Abstract
While islet transplantation is considered a useful therapeutic option for severe diabetes mellitus (DM), the outcome of this treatment remains unsatisfactory. This is largely due to the damage and loss of islets in the early transplant stage. Thus, it is important to monitor the condition of the transplanted islets, so that a treatment can be selected to rescue the islets from damage if needed. Recently, numerous trials have been performed to investigate the efficacy of different imaging modalities for visualizing transplanted islets. Positron emission tomography (PET) and magnetic resonance imaging (MRI) are the most commonly used imaging modalities for this purpose. Some groups, including ours, have also tried to visualize transplanted islets by ultrasonography (US). In this review article, we discuss the recent progress in islet imaging.
Keywords: islet transplantation, positron emission tomography (PET), magnetic resonance imaging (MRI), ultrasonography or ultrasound (US), islet, diabetes mellitus (DM)
Introduction
Islet transplantation is a useful therapeutic option for severe diabetes mellitus (DM), including type 1 DM and pancreatomized DM.1-3 The procedure involves dripping isolated islets into the portal vein to engraft them in the liver. This is a safe and relatively fast procedure that can be performed under local anesthesia. According to the most recent report from the Collaborative Islet Transplant Registry, 571 diabetic recipients received islet allotransplantation from the pancreata of 1,010 donors from 1999 to 2009 (http//www.citregistry.org). However, the outcome of islet transplantation, though improving, remains inadequate. Approximately 40% of the islet-transplanted recipients require daily insulin injection at 3 y after transplantation, and many recipients require multiple donors. The worse transplant efficacy is brought about by rejection,4 a thrombotic and inflammatory reaction called instant blood-mediated inflammatory reaction (IBMIR),5 islet toxicity due to immunosuppressants,6-9 or islet ischemia10,11 in the early transplant stage. Thus, it is important to monitor the condition of the transplanted islets. If damage to the islets can be detected, then an appropriate treatment can be selected to rescue the islets from damage. The classical monitoring parameters for assessing islet viability and function, such as blood glucose level, serum C-peptide level, glucose tolerance test, or HbA1c, are based on the metabolic function of islets. Because abnormalities in these parameters arise after actual damage to the islets, they can be considered relatively late markers of islet graft dysfunction.12 Needle biopsy of the liver is another method for monitoring transplanted islets, and can show direct evidence of islet damage such as hypoxia, apoptosis, and immune or inflammatory response by immunohistochemistry. However, needle biopsy is an invasive procedure with a low success rate for detecting islets (according to Toso and colleagues, the success rate was 31%).13
Numerous experimental trials have been performed to investigate the efficacy of different imaging modalities for visualizing transplanted islets. Bioluminescence imaging (BLI) is one of the earliest of these methodologies.14 This method visualizes transplanted islets with luciferase gene transfection as an optical image by oxidation of luciferin as an injected substrate. BLI is a good technique for evaluating islet engraftment15 and rejection.16 However, BLI is not a suitable modality for a clinical setting because it cannot visualize deep tissue. Positron emission tomography (PET) and magnetic resonance imaging (MRI), which are widely used clinical imaging modalities, are the most widely used for this purpose. Some groups, including ours, have also tried to visualize transplanted islets by ultrasonography (US). In this review article, we describe the recent progress in islet imaging using clinical imaging modalities, including our own studies.
PET and SPECT
Early PET studies
PET is a noninvasive nuclear medical imaging modality for evaluating functional processes in the body with high resolution and good sensitivity. It is used especially for detecting tumors. Images are obtained based on the cellular consumption of molecules labeled with positron-emitting isotopes. The first study on islet imaging using PET was reported by Toso and colleagues in 2005. They labeled islets with 2-[18F] fluoro-2deoxy-D-glucose (FDG) (Table 1), and certified that the radioactivity of the labeled islets was higher than that of non-labeled islets. Though the radioactivity declined at 6 h after transplantation, this was nonetheless the first successful attempt to detect islets using PET.17 Many PET trials were published in 2006. In one study, Lu and colleagues succeeded in visualizing transplanted human and rat islets that were transduced with a thymidine kinase gene using 9-[4-fluoro-3-(hydroxymethyl)butyl]guanine (FHBG) as a tracer (Table 1).18 In addition, they showed that labeled islets could be visualized for over 90 d after transplantation.19 Kim and colleagues also visualized transplanted islets by the same method; moreover, they demonstrated that the radioactivity uptake in transplanted islets was elevated when the function of the islets was improved by induction of the viral interleukin (IL)-10 gene,20 which promotes glucagon-like peptide-1 (GLP-1) expression.21
Table 1. Published PET tracers for visualizing transplanted islets.
| Method for labeling | Tracer | References | Radioactivity | Islet donor species | Comments |
| Labeling of islets directly | [18F]FDG | 17 , 33 | 4–6 h | human, rat | Available for clinical use already Short radioactivity High uncharacteristic washout |
| [18F]FHBG | 18 - 20 | 1–3 mo | human, rat, mouse | Longer radioactivity Use of viral vector |
|
| Labeling of islets via intravenous infusion | [11C]DTBZ | 23 | Approximately 30 min | rat | Specific labeling of islets via VMAT2 Short radioactivity |
| [64Cu]/[18F]-labeled exendin-4 | 26 , 36 | Over 4 h | human | Specific labeling of islets via GLP-1 receptor | |
| [18F]- labeled fallypride | 30 | Over 1.5 h | rat | Specific labeling of islets via D2/D3 receptor Available for clinical use already Binds to other organs (mainly the brain) |
|
| [68Ga]-labeled biotin | 32 | Over 30 min | human | Specific labeling of islets via avidin-biotin interaction Prevention of IBMIR |
Recent progress in PET imaging
Because these cell-labeling methods were based on a viral transduction technique, it is difficult to utilize them directly in a clinical setting. Furthermore, because the islets must be labeled before transplantation, these methods cannot be applied to post-transplant islets. To overcome these challenges, novel probes that can label islets specifically by intravenous injection have been developed. Simpson and colleagues successfully visualized islets labeled with [11C] dihydrotetrabenzine (DTBZ) via vesicular monoamine transporter 2 (VMAT2), which is specifically expressed on islets. They performed PET with normal and diabetic rats using this method, and found that radioactivity uptake was present in the normal pancreas but not in the diabetic pancreas.22 Witkowski and colleagues succeeded in visualizing intramuscular transplanted rat islets on PET using this tracer.23 In a study using a similar probe, 9-[18F]fluoropropyl-(+)DTBZ, which has a longer half-life than [11C]DTBZ (110 min in [18F] vs 20 min in [11C]), was also specifically taken up into the pancreas following intravenous injection.24
The GLP-1 receptor agonist exendin-4 has also received attention as a potentially novel and effective PET probe, because GLP-1 receptors are highly expressed in islets and a probe containing exendin-4 has been shown to bind to islets specifically. In previous studies, [64Cu]Lys40-1,4,7,10-tetraazacyclododecane-1,4,7–10-tetraacetic acid (DOTA)-NH2-exendin-4,25 and [64Cu]1,4,7-tris(acetic acid)-10-vinylsulfone-1,4,7,10-tetraazacyclododecane (DO3A-VS)-Cys40-exendin-426 have been used to specifically visualize islets in rodent models (Table 1). The other novel tracers for specific labeling of islets, such as [18F]dithizon (targeting Zn2+ ions in islets),27,28 and [11C]- and [18F]- labeled L-DOPA (catecholamine precursor),29 have been developed and used for visualization of islets in pancreatic and islet cell tumors. In particular, [18F]-labeled fallypride (dopamine D2/3 ligand) was used for labeling transplanted islets in a rat model.30
These tracers are targeted for binding with a biomarker expressed on islets, but they can also bind to other organs which have the same marker. To identify transplanted islets specifically, studies using a pretargeting approach are underway. In the pretargeting approach, the islets are visualized after receiving pretreatment with materials combined with a tracer.31 Eriksson and colleagues succeeded in labeling transplanted islets specifically by avidin-biotin interaction. They developed a [68Ga]-labeled biotin tracer and used it to visualize avidin-covered beads transplanted into the liver. They also certified that the avidin-coated human islets could uptake the tracer by means of an in vitro assay. The avidin technique might contribute to the prevention of IBMIR by binding heparin.32
Clinical trials of PET
A clinical trial in which PET was used to visualize islets was performed by Eriksson and colleagues in 2009. They performed PET in 6 patients during islet transplantation, and detected spotty radioactivity uptake in the liver of each patient. The FDG-labeled islets made up 15.0–30.2% of total islets, but there were no adverse events in any of the patients, all of whom showed good glucose tolerance at 1 mo after the transplantation.33 This was the first report to demonstrate the clinical safety of PET imaging and its usefulness for real-time quantitative and qualitative evaluation of the islet kinetics, and was also the only clinical trial using PET for this purpose. However, the results clearly suggested that PET imaging was a useful method for islet engraftment and that further clinical studies would be warranted. Especially, improvement of the probe is necessary.
SPECT studies on transplanted islets
Single-photon emission CT (SPECT) is a nuclear medicine tomographic imaging method using gamma rays. Like PET, SPECT can evaluate functional processes in the body, and thus is useful to detect the early stages of cancer. Also like PET, SPECT is useful to evaluate the conditions of islets based on tracer enhancements as a marker of radioactivity, but the spatial resolution is poor (8–10 mm) and it is impossible to visualize single islets.34 There have been few studies using SPECT to visualize islets, particularly transplanted islets, but Tai and colleagues succeeded in visualizing transplanted islet cell lines on 5-131I-iodo-1-(2-deoxy-2-fluoro-b-D-arabinofuranosyl)uracil ([131I]-FIAU)-enhanced SPECT using a mouse model in 2007.35
MRI
Early MRI studies
MRI is an important imaging modality with advantages such as high spatial resolution, good penetration depth, and strong inhibition of ionizing radiation. However, due to the similar intensity between transplanted islets and liver tissue, MRI cannot be used to visualize islets that have not been pretreated in some way. An experimental trial on the efficacy of MRI for visualizing cells and other tiny structures was started in the late 1990s.36 The structures or cells to be visualized were labeled with an iron-based MRI agent. Islets are very tiny cellular complexes (generally smaller than 400 µm), but it was expected that they could be visualized on MRI using this agent. In 2004, Jirak and colleagues were the first to succeed in visualizing transplanted islets on MRI; they used a rat model and labeled the islets with superparamagnetic iron oxide (SPIO). The islets were detected in the liver as hypointensive spotty areas on T2-weighted images (T2WI) at 7 d after transplantation, while the transplanted diabetic rats had achieved normoglycemia by that time (Table 2; Fig. 1).37 In subsequent studies, the same authors showed that the islets were also visualized at 6 weeks after transplantation,38 and that the hypointensive areas vanished when the transplanted allogeneic39 (Table 2) and xenogeneic40 islets were rejected. These data showed that the SPIO-labeled islets could be visualized by MRI, although this technique did not indicate the viability of the islets. A Harvard group developed a novel type of labeling agent that consisted of SPIO and a fluorescent agent (Cy5.5 dye) to evaluate the islet condition. Labeling islets with these SPIO magnetic nanoparticles modified with a near-infrared fluorescent (MN-NIRF) dye, they succeeded in detecting islets both by MRI and immunohistochemical examination in subrenal capsular and intraportal transplant models.41 They also described that the transplanted xenogeneic islets (human to mouse) had disappeared on T2WI MRI due to immune rejection.42 Their findings confirmed that islets could be detected on MRI by labeling with SPIO, that the labeled islets could be seen in any transplant site, and that MRI could also reveal the condition of the transplanted islets, including their engraftment status, under the certification of fluorescent stained islets immunohistochemically.
Table 2. Progress of islet imaging on MRI.
| Year | References | MRI condition | Strength in scanners | Donor | Recipient | Contrast agent | Comments |
| 2004 | 37 | T2WI | 4.7T | rat | rat | Resovist® | The first study for MRI imaging |
| 2005 | 39 | T2WI | 4.7T | rat (allo) | rat | Resovist® | Rejection model on MRI |
| 2006 | 41 | T2WI | 4.7T | human | mouse (immune deficient) |
SPIO (MN-NIRF) |
Certified islets both on MRI and in histological staining |
| 2006 | 46 | T2WI | 1.5T | rat | rat | Feridex® with poly-l-lysine | Availability of clinical MRI Few toxic agents |
| 2007 | 43 | T1WI | 7T | mouse and human | mouse (immune deficient) |
Gd | The first study of T1WI MRI |
| 2007 | 60 | T1 and T2WI | 11.7T | mouse | mouse | Feridex® and Gd | Visualized islets by Feridex® labeling and vessels around islets by Gd enhancement |
| 2007 | 64 | T2WI | 3T | human | pig | Feridex® (encapsulated islets) | MRI for large animals |
| 2008 | 66 | T2WI | 1.5T | human | human | Resovist® | The first clinical success of MRI imaging |
| 2009 | 54 | T2WI | 4.7T | rat | rat (allo) |
Feridex®, Resovist®, Endorem® | Evaluation of toxicity in various SPIOs |
| 2009 | 65 | T2WI | 1.5T | baboon | baboon (auto) | Feridex® | Autotranplantation model using non-human primates |
| 2010 | 67 | T2WI | 3T | human | human | Resovist® | Clinical MRI imaging |
| 2011 | 53 | T2WI | 1.5T | rat | mouse (immune deficient |
Feridex® with heparin | Lower toxicity Improving engraftment |

Figure 1. Syngeneic transplanted SPIO-labeled islets were seen as hypointensive spotty areas (arrow) in the livers of mice on T2WI MRI (our unpublished data).
Studies for islet imaging with MRI in a clinical setting
On the basis of this successful islet imaging by MRI in these experimental studies, numerous studies have been performed to overcome the challenges to the clinical use of this modality. First, improvement of the contrast agent was necessary because classic SPIO has some drawbacks in terms of stability, magnetic sensitivity, and toxicity.37 Biancone and colleagues tried to visualize islets using gadolinium (Gd) instead of SPIO, and succeeded in visualizing human islets transplanted into immune-deficient mice as hyperintensive areas on T1-weighted images (T1WI) MRI. They also proved that the Gd agent did not impair islets in in vitro assessments of viability and insulin-releasing function.43 Arifin and colleagues developed novel microcapsules for delivering alginate-encapsulated islet cells containing Gd chelates that could be seen as hyperintensive areas on T1WI MRI; the microcapsules conferred immunoprotection against immune-competent cells while responding to changes in blood glucose by releasing insulin.44 Leoni and colleagues tried to label human islets with a manganese (Mn) agent and performed MRI. They revealed that the Mn-enhanced MRI was useful for evaluating isolated islet functions in in vitro assessments.45 Regarding SPIO, some novel agents with high stability and low or no toxicity have also been developed. For example, Tai and colleagues used a new SPIO coated with poly-l-lysine, which has lower toxicity.46,47 Polyvinylpyrrolidone,48,49 chitosan,50-52 and heparin53 have also been used as SPIO coatings. In recent studies, clinical-grade iron nanoparticles, such as ferucarbotran (Resovist®; Bayer Schering Pharma AG), have been used as a more suitable material for labeling islets instead of the classical SPIOs, ferumoxide (Feridex®; AMAG Pharmaceuticals Inc.) and Endorem® (Guerbet). Marzola and colleagues showed that transplanted Resovist®-labeled rat islets could be detected as hypointensive spots in the liver at 42 d after transplantation. The toxicity of Resovist® for islets was weaker than that of Feridex® in an in vitro assay.54 The lower toxicity was also confirmed by the Park group.55 Ris and colleagues also compared three SPIOs, Resovist®, Endorem®, and Feridex®, in terms of their stability and function using rat syngeneic and xenogeneic (human to rat) intraportal transplant models, and found that Resovist® had better insulin-releasing ability and signal stability than Endorem® or Feridex®. They also detected Resovist®-labeled islets in the liver for 8 weeks in a syngeneic transplant model, whereas they had disappeared within 8 weeks in a xenogeneic transplant model.56 Similar data about rejection were also reported by Kriz and colleagues using an allogeneic transplant model.57
Another important question is whether a clinical-grade MRI device could visualize transplanted individual islets. A higher magnetic flux density MRI device (over 4.7 tesla [T]) was used in earlier experimental MRI studies,37,42 and there was no evidence that islets could be visualized using a clinical-grade MRI device with 1.5 T in the mid-2000s. In 2006, Tai and colleagues succeeded in visualizing SPIO-labeled rat islets that were transplanted into the subrenal capsule using a clinical-grade MRI device with 1.5 T (Table 2).46 After their success, 1.5 T became the standard magnetic flux density, and many groups applied this density condition in their experimental studies.54,58,59
MRI is useful for evaluating not only islet imaging but also neovascularization around transplanted islets. Hathout and colleagues focused on neovascularization using experimental animals. They performed syngeneic Feridex®-labeled islet transplantation to the subrenal capsule of mice and performed Gd-enhanced MRI at 3, 7, and 14 d after transplantation. They found Feridex®-labeled islets on T2WI MRI and new Gd-enhanced vessels around the islets on T1WI MRI. The Gd intensity was strongest at 14 d after transplantation, which is the time required to complete neovascularization. Finally, they confirmed the imaging of the vessel network around the islets until 28 d after transplantation60,61 (Table 2). They also performed syngeneic islet transplantation to the right lobe of the liver of diabetic mice, and performed Gd-enhanced MRI at 3, 7, 14, and 28 d after transplantation. The intensity of the right lobe was stronger at 7 d after transplantation than at 3 d, while the intensity of the left lobe had not changed. The degree of intensity was significantly correlated with the number of vessels around the islets.62 Furthermore, they showed that the intensity of the right lobe was significantly correlated with the blood glucose level, serum insulin level, and change in glucose tolerance.63 These data revealed that MRI is a useful modality for evaluating neovascularization around transplanted islets and the endocrinal function of the islets when contrast agent is applied.
MRI studies using larger animals for better approximation of the clinical setting have also been performed. The Johns Hopkins group developed encapsulated islets coated with Feridex®, called magnetocapsules. They then intraportally transplanted the magnetocapsules containing human islets into swine, and showed that the capsules could be detected in the liver as hypointensive spots on T2WI MRI at 3 weeks after transplantation, and that the serum human C-peptide level was also elevated at this time (Table 2).64 Medarova and colleagues succeeded in visualizing Feridex®-labeled baboon islets on T2WI MRI in subrenal capsular and intraportal islet autotransplant models.65
Clinical trials of MRI in islet transplantation
The first clinical trial was done by Toso and colleagues in 2008. They performed SPIO-labeled human islet transplantation in 4 patients with type 1 DM. The percentages of labeled islets were 12.6–66.6%. In 3 of the 4 patients, the islets were detected as hypointensive spots in the liver, and in one of the patients, islets were seen at 6 mo after transplantation (Table 2).66 Saudek and colleagues also performed Resovist®-labeled islet transplantation in 8 type 1 DM patients (5 of them achieved insulin independence), and found hypointensive spots on T2WI MRI at 24 weeks after transplantation.67 On the other hand, an approximately 50% signal loss was detected at 7 d after transplantation and was reduced to 30% at 168 d after transplantation in this clinical trial. This decrease in the signals might reflect early and late islet graft loss.
Summary of MRI studies
In summary, MRI is one of the most advanced modalities for visualization of islets. Some unique studies on contrast agents that function in prolonging the engraftment of islets have been performed by Wang and colleagues.68,69 Many studies have shown a positive correlation between islet engraftment and function and islet image,39,70 but there are some obstacles to promoting this methodology at the clinical level. Recently Zacharovova and colleagues confirmed that SPIOs in the transplanted islets were taken into phagocytic cells including macrophages, and that the hypointensive spots on T2WI MRI might not reflect engrafted islets alone.71 This means that the number of obtained signals might not reflect the number of engrafted islets, which could lead physicians to misinterpret the engraftment of islets (i.e., by leading to false-positive results). Moreover, labeling agents are necessary for MRI examination, and thus the islets cannot be protected from the toxicity of the labeling agents, which might impair the engraftment. The difficulty of long-term visualization of transplanted islets is also a hurdle in the clinical setting. Finally, when reduced islet graft function is observed, MRI cannot be used to evaluate the islet engraftments. All these obstacles should be overcome for the clinical setting of islet visualization using MRI.
US
US is a useful and safe imaging technique for visualizing subcutaneous body structures, and has the advantage of being performed at the bedside. If islets could be visualized by US with sufficient sensitivity, this might provide many benefits for clinicians in evaluating islet function and condition with little stress on patients. However, there have been few experimental trials.
We have investigated the visualization of islets by US. First, we attempted to visualize transplanted islets with high-frequency ultrasonography (HF-US), evaluating the correlations between HF-US findings and islet function. HF-US uses ultrasound at a high frequency (above 20 MHz), thereby producing higher-resolution images than conventional US.72 It has been used to diagnose various diseases.73,74 We transplanted syngeneic (BALB/c mice) and xenogeneic (Sprague-Dawley rats) islets into the subrenal capsular space of diabetic mice. After the transplantation, the mice were examined by HF-US (central frequency 35 MHz, axial resolution 50 µm, focal length 10 mm). In the syngeneic transplant model, a hyperechoic area was detected at the subrenal capsular space during the observation (Fig. 2). On the other hand, transplanted islets were visualized as hypoechoic areas that reflected the damage to the islets due to rejection at 3 d after transplantation; they completely disappeared by 28 d in the xenogeneic transplant model. The islet volume calculated by the HF-US device was correlated with numbers of transplanted islets, blood glucose, and serum insulin.75 These experimental data indicated that US could be used to visualize transplanted islets and to evaluate endocrinal function and condition, including rejection of the islets.
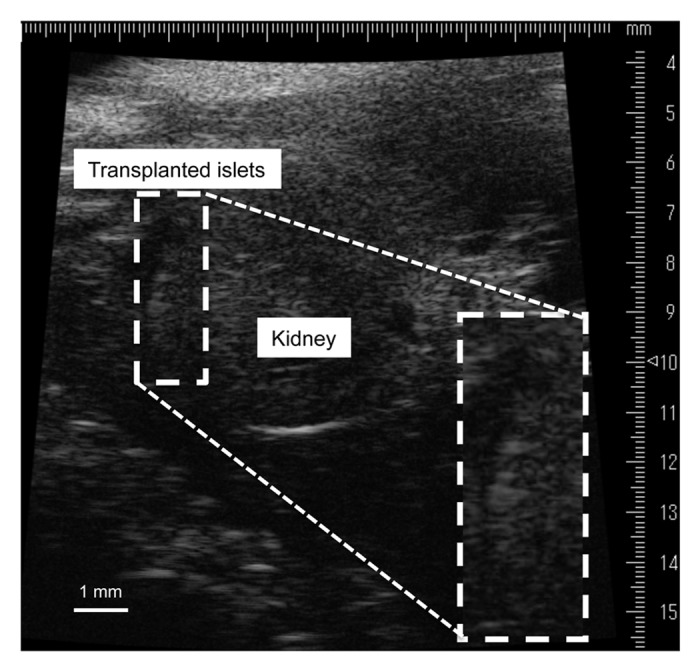
Figure 2. High-frequency ultrasonographic (HF-US) image of transplanted islets in the subrenal capsule. The islets appear as a hyperechoic area on the surface of the kidney. This is a modified version of a figure from a previous study.75
We also clarified that individual islets in the portal vein could be visualized by intraoperative US (the central frequency was 7.5 MHz) in the clinic. We performed total pancreatectomy with islet autotransplantation via the portal vein in a 39-y-old man who had chronic pancreatitis with pancreatic arteriovenous malformation. We examined the portal vein by US during the transplantation, and detected individual transplanted islets as hyperechoic clusters that flowed toward the periphery of the portal vein (Fig. 3).76 This finding and our previously described experimental data clarified some speculations about the use of US imaging for evaluating the islet condition. First, viable islets can be visualized as hyperechoic images not only in rodents but also in humans. It is conceivable that islet imaging in intraoperative US (especially echogenicity) could provide reliable information for predicting the outcome of islet transplantation. Second, islets can be visualized not only with high-frequency US but also with the usual US used for the human abdomen (central frequency of 7.5 MHz) in spite of their tiny structures. Our data also suggest that US could be an essential component in the examination of islet transplantation.

Figure 3. Intraoperative ultrasonographic (US) image for a patient who received islet autotransplantation. The transplanted islets appear as hyperechoic clusters in the portal vein. This is a modified version of a figure from a previous study.76
The next step for US is visualization of transplanted individual islets as in MRI and PET. Recently, Barnett and colleagues developed a new device that contains islets and can be visualized by multimodal imaging techniques. The device was constructed by the encapsulation of islets and a contrast agent including SPIO (perfluorocarbon)77 or gadolinium chelate44 using alginate, which is the material used to encapsulate islets. It not only functions in immune-isolation of the encapsulated islets but also can be visualized by MRI at 9.4 T, micro-CT (CT), and HF-US. These trials are considered the first to succeed in individual islet visualization. Further US studies are clearly warranted.
Conclusion
Non-invasive imaging modalities are available for evaluating islet conditions, including the success or failure of engraftment. In particular, the methodologies of MRI and PET have been rapidly improving. Because these methodologies have different advantages and disadvantages (Table 3), their use in combination is recommended for accurate assessment of the condition of transplanted islets. As one example of the combination, we consider that US can be used for detecting islets during the infusion, PET for evaluating chronic islet dysfunction and MRI for assessing islet engraftment. Moreover, the combined use of these modalities with classic examinations such as blood and urinary tests could also be used for the same purpose. And although it is difficult to apply US to the detection of islets at present, the studies are just beginning. These imaging examinations may help to improve the outcome of islet transplantation in the future.
Table 3. Advantages and disadvantages of PET, MRI, and US.
| Advantages | Disadvantages | |
| PET | Imaging of islets functionally High resolution Good sensitivity No necessity for islet labeling before transplantation |
Ionizing radiation No anatomical information Low spatial resolution (single transplanted islets cannot be visualized) |
| MRI | High resolution High spatial resolution No ionizing radiation |
Necessity of labeling islets before transplantation Toxicity of agent Difficulty of distinguishing between live and dead islets |
| US | No adverse events for patients Can be performed at bedside |
No methodology to visualize single islets at present |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This review was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (B: 22390253 [Egawa S]; B: 22390252 [Katayose Y]; Challenging Exploratory Research: 24659582 [Sakata N]), the Gonryo Medical Foundation, and the Suzuken Memorial Foundation (Sakata N).
Glossary
Abbreviations:
- BLI
bioluminescence imaging
- CT
computed tomography
- DM
diabetes mellitus
- DO3A-VS
1,4,7-tris(acetic acid)-10-vinylsulfone-1,4,7,10-tetraazacyclododecane
- DOTA
1,4,7,10-tetraazacyclododecane-1,4,7-10-tetraacetic acid
- DTBZ
dihydrotetrabenzine
- FDG
2-[18F] fluoro-2deoxy-D-glucose
- FHBG
9-[4-fluoro-3-(hydroxymethyl)butyl]guanine
- FIAU
iodo-1-(2-deoxy-2-fluoro-b-D-arabinofuranosyl)uracil
- Gd
gadolinium
- GLP-1
glucagon-like peptide-1
- HbA1c
hemoglobin A1c
- HF-US
high-frequency ultrasonography
- IBMIR
instant blood-mediated inflammatory reaction
- IL
interleukin
- Mn
manganese
- MN-NIRF
magnetic nanoparticles modified with a near-infrared fluorescent
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- SPECT
single-photon emission computed tomography
- SPIO
superparamagnetic iron oxide
- T1WI
T1-weighted images
- T2WI
T2-weighted images
- US
ultrasonography or ultrasound
- VMAT2
vesicular monoamine transporter 2
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/26980
References
- 1.Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c) Diabetes Metab Res Rev. 2012;28:338–42. doi: 10.1002/dmrr.2260. [DOI] [PubMed] [Google Scholar]
- 2.Andersen DK. The practical importance of recognizing pancreatogenic or type 3c diabetes. Diabetes Metab Res Rev. 2012;28:326–8. doi: 10.1002/dmrr.2285. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonomura N, Shimizu A, Wang S, Yamada K, Tchipashvili V, Weir GC, Yang YG. Pig islet xenograft rejection in a mouse model with an established human immune system. Xenotransplantation. 2008;15:129–35. doi: 10.1111/j.1399-3089.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 5.Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, Felldin M, Källen R, Salmela K, Tibell A, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54:1755–62. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 6.Shivaswamy V, McClure M, Passer J, Frahm C, Ochsner L, Erickson J, Bennett RG, Hamel FG, Larsen JL. Hyperglycemia induced by tacrolimus and sirolimus is reversible in normal sprague-dawley rats. Endocrine. 2010;37:489–96. doi: 10.1007/s12020-010-9332-6. [DOI] [PubMed] [Google Scholar]
- 7.Desai NM, Goss JA, Deng S, Wolf BA, Markmann E, Palanjian M, Shock AP, Feliciano S, Brunicardi FC, Barker CF, et al. Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity? Transplantation. 2003;76:1623–5. doi: 10.1097/01.TP.0000081043.23751.81. [DOI] [PubMed] [Google Scholar]
- 8.Gruessner RW, Sutherland DE, Parr E, Humar A, Gruessner AC. A prospective, randomized, open-label study of steroid withdrawal in pancreas transplantation-a preliminary report with 6-month follow-up. Transplant Proc. 2001;33:1663–4. doi: 10.1016/S0041-1345(00)02632-4. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JD, Ao Z, Ao P, Li H, Dai LJ, He Z, Tee M, Potter KJ, Klimek AM, Meloche RM, et al. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 2009;18:833–45. doi: 10.3727/096368909X471198. [DOI] [PubMed] [Google Scholar]
- 10.Sakata N, Hayes P, Tan A, Chan NK, Mace J, Peverini R, Sowers L, Pearce WJ, Chinnock R, Obenaus A, et al. MRI assessment of ischemic liver after intraportal islet transplantation. Transplantation. 2009;87:825–30. doi: 10.1097/TP.0b013e318199c7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakata N, Obenaus A, Chan N, Mace J, Chinnock R, Hathout E. Factors affecting islet graft embolization in the liver of diabetic mice. Islets. 2009;1:26–33. doi: 10.4161/isl.1.1.8563. [DOI] [PubMed] [Google Scholar]
- 12.Berney T, Toso C. Monitoring of the islet graft. Diabetes Metab. 2006;32:503–12. doi: 10.1016/S1262-3636(06)72803-8. [DOI] [PubMed] [Google Scholar]
- 13.Toso C, Isse K, Demetris AJ, Dinyari P, Koh A, Imes S, Kin T, Emamaullee J, Senior P, Shapiro AM. Histologic graft assessment after clinical islet transplantation. Transplantation. 2009;88:1286–93. doi: 10.1097/TP.0b013e3181bc06b0. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Dang H, Middleton B, Zhang Z, Washburn L, Campbell-Thompson M, Atkinson MA, Gambhir SS, Tian J, Kaufman DL. Bioluminescent monitoring of islet graft survival after transplantation. Mol Ther. 2004;9:428–35. doi: 10.1016/j.ymthe.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Virostko J, Radhika A, Poffenberger G, Chen Z, Brissova M, Gilchrist J, Coleman B, Gannon M, Jansen ED, Powers AC. Bioluminescence imaging in mouse models quantifies beta cell mass in the pancreas and after islet transplantation. Mol Imaging Biol. 2010;12:42–53. doi: 10.1007/s11307-009-0240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Zhang X, Larson C, Xia G, Kaufman DB. Prolonging islet allograft survival using in vivo bioluminescence imaging to guide timing of antilymphocyte serum treatment of rejection. Transplantation. 2008;85:1246–52. doi: 10.1097/TP.0b013e31816b66b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toso C, Zaidi H, Morel P, Armanet M, Andres A, Pernin N, Baertschiger R, Slosman D, Bühler LH, Bosco D, et al. Positron-emission tomography imaging of early events after transplantation of islets of Langerhans. Transplantation. 2005;79:353–5. doi: 10.1097/01.TP.0000149501.50870.9D. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Dang H, Middleton B, Zhang Z, Washburn L, Stout DB, Campbell-Thompson M, Atkinson MA, Phelps M, Gambhir SS, et al. Noninvasive imaging of islet grafts using positron-emission tomography. Proc Natl Acad Sci U S A. 2006;103:11294–9. doi: 10.1073/pnas.0603909103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y, Dang H, Middleton B, Campbell-Thompson M, Atkinson MA, Gambhir SS, Tian J, Kaufman DL. Long-term monitoring of transplanted islets using positron emission tomography. Mol Ther. 2006;14:851–6. doi: 10.1016/j.ymthe.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Doudet DJ, Studenov AR, Nian C, Ruth TJ, Gambhir SS, McIntosh CH. Quantitative micro positron emission tomography (PET) imaging for the in vivo determination of pancreatic islet graft survival. Nat Med. 2006;12:1423–8. doi: 10.1038/nm1458. [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Nian C, Doudet DJ, McIntosh CH. Inhibition of dipeptidyl peptidase IV with sitagliptin (MK0431) prolongs islet graft survival in streptozotocin-induced diabetic mice. Diabetes. 2008;57:1331–9. doi: 10.2337/db07-1639. [DOI] [PubMed] [Google Scholar]
- 22.Simpson NR, Souza F, Witkowski P, Maffei A, Raffo A, Herron A, Kilbourn M, Jurewicz A, Herold K, Liu E, et al. Visualizing pancreatic beta-cell mass with [11C]DTBZ. Nucl Med Biol. 2006;33:855–64. doi: 10.1016/j.nucmedbio.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witkowski P, Sondermeijer H, Hardy MA, Woodland DC, Lee K, Bhagat G, Witkowski K, See F, Rana A, Maffei A, et al. Islet grafting and imaging in a bioengineered intramuscular space. Transplantation. 2009;88:1065–74. doi: 10.1097/TP.0b013e3181ba2e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kung MP, Hou C, Lieberman BP, Oya S, Ponde DE, Blankemeyer E, Skovronsky D, Kilbourn MR, Kung HF. In vivo imaging of beta-cell mass in rats using 18F-FP-(+)-DTBZ: a potential PET ligand for studying diabetes mellitus. J Nucl Med. 2008;49:1171–6. doi: 10.2967/jnumed.108.051680. [DOI] [PubMed] [Google Scholar]
- 25.Connolly BM, Vanko A, McQuade P, Guenther I, Meng X, Rubins D, Waterhouse R, Hargreaves R, Sur C, Hostetler E. Ex vivo imaging of pancreatic beta cells using a radiolabeled GLP-1 receptor agonist. Mol Imaging Biol. 2012;14:79–87. doi: 10.1007/s11307-011-0481-7. [DOI] [PubMed] [Google Scholar]
- 26.Wu Z, Todorov I, Li L, Bading JR, Li Z, Nair I, Ishiyama K, Colcher D, Conti PE, Fraser SE, et al. In vivo imaging of transplanted islets with 64Cu-DO3A-VS-Cys40-Exendin-4 by targeting GLP-1 receptor. Bioconjug Chem. 2011;22:1587–94. doi: 10.1021/bc200132t. [DOI] [PubMed] [Google Scholar]
- 27.Sweet IR, Cook DL, Lernmark A, Greenbaum CJ, Wallen AR, Marcum ES, Stekhova SA, Krohn KA. Systematic screening of potential beta-cell imaging agents. Biochem Biophys Res Commun. 2004;314:976–83. doi: 10.1016/j.bbrc.2003.12.182. [DOI] [PubMed] [Google Scholar]
- 28.Minn H, Kauhanen S, Seppänen M, Nuutila P. 18F-FDOPA: a multiple-target molecule. J Nucl Med. 2009;50:1915–8. doi: 10.2967/jnumed.109.065664. [DOI] [PubMed] [Google Scholar]
- 29.Jager PL, Chirakal R, Marriott CJ, Brouwers AH, Koopmans KP, Gulenchyn KY. 6-L-18F-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: basic aspects and emerging clinical applications. J Nucl Med. 2008;49:573–86. doi: 10.2967/jnumed.107.045708. [DOI] [PubMed] [Google Scholar]
- 30.Garcia A, Mirbolooki MR, Constantinescu C, Pan ML, Sevrioukov E, Milne N, Wang PH, Lakey J, Chandy KG, Mukherjee J. 18F-Fallypride PET of pancreatic islets: in vitro and in vivo rodent studies. J Nucl Med. 2011;52:1125–32. doi: 10.2967/jnumed.111.088583. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson O, Alavi A. Imaging the islet graft by positron emission tomography. Eur J Nucl Med Mol Imaging. 2012;39:533–42. doi: 10.1007/s00259-011-1928-4. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson O, Carlsson F, Blom E, Sundin A, Långström B, Korsgren O, Velikyan I. Preclinical evaluation of a 68Ga-labeled biotin analogue for applications in islet transplantation. Nucl Med Biol. 2012;39:415–21. doi: 10.1016/j.nucmedbio.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson O, Eich T, Sundin A, Tibell A, Tufveson G, Andersson H, Felldin M, Foss A, Kyllönen L, Langstrom B, et al. Positron emission tomography in clinical islet transplantation. Am J Transplant. 2009;9:2816–24. doi: 10.1111/j.1600-6143.2009.02844.x. [DOI] [PubMed] [Google Scholar]
- 34.Andralojc K, Srinivas M, Brom M, Joosten L, de Vries IJ, Eizirik DL, Boerman OC, Meda P, Gotthardt M. Obstacles on the way to the clinical visualisation of beta cells: looking for the Aeneas of molecular imaging to navigate between Scylla and Charybdis. Diabetologia. 2012;55:1247–57. doi: 10.1007/s00125-012-2491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tai JH, Nguyen B, Wells RG, Kovacs MS, McGirr R, Prato FS, Morgan TG, Dhanvantari S. Imaging of gene expression in live pancreatic islet cell lines using dual-isotope SPECT. J Nucl Med. 2008;49:94–102. doi: 10.2967/jnumed.107.043430. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z, Liu S, Hassink M, Nair I, Park R, Li L, Todorov I, Fox JM, Li Z, Shively JE, et al. Development and evaluation of 18F-TTCO-Cys40-Exendin-4: a PET probe for imaging transplanted islets. J Nucl Med. 2013;54:244–51. doi: 10.2967/jnumed.112.109694. [DOI] [PubMed] [Google Scholar]
- 37.Jirák D, Kríz J, Herynek V, Andersson B, Girman P, Burian M, Saudek F, Hájek M. MRI of transplanted pancreatic islets. Magn Reson Med. 2004;52:1228–33. doi: 10.1002/mrm.20282. [DOI] [PubMed] [Google Scholar]
- 38.Koblas T, Girman P, Berkova Z, Jirak D, Kriz J, Dovolilova E, Zacharovova K, Hajek M, Saudek F. Magnetic resonance imaging of intrahepatically transplanted islets using paramagnetic beads. Transplant Proc. 2005;37:3493–5. doi: 10.1016/j.transproceed.2005.09.142. [DOI] [PubMed] [Google Scholar]
- 39.Kriz J, Jirák D, Girman P, Berková Z, Zacharovova K, Honsova E, Lodererova A, Hajek M, Saudek F. Magnetic resonance imaging of pancreatic islets in tolerance and rejection. Transplantation. 2005;80:1596–603. doi: 10.1097/01.tp.0000183959.73681.b9. [DOI] [PubMed] [Google Scholar]
- 40.Jirak D, Kriz J, Strzelecki M, Yang J, Hasilo C, White DJ, Foster PJ. Monitoring the survival of islet transplants by MRI using a novel technique for their automated detection and quantification. MAGMA. 2009;22:257–65. doi: 10.1007/s10334-009-0172-4. [DOI] [PubMed] [Google Scholar]
- 41.Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A. In vivo imaging of islet transplantation. Nat Med. 2006;12:144–8. doi: 10.1038/nm1316. [DOI] [PubMed] [Google Scholar]
- 42.Evgenov NV, Medarova Z, Pratt J, Pantazopoulos P, Leyting S, Bonner-Weir S, Moore A. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes. 2006;55:2419–28. doi: 10.2337/db06-0484. [DOI] [PubMed] [Google Scholar]
- 43.Biancone L, Crich SG, Cantaluppi V, Romanazzi GM, Russo S, Scalabrino E, Esposito G, Figliolini F, Beltramo S, Perin PC, et al. Magnetic resonance imaging of gadolinium-labeled pancreatic islets for experimental transplantation. NMR Biomed. 2007;20:40–8. doi: 10.1002/nbm.1088. [DOI] [PubMed] [Google Scholar]
- 44.Arifin DR, Long CM, Gilad AA, Alric C, Roux S, Tillement O, Link TW, Arepally A, Bulte JW. Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology. 2011;260:790–8. doi: 10.1148/radiol.11101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leoni L, Serai SD, Haque ME, Magin RL, Roman BB. Functional MRI characterization of isolated human islet activation. NMR Biomed. 2010;23:1158–65. doi: 10.1002/nbm.1542. [DOI] [PubMed] [Google Scholar]
- 46.Tai JH, Foster P, Rosales A, Feng B, Hasilo C, Martinez V, Ramadan S, Snir J, Melling CW, Dhanvantari S, et al. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes. 2006;55:2931–8. doi: 10.2337/db06-0393. [DOI] [PubMed] [Google Scholar]
- 47.Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, Bryant LH, Jr., Bulte JW. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–7. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 48.Huang H, Xie Q, Kang M, Zhang B, Zhang H, Chen J, Zhai C, Yang D, Jiang B, Wu Y. Labeling transplanted mice islet with polyvinylpyrrolidone coated superparamagnetic iron oxide nanoparticles for in vivo detection by magnetic resonance imaging. Nanotechnology. 2009;20:365101. doi: 10.1088/0957-4484/20/36/365101. [DOI] [PubMed] [Google Scholar]
- 49.Zhang B, Jiang B, Chen Y, Huang H, Xie Q, Kang M, Zhang H, Zhai C, Wu Y. Detection of viability of transplanted beta cells labeled with a novel contrast agent - polyvinylpyrrolidone-coated superparamagnetic iron oxide nanoparticles by magnetic resonance imaging. Contrast Media Mol Imaging. 2012;7:35–44. doi: 10.1002/cmmi.461. [DOI] [PubMed] [Google Scholar]
- 50.Juang JH, Wang JJ, Shen CR, Kuo CH, Chien YW, Kuo HY, Tsai ZT, Yen TC. Magnetic resonance imaging of transplanted mouse islets labeled with chitosan-coated superparamagnetic iron oxide nanoparticles. Transplant Proc. 2010;42:2104–8. doi: 10.1016/j.transproceed.2010.05.103. [DOI] [PubMed] [Google Scholar]
- 51.Juang JH, Shen CR, Wang JJ, Kuo CH, Lin MY, Wu ST, Tsai ZT, Yen TC. Magnetic resonance imaging study of mouse islet allotransplantation. Transplant Proc. 2010;42:4217–20. doi: 10.1016/j.transproceed.2010.09.089. [DOI] [PubMed] [Google Scholar]
- 52.Juang JH, Shen CR, Wang JJ, Kuo CH, Chien YW, Kuo HY, Chen FR, Chen MH, Yen TC, Tsai ZT. Magnetic resonance imaging of mouse islet grafts labeled with novel chitosan-coated superparamagnetic iron oxide nanoparticles. PLoS One. 2013;8:e62626. doi: 10.1371/journal.pone.0062626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung MJ, Lee SS, Hwang YH, Jung HS, Hwang JW, Kim MJ, Yoon S, Lee DY. MRI of transplanted surface-labeled pancreatic islets with heparinized superparamagnetic iron oxide nanoparticles. Biomaterials. 2011;32:9391–400. doi: 10.1016/j.biomaterials.2011.08.070. [DOI] [PubMed] [Google Scholar]
- 54.Marzola P, Longoni B, Szilagyi E, Merigo F, Nicolato E, Fiorini S, Paoli GT, Benati D, Mosca F, Sbarbati A. In vivo visualization of transplanted pancreatic islets by MRI: comparison between in vivo, histological and electron microscopy findings. Contrast Media Mol Imaging. 2009;4:135–42. doi: 10.1002/cmmi.274. [DOI] [PubMed] [Google Scholar]
- 55.Park KS, Lee HS, Kim YS, Kang TM, Lee JH, Joh JW, Kim SJ. Improved quantification of islet transplants by magnetic resonance imaging with Resovist. Pancreas. 2011;40:911–9. doi: 10.1097/MPA.0b013e31821fd66a. [DOI] [PubMed] [Google Scholar]
- 56.Ris F, Lepetit-Coiffe M, Meda P, Crowe LA, Toso C, Armanet M, Niclauss N, Parnaud G, Giovannoni L, Bosco D, et al. Assessment of human islet labeling with clinical grade iron nanoparticles prior to transplantation for graft monitoring by MRI. Cell Transplant. 2010;19:1573–85. doi: 10.3727/096368910X515863. [DOI] [PubMed] [Google Scholar]
- 57.Kriz J, Jirak D, Berkova Z, Herynek V, Lodererova A, Girman P, Habart D, Hajek M, Saudek F. Detection of pancreatic islet allograft impairment in advance of functional failure using magnetic resonance imaging. Transpl Int. 2012;25:250–60. doi: 10.1111/j.1432-2277.2011.01403.x. [DOI] [PubMed] [Google Scholar]
- 58.Malosio ML, Esposito A, Poletti A, Chiaretti S, Piemonti L, Melzi R, Nano R, Tedoldi F, Canu T, Santambrogio P, et al. Improving the procedure for detection of intrahepatic transplanted islets by magnetic resonance imaging. Am J Transplant. 2009;9:2372–82. doi: 10.1111/j.1600-6143.2009.02791.x. [DOI] [PubMed] [Google Scholar]
- 59.Kim HS, Kim H, Park KS, Moon WK. Evaluation of porcine pancreatic islets transplanted in the kidney capsules of diabetic mice using a clinically approved superparamagnetic iron oxide (SPIO) and a 1.5T MR scanner. Korean J Radiol. 2010;11:673–82. doi: 10.3348/kjr.2010.11.6.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hathout E, Sowers L, Wang R, Tan A, Mace J, Peverini R, Chinnock R, Obenaus A. In vivo magnetic resonance imaging of vascularization in islet transplantation. Transpl Int. 2007;20:1059–65. doi: 10.1111/j.1432-2277.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hathout E, Chan NK, Tan A, Sakata N, Mace J, Pearce W, Peverini R, Chinnock R, Sowers L, Obenaus A. In vivo imaging demonstrates a time-line for new vessel formation in islet transplantation. Pediatr Transplant. 2009;13:892–7. doi: 10.1111/j.1399-3046.2008.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan N, Obenaus A, Tan A, Sakata N, Mace J, Peverini R, et al. Monitoring neovascularization of intraportal islet grafts by dynamic contrast enhanced magnetic resonance imaging. Islets. 2009;1:249–55. doi: 10.4161/isl.1.3.9862. [DOI] [PubMed] [Google Scholar]
- 63.Sakata N, Obenaus A, Chan NK, Hayes P, Chrisler J, Hathout E. Correlation between angiogenesis and islet graft function in diabetic mice: magnetic resonance imaging assessment. J Hepatobiliary Pancreat Sci. 2010;17:692–700. doi: 10.1007/s00534-010-0269-1. [DOI] [PubMed] [Google Scholar]
- 64.Barnett BP, Arepally A, Karmarkar PV, Qian D, Gilson WD, Walczak P, Howland V, Lawler L, Lauzon C, Stuber M, et al. Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat Med. 2007;13:986–91. doi: 10.1038/nm1581. [DOI] [PubMed] [Google Scholar]
- 65.Medarova Z, Vallabhajosyula P, Tena A, Evgenov N, Pantazopoulos P, Tchipashvili V, Weir G, Sachs D, Moore A. In vivo imaging of autologous islet grafts in the liver and under the kidney capsule in non-human primates. Transplantation. 2009;87:1659–66. doi: 10.1097/TP.0b013e3181a5cbc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toso C, Vallee JP, Morel P, Ris F, Demuylder-Mischler S, Lepetit-Coiffe M, Marangon N, Saudek F, James Shapiro AM, Bosco D, et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant. 2008;8:701–6. doi: 10.1111/j.1600-6143.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 67.Saudek F, Jirák D, Girman P, Herynek V, Dezortová M, Kríz J, Peregrin J, Berková Z, Zacharovová K, Hájek M. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation. 2010;90:1602–6. doi: 10.1097/TP.0b013e3181ffba5e. [DOI] [PubMed] [Google Scholar]
- 68.Wang P, Yigit MV, Medarova Z, Wei L, Dai G, Schuetz C, Moore A. Combined small interfering RNA therapy and in vivo magnetic resonance imaging in islet transplantation. Diabetes. 2011;60:565–71. doi: 10.2337/db10-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang P, Yigit MV, Ran C, Ross A, Wei L, Dai G, Medarova Z, Moore A. A theranostic small interfering RNA nanoprobe protects pancreatic islet grafts from adoptively transferred immune rejection. Diabetes. 2012;61:3247–54. doi: 10.2337/db12-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim JH, Jin SM, Oh SH, Lee S, Oh BJ, Kim SK, Suh S, Lee JH, Jung HS, Lee MS, et al. Counting small hypointense spots confounds the quantification of functional islet mass based on islet MRI. Am J Transplant. 2012;12:1303–12. doi: 10.1111/j.1600-6143.2011.03941.x. [DOI] [PubMed] [Google Scholar]
- 71.Zacharovová K, Berková Z, Jirák D, Herynek V, Vancová M, Dovolilová E, Saudek F. Processing of superparamagnetic iron contrast agent ferucarbotran in transplanted pancreatic islets. Contrast Media Mol Imaging. 2012;7:485–93. doi: 10.1002/cmmi.1477. [DOI] [PubMed] [Google Scholar]
- 72.Chen JY, Chen HL, Wu SH, Tsai TC, Lin MF, Yen CC, Hsu WH, Chen W, Chen CM. Application of high-frequency ultrasound for the detection of surgical anatomy in the rodent abdomen. Vet J. 2012;191:246–52. doi: 10.1016/j.tvjl.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 73.Lin YH, Huang CC, Wang SH. Quantitative assessments of burn degree by high-frequency ultrasonic backscattering and statistical model. Phys Med Biol. 2011;56:757–73. doi: 10.1088/0031-9155/56/3/014. [DOI] [PubMed] [Google Scholar]
- 74.Koenig RW, Schmidt TE, Heinen CP, Wirtz CR, Kretschmer T, Antoniadis G, Pedro MT. Intraoperative high-resolution ultrasound: a new technique in the management of peripheral nerve disorders. J Neurosurg. 2011;114:514–21. doi: 10.3171/2010.9.JNS10464. [DOI] [PubMed] [Google Scholar]
- 75.Sakata N, Kodama T, Chen R, Yoshimatsu G, Goto M, Egawa S, Unno M. Monitoring transplanted islets by high-frequency ultrasound. Islets. 2011;3:259–66. doi: 10.4161/isl.3.5.17058. [DOI] [PubMed] [Google Scholar]
- 76.Sakata N, Goto M, Gumpei Y, Mizuma M, Motoi F, Satomi S, Unno M. Intraoperative ultrasound examination is useful for monitoring transplanted islets: a case report. Islets. 2012;4:339–42. doi: 10.4161/isl.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barnett BP, Ruiz-Cabello J, Hota P, Liddell R, Walczak P, Howland V, Chacko VP, Kraitchman DL, Arepally A, Bulte JW. Fluorocapsules for improved function, immunoprotection, and visualization of cellular therapeutics with MR, US, and CT imaging. Radiology. 2011;258:182–91. doi: 10.1148/radiol.10092339. [DOI] [PMC free article] [PubMed] [Google Scholar]


