surg. 1994;168 (5):494–498. doi: 10.1016/s0002-9610(05)80109-6. [DOI] [PubMed] [Google Scholar]
- 9.Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, Joneberg J, Creson N, Lindholm J, Ye W, Dalianis T, Munck-Wikland E. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119 (11):2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 10.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356 (19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 11.Ernster JA, Sciotto CG, O’Brien MM, Finch JL, Robinson LJ, Willson T, Mathews M. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. The Laryngoscope. 2007;117 (12):2115–2128. doi: 10.1097/MLG.0b013e31813e5fbb. [DOI] [PubMed] [Google Scholar]
- 12.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15 (22):6758–6762. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 13.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. JNCI Journal of the National Cancer Institute. 2008;100 (4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 14.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92 (4):805–813. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363 (1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29 (32):4294–4301. doi: 10.1200/JCO.2011.36.4596. Epub 2011 Oct 4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Cancer Institute. [Accessed February 25 2011];About the SEER Program. http://seer.cancer.gov/about/
- 18.El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients - A distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2003;27 (11):1463–1470. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Ryerson AB, Peters ES, Coughlin SS, Chen VW, Gillison ML, Reichman ME, Wu XC, Chaturvedi AK, Kawaoka K. Burden of Potentially Human Papillomavirus-associated Cancers of the Oropharynx and Oral Cavity in the US, 1998–2003. Cancer. 2008;113 (10):2901–2909. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 20.Bernier J, Cooper J, Pajak T, van Glabbeke M, Bourhis J, Forastiere A, Ozsahin E, Jacobs J, Jassem J, Ang K, Lefebvre J. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27 (10):843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]