Abstract
BACKGROUND:
Stored red cells release hemoglobin that leads to oxidative damage, which may contribute to thrombosis in susceptible transfusion recipients. Oxidative stress stimulates the generation of a new class of lipid mediators called F2-isoprostanes (F2-IsoPs) and isofurans (IsoFs) that influence cellular behavior. The present study investigated red cell-derived F2-IsoPs and IsoFs during storage, and their influence on human platelets.
STUDY DESIGN AND METHODS:
F2-IsoP and IsoF levels in red cell supernatants were measured by mass spectrometry during storage and after washing. The effects of stored supernatants, cell-free hemoglobin or a key F2-IsoP, 8-iso-PGF2α, on platelet function were examined in vitro.
RESULTS:
F2-IsoPs, IsoFs and hemoglobin accumulated in stored red cell supernatants. Pre-storage leukoreduction reduced supernatant F2-IsoPs and IsoFs levels, which increased again over storage time. Stored red cell supernatants and 8-iso-PGF2α induced platelet activation marker CD62P (P-selectin) expression and prothrombotic thromboxane A2 release. Cell-free hemoglobin did not alter platelet mediator release, but did inhibit platelet spreading. Post-storage red cell washing reduced F2-IsoP and IsoF levels up to twenty-four hours.
CONCLUSIONS:
F2-IsoPs and IsoFs are produced by stored red cells and induce adverse effects on platelet function in vitro, supporting a potential novel role for bioactive lipids in adverse transfusion outcomes. F2-IsoP and IsoF levels could be useful biomarkers for determining the suitability of blood components for transfusion. A novel finding is that cell-free hemoglobin inhibits platelet spreading and could adversely influence wound healing. Post-storage red cell washing minimizes harmful lipid mediators, and its use could potentially reduce transfusion complications.
Keywords: Red blood cell, Platelet, Transfusion, Storage lesion, Lipid mediators, F2-isoprostanes, Isofurans, Hemoglobin, Oxidative stress
INTRODUCTION
Blood transfusion is one of the oldest therapeutics in medical practice with 3-4 million patients per year receiving treatment in the United States. Transfusions are an important and indispensable procedure during catastrophic emergencies that include life-threatening hemorrhage or anemia. However, the majority of red blood cell (RBC) transfusions are given prophylactically to anemic patients to prevent complications of severe anemia or improve quality of life1. This practice raises concerns, as not all patients benefit from transfusion. There is growing clinical evidence correlating adverse transfusion outcomes with storage-induced changes in RBC concentrates2. Data supporting the efficacy and safety of transfusion practice is generally weak. Recent research demonstrates that blood transfusions are associated with morbidity and mortality3-7. RBC concentrates are stored in the USA for up to 42 days and undergo storage-induced changes in red cell structure and function that reduce RBC viability and efficacy following transfusion8, 9. While modifications of stored RBCs, such as leukoreduction or washing can improve clinical outcomes, transfusions are still associated with increased mortality10-12. Clearly, additional clinical and laboratory studies are needed to elucidate mechanisms that contribute to blood transfusion toxicity13.
Isoprostanes (IsoPs) and isofurans (IsoFs) are isomers of prostaglandins formed in vivo, predominately through nonenzymatic free radical-induced peroxidation of arachidonic acid following esterification to phospholipids14, 15. They are subsequently released in plasma by phospholipase activity15 and the ratio of IsoPs versus IsoFs generated is determined by low and high oxygen tension, respectively16. The most widely studied IsoPs are the F-ring prostanoids or F2-IsoPs, which are used to assess oxidative stress in vivo17, 18. Recently, it was suggested that measurement of both F2-IsoPs and IsoFs provides a comprehensive method of assessing cellular oxidative stress status19. Further, these lipids are key bioactive markers and likely mediators in vascular pathophysiology in many acute and chronic diseases. For example, elevated circulating levels of 8-iso-PGF2α, the most widely studied F2-IsoP, occur in various diseases, such as atherosclerosis20-23, Alzheimer’s disease24, diabetes25, and in smokers26, to list a few. Interestingly, Schwedhelm et al.27 demonstrated that F2-IsoPs may increase platelet activity in stable coronary heart disease patients that are aspirin-insensitive, and these effects are at least in part, thromboxane A2 receptor mediated.
While several factors underlie the red cell storage lesion, oxidative injury and the release of free hemoglobin is a major contributing factor. We hypothesized that adverse red cell transfusion outcomes could be caused, in part, by the oxidative-induced generation and accumulation of prostanoids derived from lipid peroxidation, either individually or in combination, in stored RBC concentrates. Our goal herein is to investigate F2-IsoPs and IsoFs to determine: 1) their levels in RBC concentrates over storage, as they may represent a means to assess oxidative stress status prior to transfusion, and 2) their influence on platelets, as these compounds could inhibit or stimulate platelet function in transfusion recipients. Either mechanism could contribute to poor transfusion outcomes. We also compared lipid and cell-free hemoglobin effects on platelets in vitro to determine how each may affect platelet function. Additionally, we investigated whether washing red cells could reduce the supernatant levels of potentially harmful lipid mediators and hemoglobin, and thus speculatively, improve transfusion safety and efficacy.
MATERIALS AND METHODS
Blood collection and donors
Red cell storage studies
AS-1 red cell concentrates were obtained from the American Red Cross, and stored according to AABB and FDA standards.
Platelet studies
Human blood was obtained from consenting donors in accordance with the Declaration of Helsinki under an approved University of Rochester IRB protocol via venipuncture into vacutainer tubes containing 0.105 M sodium citrate (BD, Franklin Lakes, NJ) from healthy donors who had not taken aspirin or other non-steroidal anti-inflammatory drugs for 10 days prior to donation. Platelet rich plasma (PRP) was prepared by centrifugation (150g for 10 minutes at 20°C). Washed platelets were prepared by centrifugation of PRP (1000g for 10 minutes) in the presence of 1 μg/mL prostacyclin followed by resuspension in Tyrode’s/ACD (25:3 v/v) solution and centrifugation (1000g for 10 minutes) in the presence of 0.1 μg/mL prostacyclin.
In vitro simulated transfusion assays
RBC supernatants were isolated from leukoreduced or non-leukoreduced AS-1 red cell concentrates stored up to 47 days. AS-1 red cell concentrates were leukoreduced with a Pall Purecell BPF Leukocyte Reduction Filtration System (Pall Corporation, East Hills, NY). Supernatants were isolated by centrifugation of stored RBCs (4000g for 15 minutes at 4°C). Supernatants were removed and centrifuged a second time (4000g for 15 minutes at 4°C) to remove residual debris. Stored RBC supernatant was mixed with freshly isolated PRP (50:50 v/v) for in vitro transfusion simulation assays.
Aggregation Studies
To examine the effect of stored RBCs on recipient platelet function, in vitro platelet activity of normal donors was assessed utilizing whole blood aggregation. Citrated whole blood was collected and PRP was prepared. Platelet count was adjusted to 3 x 105/mL using autologous plasma. PRP samples were mixed at a 1:1 ratio with freshly prepared RBCs (FP), fresh (< 8d) or stored (> 20d) leukoreduced (LR), or nonleukoreduced (NLR) PRBC concentrates. Following a 10-minute incubation (37°C), 20 μM, ADP was added to initiate aggregation, using a Chrono-log Lumiaggregometer (Chrono-log, Havertown, PA) according to the manufacturer recommendations. Donor baseline whole blood aggregation was used as a control and tests were performed within 3 hours of blood collection.
Mass Spectometry
F2-IsoPs were measured using gas chromatography-mass spectrometry as previously described28.
Immunoassays
TXB2 and PGE2 were assayed by EIA (Cayman Chemical). Soluble CD40L release was assayed by a previously published ELISA developed in our laboratory29.
Washed platelet activation assays
Washed platelets (108/mL) were treated with vehicle (0.2% DMSO), 8-iso-PGF2α (Cayman Chemical) or hemoglobin (Sigma) for 30 minutes followed by activation with either 5 μM ADP, 5 μg/mL collagen or 0.8 U/mL thrombin. Platelet supernatants were collected by centrifugation (1000g for 10 minutes) and stored at −80°C.
Platelet Spreading
Washed human platelets (3x108 platelets/mL) were pre-treated with vehicle, 8-iso-PGF2α, hemoglobin or stored RBC supernatants for 30 minutes, and spread on fibrinogen coated coverslips (100 μg/mL) for 45 minutes. The coverslips were washed with PBS and fixed with 4% paraformaldehyde for 15 minutes. Platelet spreading was visualized by differential interface contrast (DIC) optics (Olympus BX51 microscope, Melville, NY)) and SPOT RT software (New Hyde Park, NY). The percentage of fully spread platelets was determined by manually counting 4 fields of view per treatment condition.
Hemoglobin Assays (Clinical and Spectrophotometric)
Hemoglobin levels were quantified using the Quantichrom Hemoglobin Assay Kit (Bioassay Systems). A standard curve was generated using serial dilutions of the provided calibrator. 100 μL of Quantichrom Reagent was added to 25 μL of sample and incubated 5 minutes at 37°C. OD values were read at 405 nm in a plate spectrophotometer and concentration calculated in mg/dL.
Reactive Oxygen Species Assay
Washed platelets were treated with vehicle (Adsol), lyophilized human hemoglobin (Sigma H7379) reconstituted in Adsol, or RBC supernatant for 15 minutes. Dichlorodihydrofluorescein diacetate (H2DCFDA) (Molecular Probes #C400) was reconstituted in DMSO, diluted in Tyrode’s buffer and added to platelets to a final concentration of 5 μM. Fluorescence was measured at 490/520 excitation/emission wavelengths using a Varioskan Flash Multimode Reader.
Washed Red Blood Cells
Leukoreduced RBC concentrates were centrifuged to remove residual plasma and Adsol solution, and washed with an isotonic solution at 4°C. RBCs were stored for an additional 24 hours at 4°C in isotonic solution at the same hematocrit as in the original concentrate (60-70%).
Statistics
Results are expressed as the mean, plus or minus standard deviation (SD) or standard error (SEM). Statistical analyses on normally distributed data were performed using a two-tailed Student t-test or ANOVA with Bonferroni’s posttest correction for multiple comparisons. Non-parametric equivalents were employed for non-normally distributed data. p values <0.05 were deemed statistically significant. Simple linear regression was calculated by the least squares method. Linear association between two or more variables was performed to determine positive or negative correlations. All experiments were done in triplicate unless stated otherwise.
RESULTS
Leukoreduction of RBC concentrates reduces platelet aggregation after exposure to stored supernatant and reduces accumulation of selected inflammatory mediators
Stored RBC supernatants accumulate bioactive protein and lipid mediators. To determine the effects of stored RBC supernatants on platelet function, whole blood aggregation was performed following exposure to stored leukoreduced or non-leukoreduced RBC supernatants (Fig. 1A). Whole blood exposed to stored non-leukoreduced RBC supernatants (<8 days or >20 days) demonstrated a significant increase in platelet aggregation compared to freshly prepared RBC supernatants, while leukoreduced stored RBC supernatants did not alter platelet aggregation, even at a longer storage time.
Fig. 1. Leukoreduction of RBC concentrates reduces platelet aggregation after exposure to stored supernatant and reduces accumulation of selected inflammatory mediators.
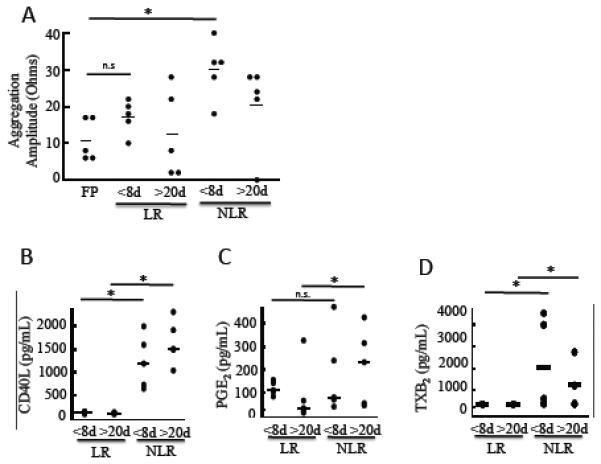
RBCs were obtained from leukoreduced (LR) or nonleukoreduced (NLR) red cell concentrates stored for <8 days or >20 days. (A) Whole blood aggregation was quantified as maximum amplitude (ohms) following addition of stored RBCs to fresh PRP samples v/v for 10 minutes at 37°C and then activated with ADP (20 μM). Freshly prepared (FP) RBCs were used as a control. (B-D) Cell-free supernatant was isolated from stored RBC concentrates by centrifugation and analyzed by competitive enzyme immunoassay (EIA) for (B) sCD40L, (C) PGE2 and (D) TXB2. Mean +/− SEM, n=5, Statistical significance was determined by one-way ANOVA. *p<0.05, **p<0.01.
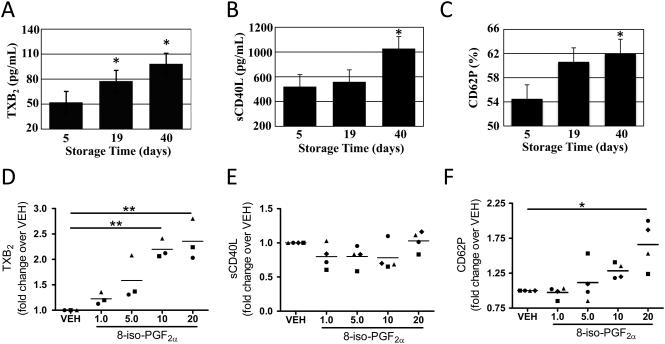
Next, we assessed whether pre-storage leukoreduction reduced protein levels and lipid mediators commonly found in stored RBCs. As shown in Fig. 1B, sCD40L levels increased during storage of nonleukoreduced RBC concentrates, which contain platelets and white blood cells. Leukoreduction reduced sCD40L to very low levels (Fig. 1B), as well as levels of vascular endothelial growth factor (VEGF), a mediator of angiogenesis and vascular permeability (data not shown). Little is known about the accumulation of non-protein mediators, such as lipids. We measured levels of a proinflammatory eicosanoid, prostaglandin E2 (PGE2), a prothrombotic eicosanoid, thromboxane B2 (TXB2) (a stable metabolite of TXA2) and an oxidative stress eicosanoid, 8-iso-PGF2α (an F2-IsoP) in stored RBC concentrates before and after leukoreduction following short (<8 days) and long (>20 days) storage times (Fig. 1C and 1D). Leukoreduction prior to storage abrogated accumulation of TXB2, in contrast to PGE2, which was significantly reduced only at the later time point. Removed Figure 1E.
F2-IsoPs, IsoFs and cell-free hemoglobin accumulate in parallel during RBC storage
Because at least 64 different F2-IsoPs and 8 regioisomers of IsoFs exist, we investigated the levels of the total F2-IsoPs and IsoFs population during storage30. Leukoreduced RBC concentrates were stored and accumulation of F2-IsoP and IsoF lipid mediators measured by mass spectrometry (Fig. 2A and 2B). Both IsoPs and IsoFs significantly increased linearly over time, demonstrating that the oxidative stress status of RBC concentrates increases with storage duration. Cell-free hemoglobin levels were measured and found to also increase linearly over storage time (Fig. 2C).
Fig. 2. F2-IsoPs, IsoFs and cell-free hemoglobin accumulate in parallel during RBC storage.
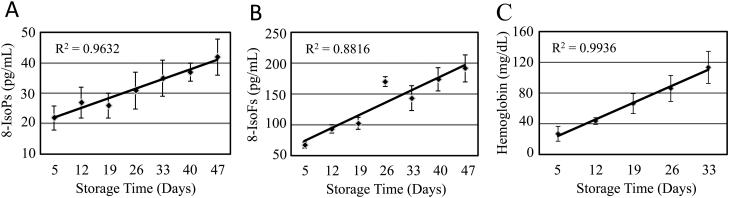
Four leukoreduced RBC concentrates were stored and evaluated on the indicated days for levels of (A) F2-isoPs and (B) Iso-Fs by mass spectrometry, and (C) hemoglobin levels by a hemoglobin colorimetric assay. Mean +/− SEM, n=4; Regression analysis demonstrates a linear and statistically significant correlation between F2-isoPs, Iso-Fs, and hemoglobin accumulation and RBC storage time.
Supernatants from stored leukoreduced RBCs and 8-iso-PGF2α cause platelet activation and release of proinflammatory and prothrombotic mediators from platelets
Having demonstrated that supernatants of stored RBCs can influence platelet aggregation, we investigated in detail the effects of stored leukoreduced RBC supernatants on platelet activation. RBC supernatants were added to freshly prepared PRP in equal volumes. RBC supernatants promoted the release of TXB2 and sCD40L from platelets over time (Fig. 3A and 3B). CD62P (P-selectin) expression, a surface marker of platelet activation, was also increased in response to stored RBC supernatants (Fig. 3C). To test whether the increase in platelet activation was due to IsoP accumulation, we exposed washed fresh platelets to increasing concentrations of 8-iso-PGF2α. 8-iso-PGF2α elicited a dose dependent release of TXB2 (Fig. 3D), but did not affect sCD40L release from washed platelets (Fig. 3E). There was also a significant increase in CD62P surface expression in response to higher concentrations of 8-iso-PGF2α. These data demonstrate that the presence of 8-iso-PGF2α in RBC supernatants is one potential stimulus to platelet activation and release of prothrombotic mediators following transfusion.
Fig. 3. Supernatants from stored leukoreduced RBCs and 8-iso-PGF2α cause platelet activation and release of proinflammatory and prothrombotic mediators from platelets.
(A-C) Freshly prepared PRP was isolated from two independent donors. PRP was incubated with stored RBC supernatants (Days 5, 19 and 40) from three independent donors for 2 hours. Cell-free plasma was collected by centrifugation and analyzed by immunoassay for TXB2 and sCD40L or by flow cytometry for CD62P expression. (D-E) Washed platelets were prepared and incubated with 8-iso-PGF2α (1-20 μM) or vehicle control (VEH). Results are displayed as fold change over VEH for three donors, where each donor is indicated by a different symbol. Mean +/− SEM, n=3-4 for each group. Statistical significance was determined by one-way ANOVA. *p<0.05, **p<0.01.
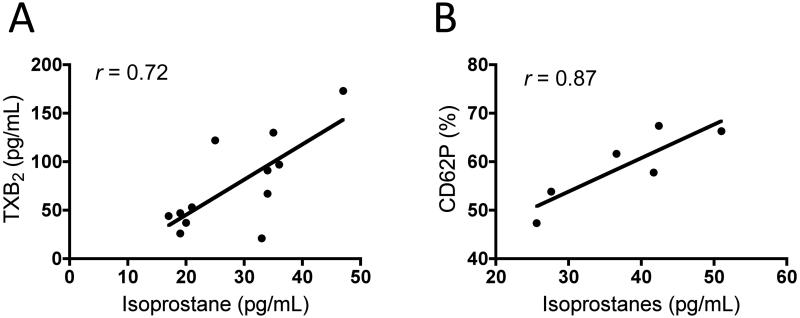
F2-IsoP levels in stored leukoreduced RBC supernatants correlate with increased platelet activation upon in vitro simulated transfusion
To determine whether F2-IsoPs and Iso-Fs could potentially play a role in platelet activation following transfusion, correlation analysis was performed to assess whether increased platelet activation correlated with F2-IsoP concentrations found in stored RBC supernatants. Our findings demonstrate that as F2-IsoPs levels increased in stored RBC supernatants over time, both platelet TXB2 release and CD62P surface marker expression increased significantly and proportionately in platelets exposed to these supernatants. Fig. 4A and 4B demonstrate a positive correlation between F2-IsoPs concentration and platelet activation.
Fig. 4. F2-IsoP levels in stored leukoreduced RBC supernatants correlate with increased platelet activation upon in vitro simulated transfusion.
IsoP concentration in RBC supernatants is plotted against (A) TXB2 release or (B) CD62P expression from freshly isolated platelets from 3 donors exposed to these supernatants. (A) Average TXB2 expression from 1 platelet donor treated with RBC supernatants from 4 different donors at three storage time points. (B) Average CD62P expression from 3 different platelet donors treated with RBC supernatants from 2 different donors. Correlation analysis demonstrates a significant positive correlation between IsoP and IsoF accumulation, and RBC storage time (Correlation coefficient (r): 0.72 for TXB2 and 0.87 for CD62P); both p<0.05.
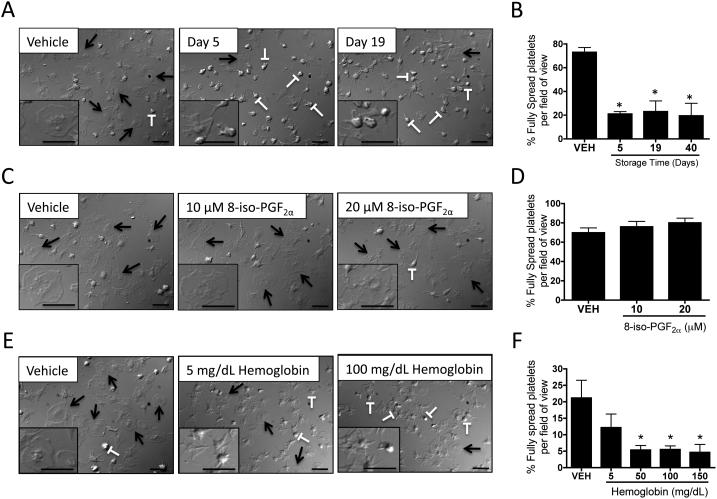
Stored leukoreduced RBC supernatants and hemoglobin, but not 8-iso-PGF2α, inhibit platelet spreading
To determine whether mediators found in stored RBC supernatants affect platelet function, freshly washed platelets were exposed to stored RBC supernatants and platelets were spread on a fibrinogen matrix. Upon attachment to the fibrinogen matrix, platelets extend filipodia and form lamellipodia (flattened appearance-see inset in Fig. 5A), increasing the platelet surface area. Platelet spreading was inhibited by exposure to RBC supernatants (Fig. 5A, see arrows), resulting in more unspread platelets and rounded forms with filipodial extensions, but lacking lamellipodia. This inhibition occurred as early as day 5 of RBC storage (Fig. 5B).
Fig. 5. Stored leukoreduced RBC supernatants and hemoglobin, but not 8-iso-PGF2α inhibit platelet spreading.
Freshly isolated platelets (3.3x 107 cells/mL) were treated 2 hours with Adsol (vehicle) or stored RBC supernatants (Days 5, 19 and 40) (A-B), or vehicle control (DMSO), 8-iso-PGF2α (C-D) or hemoglobin (E-F) and then spread on fibrinogen-coated coverslips. Coverslips were fixed and imaged by differential interference contrast (DIC) microscopy. Measurement bars =10 μm. One representative donor of three is shown (A, C, E). Arrows identify fully spread platelets and T-bars unspread platelets. Mean +/− SEM. Statistical significance was determined by one-way ANOVA. *p<0.01 compared with vehicle.
We further examined the effects of 8-iso-PGF2α on platelet spreading (Fig. 5C and 5D) to determine whether its presence in stored RBCs could influence platelet spreading in vitro and saw no effect (Fig. 5C, see arrows). To determine the mediator responsible for decreased platelet spreading in stored RBC supernatants, we investigated whether hemoglobin, the most abundant supernatant molecule, could also contribute to altered platelet spreading. Hemoglobin accumulated to high levels (>120 mg/mL) in stored RBCs (Fig. 2C) and inhibited platelet spreading in a dose-dependent manner within this range (Fig. 5E and 5F).
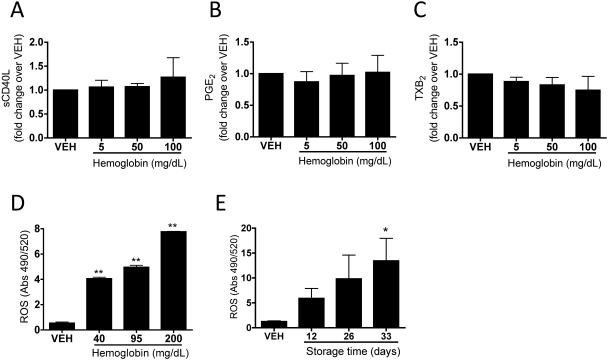
Hemoglobin has no effect on mediator release, but increases reactive oxygen species production in platelets
To determine whether supernatant hemoglobin influences platelet mediator release, we incubated washed human platelets with hemoglobin and observed no statistically significant hemoglobin-induced release of sCD40L, PGE2 or TXB2, at levels as high as 100 mg/dL (Fig. 6A, 6B and 6C, respectively). Due to the oxidizing potential of hemoglobin, we assessed the levels of reactive oxygen species (ROS) produced by platelets in response to purified hemoglobin or stored RBC supernatants. As shown in Fig. 6D, hemoglobin dose-dependently increased ROS accumulation in platelets, which mimicked the trend that is evidenced in platelets exposed to stored RBCs, whereby older stored units evoke increased ROS accumulation (Fig. 6E).
Fig. 6. Hemoglobin has no effect on mediator release, but increases reactive oxygen species production in platelets.
(A-C) Washed human platelets were treated for 2 hours at 37°C with hemoglobin in Adsol. (A) sCD40L, (B) PGE2 and (C) TXB2 levels in supernatants were measured by immunoassay. (D-E) ROS levels were measured in platelet suspsensions treated with Adsol (vehicle) or stored RBC supernatants (Days 12, 26 and 33) (D) or hemoglobin (E). Mean +/− SEM. Statistical significance was determined by one-way ANOVA. *p<0.05, **p<0.01.
Post-storage washing of RBCs reduced supernatant lipid levels for 24 hours
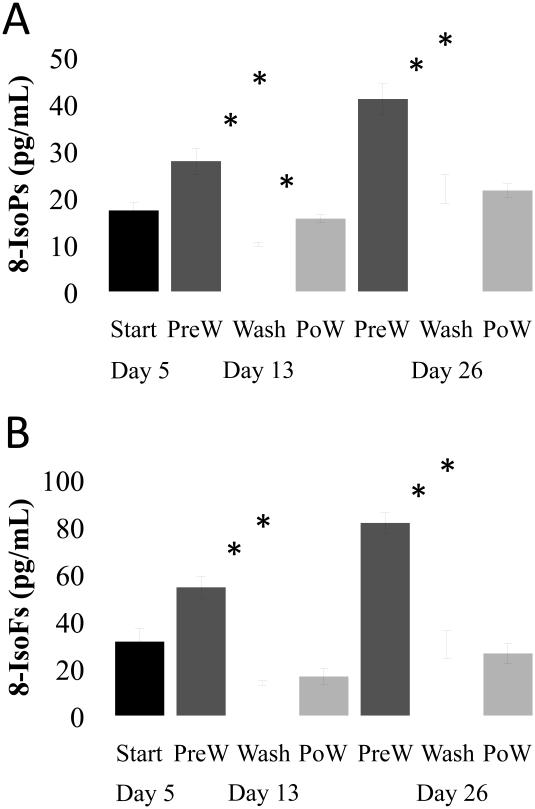
To determine whether potentially harmful lipid mediators could be removed from RBC concentrates, units prepared similarly to those for transfusion were stored, and F2-IsoPs and IsoFs were measured by mass spectometry at short (<14 days) or longer (>26 days) storage time points. The RBCs were then washed and stored for an additional twenty-four hours. Levels of F2-IsoPs and IsoFs were measured immediately following washing and twenty-four hours after washing. Both F2-IsoPs and IsoFs were significantly reduced immediately following washing (Fig. 7A and 7B). In early stored RBC concentrates, these lipids begin to reaccumulate, but levels do not increase above that measured in RBCs supernatants stored for 5 days. Similarly, post-storage washing reduces F2-IsoP and IsoF levels in RBCs supernatants stored for longer periods of time (25 days) and these mediators do not increase within 24 hours, but remain at a level similar to 5-day stored unwashed RBC supernatants.
Fig. 7. Post-storage washing of RBCs reduced supernatant lipid levels for 24 hours.
Supernatants of five day stored RBC units were measured by GC/mass spectrometry for the accumulation of (A) F2-IsoPs and (B) IsoFs and again just prior to washing (PreW) at 13 or 26 days. F2-IsoPs and IsoFs level were measured immediately after washing (Wash) and after an additional 24 hours of storage (PoW) for each storage duration. Mean +/− SEM, n = 4; *p < 0.01.
DISCUSSION
IsoPs, related lipid mediators and their metabolites exert potent biological activity, and are emerging as key mediators of vascular pathology20-23, 27. Employing in vitro modeling, we demonstrate that some adverse transfusion outcomes could, in part, be explained by the presence, generation and accumulation in RBC supernatants of F2-IsoPs and Iso-Fs derived from lipid peroxidation of the red cell membrane. Our data demonstrate that nonleukoreduced stored RBC concentrates induce platelet aggregation and that leukoreduction of RBC concentrates effectively reduces platelet aggregation. It is known that certain F2-IsoPs can dampen platelet aggregation. Our group and others have shown that 8-iso-PGF2α dampens aggregation (data not shown and 31). As RBCs are stored for longer times (>20 days), the ability of platelets to aggregate is reduced, consistent with the accumulation of F2-IsoP and IsoF compounds, such as 8-iso-PGF2α. Leukoreduction also reduces the levels of proinflammatory and prothrombotic mediators produced by platelets and white blood cells, such as sCD40L and some prostaglandins. A key finding was that leukoreduction initially lowered 8-iso-PGF2α levels, but did not completely remove this lipid mediator (data not shown). We demonstrate that F2-IsoPs and IsoFs continue to accumulate throughout the entire 6 weeks of storage in leukoreduced RBCs. These changes have the potential to stimulate the release of bioactive mediators with inflammatory potential from vascular cells in transfusion recipients, including platelets.
Increasing F2-IsoPs and IsoFs levels during RBC storage correlate with in vitro changes in platelet reactivity to RBC supernatants. We show a direct correlation between increasing F2-IsoP concentration, and induction of a surface marker of activation (CD62P) and pro-inflammatory mediator release (TXA2). Further, 8-iso-PGF2α can elicit platelet activation in PRP (data not shown) or washed platelets to signal the release of TXA2 and upregulation of CD62P. Previously, it was shown that 8-iso-PGF2α effects are partially mediated through TXA2 receptor activation15, 32. Thus 8-iso-PGF2α and other isoprostanes represent important stable ligands of the TXA2 receptor or other as yet unidentified receptors that could contribute to pathological activation of platelets33, 34 and other vascular cells35 following transfusion. This could have important consequences with respect to nitric oxide (NO) bioavailability36, 37. TXA2 and 8-iso-PGF2α are potent vasoconstrictors and dysregulated signaling via 8-iso-PGF2α could potentially decrease NO availability, leading to impaired vasodilation.
Notably, EC50 (half maximal effective concentration) values of F2-IsoPs are much higher than the measured basal plasma concentrations (1-5x10−10 nM range) and it is unlikely that basal plasma levels would induce a systemic vascular effect. However, F2-IsoPs and IsoFs are abundantly released at the site of injury or in pathological conditions and therefore, local concentrations could be sufficiently high enough to induce biological effects38. Platelet activation and enhanced free radical formation within vascular microenvironments have been postulated to be a central mechanism in atherosclerosis22. We speculate that transfusion of an increased load of bioactive F2-IsoPs and Iso-Fs to critically ill patients or individuals with chronic inflammatory conditions could be a mechanism contributing to adverse transfusion outcomes. Additionally, Silliman et al. demonstrated that the precursor to F2-IsoPs and Iso-Fs, arachidonic acid (AA), accumulates in RBC plasma throughout the allowed 42 days of RBC storage in both NLR and LR RBCs39. However, it should be noted that there are important differences between the synthetic pathways for prostaglandins and leukotrienes, as compared to F2-IsoPs and IsoFs. Prostaglandins and leukotrienes are generated only from free arachidonic acid, whereas, F2-IsoPs and IsoFs are initially esterified to phospholipids, and subsequently hydrolyzed by phospholipases to their free form14. It is unlikely that F2-IsoP and IsoF accumulation will directly correlate with free AA in stored RBC supernatants. Thus, free AA may not be an accurate indicator of peroxidated lipids levels, while F2-IsoPs and IsoFs may represent a more accurate biomarker.
Because F2-IsoPs and IsoFs progressively accumulate during storage, we saline washed RBCs, as a means to remove these bioactive lipid mediators. Two recent studies in adults with leukemia or children having cardiopulmonary surgery demonstrated that washing stored RBCs and platelets prior to transfusion resulted in decreased inflammatory responses, improved clinical outcomes and increased survival10, 12. Our findings show that F2-IsoPs and IsoFs are reduced following RBC washing. These mediators do not significantly recover beyond starting levels within twenty-four hours of washing, especially for longer stored RBCs. These results suggest that removing biolipids that can potentially elicit transfusion complications may be worthy of study in critically ill transfusion recipients.
While the mechanisms underlying adverse responses to transfusion are undoubtedly multifactorial, a possible approach suggested by these studies is that F2-IsoPs and IsoFs represent a biomarker to assess the quality or suitability of RBC concentrates for transfusion. Oxidative damage in RBC supernatants correlates with increasing F2-IsoPs levels. As RBCs age during storage, the frequency of superoxide formation increases the probability of RBC membrane damage via Fenton reaction and hydroxyl radical formation. In vivo, RBCs have a more robust anti-oxidant compensatory ability to counter this damage, but during storage, concentrations of anti-oxidant proteins, such as glutathione, decrease40. This process increases auto-oxidation of hemoglobin and ROS, promoting lipid peroxidation. We confirmed that cell-free hemoglobin levels increase significantly over storage time in our systems. This accumulation is likely an important mechanism contributing to the generation of F2-IsoPs in stored RBCs that can affect platelet function.
Our findings also demonstrated a correlation between the generation of ROS in platelets induced by 1) increasing levels of cell-free hemoglobin and 2) RBC storage time. While 8-iso-PGF2α did not inhibit platelet spreading, it promoted release of bioactive platelet mediators. In contrast, hemoglobin did not alter platelet inflammatory mediator release, but did interfere with platelet spreading. To our knowledge, this is the first report of hemoglobin effects to platelet spreading, vital for wound healing. This represents a new role for hemoglobin in modulating platelet function and is important, as platelet spreading on a fibrinogen matrix provides a measure of platelet adherence function and as vascular sealants. Abnormal spreading could indicate defects in wound healing capacity, abnormal interactions with endothelial cells and/or aberrant platelet to platelet interaction. It is not unexpected that the storage lesion affects platelet function via multiple mechanisms. Our data support the concept that lipids and hemoglobin have different mechanisms of action on platelet function and will likely have overlapping functions, as well. Regardless, all of these effects are of concern, as RBC transfusion during traumatic injury or surgical procedures is necessary to stop excessive blood loss and provide oxygen to tissues. Storage altered RBC and supernatant oxidative and proinflammatory properties may be harmful to critically ill patients.
It is important to measure both F2-IsoPs and IsoFs, as these compounds are formed via a shared pathway intermediate, but are ultimately derived from parallel mechanisms. F2-IsoP formation requires an oxygen exclusion step, making this class of compounds a sensitive measure of oxidative stress at O2 tension <21%. As oxygen tension increases above this level, isoprostane formation is disfavored and compounds that have a substituted tetrahydrofuran ring (IsoFs) are generated19. Thus, IsoFs are an important measure of oxidant stress at elevated O2 levels (21-100%). Interestingly, a recent study undertaken to reduce the storage lesion associated with oxidative damage showed that anaerobically stored RBC units had extended storage life (9 weeks), and a significant improvement in RBC quality and recovery of function following transfusion41. The authors postulated that the anaerobic environment reduced oxidative injury to the RBC membrane. This could correlate to a reduction in lipid peroxidation and supports a role for these bioactive lipids in the red cell storage lesion.
To date, most studies have focused on F2-IsoPs, but many other related compounds can be generated and are most likely present in stored RBCs (i.e. isoprostanes from prostaglandin A, D, E and J series)16 . Much effort is in progress to develop sensitive methods to better detect and identify these compounds. This is important, as most IsoPs and IsoFs can adduct to and modulate protein function. It is certain that IsoPs and IsoFs singly and in combination act to stimulate or inhibit cellular function. Our data demonstrate that differences in the levels of these compounds in stored RBC concentrates are likely donor specific, which raises the question of the safety of certain individuals as RBC donors. For example, individuals with a high body mass index (obese) have increased plasma levels of F2-IsoPs42 and further, type 2 diabetics have increased systemic lipid peroxidation and overproduction of 8-iso-PGF2α25, 43. Cigarette smokers also have significantly higher free F2-IsoPs levels in plasma26. This suggests that life style could potentially be an indicator of blood donor suitability. A better understanding of the vascular composition and mechanisms of action of these compounds will greatly benefit our understanding of disease pathology, as well as their possible role in transfusion therapy.
Overall, our study reveals that F2-IsoPs and IsoFs accumulate in stored RBC supernatants and this accumulation correlates with unwanted effects on platelet function in vitro, supporting a possible role for lipid peroxidation products in adverse transfusion responses. Moreover, F2-IsoPs and IsoFs levels could represent a novel benchmark for determining blood component suitability for transfusion, leading to improved efficacy, safety and cost efficiency of a frequently performed medical therapy. We further demonstrate that bioactive lipid mediators and cell-free hemoglobin can perturb platelet function via independent mechanisms. Washing RBCs is one modality that could mitigate adverse reactions in susceptible patient populations, as we demonstrate that F2-IsoPs and IsoFs levels are reduced during the allowed 24 hours of post-wash storage.
ACKNOWLEDGEMENTS
Analyses of eicosanoids (isoprostanes and isofurans) were performed in the Vanderbilt University Eicosanoid Core Laboratory.
We are grateful for the following assistance: The Blood Bank services; Brian H. Smith for expert aggregometry assistance.
Grant support: This work was supported by ES10247, T32A1007285-25, ES007026, RC1HL100051, HL095467 and UL1RR024160 from the National Center for Research Resources (The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the NIH), PhRMA Foundation Predoctoral Fellowship in Pharmacology/Toxicology and a grant to the University of Rochester School of Medicine and Dentistry from the Howard Hughes Medical Institute through the Med into Grad Initiative.
Footnotes
Conflict of Interest Disclosure:
NB has received lecture honoraria and consulting fees from Antek, Inc., Fenwal, Pall BioMedical and Caridian (Terumo), manufacturers of leukocyte reduction filters, blood component collection equipment and cell washing devices. The other authors have nothing to disclose.
REFERENCES
- 1.Carson JL, Duff A, Berlin JA, Lawrence VA, Poses RM, Huber EC, O'Hara DA, Noveck H, Strom BL. Perioperative blood transfusion and postoperative mortality. JAMA. 1998;279:199–205. doi: 10.1001/jama.279.3.199. [DOI] [PubMed] [Google Scholar]
- 2.Schrier SL, Sohmer PR, Moore GL, Ma L, Junga I. Red blood cell membrane abnormalities during storage: correlation with in vivo survival. Transfusion. 1982;22:261–5. doi: 10.1046/j.1537-2995.1982.22482251202.x. [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–81. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson KR, Berenholtz SM, Garrett-Mayer E, Dorman T, Klag MJ, and Pronovost PJ. Association between venous thromboembolism and perioperative allogeneic transfusion. Arch Surg. 2007;142:126–32. doi: 10.1001/archsurg.142.2.126. discussion 133. [DOI] [PubMed] [Google Scholar]
- 5.Popovsky MA, Moore SB. Mechanism of transfusion-related acute lung injury. Blood. 1991;77:2299. [PubMed] [Google Scholar]
- 6.Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, Moliterno DJ, Lindblad L, Pieper K, Topol EJ, Stamler JS, and Califf RM. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–62. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 7.van Hilten JA, van de Watering LM, van Bockel JH, van de Velde CJ, Kievit J, Brand R, van den Hout WB, Geelkerken RH, Roumen RM, Wesselink RM, Koopman-van Gemert AW, Koning J, Brand A. Effects of transfusion with red cells filtered to remove leucocytes: randomised controlled trial in patients undergoing major surgery. BMJ. 2004;328:1281. doi: 10.1136/bmj.38103.735266.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonaventura J. Clinical implications of the loss of vasoactive nitric oxide during red blood cell storage. Proc Natl Acad Sci U S A. 2007;104:19165–6. doi: 10.1073/pnas.0708871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moroff G, Holme S, Keegan T, Heaton A. Storage of ADSOL-preserved red cells at 2.5 and 5.5 degrees C: comparable retention of in vitro properties. Vox Sang. 1990;59:136–9. doi: 10.1111/j.1423-0410.1990.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 10.Cholette JM, Henrichs KF, Alfieris GM, Powers KS, Phipps R, Spinelli SL, Swartz M, Gensini F, Daugherty LE, Nazarian E, Rubenstein JS, Sweeney D, Eaton M, Lerner NB, Blumberg N. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: Results of a prospective, randomized, controlled clinical trial*. Pediatr Crit Care Med. 2012;13:290–9. doi: 10.1097/PCC.0b013e31822f173c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberg JA, McGwin G, Jr., Griffin RL, Huynh VQ, Cherry SA, 3rd, Marques MB, Reiff DA, Kerby JD, and Rue LW., 3rd Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65:279–82. doi: 10.1097/TA.0b013e31817c9687. discussion 282-4. [DOI] [PubMed] [Google Scholar]
- 12.Blumberg N, Heal JM, Liesveld JL, Phillips GL, Rowe JM. Platelet transfusion and survival in adults with acute leukemia. Leukemia. 2008;22:631–5. doi: 10.1038/sj.leu.2404920. [DOI] [PubMed] [Google Scholar]
- 13.Grimshaw K, Sahler J, Spinelli SL, Phipps RP, Blumberg N. New frontiers in transfusion biology: identification and significance of mediators of morbidity and mortality in stored red blood cells. Transfusion. 2011;51:874–80. doi: 10.1111/j.1537-2995.2011.03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–7. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow JD, Minton TA, Roberts LJ., 2nd The F2-isoprostane, 8-epi-prostaglandin F2 alpha, a potent agonist of the vascular thromboxane/endoperoxide receptor, is a platelet thromboxane/endoperoxide receptor antagonist. Prostaglandins. 1992;44:155–63. doi: 10.1016/0090-6980(92)90077-7. [DOI] [PubMed] [Google Scholar]
- 16.Roberts LJ, 2nd and Fessel JP. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Chem Phys Lipids. 2004;128:173–86. doi: 10.1016/j.chemphyslip.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–13. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 18.Pratico D. F(2)-isoprostanes: sensitive and specific non-invasive indices of lipid peroxidation in vivo. Atherosclerosis. 1999;147:1–10. doi: 10.1016/s0021-9150(99)00257-9. [DOI] [PubMed] [Google Scholar]
- 19.Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ., 2nd Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc Natl Acad Sci U S A. 2002;99:16713–8. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukunaga M, Yura T, Badr KF. Stimulatory effect of 8-Epi-PGF2 alpha, an F2-isoprostane, on endothelin-1 release. J Cardiovasc Pharmacol. 1995;26(3):S51–2. [PubMed] [Google Scholar]
- 21.Lahaie I, Hardy P, Hou X, Hassessian H, Asselin P, Lachapelle P, Almazan G, Varma DR, Morrow JD, Roberts LJ, 2nd, Chemtob S. A novel mechanism for vasoconstrictor action of 8-isoprostaglandin F2 alpha on retinal vessels. Am J Physiol. 1998;274:R1406–16. doi: 10.1152/ajpregu.1998.274.5.R1406. [DOI] [PubMed] [Google Scholar]
- 22.Pratico D, Iuliano L, Mauriello A, Spagnoli L, Lawson JA, Rokach J, Maclouf J, Violi F, FitzGerald GA. Localization of distinct F2-isoprostanes in human atherosclerotic lesions. J Clin Invest. 1997;100:2028–34. doi: 10.1172/JCI119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yura T, Fukunaga M, Khan R, Nassar GN, Badr KF, Montero A. Free-radical-generated F2-isoprostane stimulates cell proliferation and endothelin-1 expression on endothelial cells. Kidney Int. 1999;56:471–8. doi: 10.1046/j.1523-1755.1999.00596.x. [DOI] [PubMed] [Google Scholar]
- 24.Pratico D, Clark CM, Lee VM, Trojanowski JQ, Rokach J, FitzGerald GA. Increased 8,12-iso-iPF2alpha-VI in Alzheimer's disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol. 2000;48:809–12. [PubMed] [Google Scholar]
- 25.Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F, Capani F, Patrono C. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–9. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 26.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 27.Schwedhelm E, Bierend A, Maas R, Trinks R, Kom GD, Tsikas D, Boger RH. Redox-generated isoprostanes are associated with residual platelet activity in aspirin-treated patients with stable coronary heart disease. J Thromb Haemost. 2010;8:2662–70. doi: 10.1111/j.1538-7836.2010.04117.x. [DOI] [PubMed] [Google Scholar]
- 28.Milne GL, Gao B, Terry ES, Zackert WE, Sanchez SC. Measurement of F(2-) isoprostanes and isofurans using gas chromatography-mass spectrometry. Free Radic Biol Med. 2012 doi: 10.1016/j.freeradbiomed.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman J, Spinelli SL, Schultz E, Blumberg N, Phipps RP. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007;5:788–96. doi: 10.1111/j.1538-7836.2007.02412.x. [DOI] [PubMed] [Google Scholar]
- 30.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–6. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 31.Khasawneh FT, Huang JS, Mir F, Srinivasan S, Tiruppathi C, Le Breton GC. Characterization of isoprostane signaling: evidence for a unique coordination profile of 8-iso-PGF(2alpha) with the thromboxane A(2) receptor, and activation of a separate cAMP-dependent inhibitory pathway in human platelets. Biochem Pharmacol. 2008;75:2301–15. doi: 10.1016/j.bcp.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Audoly LP, Rocca B, Fabre JE, Koller BH, Thomas D, Loeb AL, Coffman TM, FitzGerald GA. Cardiovascular responses to the isoprostanes iPF(2alpha)-III and iPE(2)-III are mediated via the thromboxane A(2) receptor in vivo. Circulation. 2000;101:2833–40. doi: 10.1161/01.cir.101.24.2833. [DOI] [PubMed] [Google Scholar]
- 33.Ting HJ, Khasawneh FT. Platelet function and Isoprostane biology. Should isoprostanes be the newest member of the orphan-ligand family? J Biomed Sci. 17:24. doi: 10.1186/1423-0127-17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin K, Halushka PV, Yan YT, Wong PY. Antiaggregatory activity of 8-epi-prostaglandin F2 alpha and other F-series prostanoids and their binding to thromboxane A2/prostaglandin H2 receptors in human platelets. J Pharmacol Exp Ther. 1994;270:1192–6. [PubMed] [Google Scholar]
- 35.Benndorf RA, Schwedhelm E, Gnann A, Taheri R, Kom G, Didie M, Steenpass A, Ergun S, Boger RH. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: a potential link between oxidative stress and impaired angiogenesis. Circ Res. 2008;103:1037–46. doi: 10.1161/CIRCRESAHA.108.184036. [DOI] [PubMed] [Google Scholar]
- 36.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roback JD, Neuman RB, Quyyumi A, Sutliff R. Insufficient nitric oxide bioavailability: a hypothesis to explain adverse effects of red blood cell transfusion. Transfusion. 2011;51:859–66. doi: 10.1111/j.1537-2995.2011.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cracowski JL. Isoprostanes: an emerging role in vascular physiology and disease? Chem Phys Lipids. 2004;128:75–83. doi: 10.1016/j.chemphyslip.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Silliman CC, Moore EE, Kelher MR, Khan SY, Gellar L, Elzi DJ. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–54. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumaswala UJ, Zhuo L, Jacobsen DW, Jain SK, Sukalski KA. Protein and lipid oxidation of banked human erythrocytes: role of glutathione. Free Radic Biol Med. 1999;27:1041–9. doi: 10.1016/s0891-5849(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida T, AuBuchon JP, Tryzelaar L, Foster KY, Bitensky MW. Extended storage of red blood cells under anaerobic conditions. Vox Sang. 2007;92:22–31. doi: 10.1111/j.1423-0410.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 42.Dietrich M, Block G, Hudes M, Morrow JD, Norkus EP, Traber MG, Cross CE, Packer L. Antioxidant supplementation decreases lipid peroxidation biomarker F(2)-isoprostanes in plasma of smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:7–13. [PubMed] [Google Scholar]
- 43.Cangemi R, Pignatelli P, Carnevale R, Nigro C, Proietti M, Angelico F, Lauro D, Basili S, Violi F. Platelet isoprostane overproduction in diabetic patients treated with aspirin. Diabetes. 2012;61:1626–32. doi: 10.2337/db11-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]