Abstract
Background
Coagulation disorders remain barriers to successful pig-to-primate organ xenotransplantation. In vitro, we investigated the impact of pig genetic modifications on human platelet aggregation in response to pig aortic endothelial cells (pAEC).
Methods
In comparison to human (h)AEC and wild-type (WT) pAEC, the expression of human complement- (CD46, CD55) or coagulation (thrombomodulin [TBM], endothelial protein C receptor [EPCR]) -regulatory proteins on pAEC from WT or α1,3-galactosyltransferase gene-knockout (GTKO) pigs was studied by flow cytometry. Using platelet-aggregometry, human whole blood platelet aggregation was evaluated after co-incubation with various AEC. Further, the inhibitory effect on aggregation of heparin, low molecular weight heparin, and hirudin was assessed.
Results
Heparin, low molecular weight heparin and hirudin almost completely prevented platelet aggregation induced by WT pAEC. The level of expression of human CD46, CD55, TBM and EPCR on pAEC was comparable to that on hAEC. Platelet aggregation induced by all genetically-modified pAEC was significantly less (p<0.05) than that by WT pAEC (which was 54%). GTKO/CD46/TBM pAEC induced the least platelet aggregation (27%) – a reduction of almost 50% - but this remained significantly greater (p<0.01) than aggregation induced by hAEC (4%). There was significant positive correlation between reduction of aggregation and TBM or EPCR expression on pAEC (r2=0.89 and r2=0.86, respectively; p<0.01). Platelet aggregation induced by GTKO/CD46/TBM pAEC in the presence of hirudin (1IU/ml) was comparable to platelet aggregation induced by hAEC.
Conclusions
Genetic-modification of pAEC is associated with significant reduction of human platelet aggregation in vitro. With concomitant thrombin inhibition, platelet aggregation was comparable to that stimulated by hAEC.
Keywords: endothelial cells, platelet aggregation, pigs, genetically-modified, xenotransplantation
INTRODUCTION
The increasing shortage of human organs for transplantation has focused research on the possibility of transplanting animal organs and cells, especially from pigs, into humans (1, 2). The current major barrier to successful transplantation of pig organs and cells in humans is coagulation dysregulation, which results in the development of thrombotic microangiopathy (TM) in α1,3-galactosyltransferase gene-knockout (GTKO) pig xenografts (3–5) and consumptive coagulopathy (CC) in the recipient (6–8).
Coagulation dysregulation is at least in part related to molecular incompatibilities between pigs and primates (9–11), which result in impaired anticoagulation (12). This may relate to expression of pig (rather than human) tissue factor pathway inhibitor (TFPI), thrombomodulin (TBM), endothelial protein C receptor (EPCR), CD39, and/or heparan sulfate on the surface of pig endothelial cells (EC), particularly when these cells are activated, e.g., by reperfusion or acute humoral xenograft rejection (12).
Pig TBM can bind human thrombin, but cannot effectively convert human protein C into activated protein C (13,14), which inactivates factors (F) Va and VIIIa (15). Furthermore, pig aortic endothelial cells (pAEC) can directly interact with human prothrombin without anti-pig antibody binding or complement activation (16) or with human platelets (17). It is well-known that complement is associated with inflammatory and coagulation responses (17–19). For example, in vitro experiments have demonstrated that complement can regulate tissue factor (TF) activity in ECs (20).
In pig-to-baboon kidney transplantation, CC (steadily decreasing fibrinogen and platelet count) developed within 6 to 10 days (6–8) before histopathological evidence of rejection was advanced (7, 8). Hearts from GTKO pigs or pigs expressing a human complement-regulatory protein transplanted into baboons developed TM, leading to myocardial fibrosis (3, 4). The histopathology was different from typical acute humoral xenograft rejection, and revealed microvascular thrombosis in arterioles, capillaries, and venules, with only rare interstitial mononuclear cells (5).
Although platelet activation and aggregation have been shown to be important factors in the development of TM after xenotransplantation (21), the effect of pig genetic modification on pAEC-induced platelet aggregation has not been fully studied. Therefore, we examined the impact of genetically-modified pAEC and the expression of human complement- and/or coagulation-regulatory proteins (Table 1) on human platelet aggregation by using whole blood platelet aggregometry in vitro (22).
Table 1.
Genetically-modified pAEC tested in the human platelet aggregation assay
| Genetic modification | Effect | Promoter |
|---|---|---|
| GTKO | Deletion of Gal antigen | |
| CD46 | Transgenic expression of a human complement- regulatory protein | Endogenous |
| CD55 | Transgenic expression of a human complement- regulatory protein | CAG promoter |
| TFPI | Transgenic expression of human TFPI antagonizes the function of human tissue factor (inhibits FXa, thrombin, FVIIa-TF complex) | TIE2 |
| TBM | Transgenic expression of human TBM activates protein C | Endogenous or ICAM-2 promoter |
| EPCR | Transgenic expression of human EPCR enhances the activation of protein C | CAG promoter |
EPCR= endothelial protein C receptor
GTKO= a1,3-galactosytransferase gene-knockout
TBM= thrombomodulin
TFPI= tissue factor pathway inhibitor
In xenotransplantation experiments, anticoagulants have been used for preventing TM and/or CC. We also examined the inhibitory effect of anticoagulants (heparin, low molecular weight heparin [LMWH], and hirudin) on pAEC-induced human platelet aggregation.
MATERIALS AND METHODS
Cell sources
pAEC were collected from wild-type (WT) pigs and from different genetically-modified pigs (Table 1) provided by Revivicor, Inc., Blacksburg, VA (2, 23, 24). All pigs were of blood type O (non A) and of Large White/Landrace/Duroc cross-breed (but were not from identical clones), except the CD46 transgenic pigs which were derived from a different herd of Large White pigs (25).
Vectors were constructed to provide either endothelial-specific expression of human TBM using the porcine ICAM-2 enhancer/promoter region (n=2), or human TBM expression from the endogenous pig TBM promoter region (n=2). Two TBM expression vectors were constructed. Endothelium-specific expression of the human TBM coding DNA sequence (CDS) was driven by a 0.9kb porcine ICAM-2 promoter fragment, preceded by a 1.4kb porcine ICAM-2 enhancer originating from intron 1 of the pig ICAM-2 gene. The expression cassette was flanked by multiple copies (two copies at the 5′ end and 4 copies at the 3′ end) of chicken beta-globin insulator. An additional TBM expression vector was built using an 8.9kb region upstream of the porcine TBM gene as promoter for expression of the human TBM CDS. This vector also contained a neomycin-resistance cassette located downstream of the bovine growth hormone polyadenylation cassette inserted behind the hTBM CDS.
Human CD55 and EPCR CDS were expressed using the ubiquitously-expressing CAG enhancer/promoter. The EPCR expression cassette was also flanked by insulator/MAR regions to optimize expression.
Linear plasmid fragments were prepared and used to transfect GTKO/CD46 porcine fibroblast lines, in which human CD46 is expressed as a minigene under control of the endogenous promoter (25). Transfected pig fibroblasts were sorted by flow cytometry or selected and screened for the presence of the transgene by polymerase chain reaction, then used for nuclear transfer. Derived fetuses or live pigs were screened by Southern analysis for the presence of the transgenes. Southern-positive fetuses or pigs were screened for transgene expression by RT-PCR, immunofluorescence, and/or flow cytometry.
Two high-expressing ICAM-2-TBM lines, and one moderate-expressing TBM-TBM line were used to produce the pig lines used in these studies. EPCR pigs were founder animals, such that while EPCR expression was relatively high across cloned pigs, differences in expression from pig-to-pig from integration site effects were both expected, and observed.
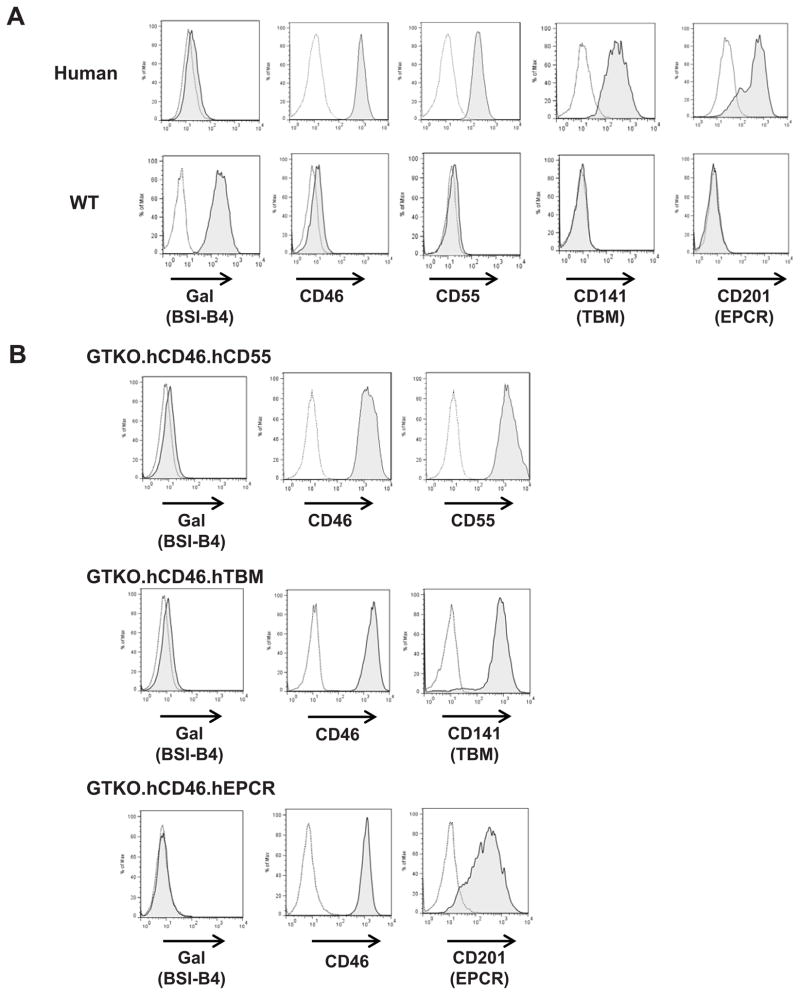
All pigs, including those expressing the human coagulation-regulatory proteins (TBM, TFPI, and EPCR), were born alive and survived without hemorrhagic complications until euthanized for procurement of pAEC and gene expression analysis (Ayares D, personal communication). Human aortic endothelial cells (hAEC) (as an allograft control) were purchased from Cambrex (Walkersville, MD). pAECs from the pigs with the highest expression of hTBM and hEPCR were used for the platelet aggregation assay in Figures 1, 4B, and 6.
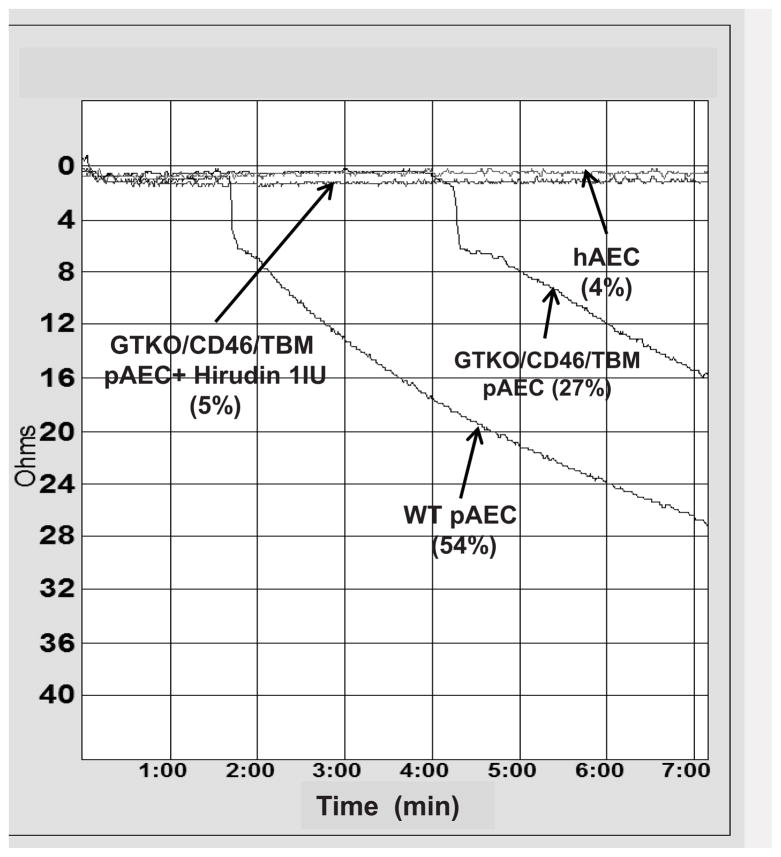
Figure 1. Human platelet aggregation induced by wild-type (WT) pAEC, human AEC (hAEC) and genetically modified pAEC.
hAEC- and pAEC-induced human platelet aggregation were measured by aggregometry (Chrono-log). Representative curves of human platelet aggregation induced by hAEC and pAEC. After human whole blood was incubated with WT pAEC, removed, diluted, and recalcified, aggregation of human platelets occurred. Incubation of blood with hAEC resulted in minimal aggregation. Platelet aggregation was reduced when whole blood was incubated with GTKO/CD46/TBM pAEC. Addition of hirudin (1U/mL) to GTKO/CD46/TBM pAEC almost completely prevented platelet aggregation in whole blood.
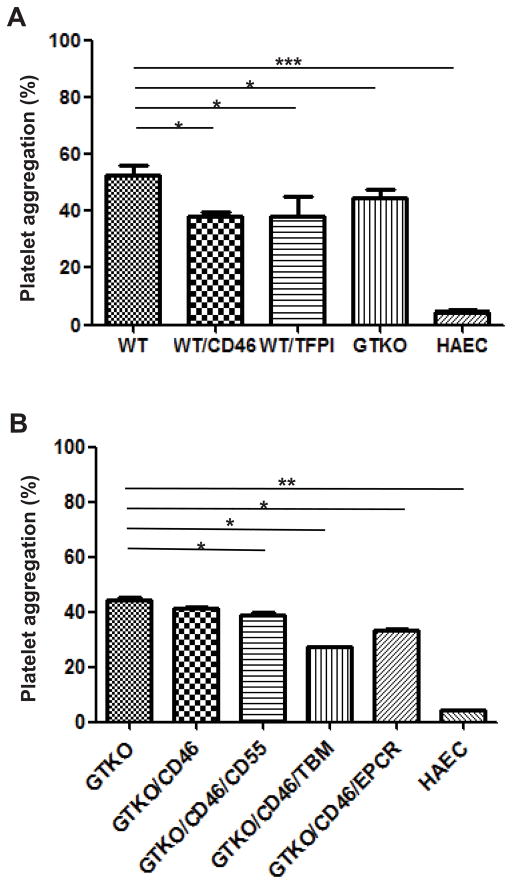
Figure 4. The effect of various genetic modifications on pAEC-induced human platelet aggregation.
(A) When human platelet aggregation associated with WT pAEC (54%) was compared with that to hAEC (4%), a significant difference was observed (p<0.001). WT/CD46 (38%), WT/TFPI (38%), and GTKO (44%), and pAEC significantly reduced the aggregation associated with WT pAEC (all p<0.05).
(B) GTKO/CD46/CD55 (39%), GTKO/CD46/TBM (27%), and GTKO/CD46/EPCR (33%) pAEC induced significantly less aggregation than GTKO pAEC (44%) (all p<0.05). (*p<0.05, **p<0.01, ***p<0.001), but did not reduce aggregation to that associated with hAEC.
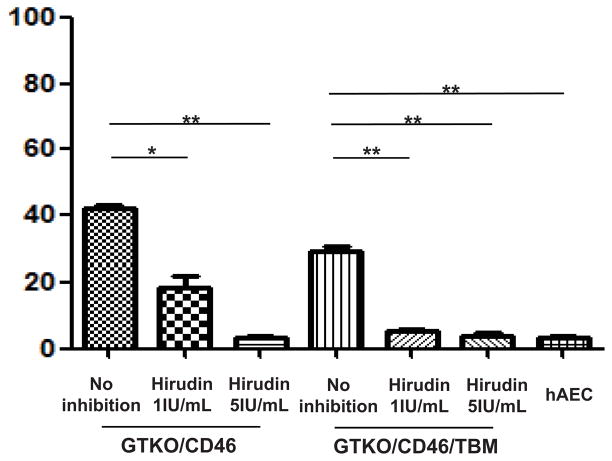
Figure 6. Anti-thrombin inhibit human platelet aggregation induced by GTKO/CD46 and GTKO/CD46/TBM pAEC.
Hirudin at 5IU/ml completely inhibited GTKO/CD46 pAEC-induced platelet aggregation in human blood (4%) while it had a partial effect at 1IU/ml (18%) when compared with control (41%; *p<0.05, **p<0.01). Platelet aggregation induced by GTKO/CD46/TBM pAEC was almost completely prevented by hirudin (1 or 5IU/ml; 5% and 4% aggregation, respectively) (**p<0.01), which was comparable to that induced by hAEC (4%).
Endothelial cell culture
pAEC were isolated from fresh aortas and cultured in collagen I-coated tissue culture flasks in pAEC culture medium (10% heat-inactivated FBS [Sigma, St. Louis, MO], antibiotic–antimycotic [Invitrogen, Carlsband, CA] and endothelial growth factor [30 lg/ml, BD Biosciences (BD), San Jose, CA) at 37°C in a humidified atmosphere of 5% CO2, as previously described (17). Cell aliquots were frozen and stored in 10% DMSO culture storing medium (Invitrogen) at −80°C until used for in vitro assays. Cryopreserved pAEC were thawed, washed twice with washing medium, and cultured for in vitro assay. EC suspensions had a viability of >90%. hAEC were cultured to monolayers in EBM-2 medium (Lonza, Walkersville, MD, which included endothelial growth medium-2 [Cambrex]) under the same conditions. Confluent cultures of both pAEC and hAEC were characterized by their cobblestone morphology and were used before passage 7 in all experiments.
Flow cytometry for Gal and human protein expression
In vitro characterization of expression on EC has been described previously (19, 26–28). Surface expression of Gal, CD31 (EC), hCD46, hCD55, hTFPI, hTBM, and hEPCR were detected by BD™ LSR II flow cytometer (BD Biosciences). pAEC and hAEC were diluted to 105 cells per tube in FACS buffer (PBS [Invitrogen] containing 1%BSA and 0.1%NaN3) (12). The following lectins and monoclonal antibodies (mAbs) were used for surface expression:- B4 from Bandeiraea simplicifolia (BS-IB4, Sigma), anti-hCD46 (clone MEM-258, Serotec, Raleigh, NC); anti-hTFPI (American Diagnostica, Stamford, CT); anti-hCD55 (clone IA10, BD); anti-hCD141 (hTBM; clone 1A4, BD); anti-hCD201 (hEPCR; clone RCR-252, BD). Purity of pAEC was confirmed to be >90% by staining with PE-conjugated mouse anti-rat CD31mAb (clone TLD-3A12, BD)
Incubation of pAEC or hAEC with human whole blood
The appropriate numbers (1×106) of pAEC and hAEC were cultured in 6-well collagen-coated culture plates. After the cells had grown to confluence in their respective media, 3.2% trisodium citrated fresh whole blood (1ml) drawn from a single healthy human volunteer was added to the adherent monolayers of pAEC and hAEC and incubated for 2h at 37°C. (Preliminary studies indicated that 2h was the optimum time for peak aggregation to occur.) Supernatant fluid (500μl) from co-incubation of blood and AEC was collected, mixed with saline (500μl) in a plastic cuvette, and used in the platelet aggregation assay. A single human volunteer was used as a blood donor for all studies. (When platelets are being isolated, e.g., as platelet-rich plasma, the initial 3–5mL of blood drawn from the donor are discarded. When whole blood is being used, some groups do discard 1.5mL, but the manufacturer of the Chronolog device does not recommend this. We therefore did not discard any blood. As we drew approximately 25mL of blood from the single donor on each occasion, of which only 1mL was used in each assay, we do not believe discarding any blood would have significantly influenced the results.)
Human platelet aggregation assay
Platelet aggregation in whole blood (500μl) incubated with pAEC/hAEC for 2h (which preliminary studies indicated was the period associated with maximal aggregation) was measured with the addition of calcium (15μl, 9.3 mg Ca++ per ml), in the absence of any platelet agonist by platelet aggregometry (two-sample, four-channel, model 592 Whole Blood Aggregometer, Chrono-log, Harvertown, PA), as previously described (22). No aggregation occurred in the absence of calcium.
Briefly, after co-incubation with pAEC/hAEC, 500μl of the blood was mixed with 500μl saline in siliconized cuvettes (Corning, Corning, NY) containing stirring bars (pre-warmed at 37°C for 5min). By adding a small rotating magnet to each sample, shear stress was created to simulate intravascular flow conditions. The extent of platelet aggregation in 6min (based on the manufacturer’s instructions) was evaluated. (In our previous studies exposing primate blood to platelet agonists (22), the peak rate of aggregation frequently occurred 6 minutes after the initiation of aggregometry. Thereafter, there was variability in the rate of aggregation.) Aggregation in the whole blood aggregometer is measured in ohms (from 0 to 40), and the maximum change of ohms from baseline is indicated, and is also presented as the percentage change from baseline. As in our previous study (22), we have chosen to report our data as percentage change. Importantly, no agonists were used to induce platelet aggregation. Supernatants of whole blood obtained after incubation (in the absence of any cells) were used at the beginning of each experiment as a negative control (0% aggregation). Aggregation associated with each type of EC was tested at least x3 using blood from the same human donor either on the same day or on different days using a different culture of the same EC.
Anticoagulants used to inhibit WT, GTKO/CD46, and GTKO/CD46/TBM pAEC-induced human platelet aggregation
Experiments were performed by co-incubating whole blood with WT, GTKO/CD46, and GTKO/CD46/TBM pAEC with and without anticoagulants (heparin #504011, APP Pharmaceuticals, Schaumburg, IL; low molecular weight heparin [LMWH] Fragmin®, Eisai, Woodcliff Lake, NJ; hirudin; #H7016, Sigma) at 1 or 5IU/ml for 2h at 37°C.
Statistical analysis
Data are presented as mean ± SEM. Significance of the difference was determined by paired Student’s t-test or the Mann-Whitney U test for two groups, and nonparametric statistical analysis of variance by Kruskal-Wallis test for multiple comparisons. Monotonic association analysis was evaluated using Spearman’s rank-order correlation. Statistical analysis was performed using social sciences software GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). Values of p<0.05 were considered statistically significant.
RESULTS
Human platelet aggregation induced by wild-type (WT) pAEC is reduced by anticoagulants
Human whole blood co-incubation with WT pAEC resulted in 54% aggregation, but with only 4% aggregation with hAEC (Figure 1). (NB. Even the addition of the platelet agonist thrombin [in the absence of any EC] does not result in 100% aggregation.)
When anticoagulants (heparin, LMWH, or hirudin) were added to coculture of WT pAEC and human whole blood, all three anticoagulants reduced WT pAEC-induced platelet aggregation (Figure 2). In the presence of heparin at 1IU/ml, LMWH at 5IU/ml, and hirudin at 5IU/ml, the aggregation of human platelets was almost completely inhibited (3%, 3%, and 4%, respectively), whereas LMWH and hirudin (both at 1IU/ml) only partially inhibited aggregation (15% and 29%, respectively).
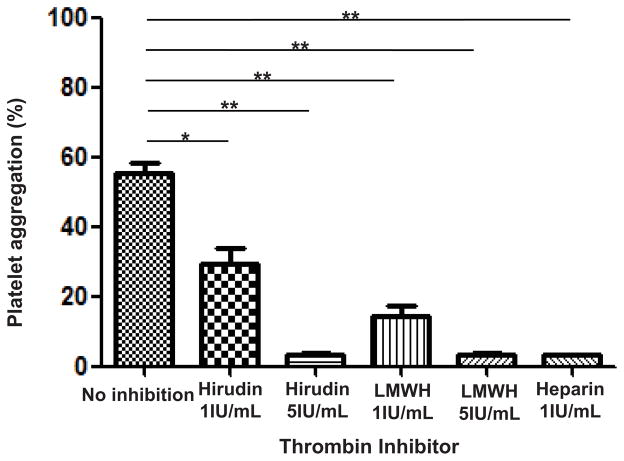
Figure 2. Anti-coagulant agents inhibit human platelet aggregation induced by WT pAEC.
Preincubation of human whole blood and WT pAEC with an anti-thrombin agent (heparin, LMWH at 1 or 5IU/mL, or hirudin at 1 or 5IU/mL) reduced platelet aggregation. Heparin at 1IU/mL (3% aggregation), LMWH at 5IU/ml (3% aggregation), and hirudin at 5IU/mL (4% aggregation) completely prevented aggregation, while LMWH (15%) and hirudin (29%) at 1IU/mL had a partial effect when compared with control (54%). (*p<0.05, **p<0.01).
Expression of Gal and human proteins on genetically-modified pAEC
The purity of isolated EC was assessed by the expression of CD31, and was consistently >90%. Human (h) AEC, WT pAEC, and genetically modified pAEC were examined by flow cytometry for expression of the Galα1,3 Gal (Gal) antigen and for expression of human transgenes (CD46, CD55, TFPI, TBM, EPCR) (Figure 3). Gal expression was absent on all types of GTKO pAEC. The expression of hCD46 on WT/CD46, GTKO/CD46, GTKO/CD46/CD55, GTKO/CD46/TBM, and GTKO/CD46/EPCR pAEC was comparable on hAEC. The expression of hCD55 on GTKO/CD46/CD55 pAEC was comparable on hAEC. Expression of TBM and EPCR on hAEC and pAEC expressing TBM or EPCR were comparable. Human TFPI expression on WT pAEC has been previously reported (19). Since TFPI expression was low (as it was also on hAEC, with only 6–15% expression [not shown]), the pig with the best expression of TFPI on pAEC was selected for platelet aggregation assays.
Figure 3. Expression of Gal and human proteins on genetically-modified pAEC.
(A) Flow cytometry was used to evaluate Gal and human transgene protein expression on human AEC in comparison to WT pAEC. Human AEC did not express Gal, but expressed high levels of CD46, CD55, CD141 (TBM), and CD201 (EPCR). In contrast, WT pAEC expressed Gal, but no human CD46, CD55, TBM, or EPCR. No GTKO pAEC expressed Gal. (B) In addition to lack of Gal expression, GTKO/CD46 pAEC expressed high levels of CD46, GTKO/CD46/CD55 pAEC expressed high levels of CD46 and CD55. GTKO/CD46/TBM pAEC expressed TBM at a level comparable to hAEC. Similarly, GTKO/CD46/EPCR pAEC expressed EPCR at a level comparable to hAEC.
Effect of genetic modification of pAEC on human platelet aggregation
Human whole blood co-incubated with WT pAEC resulted in a prompt aggregation of platelets (54%) (Figure 4A). In contrast, hAEC induced minimal platelet aggregation (4%). The difference in platelet aggregation between WT pAEC and hAEC was statistically significant (p<0.001).
Human platelet aggregation induced by pAEC is reduced by absence of expression of Gal antigens
Human platelet aggregation induced by GTKO pAEC was significantly less than that induced by WT pAEC (44% vs. 54%, p<0.05) (Figure 4A).
Human platelet aggregation induced by pAEC is reduced by expression of human complement-regulatory proteins
To determine whether pAEC expressing a human complement-regulatory protein are associated with reduced platelet aggregation, WT/CD46, GTKO/CD46, and GTKO/CD46/CD55 pAEC were incubated with blood. WT/CD46 pAEC induced significantly less platelet aggregation than WT pAEC (38% vs. 54%, p<0.05) (Figure 4A). GTKO/CD46/CD55 induced significantly less platelet aggregation in comparison to GTKO pAEC (39% vs. 44%, p<0.05) (Figure 4B), but there was no significant difference in platelet aggregation induced by GTKO/CD46 (41%) as compared to GTKO (Figure 4B).
Human platelet aggregation induced by pAEC is reduced by expression of human coagulation-regulatory proteins
The expression of hTFPI on WT (WT/TFPI) pAEC was associated with significant reduction of human platelet aggregation as compared to WT pAEC (54% vs. 38%, p<0.05) (Figure 4A).
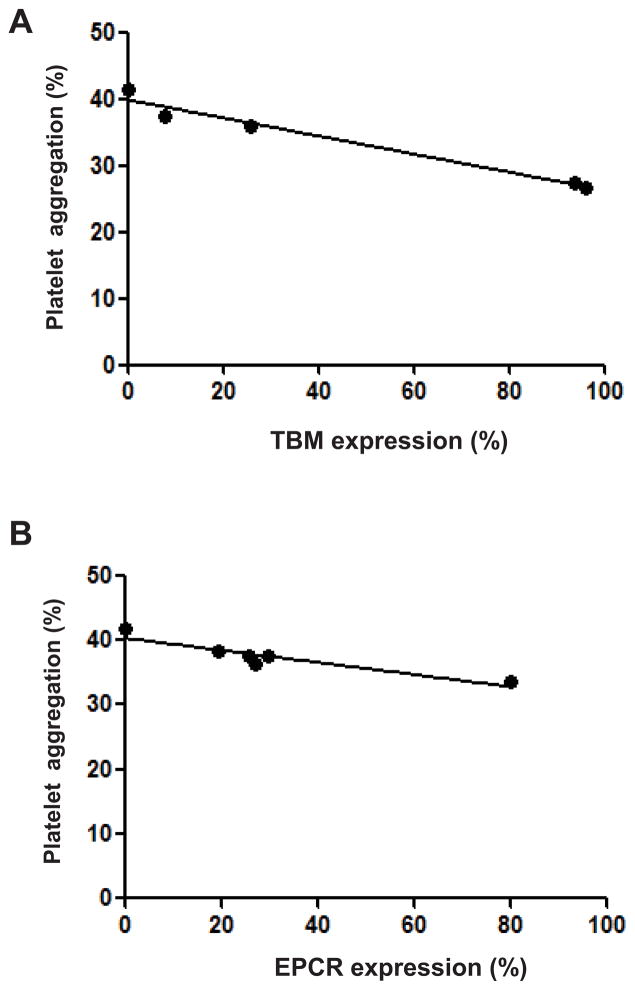
Human platelet aggregation induced by GTKO/CD46/TBM pAEC was significantly less than that induced by GTKO pAEC (27% vs. 44%, p<0.05) (Figure 4B) or by GTKO pAEC expressing one or two complement-regulatory proteins (CD46 [41%] or CD46+CD55 [39%], both p<0.05). The platelet aggregation induced by GTKO/CD46/TBM pAEC correlated significantly with the cell surface expression of TBM on the pAEC (r2=0.89, p<0.01) (Figure 5A).
Figure 5. Correlation between human platelet aggregation and expression levels of human TBM or EPCR on GTKO/CD46 pAEC.
There was a significant correlation between human platelet aggregation after exposure to (A) GTKO/CD46/TBM (n=4) or (B) GTKO/CD46/EPCR (n=5) pAEC and the expression levels of human TBM or EPCR on the pAEC (both p<0.01). The pigs transgenic for TBM were from two different lines (high- and low-expressing), whereas those transgenic for EPCR were from a single line.
Human platelet aggregation induced by GTKO/CD46/EPCR pAEC (33%) was significantly less than that induced by GTKO (44%) pAEC (p<0.05) (Figure 4B), but not significantly different from aggregation induced by GTKO/CD46/TBM pAEC (27%). There was a significant correlation between platelet aggregation induced by GTKO/CD46/EPCR pAEC and the expression level of EPCR on the pAEC (r2 = 0.86, p<0.01) (Figure 5B).
TBM expression and thrombin inhibition efficiently blocks human platelet aggregation
GTKO/CD46 and GTKO/CD46/TBM pAEC were cocultured with human whole blood in the absence or presence of 1IU/ml or 5IU/ml hirudin (Figure 6). The aggregation of human platelets induced by GTKO/CD46 pAEC was almost completely inhibited in the presence of hirudin at 5IU/ml (4%), whereas hirudin at 1IU/ml only partially inhibited aggregation (18%). In comparison, human platelet aggregation induced by GTKO/CD46/TBM pAEC was almost completely prevented in the presence of 1IU/ml (Figure 1), or 5IU/ml of hirudin (5% and 4%, respectively), which was comparable to human platelet aggregation induced by hAEC (4%).
In an effort to understand whether the concentrations of hirudin used in these studies were clinically relevant, we carried out some in vitro measurements. Recombinant hirudin (lepirudin) is occasionally administered to patients with thrombocytopenia associated with heparin therapy. The dosage (infusion rate) is adjusted according to the activated partial thromboplastin time (aPTT) ratio (i.e., the patient’s aPTT during hirudin infusion relative to the baseline [or reference] aPTT). The target ratio is 1.5 to 2.5, as a higher ratio carries a higher risk of bleeding. In our in vitro assay, when hirudin was added to human blood at 1IU/ml, the ratio was 2.2. At 5IU/ml, the ratio rose to 3.0. These data suggested to us that the concentration of 1IU/ml used in our studies was clinically-applicable but that the concentration of 5IU/ml might be moderately excessive. It is important to note that platelet aggregation induced by GTKO/CD46/TBM pAEC in the presence of 1IU/ml of hirudin reduced aggregation to the level seen when human platelets were incubated with hAEC, namely approximately 4–5%.
DISCUSSION
To date, xenotransplantation in the pig-to-nonhuman primate organ transplantation model has invariably resulted in the development of TM and/or CC, even before rejection is histopathologically advanced (3, 4, 6–8, 29). Long-term survival of discordant xenografts may depend on the successful inhibition of TM and/or CC.
Platelets have long been suspected of playing a major role in xenotransplantation through their interaction with endothelium and leukocytes in the form of platelet microthrombi (30–33). Inhibition of platelet aggregation will almost certainly be beneficial in preventing or reducing the thrombotic disorders associated with xenotransplantation. We therefore investigated the effect of various genetic modifications of pAEC and/or anticoagulant/anti-thrombin agents on human platelet aggregation by establishing a reproducible in vitro assay.
In our studies, pAEC, but not hAEC, were associated with human platelet aggregation, consistent with other reports (34–36). Although statistically significant reductions were consistently recorded in the absence of Gal expression on the pAEC, or when a human complement-regulatory or coagulation-regulatory protein was expressed, whether these reductions are biologically relevant remains uncertain. However, the expression of TBM on GTKO/CD46 pAEC reduced platelet aggregation by 50%, and hearts from these pigs showed delayed features of TM and CC after transplantation into baboons (37).
When WT pAEC were co-incubated with human blood containing an anticoagulant (heparin, LMWH, or hirudin), human platelet aggregation was inhibited, with heparin being the most effective. Hirudin reduced human platelet aggregation after co-incubation of blood with WT pAEC, suggesting that thrombin plays an important role in the platelet aggregation (38) due to the interaction of pAEC with human plasma, platelets, or monocytes, in accordance with previous reports (39), and following pig organ xenotransplantation (40) although mechanisms were not directly examined. Thrombin inhibitors have been shown to have a potent inhibitory effect on platelet aggregation in humans and nonhuman primates (21, 22).
Following incubation of whole blood with pAEC, a series of events leads to platelet activation. During the incubation period, we suggest that there is antibody binding and complement activation, leading to EC activation, promoting tissue factor and fibrinogen-like protein 2 (Fgl2) expression, loss of natural anticoagulants, and eventually resulting in the generation of thrombin. Our previous studies indicate that platelet interaction with WT pAEC (17,19) or genetically-modified pAEC (Ezzelarab M, et al, manuscript under revision) induces platelet activation (and tissue factor expression) irrespective of antibody binding and complement activation. The amount of thrombin generated during the incubation period is likely to be reduced when there are no antigenic targets for binding of anti-Gal antibodies and by the expression of human complement- and/or coagulation-regulatory proteins. Additionally, direct (hirudin) or indirect (heparin, low molecular weight heparin) thrombin inhibition is likely to downregulate further thrombin generation. Consequently, the amount of thrombin that has been produced during incubation of the blood with the pAEC will determine the degree of coagulation and platelet aggregation. We plan to measure thrombin generation during the incubation phase to determine whether this hypothesis is correct.
The Chronolog technology involves mechanical stirring of whole blood (platelets), which additionally enhances platelet aggregation and thrombus formation, which may require some “lag” time, as we have shown in our previous studies. Using platelet agonists, a one minute lag time before aggregation was observed. Also, direct activation of platelets may lead to upregulation of adhesion molecules that further enhances platelet adhesion and aggregation. In the current study, the whole blood sample was incubated for two hours (of activation) prior to Chronolog measurement, which may result in slow induction of platelet activation (with pAEC), rather than strong, rapid activation of platelets.
Furthermore, the delay of approximately two minutes suggests that this period of time may be required for sufficient thrombin to be produced for aggregation to become obvious. In this respect, our studies have shown that aggregation begins rather more quickly when the blood is exposed to wild-type pig EC than to EC expressing human TBM, indicating that thrombin generation takes longer when the EC express TBM, presumably because the thrombin being generated is partially being neutralized.
In our study, several mechanisms may contribute to human platelet aggregation. First, anti-Gal and/or anti-nonGal xenoantibodies and complement could activate pAEC to express TF (35). Furthermore, anti-pig antibodies may also activate pAEC through a complement-independent pathway (8, 12). Incubation of blood with GTKO pAEC induced less platelet aggregation, suggesting the absence of binding of anti-Gal antibody contributes to reduced platelet aggregation.
Second, previous studies suggested that activation of EC as a trigger to thrombotic graft failure is dependent on the involvement of complement, mediated by binding of xenoreactive antibodies (18). Robson et al. demonstrated that human platelet-pAEC aggregation occurred only in the presence of complement components, with the consequent generation of thrombin (35). Furthermore, membrane-attack complex (MAC) formation after complement activation has been associated with thrombin formation (41). Therefore, the EC of pigs expressing one or more complement-regulatory protein, e.g., CD46 and/or CD55, would be expected to be protected to some extent. Miwa et al. (42) demonstrated that expression of human CD55 is associated with a reduction in the up-regulation of TF when pAEC are activated by complement, inhibiting thrombin generation by 59%, through classical prothrombinase activity. However, even high levels of CD46 and CD55 will not completely inhibit formation of the membrane attack complex (42).
Third, molecular incompatibilities of the coagulation-anticoagulation system between pig and primate may accentuate thrombin generation (11) and contribute to platelet aggregation and the development of TM and/or CC. Vascular EC possess the capacity to inhibit platelet activation/aggregation through several mechanisms, e.g., TFPI (9), TBM (43, 44), EPCR (45), CD39 (36), prostacyclin (PGI2) (46), and nitric oxide (30, 47). The loss of these anticoagulant properties after activation of pig EC, e.g., by inflammation or rejection (48), promotes platelet aggregation. Furthermore, pig von Willebrand Factor aggravates the thrombotic process. Pig Fgl2, unlike human Fgl2, has been reported to have prothrombinase activity that generates thrombin directly from prothrombin in the absence of a prothrombinase (i.e., FVa-Xa complex) (16).
Endothelial cells and monocytes constitute the main sources of circulating TF, as shown in inflammation and sepsis models (49), and transfer TF to platelets (50). The expression of TF on pAEC is up-regulated by xenoreactive antibodies and complement activation (12, 51) and by activated platelets (51). Lin et al. demonstrated that resting pAEC activated monocytes and platelets to express TF independent of a humoral immune response (15). In our in vitro study, thrombin generation induced by TF from pAEC, human platelets, and/or monocytes/macrophages may be a factor in the development of platelet aggregation (52), which in turn can be an important factor in the development of TM in vivo following xenotransplantation.
In contrast, pig TFPI and TBM are unable to down-regulate the propagation of thrombus (10, 53), which leads to uncontrolled thrombin generation and ultimately promotes platelet aggregation. TFPI is a crucial regulator of the coagulation pathway initiated by TF (26). In vivo, when transplanted into a rat, overexpression of human TFPI in a transplanted mouse heart completely inhibited intragraft thrombosis (27). Hirudin was also reported to show efficacy in a rodent heart xenotransplantation model (27). These and our current data suggest that expression of human TFPI reduces platelet aggregation by inhibiting the generation of thrombin through the TF pathway, which correlates with the results of others (54).
Human TBM is considered to be a potential regulator of thrombin generation through the capture of thrombin as well as production of activated protein C (55, 56). In our experiments, pAEC expressing human TBM induced significantly less platelet aggregation. Miwa et al. (42) showed that expression of human TBM on pAEC was associated with significant reduction of thrombin production.
Another key player in the protein C anticoagulant system is EPCR, which binds protein C and presents it to the TBM/thrombin complex for activation, enhancing the generation of activated protein C approximately 20-fold in vivo (57). In the present study, expression of EPCR reduced platelet aggregation almost as much as TBM, presumably by enhancing the effect of pig TBM (as the pig cells did not express human TBM). We would anticipate, however, that, because of its mechanism of action in enhancing the generation of activated protein C, EPCR would have a greater effect when expressed on pAEC that also express human TBM. GTKO/CD46/TBM/EPCR pigs are anticipated to be available soon.
TBM inhibits not only the effects of thrombin by binding thrombin, but also further thrombin generation by inactivating factors Va and VIIIa through activation of protein C. In contrast, hirudin (a direct thrombin inhibitor) inhibits thrombin irreversibly through binding directly to thrombin, which leads to reduction of platelet aggregation. In the present study, in response to pAEC, TBM expression together with a low concentration of hirudin (1IU/ml, which would be clinically-applicable) efficiently inhibited human platelet aggregation to a level that was comparable to that induced by hAEC.
Our studies were carried out using pAEC. Whether these are representative of microvascular EC from various organs is uncertain. Nor is it clear whether AEC are representative of EC from other major blood vessels, e.g., pulmonary artery or portal vein.
In conclusion, our data indicate that genetic modification of pAEC, including expression of complement- and/or coagulation-regulatory proteins, is associated with significant reduction of human platelet aggregation initiated by their contact with pAEC, which is at least partially through thrombin regulation. Whether platelet aggregation can be reduced further by expression of additional complement- or coagulation-regulatory proteins remains uncertain, but, as TBM and EPCR have proved to be beneficial in the current study, we are optimistic that additional expression of hTFPI and/or CD39 will also be beneficial. However, in addition to genetic modification, systemic approaches, e.g., therapy with anti-thrombotic/anti-platelet agents, may be necessary to regulate platelet aggregation completely.
Acknowledgments
Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants #U19 AI090959-01, #U01 AI068642, # R21 A1074844, and # 1PO1 HL107152 and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Blacksburg, VA. Burcin Ekser, MD, is a recipient of a NIH NIAID T32 AI 074490 Training Grant. Mohamed Ezzelarab, MD, is a recipient of a Joseph A. Patrick Research Fellowship in Transplantation at the Thomas E. Starzl Transplantation Institute.
Abbreviations
- AEC
aortic endothelial cells
- aPTT
activated partial thromboplastin time
- CC
consumptive coagulopathy
- EC
endothelial cells
- EPCR
endothelial protein C receptor
- Fgl2
fibrinogen-like protein 2
- GTKO
α1,3-galactosyltransferase gene-knockout
- LMWH
low molecular weight heparin
- TBM
thrombomobulin
- TFPI
tissue factor pathway inhibitor
- TM
thrombotic microangiopathy
- WT
wild-type
Footnotes
CONFLICT OF INTEREST
DA and CP are employees of Revivicor, Inc., Blacksburg, VA. None of the other authors has a conflict of interest.
References
- 1.Cooper DKC, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133–147. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- 2.Ekser B, Ezzelarab M, Hara H, et al. Clinical xenotransplantation – the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 3.Houser SL, Kuwaki K, Knosalla C, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11:416–425. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 4.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors:initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferasegene-knockout pigs in baboons. Am J Pathol. 2008;172:1471–1481. doi: 10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozlowski T, Shimizu A, Lambrigts D, et al. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation. 1999;67:18–30. doi: 10.1097/00007890-199901150-00004. [DOI] [PubMed] [Google Scholar]
- 7.Buhler L, Basker M, Alwayn I. Coagulation and thrombotic disorders associated with pig organ and hemopoietic cell transplantation in non-human primates. Transplantation. 2000;70:1323–1331. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 8.Lin CC, Ezzelarab M, Shapiro R, et al. Tissue factor expression on recipient platelets is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10:1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp CW, Siegel JB, Hancock WW, et al. Effect of porcine endothelial tissue factor pathway inhibitor on human coagulation factors. Transplantation. 1997;63:749–758. doi: 10.1097/00007890-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 10.Cowan PJ, Robson SC, d’Apice AJ. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16:214–221. doi: 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robson SC, Cooper DKC, d’Apice AJF. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 2000;7:166–176. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 12.Gollackner B, Goh SK, Qawi I, et al. Acute vascular rejection of xenografts: roles of natural and elicited xenoreactive antibodies in activation of vascular endothelial cells and induction of procoagulant activity. Transplantation. 2004;77:1735–1741. doi: 10.1097/01.tp.0000131167.21930.b8. [DOI] [PubMed] [Google Scholar]
- 13.Kopp CW, Grey ST, Siegel JB, et al. Expression of human thrombomodulin cofactor activity in porcine endothelial cells. Transplantation. 1998;66:244–251. doi: 10.1097/00007890-199807270-00019. [DOI] [PubMed] [Google Scholar]
- 14.Roussel JC, Moran CJ, Salvaris EJ, Nandurkar HH, d’Apice AJ, Cowan PJ. Pig thrombomodulin binds human thrombin but is a poor cofactor for activation of human protein C and TAFI. Am J Transplant. 2008;8:1101–1112. doi: 10.1111/j.1600-6143.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- 15.Fulcher C, Gardiner J, Griffin J, Zimmerman T. Proteolytic inactivation of human factor VIII procoagulant protein by activated human protein C and its analogy with factor V. Blood. 1984;63:486–489. [PubMed] [Google Scholar]
- 16.Ghanekar A, Mendicino M, Liu H, et al. Endothelial induction of fgl2 contributes to thrombosis during acute vascular xenograft rejection. J Immunol. 2004;172:5693–5701. doi: 10.4049/jimmunol.172.9.5693. [DOI] [PubMed] [Google Scholar]
- 17.Lin CC, Chen D, McVey JH, Cooper DKC, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86:702–709. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muhlfelder TW, Niemetz J, Kreutzer D, Beebe D, Ward PA, Rosenfeld SI. C5 chemotactic fragment induces leukocyte production of tissue factor activity: a link between complement and coagulation. J Clin Invest. 1979;63:147–150. doi: 10.1172/JCI109269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin CC, Ezzelarab M, Hara H, et al. Atorvastatin or transgenic expression of TFPI inhibits coagulation initiated by anti-nonGal IgG binding to porcine aortic endothelial cells. J Thromb Haemost. 2010;8:2001–2010. doi: 10.1111/j.1538-7836.2010.03950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saadi S, Holzknecht R, Patte C, Stern D, Platt J. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995;182:1807–1814. doi: 10.1084/jem.182.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcus AJ, Safier LB. Thromboregulation: multicellular modulation of platelet reactivity in hemostasis and thrombosis. FASEB J. 1993;7:516–522. doi: 10.1096/fasebj.7.6.8472890. [DOI] [PubMed] [Google Scholar]
- 22.Iwase H, Ekser B, Zhou H, Dons EM, Cooper DKC, Ezzelarab MB. Platelet aggregation in humans and nonhuman primates: relevance to xenotransplantation. Xenotransplantation. 2012;19:233–243. doi: 10.1111/j.1399-3089.2012.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayares D, Phelps CJ, Vaught TD, Ball S. Genetic engineering of pigs for improved transplant outcomes. Xenotransplantation. 2007;14:428. (# 404.2) [Google Scholar]
- 24.Ayares D. Multi-transgenic pigs for xenotransplantation. Reprod Fertil Dev. 2012;25:320. Doi. 10. 1071/RDv25n1Ab344. [Google Scholar]
- 25.Loveland BE, Milland J, Kyriakou P, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11:171–183. doi: 10.1046/j.1399-3089.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen D, Riesbeck K, McVey JH, et al. Regulated inhibition of coagulation by porcine endothelial cells expressing P-selectin-tagged hirudin and tissue factor pathway inhibitor fusion proteins. Transplantation. 1999;68:832–839. doi: 10.1097/00007890-199909270-00016. [DOI] [PubMed] [Google Scholar]
- 27.Chen D, Weber M, McVey JH, et al. Complete inhibition of acute humoral rejection using regulated expression of membrane-tethered anticoagulants on xenograft endothelium. Am J Transplant. 2004;4:1958–1963. doi: 10.1111/j.1600-6143.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 28.Hara H, Long C, Lin YJ, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1674. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 29.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in α1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bach FH, Robson SC, Winkler H, et al. Barriers to xenotransplantation. Nat Med. 1995;1:869–873. doi: 10.1038/nm0995-869. [DOI] [PubMed] [Google Scholar]
- 31.Appel JZ, III, Alwayn IPJ, Correa LE, Cooper DKC, Robson SC. Modulation of platelet aggregation in baboons: implications for mixed chimerism in xenotransplantation. I. Roles of conditioning regimen and pig peripheral blood progenitor cells. Transplantation. 2001;72:1299–1305. doi: 10.1097/00007890-200110150-00020. [DOI] [PubMed] [Google Scholar]
- 32.Alwayn IP, Appel JZ, Goepfert C, Buhler L, Cooper DKC, Robson SC. Inhibition of platelet aggregation in baboons: therapeutic implications for xenotransplantation. Xenotransplantation. 2000;7:242–257. doi: 10.1034/j.1399-3089.2000.00965.x. [DOI] [PubMed] [Google Scholar]
- 33.Alwayn IP, Buhler L, Appel JZ, 3rd, et al. Mechanisms of thrombotic microangiopathy following xenogenic hematopoietic progenitor cell transplantation. Transplantation. 2001;71:1601–1609. doi: 10.1097/00007890-200106150-00020. [DOI] [PubMed] [Google Scholar]
- 34.Marcus AJ, Safier LB, Hajjar KA, et al. Inhibition of platelet function by an aspirin-insensitive endothelial cell ADPase. J Clin Invest. 1991;88:1690–1696. doi: 10.1172/JCI115485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robson SC, Siegel JB, Lesnikoski BA. Aggregation of human platelets induced by porcine endothelial cells is dependent upon both activation of complement and thrombin generation. Xenotransplantation. 1996;3:24–34. [Google Scholar]
- 36.Robson SC, Kaczmarek E, Siegel JB, et al. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997;185:153–163. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwase H, Satyananda V, Ekser B, et al. Human platelet aggregation and thrombotic microangiopathy (TM) in pig cardiac xenografts is reduced by expression of human thrombomodulin (TBM) J Heart and Lung Transplant. 2013;32:S137. (#355) [Google Scholar]
- 38.Radomski MW, Palmer RMJ, Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987;92:639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benatuil L, Fernandez AZ, Romano E. Aggregation of human platelets in plasma by porcine blood cells in vitro is probably mediated by thrombin generation. Xenotransplantation. 2003;10:454–459. doi: 10.1034/j.1399-3089.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- 40.Ezzelarab M, Cortese-Hassett A, Cooper DKC, Yazer MH. Extended coagulation profiles of healthy baboons and of baboons rejecting GT-KO pig heart grafts. Xenotransplantation. 2006;13:522–528. doi: 10.1111/j.1399-3089.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton K, Hattori R, Esmon C, Sims P. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J Biol Chem. 1990;265:3809–3814. [PubMed] [Google Scholar]
- 42.Miwa Y, Yamamoto K, Onishi A, et al. Potential value of human thrombomodulin and DAF expression for coagulation control in pig-to-human xenotransplantation. Xenotransplantation. 2010;17:26–37. doi: 10.1111/j.1399-3089.2009.00555.x. [DOI] [PubMed] [Google Scholar]
- 43.Esmon CT. Protein-S and protein-C biochemistry, physiology, and clinical manifestation of deficiencies. Trends Cardiovasc Med. 1992;2:214–219. doi: 10.1016/1050-1738(92)90027-P. [DOI] [PubMed] [Google Scholar]
- 44.Wrighton CJ, Kopp CW, McShea A, Vetr H, Bach FH. High level of functional human thrombomodulin in cultured porcine aortic endothelial cells. Transplant Proc. 1995;27:288–289. [PubMed] [Google Scholar]
- 45.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci. 1996;93:10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nosaka S, Nakayama K, Hashimoto M, et al. Inhibition of platelet aggregation by endocardial endothelial cells. Life Sci. 1996;59:559–564. doi: 10.1016/0024-3205(96)00336-0. [DOI] [PubMed] [Google Scholar]
- 47.Broekman MJ, Eiora AM, Marcus AJ. Inhibition of human platelet reactivity by endothelium-derived relaxing factor from human umbilical vein endothelial cells in suspension: blockade of aggregation and secretion by an aspirin insensitive mechanism. Blood. 1991;178:1033–1040. [PubMed] [Google Scholar]
- 48.Magee JC, Platt JL, Oldham KT, Guice KS. Oxidant stress increases susceptibility of porcine endothelial cells to injury by xenoreactive antibody and complement. Transplant Proc. 1994;26:1170. [PubMed] [Google Scholar]
- 49.Warr TA, Rao LV, Rapaport SI. Disseminated intravascular coagulation in rabbits induced by administration of endotoxin or tissue factor: effect of anti-tissue factor antibodies and measurement of plasma extrinsic pathway inhibitor activity. Blood. 1990;75:1481–1489. [PubMed] [Google Scholar]
- 50.Giesen PL, Rauch U, Bohrmann B, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci USA. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu H, Arnaud F, Tadaki DK, Burkly LC, Harlan DM, Kirk AD. Human platelets activate porcine endothelial cells through a CD154-dependent pathway. Transplantation. 2001;72:1858–1861. doi: 10.1097/00007890-200112150-00029. [DOI] [PubMed] [Google Scholar]
- 52.Savage B, Cattaneo M, Ruggeri Z. Mechanisms of platelet aggregation. Curr Opin Haematol. 2001;8:270–276. doi: 10.1097/00062752-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Schulte am Esch J, 2nd, Rogiers X, Robson SC. Molecular incompatibilities in hemostasis between swine and men--impact on xenografting. Ann Transplant. 2001;6:12–16. [PubMed] [Google Scholar]
- 54.Davidson MH, Stein EA, Dujovne CA, et al. The efficacy and six week tolerability of simvastatin 80 and 160 mg/day. Am J Cardiol. 1997;79:38–42. doi: 10.1016/s0002-9149(96)00742-4. [DOI] [PubMed] [Google Scholar]
- 55.Esmon C, Esmon N, Harris K. Complex formation between thrombin and thrombomodulin inhibits both thrombin-catalyzed fibrin formation and factor V activation. J Biol Chem. 1982;257:7944–7947. [PubMed] [Google Scholar]
- 56.Esmon N, Carroll R, Esmon C. Thrombomodulin blocks the ability of thrombin to activate platelets. J Biol Chem. 1983;258:12238–12242. [PubMed] [Google Scholar]
- 57.Taylor FB, Jr, Peer GT, Lockhart MS, Ferrell G, Esmon CT. Endothelial cell protein C receptor plays an important role in protein C activation in vivo. Blood. 2001;97:1685–1688. doi: 10.1182/blood.v97.6.1685. [DOI] [PubMed] [Google Scholar]