Abstract
Aim: To study the protective effect of a fibrin scaffold toward embedded young porcine endocrine pancreatic islets from hydrogen peroxide within the context of islet encapsulation in transplantation. Methods: After isolation and in vitro maturation, groups of 200 young porcine islet equivalents (IEQ) were embedded in a 200 µL fibrin gel and exposed to 2 concentrations (10 and 100 µM) of hydrogen peroxide (H2O2) to investigate the ability of fibrin to protect islets against apoptotic stimuli. As a control, young porcine islets were seeded in tissue culture polystyrene (TCPS) well plates and exposed to the same H2O2 concentrations. Islet integrity, viability and function were then investigated. Results: Morphologically, the integrity of islets embedded in fibrin gels was better preserved compared with that of islets cultured in TCPS plates, when exposed to H2O2. Immunofluorescence staining showed that insulin and glucagon expression was higher in islets cultured in fibrin. Overall, H2O2 incubation led to decreased insulin and glucagon expression. A TUNEL assay revealed elevated numbers of apoptotic cells for islets cultured in TCPS plates when compared with those embedded in fibrin. Islets cultured in TCPS plates and exposed to H2O2 had diminished ability to secrete insulin in response to glucose stimulation, whereas islets embedded in fibrin maintained their glucose responsiveness. Insulin trapped in fibrin was extracted and quantified, revealing insulin in the extract. Conclusions/Interpretation: Fibrin has a protective effect on young porcine endocrine pancreatic islets exposed to hydrogen peroxide.
Keywords: endocrine pancreatic islet culture, encapsulation, extracellular matrix, fibrin, hydrogen peroxide, porcine pancreatic islets
Introduction
This study follows the hypothesis that cell-extracellular matrix interactions improve islet integrity, viability and function after exposure to immune system-derived apoptotic stimuli, such as hydrogen peroxide (H2O2). Apoptotic stimuli such as H2O2 are present during type 1 diabetes mellitus (T1DM) onset, autoimmune β-cell destruction, and after islet transplantation. Islet transplantation could offer a cure for T1DM as the islet graft could restore adequate endogenous insulin secretion for the patient. Even after the introduction of the Edmonton’s protocol, islet transplantation is still attempting to overcome the low success rates associated with cellular or functional loss of the graft.1 Two reasons for loss of such a graft include damage to the native extracellular matrix (ECM) after isolation and transplantation, and later, rejection of the donor graft by the host’s immune system.2 One of the macrophage-secreted mediators of the immune system response is H2O2, a small and uncharged molecule with the capacity to be microbicidal, cytotoxic, and tissue damaging, which can easily pass through the cell membrane.3 Physiological H2O2 concentrations during inflammation can range between 5 and 15 µM.4 Recent studies have shown that the islet ECM, and more specifically, cell-integrin interactions, mediate apoptosis resistance and support viability and function of pancreatic β cells.5,6 Fibrin, a transient in vivo ECM, has been used to improve culture conditions for mouse and human pancreatic islets before transplantation.7,8 Mouse islets pre-cultured in fibrin and implanted in mice have shown improved survival, increased insulin content and yielded a larger population of functional islets.7 Human islets embedded in fibrin gels enriched with perfluorodecalin showed less apoptosis and maintained their insulin secretion in response to glucose.8
It is hypothesized that fibrin protects young porcine endocrine pancreatic islets from hydrogen peroxide. In accordance, culturing young porcine endocrine pancreatic islets embedded in fibrin could decrease pre- and post-transplantation-related complications, thus improving islet integrity, viability and insulin secretion. This study is a first step toward improved xeno-islet grafts for human transplantation.
Results
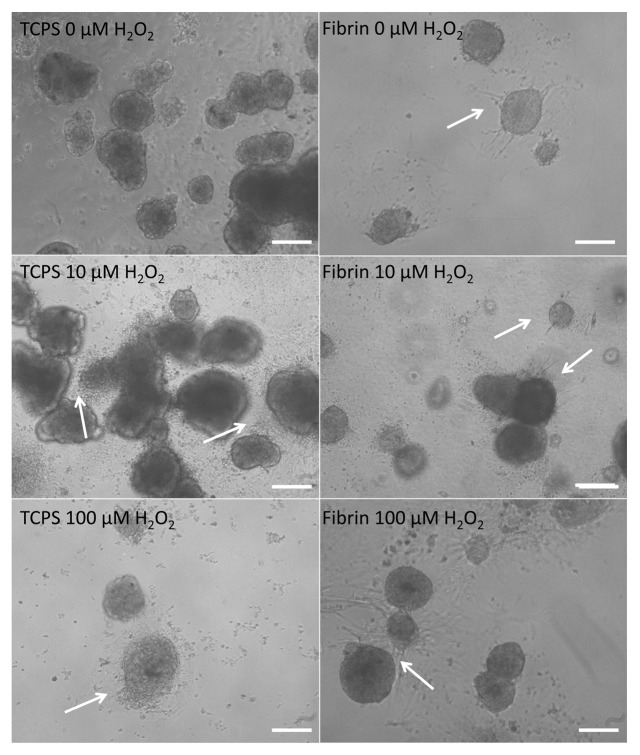
Islets in fibrin maintained their typical morphology whereas islets cultured in TCPS plates dispersed
After the overnight culture and 12 h incubation with 0, 10, and 100 µM H2O2, islets cultured in fibrin showed the first signs of sprouting, forming capillary-like structures (Fig. 1D–E). The occurrence of sprouts decreased with increasing H2O2 concentrations. Islets in fibrin were viable with little or no effect from H2O2. Almost no single cells could be observed in proximity of the islets, indicating that islets in fibrin did not start to disintegrate. Islets cultured in TCPS plates were dispersed after incubation with 100 µM H2O2. The grade of dispersion increased with the H2O2 concentration (Fig. 1A–C).
Figure 1. Morphology of young porcine endocrine pancreatic islets cultured for 12 h in TCPS plates with no H2O2, 10 µM H2O2 and 100 µM H2O2. Morphology of islets cultured for 12 h in fibrin with no H2O2, 10 µM H2O2 and 100 µM H2O2. 10x magnification. Bars represent 50 µm.
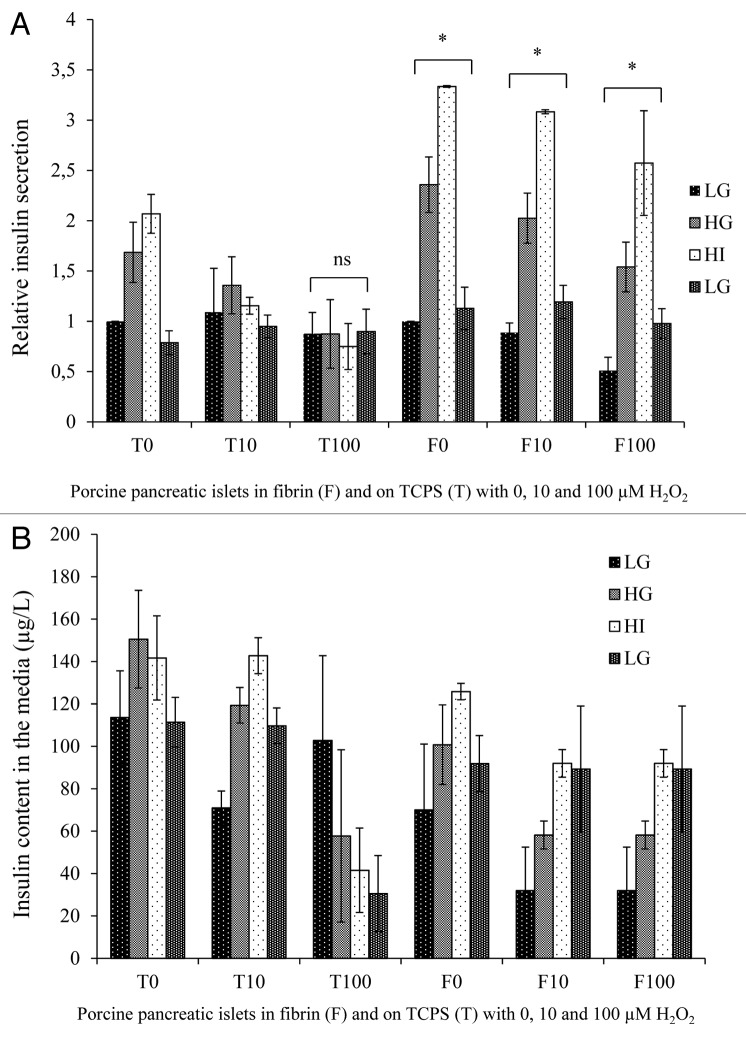
Islets cultured in fibrin maintained their responsiveness to glucose concentrations and secreted more insulin than islets in TCPS plates
The results of the glucose-stimulated insulin secretion (GSIS) assay performed after the 12 h H2O2 incubation indicated that islets in fibrin not only maintained their responsiveness to different glucose concentrations but also secreted relatively more insulin when compared with their respective controls in TCPS (Fig. 2A). As shown in Figure 2, the insulin secretion of islets in TCPS exposed to 10 and 100 µM H2O2 were normalized to the insulin secretion of islets in TCPS cultured without H2O2 in response to the initial low glucose concentration (LG) conditions. Similarly, the insulin secretion of islets embedded in fibrin exposed to 10 and 100 µM H2O2 were normalized to the insulin secretion of islets in fibrin cultured without H2O2 in response to the initial low glucose concentration (LG) conditions. This allows better comparing the effect of culture conditions on insulin secretion. Absolute insulin secretion of 3 islet batches in 3 repeats is shown in Figure 2B.
Figure 2. (A) Relative insulin secretion of young porcine endocrine pancreatic islets after 1 h stimulation with low glucose concentration (LG, 2.8 mM), high glucose concentration (HG, 28 mM), high glucose concentration + 3-isobutyl-1-methylxanthine (IBMX) (HI, 28 mM + IBMX) and a second low glucose concentration (LG2, 2.8 mM) concentration. Insulin secretion of islets in TCPS/fibrin was normalized to the insulin secretion of islets in TCPS/fibrin with no H2O2 in response to low glucose concentration. (B) Three repeats of a glucose-stimulated insulin secretion assay with LG, HG, HI and a final LG. Shown are the insulin contents in the media (microgram per liter) of porcine pancreatic islets (200 IEQ) cultured on TCPS (T) or in fibrin gels (F) with 0, 10 or 100 µM H2O2.’*’ represents a value of P < 0.05, ‘ns‘ means no statistical significance.
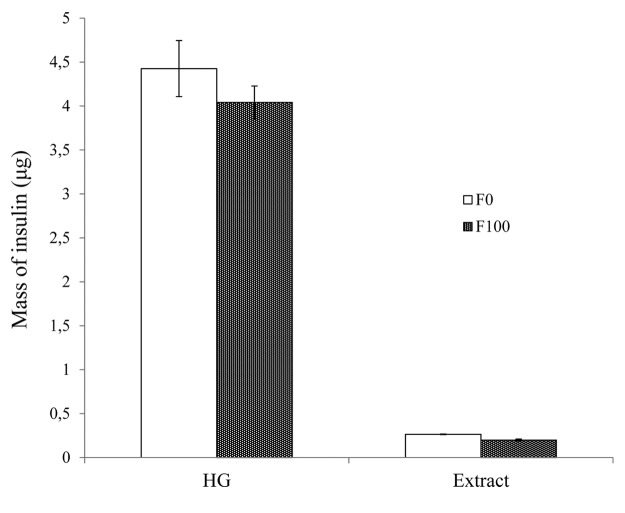
Analysis of the extract revealed that some insulin remained trapped within the fibrin gels (Fig. 3). The mass of insulin secreted by 200 IEQ in fibrin with no exposition to H2O2 and by those in fibrin exposed to 100 µM H2O2 was 0.26 and 0.20 µg, respectively. Therefore, approximately 5–6% of the total mass of insulin remained trapped in the fibrin mesh. Further development is needed to develop an extraction protocol to quantify the insulin trapped in the fibrin.
Figure 3. Mass of insulin (in micrograms) secreted into the media by 200 IEQ embedded in fibrin and exposed to 0 (F0) and 100 µM H2O2 (F100) in a high glucose concentration condition (HG) and the mass of insulin (in micrograms) in the extract of fibrin gels exposed to the same conditions.
Insulin and glucagon expression is diminished in islets cultured in TCPS plates
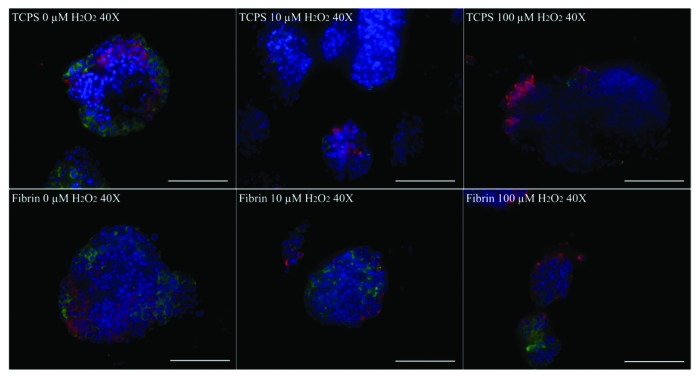
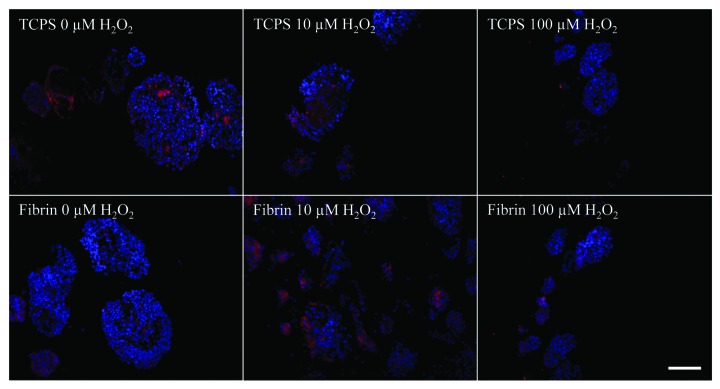
Immunofluorescence staining for insulin and glucagon was performed (Fig. 4). Cells staining positively for insulin were manually counted. At least 10 random fields per condition were examined, containing an average of 3 islets per field. Islets cultured in fibrin and on TCPS plates showed decreasing insulin and glucagon expression with increasing H2O2 concentration when compared with the ‘Time Zero’ control (Table 1). On TCPS, islets had a lower number of insulin-positive cells than glucagon-positive cells and overall lower levels of expression of both molecules as opposed to fibrin. Islet cells cultured in fibrin had higher numbers of insulin-positive cells than glucagon-positive cells but overall lower expression of both molecules when compared with islets at ‘Time Zero’.
Figure 4. Immunofluorescence staining for insulin (green) and glucagon (red) of young porcine endocrine pancreatic islets. Nuclei are DAPI stained in blue. 40x magnification. Bars represent 100 µm.
Table 1. Number of cells stained positively for glucagon, insulin, and both glucagon and insulin, counted on immunofluorescence images of porcine pancreatic islets at ‘Time Zero’, in fibrin with no (F0), 10 (F10), and 100 (F100) µM H2O2, and on TCPS with no (T0), 10 (T10), and 100 (T100) µM H2O2. Percentages of glucagon- and insulin-positive cells are shown in brackets.
| Condition | Total number of cells counted* (100%) |
Glucagon-positive cells | Insulin-positive cells | Positive cells |
| Time Zero | 1925 | 443 (23.0%) | 548 (28.5%) | 991 (51.5%) |
| F0 | 2332 | 403 (17.3%) | 482 (20.7%) | 885 (38.0%) |
| F10 | 2348 | 319 (13.6%) | 433 (18.4%) | 752 (32.0%) |
| F100 | 2746 | 164 (6.0%) | 301 (11.0%) | 465 (17.0%) |
| T0 | 4174 | 735 (17.6%) | 368 (8.8%) | 1103 (26.4%) |
| T10 | 3725 | 314 (8.4%) | 194 (5.2%) | 508 (13.6%) |
| T100 | 2908 | 144 (5.0%) | 104 (3.6%) | 248 (8.5%) |
Variations in the cell numbers counted depend on sample sizes, which vary from one 4-μm-thick paraffin section to another.
Integrin α5 is strongly expressed in fibrin-embedded islets
Islets were stained for the αVβ3 and the α5 integrins. The staining for αVβ3 was overall very weak (data not shown) and showed no differences between the culture conditions. In contrast, the integrin α5 was expressed more strongly in islets cultured in fibrin compared with islets on TCPS (Fig. 5). A decrease in integrin α5 could be noted with increasing H2O2 concentration. Table 2 lists a manual count of at least 5 fields with an average of 3 islets per field. A higher percentage of cells expressed α5 integrin in the fibrin set when compared with islets on TCPS.
Figure 5. Immunofluorescence staining for integrin α5 (red) of young porcine endocrine pancreatic islets. Nuclei are DAPI stained in blue. 20× magnification. Bars represent 50 µm.
Table 2. Number of cells stained positively for integrin α5, counted on immunofluorescence images of porcine pancreatic islets at ‘Time Zero’, in fibrin with no (F0), 10 (F10), and 100 (F100) µM H2O2, and on TCPS with no (T0), 10 (T10), and 100 (T100) µM H2O2.
| Condition | Total number of cells counted* (100%) |
Integrin α5-positive cells | Percentage (positive) |
| Time Zero | 2644 | 779 | 29.5% |
| F0 | 2541 | 1790 | 70.4% |
| F10 | 2253 | 1206 | 53.5% |
| F100 | 2300 | 1116 | 48.5% |
| T0 | 2798 | 836 | 29.9% |
| T10 | 2236 | 398 | 17.8% |
| T100 | 2591 | 332 | 12.8% |
Variations in the cell numbers counted depend on sample sizes, which vary from one 4-μm-thick paraffin section to another.
Fibrin embedding protects islets against apoptosis
Paraffin sections of islets in each condition were treated with a TUNEL assay and then imaged and counted. At least 10 random fields of each condition performed in triplicate were counted manually (Fig. 6A and B). All brownish and black spots within the cell nuclei were counted. Fibrin-embedded islet cells were significantly less apoptotic than the islet cells in TCPS plates. The incidence of apoptosis increased with increasing H2O2 concentrations (Fig. 6C).
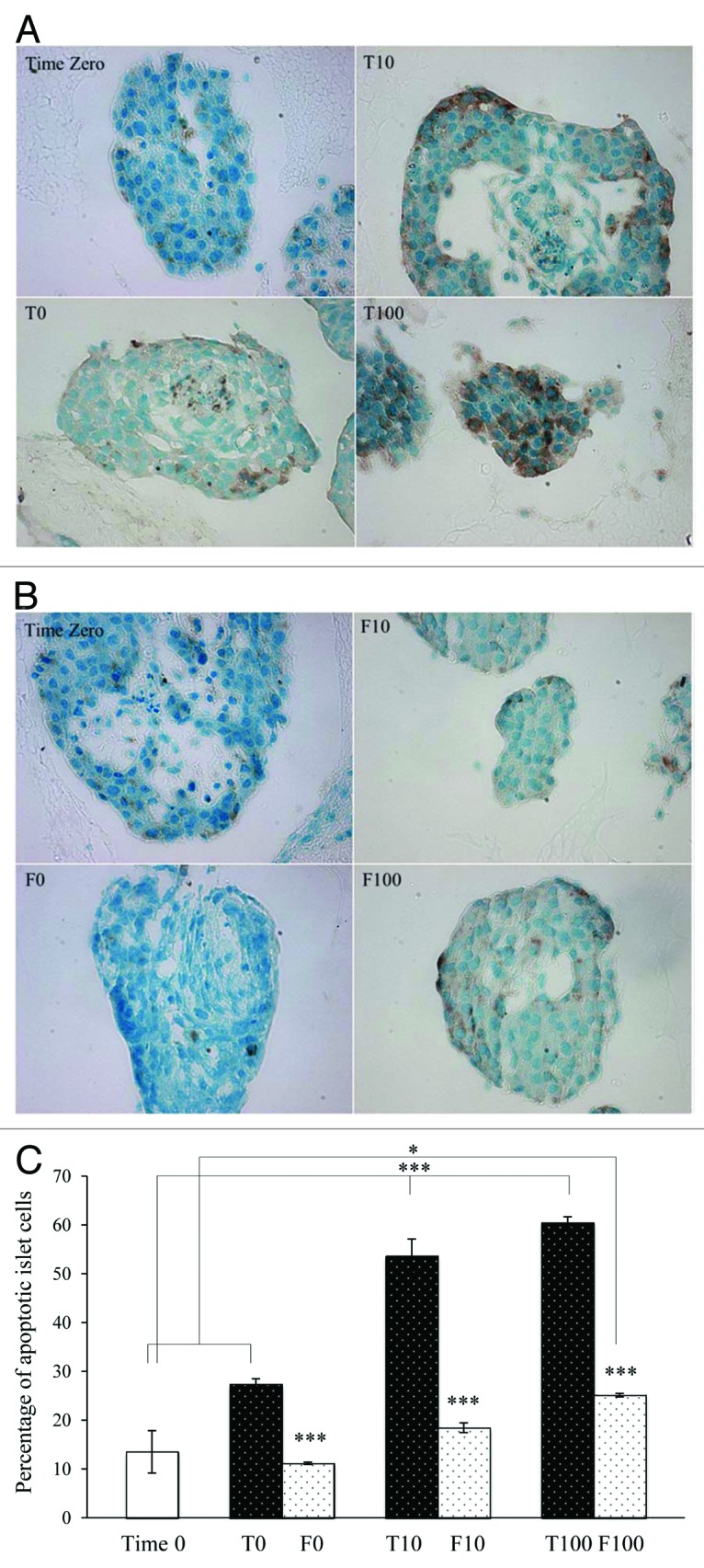
Figure 6. Light microscopy images of young porcine endocrine pancreatic islets treated with the APO-BrdU IHC staining kit. 40× magnification. Islets cultured in TCPS plates with 0, 10 or 100 µM H2O2 compared with Time Zero (A) and islets embedded in fibrin with 0, 10, or 100 µM H2O2 compared with Time Zero (B) are shown. The percentage of apoptotic cells (brown) in the total cell population (blue and brown) is shown in Figure 6C. ’*’ represents a value of P < 0.05, ‘***’ represents a value of P < 0.001. *** compared Time Zero with T10 and Time Zero with T100 and each T-set compared with the corresponding F-set.
Discussion
This study confirms fibrin has a protective effect when used to embed young porcine endocrine pancreatic islets after exposure to H2O2 (10 and 100 µM). It is known that pancreatic islets are separated from their ECM after isolation and consequently lose their typical spherical morphology and disperse if cultured in suspension.7 Islet cells may suffer from anoikis, a form of apoptosis induced by cell detachment from its ECM.9 Additionally, in this study, elevated islet dispersion in suspension was observed with increasing concentrations of H2O2 whereas islets cultured in fibrin exhibited sprouting formation. It has been shown that fibrin gels promote microvessel formation and re-vascularisation of pancreatic porcine islet grafts in mice when transplanted subcutaneously.10
Recent studies showed that interactions between pancreatic islets and their ECM regulate insulin secretion and cell proliferation, and prevent apoptosis.11,12 In this study, we showed that islets cultured on TCPS lost their glucose responsiveness when exposed to H2O2, whereas islets embedded in fibrin maintained it and even secreted relatively more insulin in response to glucose (Fig. 2). Without the provision of a three-dimensional environment, isolated pancreatic islets undergo more apoptosis than islets supported by fibrin, agarose, collagen and/or other ECM-like networks during a given culture period before being transplanted. When recovered from those matrices and transplanted, islets showed improved resistance toward apoptosis and improved survival when exposed to the immune system of the host. A higher insulin secretion in response to glucose was also observed in those islets.6-8,11-13 In our cultures, relative insulin secretion was also higher in the fibrin set with islets maintaining their glucose responsiveness whereas non-embedded TCPS cultures did secrete insulin but were not able to respond to higher glucose concentrations (Fig. 2A). Our results confirm that fibrin-embedded islets are significantly less apoptotic even after exposure to 10 or 100 µM H2O2 compared with islets cultured in TCPS plates. The two different H2O2 concentrations were chosen to represent a physiological concentration i.e., 10 µM, and a higher concentration i.e., 100 µM, shown to induce elevated levels of apoptosis.4,14
Insulin and glucagon expression was better preserved in islets cultured in fibrin (Table 1) but diminished with increasing H2O2 concentrations. Overall, numbers of glucagon- and insulin-positive cells appeared to be low, with just 52.5% of all cells at ‘Time Zero’ staining positively for both. After islet culture, decreasing percentages were observed. These relatively low numbers could be due to the time passed between isolation and culture, the culture time itself, the varying cell composition of pancreatic islets, and/or even the region of origin of the islets within the pancreas.15
One possible explanation for the beneficial effects of fibrin on the survival of pancreatic islets after H2O2 exposure could be the cell-integrin-fibrin interactions.11 It has been shown that integrins are essential for islet survival, resistance to apoptosis, and islet cell maintenance.5,6,16 Porcine pancreatic islets express several integrins e.g., αV and α5.17 We found evidence that islets in fibrin and on TCPS expressed integrins, αVβ3 and α5, with a much weaker overall αVβ3 expression. Integrin α5 was expressed more strongly in islets cultured in fibrin than in islets on TCPS and expression decreased with increasing H2O2, indicating a possible integrin-dependent protective effect of fibrin toward pancreatic islet cultures (Table 2). After a 12 h culture in fibrin, integrin α5 expression was stronger in fibrin cultures than in islets fixed, processed and stained right after arrival at ‘time zero’, suggesting that fibrin triggers integrin expression in islets. This is supported by recent findings, that the postulated protective effect of fibrin could be related to the available binding sites in the fibrin mesh for the islet cell integrins.5,9,11 Immunofluorescence techniques are one of the few methods currently available to investigate integrin expression of islets in fibrin gels. Other traditional methods such as RT-PCR and Western blotting pose several problems when working with tissues or cells embedded in gels, as the extraction of the cell materials from the tissues (here, the islets) embedded in fibrin, without affecting the cells, is challenging.
Finally, it can be argued that fibrin could simply constitute a mechanical barrier protecting the islets by delaying the H2O2 diffusion. It has previously been shown that rhodamine B, a fluorescent dye with a molar mass of 479.02 g·mol−1, diffuses quite rapidly in fibrin gels (2 mg fibrinogen per mL)18 considering the time scale (12 h) of the H2O2 experiments. Therefore, it can be assumed that the diffusion of H2O2, with a molar mass of 34 g·mol−1, is not hindered through the fibrin matrix. Interestingly, fibrin was found to retain small amounts of insulin. Theoretically, more insulin could be trapped inside the gels because the extraction method used to extract insulin does not guarantee that all insulin was recovered and/or remained stable during the extraction process. Not measuring all insulin trapped in the gels could yield a lower apparent insulin secretion in 3D cultures.
This study revealed that fibrin, when used to embed young porcine endocrine pancreatic islets, protects those islets from the destructive effects of H2O2. In the context of islet transplantation, these results suggest that fibrin could provide protection for such a graft. Further studies with endocrine pancreatic islets embedded in fibrin will include immune cells and immune system-derived effectors to further investigate the potential protective effects of fibrin.
Materials and Methods
Isolation of young porcine endocrine pancreatic islets
Young porcine endocrine pancreatic islets were provided by the islet isolation facility at the Department of Surgery, University California Irvine, under the ethical protocol IACUC #2008-2823. Pancreata from young Yorkshire pigs (14–22 d) were removed using rapid surgical procurement and placed in University of Wisconsin (UW) solution for organ preservation.
Pancreata were minced and digested in a low dose collagenase MA/BP protease enzyme mixture (001-2020, Vitacyte) for 15 ± 2 min in a 37 °C water bath and mixing at 60 rpm. Cold (4 °C) HBSS supplemented with HEPES and 10% BSA was added to the tissue-enzyme mixture to stop the enzymatic digestion and the partially digested tissue was force-filtered through a 500 µm metal mesh.
Tissue clusters were then cultured at 37 °C, 5% CO2 in T-175 suspension flasks in media. The medium used was a novel culture medium supplemented with proteins and enzymes designed to improve β-cell function (GLP-1 antagonists, insulin, etc.) and exclusion of exocrine tissue, and containing 10% porcine serum.19 The islets were allowed to mature for 8 d with media changed every 48 h, which has been shown to further decrease exocrine tissue.20 After which, samples for islet counts as well as for islet viability and function were taken and aliquots were collected, packaged in a 50 mL conical tube with culture medium and shipped via overnight express shipment at room temperature. The total number of IEQ has been calculated as described elsewhere.21
Young endocrine porcine islet culture
Upon arrival, porcine endocrine pancreatic islets were inspected, washed and placed in fresh media made of CMRL-1066 medium (11530, Invitrogen) containing 10% fetal bovine serum (FBS, 12483, Invitrogen), 25 mM HEPES (H3375, Sigma-Aldrich), 1% penicillin-streptomycin (15140-122, Invitrogen) and 2 mM L-glutamine (25030-149, Invitrogen) at pH 7.4. Islets were either embedded and cultured in fibrin gels or cultured in tissue culture polystyrene (TCPS) plates. Fibrin gels with a total volume of 200 µL (2 mg∙mL−1 bovine fibrinogen, F8630, Sigma-Aldrich) containing 200 IEQ were prepared in a 48-well TCPS plate as described previously.22 After jellification with 1 U∙mL−1 bovine thrombin (T9549, Sigma-Aldrich), the gels were covered with 200 µL of medium. An equal number of islets was re-suspended in 200 µL of medium and seeded in a 48-well TCPS plate.
Islet incubation with hydrogen peroxide (H2O2)
After the overnight culture, media were removed and replaced with 200 µL of either fresh medium with no H2O2, 200 µL of fresh medium containing 10 µM H2O2 (H3410, Sigma-Aldrich), or 200 µL of fresh medium containing 100 µM H2O2. The islets were then incubated for 12 h.
Glucose-stimulated insulin secretion (GSIS) and insulin entrapment in fibrin gels
As described in detail elsewhere, a GSIS test23 was performed after the 12 h H2O2 incubation. 2.8 mM glucose solutions served as the low glucose (LG and LG2) steps and 28 mM glucose solutions were used to represent high glucose (HG) concentrations. An additional incubation step with 28 mM glucose + 50 μM 3-isobutyl-1-methyl-xanthine (IBMX; I5879, Sigma-Aldrich) was performed. Islets were incubated with the different glucose solutions for 1 h each in the sequence LG, HG, HI, and LG2. Insulin contents were measured using either an Insulin Porcine High Range ELISA kit (0.313 µg∙L−1 sensitivity; 10-1223-01, Mercodia Inc.) or an Insulin Porcine ELISA kit (0.01 µg∙L−1 sensitivity; 10-1200-01, Mercodia Inc.). Insulin content of the media was normalized to the volume of medium for each condition.
Insulin was extracted from fibrin gels containing the islets by rinsing the gels with 500 µL PBS and centrifuging them for 20 s (12,800 rpm). The insulin content of the fibrin extract was expressed as µg per 200 IEQ. Absorbance was measured using a Safire2 microplate reader (Tecan Group Ltd.).
Immunofluorescence
Islets in TCPS plates and in fibrin gels were collected into centrifuge tubes, fixed in 4% paraformaldehyde and processed for paraffin embedding. Immunofluorescence staining was performed according to standard protocols with paraffin sections consecutively cut at a thickness of 4 μm. Primary antibodies to insulin (ab7842), glucagon (ab18461) and αVβ3 integrin (ab7166) were purchased from Abcam. The primary antibody for the α5 integrin (sc-10729) was purchased from Santa Cruz. As secondary antibodies a goat anti-mouse IgG Alexa Fluor® 488 (A11001, Invitrogen) and a goat anti-rabbit IgG Alexa Fluor® 555 (A21428, Invitrogen) were used. All sections were blocked in 10% goat serum and counterstained with DAPI (D1306, Invitrogen). Manufacturer recommendations were used for pre-treatment, dilutions, incubation time and temperature. Samples were imaged using an epifluorescence microscope (Nikon Eclipse).
Insulin and integrin α5-positive cells were counted manually. For each condition, at least 5 fields with an average of 3 islets on each were counted. The total cell population was represented by DAPI-positive cells. The percentage of cells staining positively for insulin or integrin α5 over the total cell population was assessed.
TUNEL apoptosis assay
Paraffin sections of pancreatic islets cultured in fibrin or in TCPS plates were treated with an APO-BRDU-IHCTM* kit (AH1001, Phoenix Flow Systems) to measure apoptosis by dual color immunohistochemistry. Since apoptosis is leading to a multitude of 3′-hydroxyl ends in the fragmented DNA, those ends can be labeled with Br-dUTP. A combination of biotin-labeled anti-BrdU antibody, horseradish peroxidase streptavidin conjugate, DAB and H2O2/Urea results in a brownish-black color generation in apoptotic nuclei whereas a methyl green solution for counter staining results in a light blue color of all cells within the population. After deparaffinization, samples were rehydrated and permeabilized with Proteinase K (1:100 in 10 mM Tris pH 8) at room temperature (RT) for 20 min. Following a PBS rinsing step, endogenous peroxidases were inactivated using 3% H2O2 in methanol (at RT for 5 min). After rinsing in PBS, samples were equilibrated with a reaction buffer provided with the kit, diluted in distilled water, at RT for 30 min. Immediately after equilibration, the DNA labeling solution containing the reaction buffer, TdT enzyme, Br-dUTP and distilled water, was applied for 90 min at 37 °C with samples placed in a humid chamber. All samples were then rinsed with PBS. This was followed by 10 min incubation with a blocking buffer at RT. After, antibody solution containing anti-BrdU-biotin and blocking buffer was applied to each sample for 90 min at RT in a humid chamber. Samples were covered with blocking buffer after rinsing with PBS. A conjugate solution made in blocking buffer was then applied for 30 min at RT, followed by a PBS rinse. DAB solution (DAB/H2O2/Urea in tap water) was added and incubated for 5 min at RT. Samples were rinsed with tap water and immediately after, covered with a methyl green counterstain solution at RT for 5 min. Slides were dipped in 100% ethanol and later, xylene, before being mounted with a Permount solution (SP15100, Fisher Scientific). Samples were imaged with a Leica DMR HCS microscope system.
Statistical analysis
Data are presented as means ± SD for 3 independent experiments. Differences in results were assessed by a single factor ANOVA. A probability (P) value < 0.05 was considered statistically significant, P < 0.001 was considered highly statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Professor Joël Sirois and Jocelyne Ayotte at the Université de Sherbrooke for the provision and support of the microplate reader. We are grateful to Michael Alexander for his technical assistance at UC Irvine. We appreciate the contribution of Jamie Sharp who provided language support in proof reading the article. This research project was supported by the Université de Sherbrooke and NSERC through a Discovery Grant awarded to Vermette P (grant # 250296-07). Funding was also provided through the Department of Surgery, University of California, Irvine.
Glossary
Abbreviations:
- Br-dUTP
5-bromo-2´-deoxyuridine 5′-triphosphate
- BSA
bovine serum albumin
- DAB
diaminobenzidine
- ECM
extracellular matrix
- ELISA
enzyme-linked immunosorbent assay
- GSIS
glucose-stimulated insulin secretion
- H2O2
hydrogen peroxide
- HBSS
Hank’s balanced salt solution
- IBMX
3-isobutyl-1-methylxanthine
- IEQ
islet equivalents
- PBS
phosphate buffered saline
- RT
room temperature
- T1DM
type 1 diabetes mellitus
- TCPS
tissue culture polystyrene
- TdT
terminal deoxynucleotidyl transferase
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labelling
- UW
University of Wisconsin
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/26989
References
- 1.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JRT, Shapiro AMJ. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–9. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 2.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587–97. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 3.Rebelato E, Abdulkader F, Curi R, Carpinelli AR. Low doses of hydrogen peroxide impair glucose-stimulated insulin secretion via inhibition of glucose metabolism and intracellular calcium oscillations. Metabolism. 2010;59:409–13. doi: 10.1016/j.metabol.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 4.de Oliveira-Marques V, Cyrne L, Marinho HS, Antunes F. A quantitative study of NF-kappaB activation by H2O2: relevance in inflammation and synergy with TNF-alpha. J Immunol. 2007;178:3893–902. doi: 10.4049/jimmunol.178.6.3893. [DOI] [PubMed] [Google Scholar]
- 5.Hammar E, Parnaud G, Bosco D, Perriraz N, Maedler K, Donath M, Rouiller DG, Halban PA. Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes. 2004;53:2034–41. doi: 10.2337/diabetes.53.8.2034. [DOI] [PubMed] [Google Scholar]
- 6.Wang RN, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol. 1999;163:181–90. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- 7.Beattie GM, Montgomery AMP, Lopez AD, Hao E, Perez B, Just ML, Lakey JRT, Hart ME, Hayek A. A novel approach to increase human islet cell mass while preserving β-cell function. Diabetes. 2002;51:3435–9. doi: 10.2337/diabetes.51.12.3435. [DOI] [PubMed] [Google Scholar]
- 8.Maillard E, Juszczak MT, Clark A, Hughes SJ, Gray DRW, Johnson PRV. Perfluorodecalin-enriched fibrin matrix for human islet culture. Biomaterials. 2011;32:9282–9. doi: 10.1016/j.biomaterials.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 9.Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve β-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A. 2008;14:1959–68. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J-S, Lim J-H, Nam H-Y, Lim H-J, Shin J-S, Shin J-Y, Ryu J-H, Kim K, Kwon I-C, Jin S-M, et al. In situ application of hydrogel-type fibrin-islet composite optimized for rapid glycemic control by subcutaneous xenogeneic porcine islet transplantation. J Control Release. 2012;162:382–90. doi: 10.1016/j.jconrel.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Pinkse GGM, Bouwman WP, Jiawan-Lalai R, Terpstra OT, Bruijn JA, de Heer E. Integrin signaling via RGD peptides and anti-beta1 antibodies confers resistance to apoptosis in islets of Langerhans. Diabetes. 2006;55:312–7. doi: 10.2337/diabetes.55.02.06.db04-0195. [DOI] [PubMed] [Google Scholar]
- 12.Navarro-Alvarez N, Rivas-Carrillo JD, Soto-Gutierrez A, Yuasa T, Okitsu T, Noguchi H, Matsumoto S, Takei J, Tanaka N, Kobayashi N. Reestablishment of microenvironment is necessary to maintain in vitro and in vivo human islet function. Cell Transplant. 2008;17:111–9. doi: 10.3727/000000008783907125. [DOI] [PubMed] [Google Scholar]
- 13.Korbutt GS, Mallett AG, Ao Z, Flashner M, Rajotte RV. Improved survival of microencapsulated islets during in vitro culture and enhanced metabolic function following transplantation. Diabetologia. 2004;47:1810–8. doi: 10.1007/s00125-004-1531-3. [DOI] [PubMed] [Google Scholar]
- 14.Chen YY, Zheng MZ, Lv PP, Hu L, Wang LL, Shen YL. Hydrogen peroxide regulates glucose-regulated protein 78 expression via a cyclooxygenase-2 dependent mechanism. J Biochem Mol Toxicol. 2010;24:279–85. doi: 10.1002/jbt.20336. [DOI] [PubMed] [Google Scholar]
- 15.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–9. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber LM, Anseth KS. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol. 2008;27:667–73. doi: 10.1016/j.matbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang RN, Paraskevas S, Rosenberg L. Characterization of integrin expression in islets isolated from hamster, canine, porcine, and human pancreas. J Histochem Cytochem. 1999;47:499–506. doi: 10.1177/002215549904700408. [DOI] [PubMed] [Google Scholar]
- 18.Shkilnyy A, Proulx P, Sharp J, Lepage M, Vermette P. Diffusion of rhodamine B and bovine serum albumin in fibrin gels seeded with primary endothelial cells. Colloids Surf B Biointerfaces. 2012;93:202–7. doi: 10.1016/j.colsurfb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Lamb M, Laugenour K, Liang O, Alexander M, Foster CE, Lakey JR. In vitro maturation of viable islets from partially digested young pig pancreas. Cell Transplant. 2013 doi: 10.3727/096368912X662372. In press. [DOI] [PubMed] [Google Scholar]
- 20.Matas AJ, Sutherland DE, Steffes MW, Najarian JS. Short-term culture of adult pancreatic fragments for purification and transplantation of islets of Langerhans. Surgery. 1976;80:183–91. [PubMed] [Google Scholar]
- 21.Ricordi C. Pancreatic Islet Cell Transplantation. Austin: R.G. Landes Company; 1992.137-8. [Google Scholar]
- 22.Sabra G, Vermette P. Endothelial cell responses towards low-fouling surfaces bearing RGD in a three-dimensional environment. Exp Cell Res. 2011;317:1994–2006. doi: 10.1016/j.yexcr.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Kuehn C, Dubiel EA, Sabra G, Vermette P. Culturing INS-1 cells on CDPGYIGSR-, RGD- and fibronectin surfaces improves insulin secretion and cell proliferation. Acta Biomater. 2012;8:619–26. doi: 10.1016/j.actbio.2011.10.036. [DOI] [PubMed] [Google Scholar]