Abstract
The aim of this study was to assess recovery, cell death, and cell composition of post-thaw cultured human islets. Cryopreserved islets were provided by the Clinical Islet Transplant Program, Edmonton, Canada. Islets were processed using media prepared in accordance with Pre-Edmonton and Edmonton protocols. Cryopreserved islets were rapidly thawed and cultured for 24 h, 3 d, 5 d, and 7 d, following which they were processed for histology. Islet quantification, integrity, morphology and tissue turnover were studied via hematoxylin and eosin stained sections. Ultrastructure was studied by electron microscopy and endocrine cell composition by immunohistochemistry. Using the Pre-Edmonton protocol, islet recovery was 50.1% and islet survival was 50% at 24 h while for the Edmonton protocol, the islet recovery was 69.4% (p < 0.001) and islet survival, 50% at ≈2.5 d. With an increasing culture duration although the physical integrity was retained there was an increasing loss of cohesivity both at light microscopic and at ultrastructure level regardless of the protocols used. Percentage islet survival and tissue turnover correlated negatively with culture duration in both protocols. The Edmonton protocol appears to preserve the islets better. However, culture duration adversely affects islet survival and quality, indicating the need for more optimal cryopreservation and culture techniques.
Keywords: islet transplantation, cryopreservation, post-thaw culture, islet isolation, clinical phase, pre-clinical phase, ultrastructure TUNEL, immunohistochemistry
Introduction
Islet transplantation would be the ideal treatment for individuals with insulin dependent type 1 diabetes, particularly those who are unable to achieve adequate glycemic control despite insulin or those with frequent hypoglycemic episodes. Until the Edmonton protocol was developed in 2000,1,2 only 10% of the islet transplants enjoyed a 1 y freedom from insulin.3 Between 2000 and 2012, approximately 750 successful islet transplants have been performed worldwide. The Edmonton group have achieved a 70% 8-y graft survival rate, while worldwide the 3 y graft survival rate is around 50%.3 The key reasons for the success of the Edmonton protocol were stringent donor and recipient criteria, a critical islet mass of 9000–11,000 islet equivalents (IEQ) per kilogram of recipient weight, transplant of islets from 2 donors, and use of a steroid-free immunosuppression protocol.1-5
While a healthy pancreas contains about 1 million islets, donor islets are exposed to various stresses ranging from hypoxia related to donor health status preceding brain or cardiac death, warm ischemic stress prior to pancreas procurement, cold ischemic stress in the laboratory prior to islet isolation, and finally the enzyme and osmotic stress of the islet isolation procedure.6 These stresses result in 15–50% loss of islet mass and it also affects islet functionality, integrity and viability. Culture of islets post-isolation also accounts for at least 15% loss in islet mass on an average.6 Taking into consideration the potential loss of islet mass due to stress the “islet dosage” measured by the number of islets in IEQ/kg recipient body weight required to restore insulin independence, also termed “critical islet mass” was developed. Although the stipulated critical islet mass is 9000–11,000 IEQ/kg recipient weight,1-4,6 islet preparations that yield 5000 IEQ/kg recipient weight are considered successful and worthy of being transplanted.4
Islet preparations that fall below this critical islet mass, or those that are awaiting a suitable recipient/tissue-typing, require storage to improve their shelf life.4,7 Depending on the lag time, islets to be transplanted may be stored in short-term via tissue culture. Potentially indefinite storage can be attained using cryopreservation in liquid nitrogen. However, current protocols for cryopreservation are injurious to islets, and transplantation of preparations accrued from multiple donors carry an excessive risk of donor sensitization wherein a recipient develops high antibody levels against islets from multiple donors. Optimal islet usage is essential to ensure sustainability and viability of the clinical islet transplant program given that each human islet isolation procedure costs about $20,000 in the US.4,7,8 Thus, clinical implementation of cryopreservation requires improved protocols to optimize maintenance of islet integrity, viability and functionality as well as measures to minimize release of human leukocyte antigens (HLA). The islet preservation procedure could additionally provide an opportunity to carry out strict quality control measures, to reduce major histocompatibility complex class-I (MHC class-I) antigen expression and more importantly, to facilitate pooling of islets from several donors and islet transport between centers.7-10
Cryopreservation still has some drawbacks. As stated earlier, post-thaw islets must deal with the burden of cumulated stress: from post-mortem ischemia to the rigors of islet isolation and cryopreservation, to the thawing process itself.7-14 Indeed, several studies on post-cryo stress have reported a decline in islet viability following cryopreservation.8,11-16 The other inherent challenges of cryopreservation include the cytotoxic effect of the cryoprotectant, osmotic stress and the formation of inter and intra-cellular ice.17-20 In addition, cryopreserved islets must face the same set of challenges in reverse order during a thaw, which affects islet physical integrity.7,8,11-13 Thus, it is mandatory to culture post-thaw islets prior to transplantation to ensure complete removal of cryoprotectant in order to protect the recipient.19,20 However, the increasing culture duration has its own effect causing a steady decline in islet viability.9,10,21,22
This study deals with post-thaw cultured human islets using 2 protocols, the Pre-Edmonton protocol and the Edmonton protocol, comparing their effect on islet cell recovery, survival, and cell composition.
Results
Table 1 presents the results of the islet survival, tissue turnover, and cell composition studies performed via histomorphology. Islet recovery post-thaw was 50.1% for islets processed using the Pre-Edmonton protocol and 69.4% for islets processed using the Edmonton protocol (p < 0.001). Islet survival rate was negatively correlated with culture duration (rs = -0.764, p-value < 0.01). While there was an overall decline in islet size in terms of IEQ with increasing culture duration the proportion of islets surviving at each culture duration was significantly greater (p < 0.001) for islets processed using the Edmonton protocol (Table1) compared with islets processed using the Pre-Edmonton protocol (p < 0.001). Islets processed using the Edmonton protocol fared better than islets processed using the Pre-Edmonton protocol with islet survival (%) reaching 50% at ≈2.5 d in the former, while in the latter, islet survival fell to 50% at 24 h. We looked at the insulin stimulation index (SI) at islet survival of 50% which was at 24 h for islets processed using the Pre-Edmonton protocol which was nearly 3-fold at 2.83 while for islets processed using the Edmonton protocol the SI at islet survival of 50% (3 d) was 1.12.
Table 1. Histomorphological assessment of post-thaw isolated human islets in culture.
| Variables | Culture duration | Pre-Edmonton protocol | Edmonton protocol | p value | |
|---|---|---|---|---|---|
| Cumulative survival % measured in terms of islet mass in islet equivalents (IEQ)# | 24 h | 50.1 ± 10.6 | 69.4 ± 3.5 | 0.001 | |
| 3 d | 17.3 ± 3.7 | 31.5 ± 1.6 | 0.001 | ||
| 5 d | 2.2 ± 0.5 | 11.9 ± 0.6 | 0.001 | ||
| 7 d | 0.1 ± 0 | 2 ± 0.1 | 0.001 | ||
| In vitro tests | Viability (%) | 24 h | 8.3 | 59.1 | 0.001 |
| 3 d | 18.2 | 38.9 | 0.242 | ||
| 5 d | 66.7 | 81.0 | 0.353 | ||
| 7 d | 57.1 | 23.5 | 0.038 | ||
| Insulin stimulation index (SI) at cumulative survival of 50% | 24 h | 2.83 | 1.22 | N/A | |
| 3 d | N/A | 1.12 | |||
|
Histomorphology – islet hormones |
Insulin (%) | 24 h | 82.8 ± 10.5 | 82.9 ± 2.7 | 0.950 |
| 3 d | 89 ± 4.6 | 87.7 ± 1.4 | 0.428 | ||
| 5 d | 78.8 ± 5 | 84.2 ± 0.8 | 0.001 | ||
| 7 d | 73.2 ± 0.5 | 97 ± 0.3 | 0.001 | ||
| Overall | 83.1 ± 9.1 | 86.9 ± 5.8 | 0.006 | ||
| Overall | Glucagon (%) | 11.3 ± 3.8 | 10.7 ± 5.9 | 0.580 | |
| PP(%) | 5.8 ± 1.2 | 5.4 ± 1.7 | 0.142 | ||
| Somatostatin (%) | 3.3 ± 1.6 | 3.5 ± 1.2 | 0.527 | ||
| Islet hormone ratios## | 80: 11: 6: 3 | 82: 10: 5: 3 | N/A | ||
| Histomorphology –tissue turnover | Apoptotic index (%) | 24 h | 2.7 ± 0.8 | 2.1 ± 0.7 | 0.315 |
| 3 d | 1.5 ± 0.6 | 1.5 ± 0.9 | 0.987 | ||
| 5 d | 0.8 ± 0.5 | 1.3 ± 0.8 | 0.443 | ||
| 7 d | 1 ± 1 | 0.4 ± 0.4 | 0.554 | ||
| Overall | 1.8 ± 0.3 | 1.6 ± 0.3 | 0.572 | ||
| Necrotic index (%) | 24 h | 15 ± 13.8 | 12.5 ± 3.5 | 0.850 | |
| 3 d | 8.4 ± 4.4 | 27.6 ± 4.5 | 0.030 | ||
| 5 d | 8.5 ± 3.5 | 5 ± 2.9 | 0.474 | ||
| 7 d | 15.6 ± 8.7 | 7.8 ± 1.3 | 0.342 | ||
| Overall | 11.7 ± 4.3 | 12.3 ± 2.4 | 0.901 | ||
| Cell turnover (%) | 24 h | -17.7 ± 13.1 | -14.6 ± 4 | 0.808 | |
| 3 d | -10 ± 4.2 | -29.1 ± 5.4 | 0.036 | ||
| 5 d | -9.3 ± 3.6 | -6.3 ± 2.9 | 0.542 | ||
| 7 d | -16.5 ± 7.8 | -8.2 ± 1.7 | 0.280 | ||
| Overall | -13.2 ± 4.1 | -13.7 ± 2.6 | 0.926 | ||
# Correlation estimates of islet equivalents and culture duration were rs = -764, p < 0.001. Survival was manually computed. All values are in mean ± SE; ##Islet hormone ratios, Insulin: Glucagon: PP: Somatostatin; Mitotic index was not computed as no mitosis was seen
The next step was to observe how much of this islet quantity was affected by cell loss via tissue turnover assessed in vitro via the viability test and via histomorphology (Table 1). Islet viability was 8.3% at 24 h for islets processed using the Pre-Edmonton protocol while for islets processed using the Edmonton protocol it was 59.1% (p < 0.001). Comparing the viability with islet survival; islet processed using the Pre-Edmonton protocol had a survival 50.1% and a viability of 8.3% implying that only 8.3% of the 50.1% islet mass in islet equivalents that survived at 24 h was viable. Similarly for islets processed using the Edmonton protocol survival was 69.4% and viability was 59.1% indicating that 59.1% of the 69.1% islet mass in islet equivalents that survived at 24 h was viable. Thus, looking at the cumulative survival% and the viability together over the culture duration (24 h to 7 d), it is seen that islets processed using the Edmonton protocol fared significantly better (p < 0.001). Apoptotic index, mitotic index and necrotic index were determined by light microscopy and the cell/tissue turnover was calculated for each culture duration (Table 1). The loss of tissue peaked on day 3, declined on day 5, and increased on day 7 irrespective of the protocol used. Culture duration was negatively correlated with apoptotic index (rs = -0.450, p value < 0.01) and cell turnover (rs = -0.978, p value < 0.001).
The IHC for insulin, glucagon, and somatostatin were performed (Table 1; Fig. 1) to examine the endocrine cell composition of post-thaw islets with culture duration. Among the islet hormones indices, the insulin index declined in islets processed using the Pre-Edmonton protocol while for islets processed using the Edmonton protocol it increased slightly with culture duration. For islets processed using the Pre-Edmonton protocol the somatostatin index was negatively correlated (rs = -0.339, p < 0.05) with culture duration. For islets processed using the Edmonton protocol the glucagon index was negatively correlated (rs = -0.595, p < 0.001) and insulin index was positively correlated with culture duration (rs = 0.696, p < 0.001).
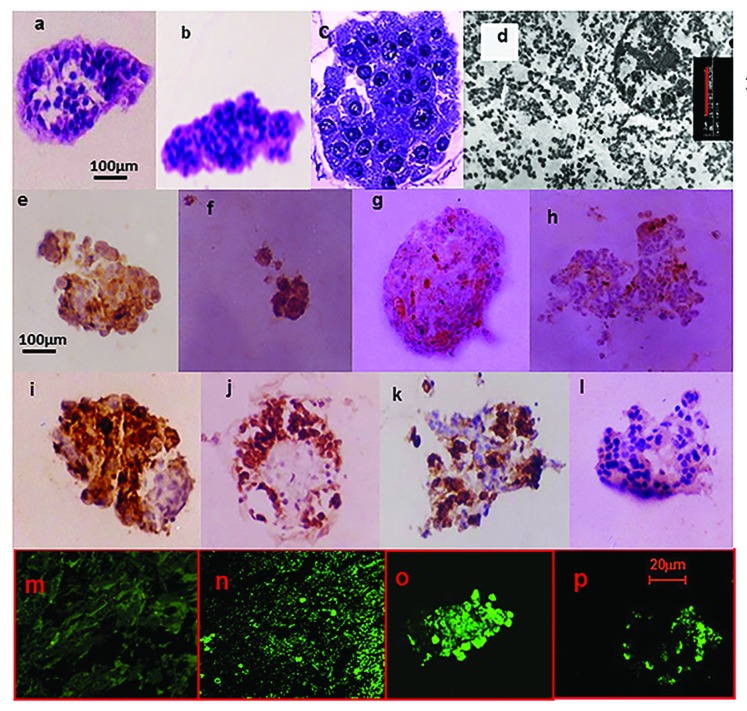
Figure 1. Histomorphology studies on post-thaw cultured isolated human islets. Physical integrity: 320× Light Microscopy 5 μm sections H&E stain of islets processed using (A) the Pre-Edmonton protocol and (B) Edmonton protocol. The islets were not contaminated by the presence of acinar tissue in both cases, illustrating the efficiency of the isolation procedure. With increasing culture duration, there was an increasing loss of cohesivity. Islets that retained their physical integrity (mantle or core was intact) exhibited cytoplasmic and nuclear appearance synonymous with normal cellular structure. While such islets were visible on all days of culture, their frequency steadily declined with culture duration. (C) 1000×—Zoom 2 semi-thin sections toluidine blue stain of islets processed using the Edmonton protocol. There was regional damage to the intra-islet extra-cellular matrix (ECM) (as illustrated). Nevertheless, cytoplasmic and nuclear integrity was retained. (D) β-cell ultra-structure uranylacetate and lead citrate stain of islets processed using the Edmonton protocol. β-cell granules were visible, though there was loss of the halo; ribosomes were present though detached; lysosomes had not yet disintegrated; nuclear, mitochondria, and plasma membrane integrity were also retained. Functionality: Immunohistochemistry (IHC) for the islet hormones (x320). By days 5 and 7, there were increasing number of ghost cells that answered positive for more than one of the islet hormones. IHC for Insulin (E); Glucagon (F); Somatostatin (G); and PP (H) islets processed using the Pre-Edmonton protocol. IHC for Insulin (I); Glucagon (J); Somatostatin (K); and PP (L) islets processed using the Edmonton protocol. Apoptosis: Confocal Microscopy In Situ Cell Death (Apoptosis) Detection Kit, Terminal Uridine Nucleotide End Labeling (TUNEL)-Roche. Fluorescein labeling: (M) negative control, (N) positive control, (O) islets processed using the Pre-Edmonton protocol, (P) islets processed using the Edmonton protocol.
Histomorphological assessment of islet integrity was estimated using standard criteria.26,30-39 Assessments of light microscopic, semi-thin structure and ultra-thin structure were performed. Histomorphological assessment of islets processed using the Edmonton protocol and islets processed using the Pre-Edmonton protocol revealed that with increasing culture duration, there was an increasing loss of cohesivity. By day 5 and day 7, there were an increasing number of ghost cells that stained positive for more than one of the islet hormones. Further, islets that retained their physical integrity (mantle was intact) retained cytoplasmic and nuclear appearance synonymous with normal cellular structure. While such stable islets were visible on all days of culture their frequency steadily declined with culture duration.
Semi-thin sections of islets processed using the Pre-Edmonton protocol and the Edmonton protocol studied at 24 h culture duration showed regional damage to the intra-islet extra-cellular matrix (ECM). Nevertheless, cytoplasmic and nuclear integrity was retained.
Islets processed using the Edmonton protocol was studied at ultra-structural level as the condition of the tissue (observed via semi-thin sections) indicated that it would withstand the rigors of tissue processing and preparation procedures for electron microscopy. The same regional loss of cohesivity was seen. The β cell granules were visible though there was loss of the halo; the ribosomes were present though detached and the lysosomes had not yet disintegrated. The nuclear, mitochondria and plasma membrane integrity were also retained. Taken together these morphological features23-26 indicate that the cells were in the reversible stage of cell injury which is further confirmed by an islet survival of 50% in ≈2.5 d (Table 1). The islets were minimally contaminated by the presence of acinar tissue in both cases, confirming the efficiency of the isolation procedure.
Discussion
This study suggests that the Edmonton protocol1,21 appears to protect post-thaw islets in culture significantly better than the Pre-Edmonton protocol. This was confirmed by hormone indices as well as the histomorphological assessment of the islets.
Post-thaw islet survival fell to 50% by 24 h for islets processed using pre-Edmonton protocol, while using Edmonton protocol it took 2.5 d to decline to 50%. The duration of culture was found to be negatively correlated with islet size in IEQ and positively correlated with the apoptotic index. This confirms previous studies that suggest larger islets are more vulnerable to damage.27,28 The degree of correlation between islet size in IEQ found in our study (r = -0.764) was similar to that reported in 2 studies performed in 1999 and 2003 (r < -0.80) which argued that while the insulin vs. glucose response could be improved by culture, culture in itself affected the volume of the functional islet mass available in a transplant with the larger islets being more vulnerable than smaller islets.27,28
Ultra-structural assessment of the islets processed using the Edmonton protocol revealed that at 24 h the cell morphology was akin to that seen in the reversible stage of cell injury. These findings showed that islet morphology was preserved post-thaw.17,27-29 Histomorphological assessment of the islets by light microscopy confirmed that with increasing duration of culture there was a decrease in islet size which was due to increasing loss of cohesivity within the islets.23-26,30-34 The loss of tissue peaked on day 3, declined on day 5, and increased on day 7 irrespective of the protocol used. The former could be due to the effect of the thaw and the latter due to culture of isolated islets that had not yet undergone revascularization. The decline in islet survival % and the changes in apoptotic and necrotic index observed with culture duration are in keeping with the findings of other studies that the process of islet isolation and cryopreservation greatly influence islet survival.6-10 A key factor that influences post-thaw islet survival is the various stresses that islets are exposed to that include: on cryopreservation islets undergo 2 transient cell shrinkages and swelling 1 during cryoprotectant permeation and the other prior to intracellular and extracellular freezing.7 On thaw, the cells experience osmotic stress during the removal of the cryoprotectant and gradually return to normal volume. The influence of culture on islet survival has been long documented both in freshly cultured and post-thaw cultured isolated islets. One study reported an 80% reduction in DNA content following 24 h culture and another reported an 18% recovery rate after 48 h culture.35,36, As to their usefulness in transplant, pooled islets from several donors consisting of both freshly and post-thaw isolated islets have successfully restored insulin independence up to roughly over 1 y.37
Though the SI for islets processed using the Edmonton protocol was lower than islets processed using the Pre-Edmonton protocol, the viability and islet survival (%) of islets processed using the Edmonton protocol was consistently higher at each culture duration than islets processed using the Pre-Edmonton protocol. The true potential potency of an islet dosage is the composite function of islet survival (%) in terms of islet mass in IEQ, islet viability, tissue turnover and islet functionality looked at via the SI and IHC. Looking at the mentioned variables together, islets processed using the Edmonton protocol was consistently better than islets processed using the Pre-Edmonton protocol.
The key role of cryopreservation in achieving islet sustainability and optimal usage of scarce insulin-producing tissue38-48 is highlighted by a study wherein out of 56 pancreata processed for islets isolation, 25 pancreata were not used owing to low islet yields in IEQ.41 This underlines the need for a better cryopreservation methods, because of the potential to combine and thus use those low-yield islet isolations. A study comparing fresh and post-thaw islet viability via transplantation found that insulin recovery after transplantation was comparable.38,47,48 Islet culture following a thaw is the key to restoring glucose-stimulated insulin release.27-29,48 Efforts to minimize the effect of the cryoprotectant include hypothermic exposure to the cryoprotectant using stepwise addition and slow cooling.10,27,28 Cultured post-thaw isolated human islets have been successful in restoring long-term euglycemia with persistent C-peptide secretion at 18 mo post-transplant12,14,15,19-21,43,47 showing that transport of post-thaw islets between centers is a feasible option.3 Studies on revascularization revealed that post-thaw islets retained the capacity to develop new vessels.27,29 Frozen-thawed islets harvested 14 d after renal subcapsular xenografting in nude mice were revascularized and well granulated.17
While there is 1 study38 comparing islets isolated using Pre-Edmonton and Edmonton protocols, there is no study to our knowledge evaluating the viability of post-thaw islets. The limitation of this study is that the study design did not include the pre-cryopreservation assessment of the islets in culture and hence the influence of the process of cryopreservation on the outcome could not be addressed. Therefore, significance of results presented is restricted to the issue of the influence of protocol used and culture duration on post-thaw islet viability.
In summary, this is one of the first studies to evaluate post-thaw cultured isolated islets and shows that the Edmonton protocol may improve islet survival and β cell preservation. More research is needed in the area of cryopreservation of islets to increase the worldwide availability and feasibility of islet banking and islet transplantation.
Methodology
Islets
We used post-thaw isolated human islets following receipt of Institutional Review Board (IRB) approval and studied them as follows:
(1) 50,000 IEQ cryopreserved human islets that had been isolated using the Pre-Edmonton protocol islet isolation procedures and (2) 50,000 IEQ cryopreserved human islets that had been isolated using the Edmonton protocol.
The Edmonton protocol procedure included use of the low endotoxin level Liberase (endotoxin level < 50 units/mg) which replaced crude collagenase (endotoxin level 300–7000 units/mg). Moreover, human serum albumin (HSA) replaced fetal bovine serum (FBS) to meet federal drug administration (FDA) and current good medical practice (cGMP) safety guidelines. Finally, L-glutamine, nicotinamide and insulin-transferrin-selenium (ITS) served as a supplement.1-4
Thawing procedure
Thawing of islets was performed based on standard protocols.7,8 ≈50,000 IEQ human islets cryopreserved at -196 °C were rapidly thawed to 37 °C in a water bath until the sample reached 0 °C (i.e., the ice melted). The samples were then transferred to an ice slush in order to maintain the temperature at 0°C until all the tubes had thawed. The supernatant was aspirated following centrifugation at 450 g (1500 rpm) so that the islets formed a loose pellet. One milliliter of 0.75 M sucrose was added to effect the removal of the cryoprotectant (2 M dimethyl-sulfoxide (DMSO)). The tubes were maintained at 0 °C in the ice slush for 30 min for equiliibration with sucrose. Sucrose removal was performed by serial dilution with CMRL1066 i.e., with a 5 min incubation period between each addition of (1 mL, then 2 mL, and a final 1 mL) medium. After a further 5 min equilibration period, the islets were pelleted at 450 g (1500 rpm) and the supernatant was removed (a total of 20 min). The islets were washed in supplemented medium and transferred to T-75cm2 (non-tissue culture treated) flasks and placed in an incubator and kept at 37 °C, 5% CO2, 95% humidity for 48 h to allow for metabolic recovery.
Islet culture procedure
All tissue culture reagents were obtained from Gibco. Glucose and dithizone were obtained from Sigma Chem. Co. Pre-Edmonton protocol media: CMRL1066 (5.6 mM glucose) supplemented with 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin, 25 mM HEPES buffer, pH.7.4.
Edmonton protocol media: CMRL1066 (5.6 mM glucose) supplemented with 10% HSA, 100 units/mL penicillin, 100 μg/mL streptomycin, 25 mM HEPES buffer, 2 mM L-glutamine, ITS supplement (5 mg/L insulin + 5 mg/L transferrin + 5mg/L selenium, Sigma Chem. Co., and 10 mM nicotinamide, pH.7.4.
During the 48 h recovery period following a thaw, media contained 20% FBS (Pre-Edmonton protocol) or 20% HSA (Edmonton protocol) after which 10% FBS or 10% HSA was used. Islets were cultured in triplets for 24 h, 3 d, 5 d, and 7 d, following which the tissue was processed for histology where barring ultrastructural studies 4 serial sections were prepared for each of the histomorphology tests performed. Ultrastructural studies were performed on islets cultured for 24 h. Regions within a semi-thin section that showed high islet concentration and indicated that the tissue was in good condition to withstand tissue processing for electron microscopy were studied.49,50 BD Falcon™ 75 cm2 Cell Culture Flask, Non-treated polystyrene, were used and ≈5000 IEQ were cultured in 30 mL media at 37 °C, 5% CO2 and 95% air with media changed every third day using standard protocols.7,8
In vitro tests on islets
Quantification of islets was performed at the end of the recovery period and at the end of each culture duration using standard dithizone staining protocols.30 Islet viability was also similarly measured using fluorescein diacetate (FDA)/propidium iodide(PI) final concentration FDA:0.46 µM and PI: 14.34 µM using the fluorescence microscope, with the filter set for fluorescein (emission ~530 nm) and rhodamine (emission > 600 nm) using standard protocols.31 FDA, a non-polar ester, passes through the cell membrane of live cells where this chromogeneic dye is hydrolized to the polar free fluroscein emitting a green color. PI cannot enter living cells but readily permeates dead cells causing them to fluoresce red. An in vitro insulin stimulation index (SI) was calculated using standard protocols by incubating of 100–150 islets with basal glucose (2.8 mM) and high-glucose (20 mM) with the supernatant then collected and frozen (−20 °C) and subsequently assayed for insulin content by ELISA. The in vitro stimulation index (SI) was calculated by dividing the insulin secreted in response to high-glucose by insulin secreted during exposure to basal glucose.51
Histology processing
At the end of the respective culture durations (24 h, 3 d, 5 d, and 7 d) pelleted islets underwent fixation for 24 h in 10% buffered formalin. The pellet of the fixed isolated islets was then pipetted onto a microscope slide with a circular concave divet in the center. The islets were dispersed throughout the agar as the 2% agar solution was added to the slide. The agar was permitted to harden and then the agar disc was dehydrated and processed to wax through a series of alcohol, xylene, and wax baths. Discs once embedded in paraffin were sectioned at 5 µm thickness.49 Hematoxylin and eosin (H&E) stained histo-processed islets were assessed for physical integrity and cell/tissue turnover (mitosis, necrosis, apoptosis) using the Olympus Compound Microscope by a pathologist who was masked to the study.
Apoptosis was also assessed by the Confocal Microscope LSM 510, Zeiss following in situ hybridization terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling (TUNEL) method50 using the In Situ Cell Death Detection Kit (Fluorescein, 1 684 795, Roche Diagnostics Corporation, Roche Applied Science). Pancreatic tumor tissue following therapy was used as the positive control as it has a naturally high apoptotic index. For the negative control the TUNEL reaction mixture contained only label (fluorescein) and for the positive control, the reaction mixture contained label and enzyme.
Endocrine cell composition was assessed by immunohistochemistry (IHC) where the primary antibodies used were: anti-insulin (raised in mouse) and anti-glucagon, anti-pancreatic polypeptide and anti-somatostatin (raised in rabbit), purchased from Zymed Laboratories Inc. The Dako LSAB 2 System Peroxidase (Universal, K0672, K0673, K0675) kit comprised of biotinylated anti-mouse or anti-rabbit with streptavidin conjugated to horseradish peroxidase, developed with 3,3′-diaminobenzidinetetrahydrochloride (Sigma-Aldrich) and counterstained with Harris’ hematoxylin (Sigma-Aldrich).
Ultra-structure via transmission electron microscopy (TEM)
Retrieval of agar and paraffin embedded islets52,53 was performed using H.E stained reference sections to locate regions of high islet concentration for removal from the paraffin blocks. The tissue was then dewaxed in xylene and rehydrated in ethanol (100% to 90% to 70%). Retrieved islets were fixed in 3% glutaraldehyde in 0.1 M sodium cacodylate buffer, equilibrated in 2% aqueous osmium tetroxide dissolved in cacodylate buffer and dehydrated in ethanol (50% to 70% to 90% to 99.9%) and embedded in propylene oxide/epoxy resin mixture (50/50).53 Semi-thin (1 μm) sections used for morphological assessment of islets and block trimming prior to ultra-structural studies were cut using the Leica Ultracut, stained with 1% toluidine blue in 1% borax (sodium tetraborate), mounted with DPX and viewed using the Karl Zeiss Image Analyzer KS300. Ultra-thin sections (90 nm) were stained using alcoholic uranyl acetate and lead citrate53 and viewed on a 200 kV Philips Tecnai 20 TEM, the negative was obtained on a 6.5 x 9.0 cm film and a digital image of the negative was captured using a Nikon camera and stored for interpretation.
Definitions used in the study
The islets were quantified in IEQ. IEQ, physical integrity, hormone indices, and cell/tissue turnover (mitotic index, mean apoptotic index, and necrotic index) were measured using standard protocols.23-26,30-34
Islet viability
Percentage of islets positive for FDA divided by islets positive for PI (%).
In vitro stimulation index (SI)
Calculated by dividing the insulin secreted in response to high-glucose by insulin secreted during exposure to basal glucose.
Post-thaw islet recovery %
Percentage of post-thaw islets quantified in IEQ divided by the pre-thaw islets quantified in IEQ.
Islet survival % at a given culture duration
Percentage of islets in IEQ at the culture duration divided by the islets in IEQ at the previous culture duration.
Hormone indices
Percentage of cells with a marker positive for an islet hormone divided by the number of cells within an islet (%).
Mitotic/necrotic/apoptotic indices
Percentage (%) of cells within islets per 1000 cells counted showing mitosis/necrosis/apoptosis divided by the number of cells counted (i.e., 1000) within islets in 20 random fields at a total magnification of x400. For both the TUNEL and light microscopy, islets were examined in 20 random fields until a minimum of 1000 cells were counted. Thus the possibility of bias due to geographical variation in apoptosis in the section examined was avoided.
Cell turnover
Mitotic Index – (Necrotic Index + Apoptotic Index).
Statistical analysis
Results are presented as mean ± standard error mean (SEM) or in percentages. The Spearman’s rank correlation, the Mann-Whitney U test and the Kruskal-Wallis H were performed where appropriate.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The post-thaw human isolated islets used in this study were kindly provided by Lakey JRT and Shapiro AMJ of the Clinical Islet Transplant Program, Edmonton, Canada. We thank CMC Vellore Fellowship, Lady Tata Memorial Fellowship, MDRF Travel and Training Fellowship and University of Alberta, Edmonton, Canada for their support. We thank Dr AB Peter, Dr Meera Govindarajan, B Murali Manohar, S Vairamuthu, and Dr Manjula Datta for their guidance and support. We thank Kimberli Sawarin for her help with the wax embedding of isolated islets in agar.
Author contributions
Lakey JRT, Priya M, and Mohan V conceived the study. Lakey JRT, Shapiro AMJ, Kin T, and Mohan V, supervised the study and revised the manuscript. Churchill TA provided guidance and support on agar and wax embedding of isolated islets. Priya M conceived, coordinated, performed the study, performed the statistical analysis and wrote the first draft of the article. Cell turnover was assessed by Ganthimathy S and she also revised the manuscript. Anjana RM gave valuable input and revised the manuscript. Thiagarajan V and S Gunasekaran revised the manuscript.
Glossary
Abbreviations:
- IEQ
islet equivalents
- MHC class I
major histocompatibility complex class I
- HLA
human leukocyte antigen
- IRB
Institutional Review Board
- HSA
human serum albumin
- FBS
fetal bovine serum
- FDA
Federal Drug Administration
- cGMP
current good medical practice
- DMSO
dimethyl-sulfoxide
- M
moles
- FDA
Fluorescein diacetate
- PI
propidium iodide
- SI
stimulation index
- ELISA
enzyme linked immunosorbent assay
- TUNEL
Terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling
- IHC
immunohistochemistry
- T.E.M
Transmission Electron Microscopy
- ECM
extra-cellular matrix
- %
Percentage
- DNA
Deoxyribonucleic acid
- ITS
insulin-transferrin-selenium
- rs
Spearman correlation
- SEM
standard error mean
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/26304
References
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–30. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 3.McCall M, Shapiro AMJ. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2:a007823. doi: 10.1101/cshperspect.a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.http://biomed.brown.edu/Courses/BI108/BI108_2004_Groups/Group09/procedure.htm (Accession date: April 5th 2013).
- 5.Lakey JR, Aspinwall CA, Cavanagh TJ, Kennedy RT. Secretion from islets and single islet cells following cryopreservation. Cell Transplant. 1999;8:691–8. doi: 10.1177/096368979900800614. [DOI] [PubMed] [Google Scholar]
- 6.Kin T. Islet isolation for clinical transplantation. Adv Exp Med Biol. 2010;654:683–710. doi: 10.1007/978-90-481-3271-3_30. [DOI] [PubMed] [Google Scholar]
- 7.Lakey JRT, Burridge PW, Rajotte RV. Cryopreservation of Human Pancreatic Islets Graft. 2002;5:266–76. [Google Scholar]
- 8.Rajotte RV, Warnock GL, Kneteman NM. Methods of islet cryopreservation. In: Ricordi C, ed. Pancreatic islet transplantation, Chap. 12. Austin, TX: Landes, 1992: 124-131. [Google Scholar]
- 9.Piemonti L, Bertuzzi F, Nano R, Leone BE, Socci C, Pozza G, Di Carlo V. Effects of cryopreservation on in vitro and in vivo long-term function of human islets. Transplantation. 1999;68:655–62. doi: 10.1097/00007890-199909150-00011. [DOI] [PubMed] [Google Scholar]
- 10.Warnock GL, Lakey JR. Cryopreservation of human islets of Langerhans. Transplantation. 1999;68:597–8. doi: 10.1097/00007890-199909150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Rich SJ, Swift S, Thirdborough SM, James RF, Bell PR, London NJ. Islet cryopreservation: a detailed study of total functional losses. Transplant Proc. 1994;26:823–4. [PubMed] [Google Scholar]
- 12.di Carlo A, Scharp DW, Gingerich RL, Giannarelli R, Ansara M, Olack BJ, Swanson CJ, Navalesi R. Insulin and glucagon release from isolated, perifused human islets following low-temperature culture and cryopreservation. Transplant Proc. 1994;26:821–2. [PubMed] [Google Scholar]
- 13.Rich SJ, Swift S, Thirdborough SM, Rumford G, James RF, London NJ. Cryopreservation of rat islets of Langerhans: a comparison of two techniques. Cryobiology. 1993;30:407–12. doi: 10.1006/cryo.1993.1040. [DOI] [PubMed] [Google Scholar]
- 14.Warnock GL, Kneteman NM, Ryan E, Seelis RE, Rabinovitch A, Rajotte RV. Normoglycaemia after transplantation of freshly isolated and cryopreserved pancreatic islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1991;34:55–8. doi: 10.1007/BF00404026. [DOI] [PubMed] [Google Scholar]
- 15.Warnock GL, Kneteman NM, Ryan EA, Rabinovitch A, Rajotte RV. Long-term follow-up after transplantation of insulin-producing pancreatic islets into patients with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:89–95. doi: 10.1007/BF00400857. [DOI] [PubMed] [Google Scholar]
- 16.Scharp DW, Lacy PE, Santiago JV, McCullough CS, Weide LG, Boyle PJ, Falqui L, Marchetti P, Ricordi C, Gingerich RL, et al. Results of our first nine intraportal islet allografts in type 1, insulin-dependent diabetic patients. Transplantation. 1991;51:76–85. doi: 10.1097/00007890-199101000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Kneteman NM, Alderson D, Scharp DW, Lacy PE. Prolonged cryopreservation of purified human pancreatic islets. Diabetes. 1989;38(Suppl 1):176–8. doi: 10.2337/diab.38.1.s176. [DOI] [PubMed] [Google Scholar]
- 18.Lawson A, Ahmad H, Sambanis A. Cytotoxicity effects of cryoprotectants as single-component and cocktail vitrification solutions. Cryobiology. 2011;62:115–22. doi: 10.1016/j.cryobiol.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajotte RV, Warnock GL, Bruch LC, Procyshyn AW. Transplantation of cryopreserved and fresh rat islets and canine pancreatic fragments: comparison of cryopreservation protocols. Cryobiology. 1983;20:169–84. doi: 10.1016/0011-2240(83)90006-8. [DOI] [PubMed] [Google Scholar]
- 20.McKay DB, Karow AM., Jr. Factors to consider in the assessment of viability of cryopreserved islets of Langerhans. Cryobiology. 1983;20:151–60. doi: 10.1016/0011-2240(83)90004-4. [DOI] [PubMed] [Google Scholar]
- 21.Lakey JR, Anderson TJ, Rajotte RV. Novel approaches to cryopreservation of human pancreatic islets. Transplantation. 2001;72:1005–11. doi: 10.1097/00007890-200109270-00005. [DOI] [PubMed] [Google Scholar]
- 22.Woods EJ, Liu J, Zieger MA, Lakey JR, Critser JK. Water and cryoprotectant permeability characteristics of isolated human and canine pancreatic islets. Cell Transplant. 1999;8:549–59. doi: 10.1177/096368979900800510. [DOI] [PubMed] [Google Scholar]
- 23.Cotran RS, Kumar V, Collins T. Cellular Pathology 1: Cell Injury and Cell Death. In: Robbin’s Pathologic Basis of Disease. Sixth Edition. Cotran RS, Kumar V, Collins T, (eds). WB Saunders Company. 1999, 260-327. [Google Scholar]
- 24.Krickhahn M, Bühler C, Meyer T, Thiede A, Ulrichs K. The morphology of islets within the porcine donor pancreas determines the isolation result: successful isolation of pancreatic islets can now be achieved from young market pigs. Cell Transplant. 2002;11:827–38. [PubMed] [Google Scholar]
- 25.Bockman DE. Microanatomy of the Pancreas. In: Cellular Inter-relationships in the Pancreas-Implications for Islet Transplantation. Rosenberg L, Duguid WP (eds). RG Landes Company. 1996, 9-27. [Google Scholar]
- 26.Bockman DE. Histology and Fine Structure. In: The Pancreas (Vol 1). Beger HG, Warshaw AL, Buchler MW, Carr-Locke DL, Neoptolemos JP, Russsel C, Sarr MG (eds). Blackwell Science Ltd. London. 1998, 19-26. [Google Scholar]
- 27.Sandler S, Andersson A. The significance of culture for successful cryopreservation of isolated pancreatic islets of Langerhans. Cryobiology. 1984;21:503–10. doi: 10.1016/0011-2240(84)90048-8. [DOI] [PubMed] [Google Scholar]
- 28.von Mach MA, Schlosser J, Weiland M, Feilen PJ, Ringel M, Hengstler JG, Weilemann LS, Beyer J, Kann P, Schneider S. Size of pancreatic islets of Langerhans: a key parameter for viability after cryopreservation. Acta Diabetol. 2003;40:123–9. doi: 10.1007/s00592-003-0100-4. [DOI] [PubMed] [Google Scholar]
- 29.Langer S, Lau D, Eckhardt T, Jahr H, Brandhorst H, Brandhorst D, Hering BJ, Federlin K, Bretzel RG. Viability and recovery of frozen-thawed human islets and in vivo quality control by xenotransplantation. J Mol Med (Berl) 1999;77:172–4. doi: 10.1007/s001090050330. [DOI] [PubMed] [Google Scholar]
- 30.Latif ZA, Noel J, Alejandro R. A simple method of staining fresh and cultured islets. Transplantation. 1988;45:827–30. doi: 10.1097/00007890-198804000-00038. [DOI] [PubMed] [Google Scholar]
- 31.Ricordi C, Hering BJ, London NJM, Rajotte RV, Gray DWR, Sutherland DER, Socci C, Alejandro R, Carroll PB, Bretzel RG, et al. Islet isolation assessment. In: In: Ricordi C, ed. Pancreatic islet transplantation, Chap. 13. Austin, TX: Landes, 132-142. [Google Scholar]
- 32.Scharp DW. Islet quality control testing and the islet isolation laboratory. In: Ricordi C, ed. Pancreatic islet transplantation, Chap. 8. Austin, TX: Landes, 82-88. [Google Scholar]
- 33.Cattan P, Berney T, Schena S, Molano RD, Pileggi A, Vizzardelli C, Ricordi C, Inverardi L. Early assessment of apoptosis in isolated islets of Langerhans. Transplant Proc. 2001;33:264–5. doi: 10.1016/S0041-1345(00)02006-6. [DOI] [PubMed] [Google Scholar]
- 34.Ricordi C. Quantitative and qualitative standards for islet isolation assessment in humans and large mammals. Pancreas. 1991;6:242–4. doi: 10.1097/00006676-199103000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Bottino R, Balamurugan AN, Bertera S, Pietropaolo M, Trucco M, Piganelli JD. Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes. 2002;51:2561–7. doi: 10.2337/diabetes.51.8.2561. [DOI] [PubMed] [Google Scholar]
- 36.Zhang G, Matsumoto S, Hyon SH, Qualley SA, Upshaw L, Strong DM, Reems JA. Polyphenol, an extract of green tea, increases culture recovery rates of isolated islets from nonhuman primate pancreata and marginal grade human pancreata. Cell Transplant. 2004;13:145–52. doi: 10.3727/000000004773301825. [DOI] [PubMed] [Google Scholar]
- 37.Hering BJ, Browatzki CC, Schultz A, Bretzel RG, Federlin KF. Clinical islet transplantation--registry report, accomplishments in the past and future research needs. Cell Transplant. 1993;2:269–82, discussion 283-305. doi: 10.1177/096368979300200403. [DOI] [PubMed] [Google Scholar]
- 38.Fraga DW, Sabek O, Hathaway DK, Gaber AO. A comparison of media supplement methods for the extended culture of human islet tissue. Transplantation. 1998;65:1060–6. doi: 10.1097/00007890-199804270-00009. [DOI] [PubMed] [Google Scholar]
- 39.Rajotte RV, Warnock GL, Kneteman NN. Cryopreservation of insulin-producing tissue in rats and dogs. World J Surg. 1984;8:179–86. doi: 10.1007/BF01655133. [DOI] [PubMed] [Google Scholar]
- 40.Coulombe MG, Warnock GL, Rajotte RV. Prolongation of islet xenograft survival by cryopreservation. Diabetes. 1987;36:1086–8. doi: 10.2337/diabetes.36.9.1086. [DOI] [PubMed] [Google Scholar]
- 41.Guignard AP, Oberholzer J, Benhamou PY, Touzet S, Bucher P, Penfornis A, Bayle F, Kessler L, Thivolet C, Badet L, et al. GRAGIL Group.Cost analysis of human islet transplantation for the treatment of type 1 diabetes in the Swiss-French Consortium GRAGIL. Diabetes Care. 2004;4:895–900. doi: 10.2337/diacare.27.4.895. [DOI] [PubMed] [Google Scholar]
- 42.Gray DW, Reece-Smith H, Fairbrother B, McShane P, Morris PJ. Isolated pancreatic islet allografts in rats rendered immunologically unresponsive to renal allografts. The effect of the site of transplantation. Transplantation. 1984;37:434–7. doi: 10.1097/00007890-198405000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Warnock GL, Lakey JRT, Ao Z, Rajotte RV. Tissue banking of cryopreserved islets for clinical islet transplantation. Transplant Proc. 1994;26:34–8. [PubMed] [Google Scholar]
- 44.Sutton R, Warnock GL, McWhinnie DL, Gray DW, Peters M, Wood KJ, Morris PJ. Expression of HLA in isolated human pancreatic islets and cryopreservation. Transplant Proc. 1987;19:220–1. [PubMed] [Google Scholar]
- 45.Cattral MS, Lakey JRT, Warnock GL, Kneteman NM, Rajotte RV. Effect of cryopreservation on the survival and function of murine islet isografts and allografts. Cell Transplant. 1998;7:373–9. doi: 10.1016/S0963-6897(98)00013-X. [DOI] [PubMed] [Google Scholar]
- 46.Cattral MS, Warnock GL, Kneteman NM, Halloran PF, Rajotte RV. The effect of cryopreservation on the survival and MHC antigen expression of murine islet allografts. Transplantation. 1993;55:159–63. doi: 10.1097/00007890-199301000-00029. [DOI] [PubMed] [Google Scholar]
- 47.Rajotte RV, Warnock GL, Kneteman NM. Transplantation of cryopreserved islets: long-term insulin independence in type 1 diabetics. Endocrinologia. 1992;39:149–52. [Google Scholar]
- 48.Rajotte RV, Scharp DW, Downing R, Preston R, Molnar GD, Ballinger WF, Greider MH. Pancreatic islet banking: the transplantation of frozen-thawed rat islets transported between centers. Cryobiology. 1981;18:357–69. doi: 10.1016/0011-2240(81)90108-5. [DOI] [PubMed] [Google Scholar]
- 49.Anderson G, Gordon KC. Tissue processing, microtomy and paraffin sections. In: Bancroft JD, Stevens A, Turner DR (eds). Theory and Practice of Histological Techniques, 4th Edition, Churchill Livingstone. 1996, 47-67. [Google Scholar]
- 50.Warford A. In situ hybridization. In: Theory and Practice of Histological Techniques, Bancroft JD, Stevens A, Turner DR (eds). 4th Edition, Churchill Livingstone. 1996, 491-512. [Google Scholar]
- 51.Marchetti P, Scharp DW, Mclear M, Gingerich R, Finke E, Olack B, Swanson C, Giannarelli R, Navalesi R, Lacy PE. Pulsatile insulin secretion from isolated human pancreatic islets. Diabetes. 1994;43:827–30. doi: 10.2337/diabetes.43.6.827. [DOI] [PubMed] [Google Scholar]
- 52.Robinson G, Gray T. Electron microscopy 2: Practical procedures. In: Theory and Practice of Histological Techniques, Bancroft JD, Stevens A, Turner DR (eds). 4th Edition, Churchill Livingstone. 1996, pp. 585-626. [Google Scholar]
- 53.Stevens A. Electron microscopy 3: Diagnostic applications. Theory and Practice of Histological Techniques, In: Bancroft JD, Stevens A, Turner DR (eds). 4th Edition, Churchill Livingstone. 1996, pp. 626-629. [Google Scholar]