Abstract
Background
This was to identify mechanisms of innate resistance to an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, erlotinib, in a panel of head and neck squamous cell carcinoma (HNSCC) cell lines. Specifically, we analyzed the role of HRAS mutations in erlotinib resistance.
Methods
Erlotinib sensitivity was determined by MTT assays. Molecular signaling pathways and somatic mutations were examined. Changes in sensitivity after modulation of HRAS expression were evaluated.
Results
All seven cell lines were wild-type for EGFR and KRAS regardless of erlotinib sensitivity; however, one erlotinib-resistant cell line (HN31) harbored an HRAS G12D mutation. Down regulation of HRAS expression by siRNA or shRNA in HN31 led to increased erlotinib sensitivity in vitro and in vivo. Transfection of activating HRAS-mutant (G12D and G12V) constructs into erlotinib-sensitive cell lines made them more resistant to erlotinib.
Conclusion
Activating HRAS mutations can confer erlotinib resistance in an HRAS mutant HNSCC cell line.
Keywords: Epidermal growth factor receptor, erlotinib, HRAS, resistance, head and neck squamous cell carcinoma
Introduction
Many recent studies have aimed to identify molecularly targeted therapeutics for head and neck squamous cell carcinoma (HNSCC). The epidermal growth factor (EGF) receptor (EGFR) is the most promising candidate molecule for targeted treatment of HNSCC (1), as most HNSCCs overexpress EGFR, and investigators have developed a number of different types of EGFR-targeted agents.
Despite extensive experience with EGFR inhibitors in treating HNSCC, the response rates(7~7.6% (2, 3) and 25% in select patients (4)) and survival durations in patients who receive these agents remain relatively low owing to intrinsic and acquired resistance to these agents. Activation of EGFR stimulates downstream signaling pathways, including the RAS/RAF/mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/phosphatase and tensin homolog (PTEN)/AKT pathways (5). Researchers have reported several mechanisms of resistance as well as sensitivity to EGFR tyrosine kinase inhibitors, including mutations and other molecular alterations of members of the EGFR pathways. However, many cancer cells exhibit resistance to these inhibitors without a clear mechanism. To predict the response of HNSCC to targeted agents and identify the most efficacious targeted agent for each patient, more markers and resistance mechanisms must be explored.
Therefore, we designed and performed this study to identify mechanisms of erlotinib resistance in HNSCC cells. Since we found the presence of HRAS mutation in a resistant cell during this research, we specifically analyzed the role of HRAS mutations in erlotinib resistance.
Materials and Methods
Cell lines
Based upon preliminary screening (data not shown) of more than 50 HNSCC cell lines for sensitivity to vandetanib, a dual inhibitor of EGFR and vascular endothelial growth factor receptor-2, highly sensitive and highly resistant cell lines were selected and further screened to determine erlotinib sensitivity using a cell proliferation assay. Three sensitive cell lines—UM-SCC-10B, UM-SCC-22A, and HN5—and four resistant cell lines—HN31, UM-SCC-19, MSK-922, and MDA1386LN—were selected for further experiments. The sources and culture conditions of these cell lines are listed in a previous article on the characteristics of the HNSCC cell lines. (6)
Reagents
Erlotinib was purchased from LC Laboratories. For in vitro experiments, stock solutions of erlotinib were prepared in dimethyl sulfoxide (Sigma-Aldrich) and diluted with a culture medium. For animal experiments, erlotinib powder was suspended in normal saline.
Cell proliferation assay
The antiproliferative activity of erlotinib in vitro was determined using a 3-(4,5--dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Two to six thousand cells per well grew in a medium containing 10% FBS in 96-well tissue culture plates. After 24 hours, the cells were treated with erlotinib at various concentrations (0.01–90.0 µM). The concentration of erlotinib producing 50% growth inhibition (GI50) in each cell line was calculated using the GraphPad Prism (version 5.04; GraphPad Software). This experiment was repeated at least twice.
Genetic screening and sequencing
Sequencing for EGFR and KRAS mutations was performed using a Sequenom machine with primers for EGFR (G719, S720, T790, Y813, T854, L858, K860, and L861) and KRAS (G10, G12, G13, A14, and Q61) (6). HRAS sequencing was performed using Sanger sequencing for two exons that included G12 and Q61.
Western blots
Western blot analysis of cultured HNSCC cells was performed to measure the expression and phosphorylation of EGFR and related signaling molecules. Western blotting was also performed to demonstrate whether erlotinib is able to inhibit the phosphorylation of EGFR. HNSCC cells were treated with 1 M erlotinib for 4 hours and stimulated with EGF (10 ng/mL; Upstate Biotechnology) for 15 minutes before harvesting protein. To compare molecular changes in the EGFR downstream signaling molecules, including AKT and MAPK, Western blot analysis was performed after 48 hours of treatment with 1 µM erlotinib. These downstream pathways were also examined after transient transfection of HRAS small interfering RNA (siRNA) into erlotinib-resistant HN31 and UM-SCC-19 cells. HRAS expression was measured using Western blotting after stable transfection of activated HRAS mutation constructs into erlotinib-sensitive HN5, UM-SCC-10B, and UM-SCC-22A cells and stable transfection of HRAS short hairpin RNA (shRNA) into HN31 cells.
The primary antibodies used in Western blotting consisted of an EGFR rabbit monoclonal antibody (mAb), an HRAS (C–20) rabbit polyclonal antibody (Santa Cruz Biotechnology), a phospho-EGFR (Tyr1068) rabbit mAb, an AKT rabbit mAb, a phospho-AKT (Ser473) rabbit mAb, a p44/42 MAPK (Erk1/2) mouse mAb, and a phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) rabbit mAb (Cell Signaling).
Transfection
To determine whether the constitutively active HRAS mutation constructs led to alterations in erlotinib sensitivity, HN5, UM-SCC-22A, and UM-SCC-10B cells were transfected with either the pBabe-HRAS G12D or pBabe-HRAS G12V vector. Control cells were transfected with the pBabe-wild-type HRAS vector and pBabe backbone vector. Because the wild-type HRAS and HRAS G12D vectors were not commercially available, they were constructed using a QuikChange site-directed mutagenesis kit (Stratagene) with the pBabe-HRAS G12V vector (Addgene) as a template. All constructs were authenticated via DNA sequencing.
Electroporation was used to deliver Silencer Select siRNAs (HRAS (Cat# 4390824-S808) and siRNA negative control (Cat# 4390843); Ambion) to the erlotinib-resistant cell lines HN31 and UM-SCC-19. Each siRNA (300 nM) was added to 2–3 × 106 cells/reaction in 100 µL of nucleofector solution (L), transferred to an cuvette, and electroporated using a Nucleofector II apparatus (Amaxa Biosystems) as per the manufacturer’s instructions. After electroporation, cells were plated for MTT assay and Western blotting.
To assess changes in the in vivo sensitivity of HN31 cell line after knockdown of HRAS expression to erlotinib, six stable HN31 cell lines with knockdown of HRAS expression were made using 6 shRNA plasmid vectors. The HRAS shRNAs were purchased from Open Biosystems (Lentivirus, pLKO.1; TRCN0000033264, TRCN0000033265, TRCN0000040090, TRCN0000010357, TRCN0000010358, and TRCN0000018336). Knockdown efficiencies were checked using Western blotting of HRAS and erlotinib sensitivity with an MTT assay following erlotinib treatment.
Animal experiments
The mouse model was used to determine the in vivo changes in erlotinib sensitivity after knockdown of HRAS in HRAS mutated cells. Animal care and procedures were carried out under regulations same as described in a previous article. (7)
Three different clones of HN31 HRAS shRNA and HN31 non-target shRNA (NT shRNA, control) cells were used. The cells (5×105 cells/100 µL phosphate-buffered saline) were inoculated subcutaneously into the flanks of nude mice. The mice were then examined three times a week to measure their tumor sizes. Tumor volumes were calculated using the formula (A)(B2)π/6, in which A is the longest dimension of the tumor and B is the dimension of the tumor perpendicular to A. When the mean tumor volume for each clone reached 40 mm3, the mice were randomly assigned to one of two groups: no treatment and erlotinib treatment. Erlotinib was administered via oral gavage 6 days a week for 2 weeks at 50 mg/kg. The mice were euthanized at the end of treatment. Data for both groups were expressed in mean tumor volume and standard deviation. The differences in the tumor volume between the two groups were analyzed with two-way repeated measure analysis of variance (RM ANOVA) using GraphPad Prism (version 5.04; GraphPad Software).
Results
Characteristics of the erlotinib-sensitive and -resistant HNSCC cell lines
To characterize erlotinib-sensitive and -resistant HNSCC cell lines, cells were treated with erlotinib and their growth quantitated using an MTT assay. The three erlotinib-sensitive cell lines (UM-SCC-10B, UM-SCC-22A, and HN5) had very low GI50s (0.067–0.184 µM erlotinib). In contrast, the GI50s of the four erlotinib-resistant cell lines (HN31, UM-SCC-19, MSK-922, and MDA1386LN) were all greater than 3.50 µM (Table 1). Genetic screening found all seven cell lines to be wild-type for both EGFR and KRAS. We found a heterozygous HRAS G12D mutation present in erlotinib-resistant HN31 cells (Table 1).
Table 1.
Erlotinib GI50s and mutational statuses of the erlotinib-resistant and -sensitive HNSCC cell lines
| Cell line | GI50 (µmol/L) | EGFR | KRAS | HRAS |
|---|---|---|---|---|
| Resistant | ||||
| MDA1386LN | >10* | WT | WT | WT |
| HN31 | 3.590 | WT | WT | G12D |
| UM-SCC-19 | 3.540 | WT | WT | WT |
| MSK-922 | 4.420 | WT | WT | WT |
| Sensitive | ||||
| UM-SCC-22A | 0.184 | WT | WT | WT |
| HN5 | 0.101 | WT | WT | WT |
| UM-SCC-10B | 0.067 | WT | WT | WT |
Could not be precisely determined because the growth inhibition of cells never reaches 50% at the concentrations of erlotinib examined; WT, wild-type.
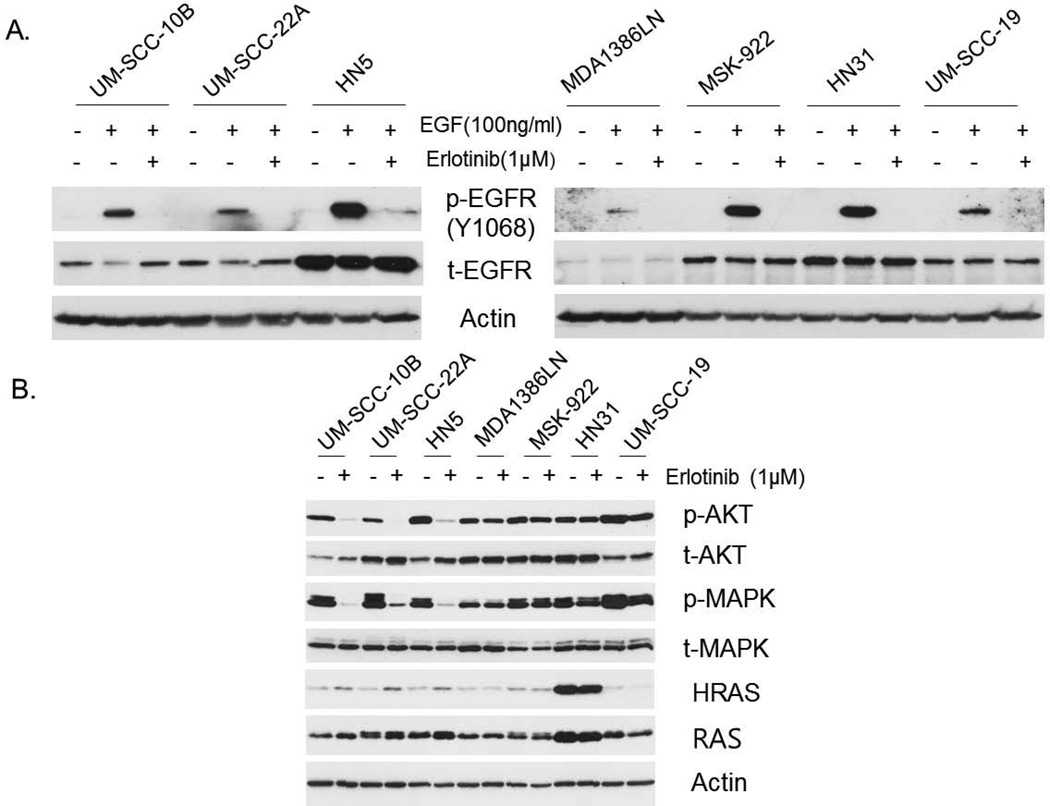
To determine the effect of erlotinib treatment on EGFR signaling pathways in these cell lines, we treated cells with erlotinib and EGF. We observed that phosphorylation of EGFR was increased by EGF stimulation and suppressed by treatment with 1 µM erlotinib in all seven cell lines, regardless of erlotinib sensitivity, as determined by Western blotting (Fig. 1A). We also determined the status of the signaling pathways downstream from EGFR in the erlotinib-resistant and -sensitive groups. Phosphorylation of AKT and MAPK after erlotinib treatment was sustained in the resistant cell lines but dramatically suppressed in the sensitive cell lines. HN31 cells, which had the HRAS G12D mutation, had much higher HRAS expression than did the other cell lines (Fig. 1B).
Figure 1.
Impact of erlotinib treatment on the expression of EGFR-signaling molecules in HNSCC cells. (A) Levels of total EGFR (t-EGFR) and phosphorylated EGFR (p-EGFR, Y1068) expression after erlotinib treatment (1 µM, 4 hours) with EGF stimulation (100 ng/mL, 15 minutes). (B) Expression of p-AKT (S475), t- AKT, p-MAPK, t-MAPK, HRAS and RAS after erlotinib treatment (1 µM, 48 hours).
Impact of decreased expression of mutant HRAS on erlotinib-resistant HNSCC cell lines
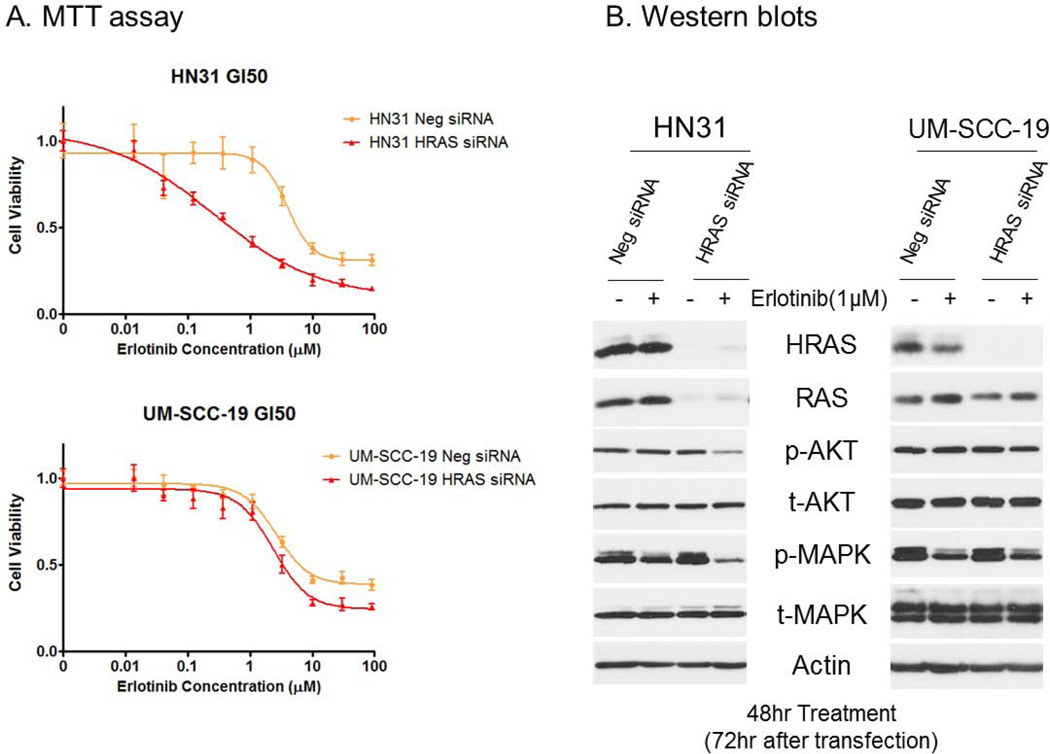
To determine the impact of decreased expression of HRAS on erlotinib-resistant HNSCC cell lines, we transfected HN31 cells, which have HRAS G12D mutation, with HRAS siRNAs. UM-SCC-19, which were wild-type for HRAS, was used as a control. Transient knockdown of HRAS expression in HN31 cells sensitized them to erlotinib. The erlotinib GI50 was 3.974 µM in HN31 cells transfected with negative control siRNA, but 0.2621 µM in those transfected with HRAS siRNA (Fig. 2A). These differences in GI50 were accompanied by decreases in phosphorylation-AKT (p-AKT) and -MAPK (p-MAPK) after erlotinib treatment in HN31 with knockdown of HRAS expression (Fig. 2B). However, transient knockdown of HRAS expression in UM-SCC-19 cells did not alter erlotinib sensitivity (Fig. 2A). Levels of p-AKT and p-MAPK in the UM-SCC-19 HRAS knockdown cells were similar to those in the UM-SCC-19 transfected with negative control siRNA (Fig. 2B).
Figure 2.
Transient knockdown of expression of HRAS in the erlotinib-resistant HNSCC cell lines HN31 and UM-SCC-19 with HRAS siRNA. (A) MTT assay results showing the erlotinib-sensitivity changes after transient knockdown of expression of HRAS. (B) Western blot analyses performed to evaluate the changes in the expression of the target molecule HRAS and related signaling molecules after transfection with HRAS siRNA and treatment with erlotinib.
Impact of activated expression of mutant HRAS on erlotinib-sensitive HNSCC cell lines
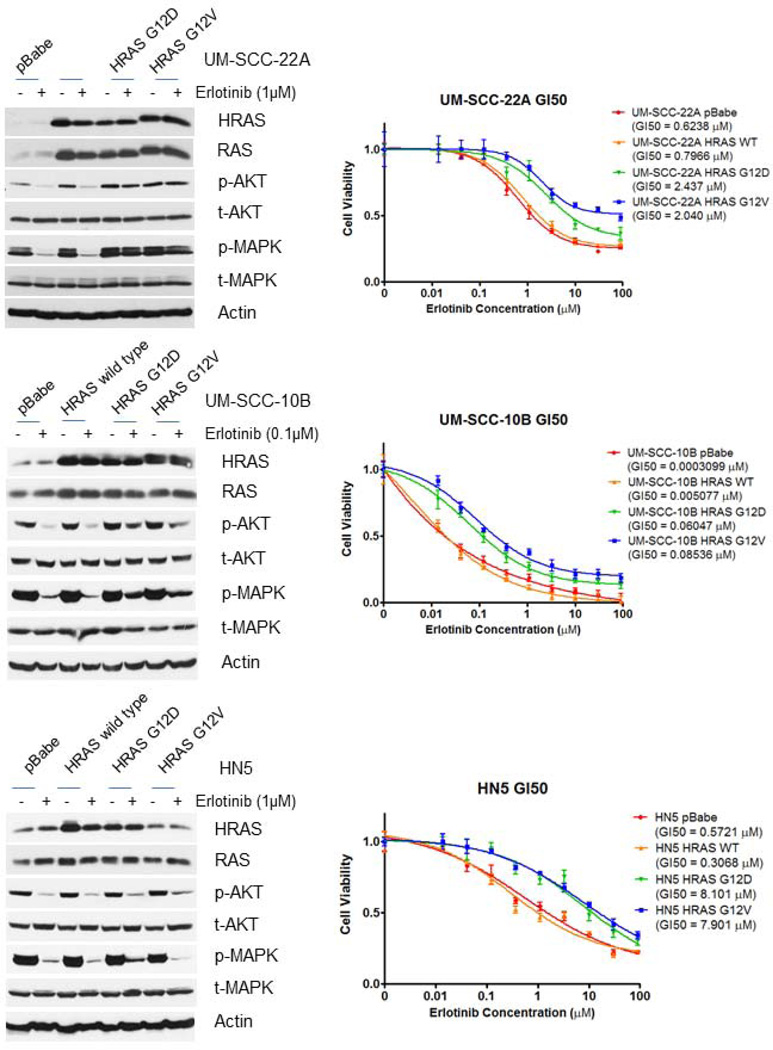
To determine whether over-expression or activation of HRAS can confer resistance to erlotinib-sensitive HNSCC cell lines, we transfected constructs expressing wild-type HRAS or HRAS mutations (G12D and G12V) into the three erlotinib-sensitive HNSCC cell lines. Stable transfection of activating HRAS mutation constructs resulted in relative resistance to erlotinib in UM-SCC-22A (Fig. 3 top), UM-SCC-10B (Fig. 3 middle), and HN5 (Fig. 3 bottom) compared to erlotinib-sensitive parental cell lines, whereas, stable transfection of a wild-type HRAS construct did not alter the erlotinib GI50s. We observed sustained expression of p-AKT and p-MAPK following erlotinib treatment of UM-SCC-22A that had been transfected with mutant, but not wild type HRAS. Furthermore, the erlotinib induced deceases in p-AKT and p-MAPK normally observed in parental UM-SCC-10B were markedly attenuated by transfection with mutant, but not wild type HRAS (Fig. 3).
Figure 3.
Stable transfection of constructs expressing wild-type HRAS and HRAS mutations (G12D and G12V) into erlotinib-sensitive UM-SCC-22A, UM-SCC-10B and HN5 cells. Transfection with the constructs expressing HRAS mutations resulted in increased erlotinib resistance in both cell lines, whereas that with the construct expressing wild-type HRAS did not alter the GI50 of erlotinib in either cell line.
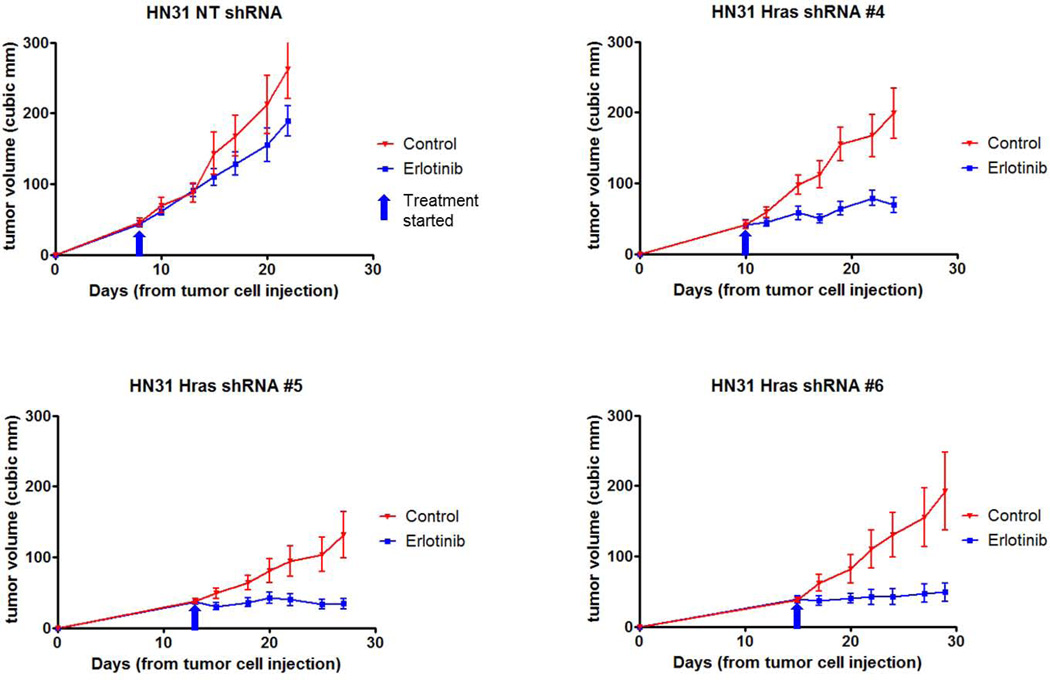
Stable knockdown of HRAS expression leads to erlotinib sensitivity in erlotinib-resistant HN31 cell lines growing in vivo
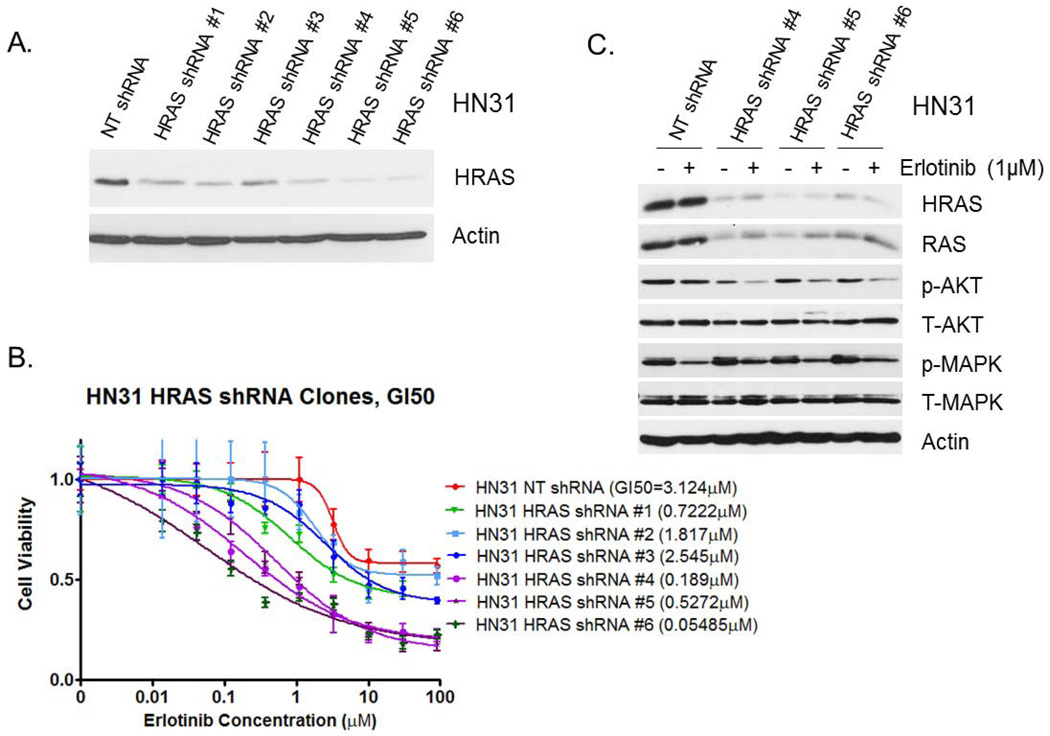
To determine whether decreased HRAS expression can enhance sensitivity to erlotinib in HN31 cells that grow in an in vivo tumor model, we established six stable clones of HN31 cells transfected with HRAS shRNA. Three (#4, #5, and #6) of the clones showed better HRAS knockdown than the others did. HRAS knockdown efficiencies of the clones correlated well with reduced in vitro sensitivity to erlotinib (Fig. 4 A and B). These three clones exhibited significantly higher sensitivity to erlotinib (GI50 ≤ 0.5272 µM) than did a control NT shRNA clone (GI50 = 3.124 µM). Western blot analysis demonstrated suppression of p-AKT expression after erlotinib treatment in these three HRAS shRNA-transfected clones (Fig. 4C), which was not observed in the control NT shRNA clone. On the contrary, p-MAPK was not affected significantly in the three HRAS shRNA clones and in the NT shRNA control clone as well.
Figure 4.
Stable transfection of HRAS shRNAs into HN31 cells. (A) Western blot analysis performed to determine the HRAS-expression knockdown efficiencies in six stable clones of HN31 cells transfected with HRAS shRNAs. (B) Results of MTT assay with these six clones and HN31 NT shRNA control. (C) Western blot analysis of downstream molecules in the EGFR pathway with three selected clones of HRAS shRNAs for our animal study of in vivo erlotinib sensitivity changes.
We next used these three clones in a study using a mouse xenograft model. Formation of tumors by three stable clones of HN31 cells transfected with HRAS shRNA (#4, #5 and #6) and their growth were delayed variably. The tumors formed by HRAS-knockdown cells demonstrated dramatic sensitivity to erlotinib in vivo and the differences in the tumor volume between the treatment and control groups were significant (P=0.0088, 0.0226 and 0.0399, respectively), whereas those formed by the NT shRNA control cells did not have significant responses to it (P=0.2584) (Fig. 5).
Figure 5.
Changes in the in vivo erlotinib sensitivity of HRAS-knockdown HN31 cells. Three stable clones of HN31 cells transfected with HRAS shRNA (#4, #5 and #6) exhibited dramatic changes in erlotinib sensitivity (P=0.0088, 0.0226 and 0.0399, respectively), whereas an NT shRNA control did not have a significant response to erlotinib treatment (P=0.2584).
Discussion
In this study, we found that sensitivity to erlotinib correlated well with the ability of the drug to inhibit the downstream signaling pathways of the EGFR. And we found that HRAS mutations can mediate erlotinib resistance in HNSCC cells.
In cells sensitive to erlotinib, the drug caused profound decreases in p-AKT and p-MAPK. In contrast, decreases in p-AKT and p-MAPK were greatly attenuated or barely detectable following erlotinib treatment of resistant cells–despite the fact that erlotinib efficiently blocked EGFR signaling as measured by EGFR phosphorylation.
Uncoupling of the AKT and MAPK signaling pathways from the EGFR in erlotinib-resistant cells prompted us to look for other mechanisms driving these pathways. RAS is capable of activating both the PI3K/AKT and MAPK (8, 9), and activating mutations in RAS could explain EGFR-independent stimulation of p-AKT and p-MAPK. Whereas KRAS mutations are well studied as mediators of resistance to EGFR tyrosine kinase inhibitors and EGFR targeting antibodies in lung cancer (10) and colorectal cancer (11) cases, KRAS is not frequently mutated in HNSCC cells (12, 13). In contrast, activating HRAS mutations reportedly occur in 5–22% of HNSCC cases (12, 14–17). Although HRAS mutations have been thought as a resistance mechanism of EGFR targeted agents, the functional impact of these mutations has yet to be well reported with experiments. Recently, one study (18) showed that a constitutively active HRAS mutation (G12V) in A431 human vulvar squamous carcinoma cells contributed to cellular resistance to the EGFR inhibitors cetuximab and gefitinib. Interestingly, we found that one of the HNSCC lines highly resistant to erlotinib in our study (HN31) carried an activating G12D HRAS mutation. We performed this study to characterize the impact of HRAS mutations on the sensitivity to erlotinib and showed the effect of transient and stable knockdown of HRAS expression in the HRAS-mutant HNSCC cell line HN31 as well as the effect of activating HRAS-mutant constructs in erlotinib-sensitive HNSCC cell lines.
In Western blotting of the EGFR downstream signaling pathways, persistent phosphorylation of AKT and MAPK after erlotinib treatment was the prominent feature of erlotinib-resistant cell lines. This was independent of the mutational status of HRAS. Several studies of cetuximab and gefitinib in treatment of breast cancer, non-small cell lung cancer, and HNSCC have demonstrated this persistent phosphorylation (19–21). We observed that in HRAS-mutant HN31 cells, but not HRAS wild-type UM-SCC-19 cells, knockdown of HRAS expression by siRNA or shRNA caused increased sensitivity to erlotinib that correlated with drug induced inhibition of p-AKT and p-MAPK, though significant inhibition of p-MAPK was not found after HRAS knockdown with shRNA. These findings suggest that mutant HRAS, but not wild type HRAS, can render HNSCC lines resistant to erlotinib. However, in the absence of mutated HRAS, other upstream signaling molecules can also drive EGFR-independent stimulation of p-AKT and p-MAPK and lead to erlotinib resistance as well. Also, after introduction of constructs expressing mutant HRAS but not those expressing wild-type HRAS into erlotinib-sensitive HNSCC cell lines, we observed persistent phosphorylation of AKT and MAPK after erlotinib treatment and that the cells had increased resistance to erlotinib. Taken together, these results suggested that HRAS mutation is a mechanism of erlotinib resistance and that both AKT and MAPK pathways are involved in that mechanism.
In conclusion, we found that HRAS mutations confer erlotinib resistance to HNSCC cells. HRAS mutations have been found in 5–22% of HNSCC cases, our finding may help identify HNSCC patients who will not benefit from EGFR-targeted therapeutics in the clinic. Even though HRAS mutations occur in a minority of patients they are still relevant because they are likely to be clinically actionable. Additionally, if an HRAS-targeting therapeutic is ever developed it would be likely to sensitize these tumors to erlotinib. To determine the clinical significance of this finding, clinical data including HRAS mutational status of the patient’s tumor and its response to erlotinib treatment should be analyzed in the future.
Acknowledgments
Grant Support
This work was supported by National Institutes of Health (NIH) Specialized Program of Research Excellence Grant P50CA097007, NIH Cancer Center Support Grant CA016672, The University of Texas MD Anderson Cancer Center PANTHEON program, a TRIUMPH Fellowship, a GSK Translational Research Fellowship, and the NIH via National Research Science Award Research Training Grant T32CA060374 (National Cancer Institute).
Footnotes
Conflicts of Interest Statement:
The authors disclose no potential conflicts of interest.
References
- 1.Frederick MJ, VanMeter AJ, Gadhikar MA, et al. Phosphoproteomic analysis of signaling pathways in head and neck squamous cell carcinoma patient samples. Am J Pathol. 2011;178:548–571. doi: 10.1016/j.ajpath.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen EE, Halpern AB, Kasza K, Kocherginsky M, Williams R, Vokes EE. Factors associated with clinical benefit from epidermal growth factor receptor inhibitors in recurrent and metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2009;45:e155–e160. doi: 10.1016/j.oraloncology.2009.05.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected] J Clin Oncol. 2009;27:1864–1871. doi: 10.1200/JCO.2008.17.0530. [DOI] [PubMed] [Google Scholar]
- 4.William WN, Jr, Weber RS, Lee JJ, et al. Randomized trial of a short course of erlotinib 150 to 300 mg daily prior to surgery for squamous cell carcinomas of the head and neck (SCCHN) in current, former, and never smokers: Objective responses and clinical outcomes. J Clin Oncol. 2011;29(suppl) abstr 5520. [Google Scholar]
- 5.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12:5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- 6.Zhao M, Sano D, Pickering CR, et al. Assembly and Initial Characterization of a Panel of 85 Genomically Validated Cell Lines from Diverse Head and Neck Tumor Sites. Clin Cancer Res. 2011;17:7248–7264. doi: 10.1158/1078-0432.CCR-11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sano D, Fooshee DR, Zhao M, et al. Targeted molecular therapy of head and neck squamous cell carcinoma with the tyrosine kinase inhibitor vandetanib in a mouse model. Head Neck. 2011;33:349–358. doi: 10.1002/hed.21455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Viciana P, Warne PH, Dhand R, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 9.De Luca A, Maiello MR, D' Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets. 2012;16(Suppl.2):S17–S27. doi: 10.1517/14728222.2011.639361. [DOI] [PubMed] [Google Scholar]
- 10.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lievre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 12.Lea IA, Jackson MA, Li X, Bailey S, Peddada SD, Dunnick JK. Genetic pathways and mutation profiles of human cancers: site- and exposure-specific patterns. Carcinogenesis. 2007;28:1851–1858. doi: 10.1093/carcin/bgm176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheu JJ, Hua CH, Wan L, et al. Functional genomic analysis identified epidermal growth factor receptor activation as the most common genetic event in oral squamous cell carcinoma. Cancer Res. 2009;69:2568–2576. doi: 10.1158/0008-5472.CAN-08-3199. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson JA, Irish JC, Ngan BY. Prevalence of RAS oncogene mutation in head and neck carcinomas. J Otolaryngol. 1992;21:321–326. [PubMed] [Google Scholar]
- 16.Anderson JA, Irish JC, McLachlin CM, Ngan BY. H-ras oncogene mutation and human papillomavirus infection in oral carcinomas. Arch Otolaryngol Head Neck Surg. 1994;120:755–760. doi: 10.1001/archotol.1994.01880310059011. [DOI] [PubMed] [Google Scholar]
- 17.Forbes SA, Bhamra G, Bamford S, et al. The Catalogue of Somatic Mutations in Cancer (COSMIC) Curr Protoc Hum Genet. 2008;Chapter 10(Unit 10.11) doi: 10.1002/0471142905.hg1011s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luwor RB, Lu Y, Li X, Liang K, Fan Z. Constitutively active Harvey Ras confers resistance to epidermal growth factor receptor-targeted therapy with cetuximab and gefitinib. Cancer Lett. 2011;306:85–91. doi: 10.1016/j.canlet.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janmaat ML, Kruyt FA, Rodriguez JA, Giaccone G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin Cancer Res. 2003;9:2316–2326. [PubMed] [Google Scholar]
- 20.Normanno N, De Luca A, Maiello MR, et al. The MEK/MAPK pathway is involved in the resistance of breast cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J Cell Physiol. 2006;207:420–427. doi: 10.1002/jcp.20588. [DOI] [PubMed] [Google Scholar]
- 21.Yamatodani T, Ekblad L, Kjellén E, Johnsson A, Mineta H, Wennerberg J. Epidermal growth factor receptor status and persistent activation of Akt and p44/42 MAPK pathways correlate with the effect of cetuximab in head and neck and colon cancer cell lines. J Cancer Res Clin Oncol. 2009;135:395–402. doi: 10.1007/s00432-008-0475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]