Abstract
Measurements of cochlear function with compound action potentials (CAPs), auditory brainstem responses, and otoacoustic emissions work well with high-frequency sounds but are problematic at low frequencies. We have recently shown that the auditory nerve overlapped waveform (ANOW) can objectively quantify low-frequency (<1 kHz) auditory sensitivity, as thresholds for ANOW at low frequencies and for CAP at high frequencies relate similarly to single auditory nerve fiber thresholds. This favorable relationship, however, does not necessarily mean that ANOW originates from auditory nerve fibers innervating low-frequency regions of the cochlear apex. In the present study, we recorded the cochlear response to tone bursts of low frequency (353, 500, and 707 Hz) and high frequency (2 to 16 kHz) during administration of tetrodotoxin (TTX) to block neural function. TTX was injected using a novel method of slow administration from a pipette sealed into the cochlear apex, allowing real-time measurements of systematic neural blocking from apex to base. The amplitude of phase-locked (ANOW) and onset (CAP) neural firing to moderate-level, low-frequency sounds were markedly suppressed before thresholds and responses to moderate-level, high-frequency sounds were affected. These results demonstrate that the ANOW originates from responses of auditory nerve fibers innervating cochlear apex, confirming that ANOW provides a valid physiological measure of low-frequency auditory nerve function.
Keywords: auditory nerve neurophonic, compound action potential, low-frequency hearing, neural synchrony, phase locking
INTRODUCTION
To quantify low-frequency auditory thresholds, we recently developed a technique that uses information about phase-locked neural firing contained in the cochlear response (CR) recorded near the round window. From CR recordings, we derived the “auditory nerve overlapped waveform” (ANOW) (Lichtenhan et al. 2013). We showed that ANOWs from low frequencies and auditory nerve compound action potentials (CAPs) from higher frequencies (>1 kHz) both have thresholds that are ∼10 dB less sensitive than the most sensitive single auditory nerve fiber thresholds. We proposed ANOW as a way to quantify cochlear neural thresholds objectively at low frequencies (<1 kHz) for which CAPs have been regarded to not yield accurate measures of threshold (e.g., Spoor and Eggermont 1976). The similarity across frequency of ANOW thresholds and single auditory nerve fiber thresholds suggests that ANOW from a given low-frequency tone (e.g., 300 Hz) originates from the corresponding cochlear frequency place. However, alternate possibilities are that ANOW originates from the excitation of low-frequency tails of high-characteristic frequency auditory nerve fibers or from distortion in the cochlear microphonic (CM). If ANOW is to be interpreted correctly, it is important to understand its origin.
The spatial origins of CRs were evaluated with a novel method in which pharmaceuticals were slowly injected from a pipette sealed into scala vestibuli at the apex of guinea pig cochleae. Solution injected in this manner flows basally along the scala tympani toward the cochlear aqueduct in the basal turn as shown by a marker injected at the apex and recovered in the base with a concentration versus time in the base appropriate for the flow through the cochlea (Fig. 3A, B of Salt et al. 2006). This controlled injection method provides a window of time when low-frequency apical regions are affected while basal regions remain unaffected, allowing the origination sites of various cochlear potentials to be demonstrated. In this study, we used tetrodotoxin (TTX), which blocks the production of action potentials while leaving other cochlear functions such as the production of CM intact. Previous studies have used TTX in the cochlea, but they did not focus on low-frequency responses and were not able to conclusively demonstrate which potentials originated from the apex (Henry 1995; He et al. 2012). The sequential apex-to-base blocking of cochlear neural responses by TTX validated the apical neural origin of ANOW.
METHODS
Animal Preparation
Experiments used 400–600 g NIH strain-pigmented guinea pigs of either sex. Guinea pigs were initially anesthetized with intraperitoneal injection of 100 mg/kg of sodium thiobutabarbital. Once anesthetized, the head and neck region was shaved and a tracheotomy was performed. The animal was then artificially ventilated with isoflurane (∼1 % in oxygen), with the respirator set to maintain an end tidal CO2 level of 5 %. A pulse oximeter (CapnoTrue AMP, bluepoint Medical, The Netherlands) was used to monitor heart rate, O2 saturation, and expired CO2 level. Adequate isoflurane level was determined using heart rate and the presence or absence of a toe pinch reflex. The right cochlea was accessed through the auditory bulla with a ventral approach. The soft tissue of the right ear canal was removed and the guinea pig was mounted in a head holder using a hollow ear bar that allowed acoustic stimuli to be delivered. Immediately prior to electrical recordings, pancuronium bromide (0.06 mg/kg) was administered through a cannula in the left jugular vein to eliminate middle ear muscle contractions. A d.c.-powered heating blanket and rectal thermometer were used to maintain temperature at 38 °C. At the end of the experiment, the animal was euthanized with 0.5 ml of 2 M KCl given through the IV cannula. Experimental protocols 20120113 and 20100135 for this study were approved by the Animal Studies Committee of Washington University.
Apical Injection of Tetrodotoxin
Tetrodotoxin binds to sodium channels and blocks neural action potentials. TTX (250 ng/ml in artificial perilymph) was injected from a pipette sealed into a fenestra at the cochlear apex. The composition of artificial perilymph, in millimolar, was NaCl (127.5), KCl (3.5), NaHCO3 (25), CaCl2 (1.3), MgCl2 (1.2), NaH2PO4 (0.75), and glucose (11) (Salt et al. 2012). Control experiments utilized artificial perilymph without TTX. The pipette sealing procedure required first removing the mucosal covering at the cochlear apex, coating the dried area with a thin layer of cyanoacrylate glue, and then applying a layer of two-part silicone elastomer (Kwik-Cast, World Precision Instruments, Sarasota, FL) to make the surface hydrophobic. A fenestra was made through the adhesive layers and was just large enough to insert a pipette with a 25–40-μm tip diameter that was pulled from 1-mm glass tubing. After insertion, the injection pipette was sealed in place with additional cyanoacrylate glue. The injection pipette was coupled to a 100-μL gas-tight syringe (1710TLL Hamilton) with a plexiglass coupler (MPH6S10 World Precision Instruments, Sarasota, FL) that was glued to the syringe to prevent fluid leaks. The syringe was mounted on a digitally controlled WPI Ultrapump (World Precision Instruments, Sarasota, FL). Apical injections caused volume flow from the injection site toward the cochlear aqueduct of scala tympani in the cochlear base. Constant rate injections were not used because they would have yielded flow along the length of the cochlea that would slow down as scala tympani cross section widens at the base. To achieve a more uniform longitudinal flow, we began injections at a rate of 50 nl/min, increasing to 100 nl/min after 10 min, and to 200 nl/min after 30 min. This combination of flow rates provided a more uniform rate of spread down the cochlea than a single, constant rate. Within ∼40 min from the start of the injection, when a total volume of 4.5 μL had been injected, TTX reached the basal cochlear regions of scala tympani. Changes in electrophysiologic responses as a function of time could therefore be translated into the origins of responses according to distance along the cochlea from the apex.
Acoustic Stimuli and Electrophysiologic Recordings
Stimulus generation and electrophysiologic recordings were made with Tucker-Davis System 3 hardware controlled by custom-written Visual Basic (Microsoft) software on a personal computer. Stimuli were generated by TD-RP2 modules, passed through TD-PA5 attenuators and TD-HB7 headphone amplifiers. Stimuli were delivered with an Etymotic ER-10C connected to the hollow ear bar to make a closed sound system. Stimuli were calibrated in each experiment using a procedure that tracked 70 dB sound pressure level (SPL) in the ear canal and established attenuation to decibel SPL relationships from 125 Hz to 26 kHz in 1/4 octave steps.
Electrical responses were recorded differentially between an Ag/AgCl ball electrode positioned near the round window and a platinum needle vertex electrode. Signals were recorded with an optically coupled amplifier (TD HB7, 1000× gain) with filters set to 5 Hz (high pass) and 15 kHz (low pass). Signals were routed to the TD-RP2 modules for digitization (48.8 kHz) and averaging. The animal was grounded by an Ag/AgCl pellet electrode connected by a fluid bridge to exposed tissues on the left side of the neck.
Endocochlear potential recordings were made in some of the experiments. Under microscopic visualization, the bony otic capsule over stria vascularis was thinned with a flap knife. A fenestra with an approximate 15-μm radius was made in the thinned bone with a pick. Endocochlear potential was recorded with electrodes made from 1-mm diameter glass pulled to a sharp tip and beveled at a 45° angle to yield a tip diameter of 4–5 μm. The pipette was filled with 500 mM KCl (to yield a low resistance) and was connected to an electrometer with an Ag/AgCl wire. The pipette was mounted on a manipulator and inserted through the fenestra while monitoring potential. When inserted, there was no leakage of cochlear fluids at the fenestration site, as the pipette filled the fenestra. Endocochlear potentials were recorded with a d.c.-coupled electrometer with 10× gain. The potential with the tip in contact with fluid of the spiral ligament established the zero level.
Terminology
We use a detailed terminology based on stimulus conditions and signal processing of the CR which allows the results to be presented while avoiding inferences about cell type or physiologic origin. This detailed terminology, while very precise, is difficult to read, so we often also include more common names (such as “CM”) with a “p” appended to indicate that the potential recorded is putatively or partly of that origin (e.g., pCM). Only when evidence is presented that a potential truly originates as the name implies, will we use the name without the appended p.
The CR is all that which is recorded from a round window electrode in response to sound and can include blends of both neural and hair cell responses. Most of our recordings were made with alternating polarity sounds, and this is assumed in our terminology. The signal processing is indicated by subscripts: CRave is the entire waveform that results from averaging responses to alternating polarity sounds, CRdif is the entire waveform that results from calculating the difference of responses to alternating polarity sounds, and CRave,onset is the portion of the CRave response waveform nearest the onset of stimulation (i.e., it is pCAP). As outlined below, our use of CRave,onset (pCAP) will be further separated according to whether the sounds used were low (<1 kHz) or high (>1 kHz) frequency. We use this complex terminology so that we can clearly present when a particular measurement and data processing corresponds to a particular physiologic variable and when it does not. We are not advocating that this terminology should normally be used.
Responses to Low-Frequency Sounds
CRs were averaged during stimulation with 40 tone bursts of alternating polarity with frequencies of 353, 500, and 707 Hz. Tone bursts were 30 ms in duration with linear rise/fall times that varied with frequency to include two cycles. Tone bursts were presented at 4.8/s. Figure 1 shows examples of CRs averaged from tone bursts (Fig. 1A) and inverted tone bursts (Fig. 1B). The waveforms resulting from subtracting (Fig. 1C, CRdif) and averaging (Fig. 1D, CRave) the response waveforms were stored online in separate buffers. The amplitude (peak voltage) in the middle of the response, called CRdif,mid (pCM), was measured by fitting a sinusoid at the sound frequency, with variable amplitude and phase, to the data in a 20- to 30-ms window (region shown as a thick line in Fig. 1C). Best fit was established using Excel Solver tool by minimizing the sum-of-square deviations between the fitted sinusoid and the waveform. For low-frequency sounds, the slowly varying potential CRave,onset,L (pCAP) (Fig. 1E) was derived by performing a two-cycle smooth of the CRave waveform (i.e., each point in the data buffer was the average of all points during one stimulus cycle before and one cycle after that point). Due to the shape of the onset response in this low-pass waveform, the amplitude of CRave,onset,L (pCAP) (vertical line in Fig. 1E) was measured as the first negative peak relative to the pre-tone baseline (dotted line in Fig. 1E). To measure the response in the middle of CRave, called CRave,mid (pANOW), the slowly occurring potential change of CRave,onset (pCAP) was subtracted from the original CRave waveform. This results in a response waveform centered around a near-zero baseline with oscillations at twice the frequency of the sound (Fig. 1F). In a similar manner to that described above for CRdif,mid (pCM), CRave,mid (pANOW) amplitude (peak voltage) was measured by fitting a sinusoid at twice the sound frequency to the 20–30-ms region of the waveform. The time domain analyses used here were validated on a few animals using the frequency domain analysis described by Lichtenhan et al. (2013) by finding that indistinguishable results were achieved by using both time and frequency domain analyses. Each statistical analysis was performed in both MATLAB and Excel.
FIG. 1.
Waveforms and manipulations used to measure cochlear responses (CRs) to low-frequency sounds. In this example, the sound was a 500-Hz, 65-dB SPL tone burst. A Average of responses to sounds of one phase. B Same as A, but waveform is from the inverted sounds. C CRdif waveform obtained by subtracting B from A. D CRave waveform obtained by averaging B and A. E Slow potential changes extracted from two-cycle smoothing of D. F The high-pass oscillating component of the CRave response obtained by subtracting E from D. Thick lines in C and F show the 20- to 30-ms regions where the amplitudes of the CRdif and CRave were measured, namely, CRdif,mid (pCM) and CRave,mid (pANOW), respectively. Hair cell currents are putatively the dominant origin for waveforms in A–C and auditory nerve action potentials for D–F. In the simplest identification, C is the cochlear microphonic (CM), E is a CAP, and F is the auditory nerve overlapped waveform (ANOW), but see the “DISCUSSION” for the limitations of these identifications.
Responses to High Frequencies
Using high-frequency sounds, we obtained CRave,onset,H (pCAP) from the responses to 2–16-kHz tone bursts of 12 ms duration, shaped with 0.5 ms raised cosine rise and fall, and presented at 10.9/s. Responses to 10 tone bursts were averaged, followed by an average of responses to 10 inverted tone bursts (alternating polarities not interleaved). The two responses were averaged to produce a waveform that was post hoc low-pass filtered at 2 kHz. The amplitude of CRave,onset,H (pCAP) was measured as the difference between the negative peak occurring at least 1 ms after sound onset (N1) and the following positive peak (P1). Peak times in the CR were initially identified visually and subsequent measurements were made automatically by measuring peak values in a window starting 0.24 ms before the N1 and ending 0.24 ms after the P1 from the previous waveform. CRave,onset,H (pCAP) thresholds were quantified by an automated algorithm that increased sound level in 5 dB steps to achieve a CRave,onset,H (pCAP) amplitude above the 10-μV criterion, followed by sound level decreases in 5 dB steps to yield a response below the criterion. The threshold was measured in decibel SPL by linear interpolation between amplitude measurements that straddled the 10-μV criterion.
Electrophysiologic Measurements During Apical Injections
During apical injections, CRs from different stimulus conditions were measured repeatedly. Measurements most often included CRave,onset,H (pCAP) thresholds recorded automatically at octave intervals of 2, 4, 8, and 16 kHz and CRave,mid (pANOW), CRdiff,mid (pCM), and CRave,onset,L (pCAP) to 353, 500, and 707 Hz at 50 and 65 dB SPL. In some cases, recordings of CR from low-frequency sounds were made from a much wider range of sound levels, such as 15 to 70 dB SPL or 50 to 90 dB SPL. Stimulus conditions were selected such that all responses could be collected within a 2-min period. The measurements were repeated at 2-min intervals throughout the duration of the apical injection procedure.
RESULTS
Sound-Level Growth Functions
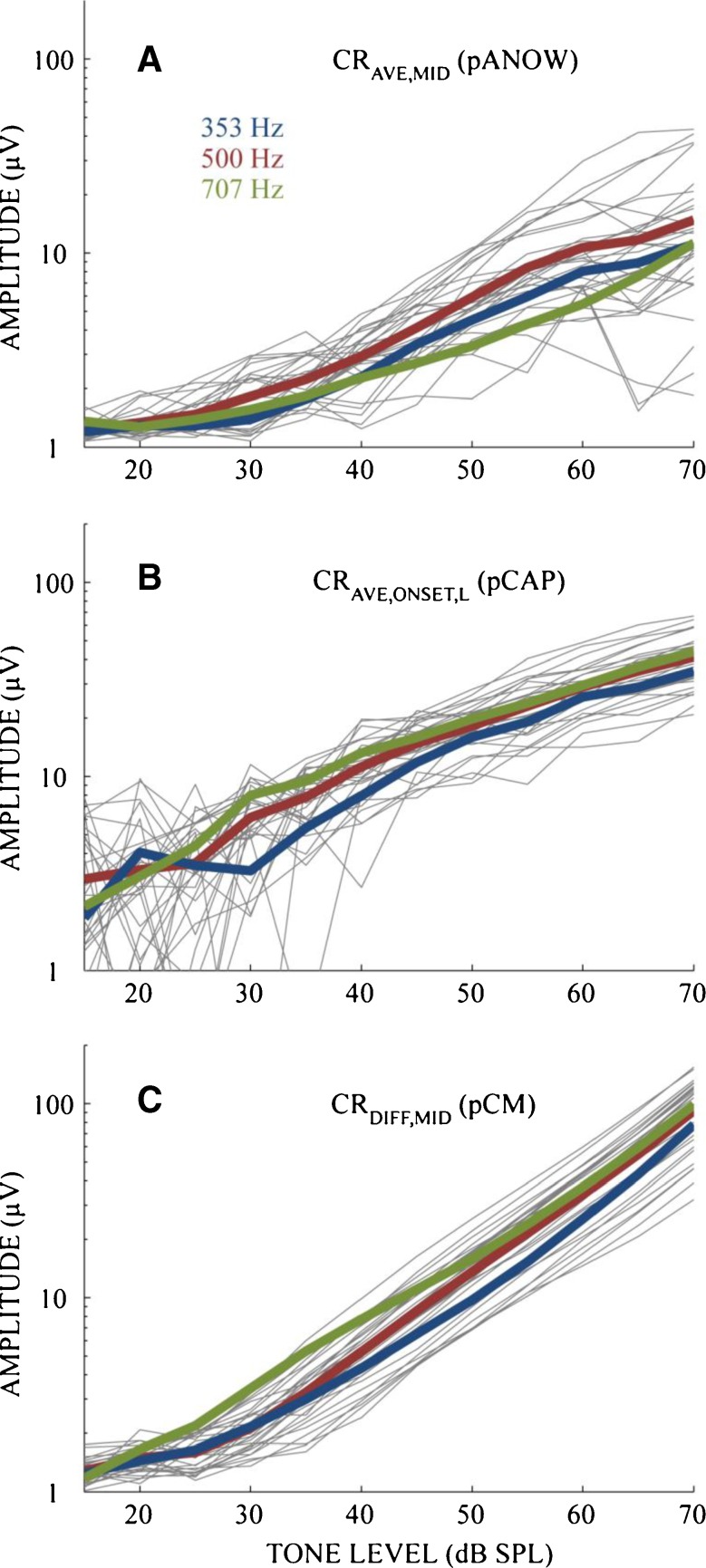
Figure 2 shows stimulus–response growth curves to tone burst frequencies 353, 500, and 707 Hz. These measurements were acquired just before the fenestra(s) were made. Some individual CRave,mid (pANOW) measurements varied non-monotonically with level and some increased monotonically. Although noise contributed to some of the non-monotonicity, dips seen at higher sound levels were often reproducible, consistent with ANOW from cat ears (Lichtenhan et al. 2013). CRave,onset,L (pCAP) could not be measured well at low levels, which is consistent with poor CAP performance at low frequencies (Spoor and Eggermont 1976). In contrast, CRdif,mid (pCM) could indeed be measured well at low levels, which is consistent with the well-known behavior of CM (e.g., Dallos 1973). The primary purpose of presenting these growth functions is because hereafter data were normalized to pre-injection measures.
FIG. 2.
Colored lines are mean CR measurements (baseline to peak voltage) from different sound levels and frequencies: 353 Hz (blue, n = 15), 500 Hz (red, n = 30), and 707 Hz (green, n = 14). Gray lines are measurements from individual animals in response to 500 Hz. The variation of measurements from 353 and 707 Hz, while not shown, was similar to that at 500 Hz.
Threshold Changes Illustrate the Slow Flow of TTX Toward the Base from Apical Injections
The changes in CRave,onset,H (pCAP) thresholds to high-frequency sounds (i.e., CAP thresholds) produced by apical injections of TTX or artificial perilymph are shown in Figure 3. Average thresholds from four animals, expressed as threshold shifts relative to the corresponding averages in the pre-injection period, are shown by gray lines in panels A and B. The baseline thresholds at 2, 4, 8, and 16 kHz, averaged from 20 measurements (five pre-injection threshold measures from each of the four TTX injection animals), were 36.6 ± 0.2 (mean ± standard error of the mean), 44.3 ± 0.1, 25.1 ± 0.1, and 34.6 ± 0.2 dB SPL, respectively. After the TTX injection started, thresholds remained relatively stable for ∼10 min, and then, the threshold at the lowest frequency tested (2 kHz) increasingly shifted while those at higher frequencies did not. As time progressed, thresholds at 4, 8, and 16 kHz shifted each in turn. These results are consistent with the CRave,onset,H (pCAP) from higher frequency sounds originating from more basal sites, so that more time, and thus more TTX solution flow toward the base, was needed to shift threshold. These threshold changes during TTX injections, and lack of threshold change during artificial perilymph injections (Fig. 3A), confirm that using apical injections of TTX can reliably demonstrate differences in spatial origins of evoked potentials from different sound frequencies. Overall, the data indicate that solutions injected through a fenestra in the scala vestibuli near the apex propagate from apex to base through the scala tympani, consistent with Salt et al. (2006).
FIG. 3.
Apical injections of tetrodotoxin (TTX) produced systematic reductions of neural responses as the TTX flowed toward the cochlear base (B), but injections of artificial perilymph produced no substantial effect (A). Data in A are normalized to pre-injection measurements from −8 to 0 min, and data in B are normalized to pre-injection measurements from −10 to 0 min. Gray lines in A and B are CRave,onset,H (pCAP) thresholds from four tone burst frequencies as a function of time during injections into the cochlear apex. TTX data are averages from four animals and artificial perilymph data are averages from four different animals. Lines are mean data and vertical bars are standard errors of the mean. Also shown in B is the normalized amplitude of the CRave,onset,H (pCAP) response to an 8-kHz fixed-level 55-dB SPL tone burst (black line, right side axis). Responses from 55 dB SPL 8 kHz are used throughout this report as a measure when TTX reached the cochlear base. Note that the sags in the responses over the first 20 min are similar for the 8-kHz fixed-level tone (∼20 %) and for the threshold shifts before their rapid decline (∼2 dB).
Also shown in Figure 3B is CRave,onset,H (pCAP) amplitude to 55 dB SPL, 8 kHz tone bursts (black line), averaged from the same four ears as the threshold data. CRave,onset,H (pCAP) amplitudes were normalized and expressed as percentages with respect to the average amplitude in the pre-injection period between −10 and 0 min (195 ± 32 μV). These voltages, which are on a linear scale, are more sensitive indicators of the initial decline than are the threshold measures on a decibel scale. Over the first 18–20 min following the injection, there was a slow, <20 %, decline in all of the responses in Figure 3, but because of the scales, the 20 % reduction in the CRave,onset,H (pCAP) amplitude of the response to the fixed-level 8 kHz tone looks much bigger than the similar-amplitude 2 dB declines in the thresholds at 2, 4, 8, and 16 kHz. The major decline of CRave,onset,H (pCAP) amplitudes to the 8 kHz tone bursts occurred at approximately the same time as the major shift of the 8 kHz CRave,onset,H (pCAP) thresholds. The CRave,onset,H (pCAP) thresholds illustrate how apical injections systematically affected cochlear thresholds. Since our low-frequency CR measurements were made at suprathreshold levels, we will compare them to a high-frequency response at suprathreshold levels. To do this, we acquired CRave,onset,H (pCAP) from 8 kHz, 55 dB SPL tone bursts on most preparations, and these data will be used throughout this report for comparisons to low-frequency CRs.
ANOW Is due to Auditory Nerve Fibers that Originate Near the Cochlear Apex
The effects of TTX and control (artificial perilymph) injections on normalized CRave,mid (pANOW) amplitudes from low-frequency (353, 500, and 707 Hz) sounds are shown in Figure 4. CRave,mid (pANOW) amplitudes to 50 dB SPL, 353 (upper panel), 500 (middle panel), and 707 Hz (lower panel) sounds began to rapidly shift within 2 to 4 min after the start of the TTX injection and were substantially reduced by 10 min—the time when thresholds from 2 kHz began to rapidly shift (Fig. 3B). During TTX injections, the CRave,mid (pANOW) to 65 dB SPL declined later in time than CRave,mid (pANOW) to 50 dB SPL, suggesting that abolishing the ANOWs from 65 dB SPL required greater TTX accumulation, or additional basal ward flow of solution. The dotted lines demonstrate that during apical injections of artificial perilymph CRave,mid (pANOW) amplitudes declined very little until the perfusion rate was increased, which was after the major ANOW decline produced by TTX. Thus, the decline of ANOW amplitude during TTX injection experiments results from the effect of TTX and not from the injection itself. For comparison to CRave,mid (pANOW) amplitudes during TTX injections, Figure 4 shows normalized amplitudes of CRave,onset,H (pCAP) to 55 dB SPL, 8 kHz tone bursts during TTX injections (from Fig. 3). CRave,mid (pANOW) to the low-frequency, 50 dB SPL tone bursts declined substantially earlier than the responses to the 55 dB SPL, 8 kHz tone bursts, confirming that ANOW (i.e., CRave,mid) originates from apical cochlear regions that are affected earliest by apical TTX injections.
FIG. 4.
Demonstration that ANOW originates from the cochlear apex. Colored lines Normalized amplitudes of CRave,mid to low-frequency sounds (ANOW) during TTX injection into the cochlear apex. Each panel shows measurements from a different tone burst frequency with responses to 50 and 65 dB SPL shown with dark and light colors, respectively. Data are from the same four animals as Figure 3. Dotted lines Normalized amplitudes (re. pre-injection measurements between −8 and 0 min) of CRave,mid to low-frequency sounds (ANOWs) during four control experiments with apical injections of artificial perilymph. When the rate of injection increased to compensate for the larger cross sectional area of scala tympani in the cochlear base, ANOW decreased somewhat. Note that this was after the major decline of ANOW produced by TTX. Black lines Normalized amplitudes of CRave,onset,H (pCAP) from 55 dB SPL 8 kHz tone bursts (from Fig. 3). TTX flowing from the cochlear apex to base abolished low-frequency ANOWs well before abolishing CAPs from 8 kHz tone bursts, thus verifying that ANOW originates in the cochlear apex. Vertical bars represent standard errors of the mean.
The Time Course of ANOW Abolition
It is apparent in Figure 4 that CRave,mid amplitudes to low-frequency sounds (ANOWs) declined with similar time courses. A more direct comparison is shown in Figure 5 by superimposing data from the different frequencies in separate plots for 50 and 65 dB SPL. Differences between the test frequencies are small, consistent with the frequencies being only 1/2 octave apart and the broad tuning of responses in the cochlear apex. Data from 65 dB SPL showed the expected trend: CRave,mid (ANOW) amplitudes to 353 Hz tone bursts were reduced first and were followed systematically by 500 Hz and then 707 Hz. At 50 dB SPL, CRave,mid (ANOW) amplitudes to 353 Hz tone bursts were reduced before responses from the other two frequencies. One-tailed t tests indicated that 12 min after the injection began (a relatively steep region of the TTX-based trends in Figs. 4 and 5), ANOW amplitudes were statistically significantly smaller for 50 than for 65 dB SPL tones at 353, 500, and 707 Hz: t(3) = 1.88, P = 0.05; t(3) = −3.60, P = 0.0057; and t(3) = −3.48, P = 0.0066. In the “DISCUSSION,” we will consider possible reasons why there were such small differences in the time courses of response abolition at different low frequencies, but larger differences at higher frequencies.
FIG. 5.
Data from Figure 4 rearranged to contrast the time courses of responses to different frequencies for 50 dB SPL tone bursts (top) and 65 dB SPL (bottom). Measurements from 65 dB SPL show the expected trend—CRave,mid (ANOWs) were reduced systematically from lowest to highest frequency as TTX flowed from the cochlear apex to base. However, 50 dB SPL data show that measurements from 353 Hz were reduced first, but measurements from 500 and 700 Hz were not reduced in the order expected (although the time difference was small). For visualization of the standard errors of the mean, vertical bars were displaced slightly to the right for 707 Hz and to the left for 353 Hz.
Comparing CRave,mid (ANOW) and CRave,onset,L (pCAP) from Low-Frequency Sounds
Changes in CRave,mid (ANOW) and CRave,onset,L (pCAP) collected simultaneously from the same low-frequency sounds are compared in Figure 6. Responses from 50 dB SPL were reduced before those from 65 dB SPL for both CRave,mid (ANOW) and CRave,onset,L (pCAP). When obtained at the same tone burst levels, CRave,onset,L (pCAP) declined before CRave,mid (ANOW). These patterns can be seen at all three sound frequencies. CRave,onset,L (pCAP) is a response to the sound onset. At the tone burst onset, the rapid rise in the amplitude of the sinusoidal signal spreads the energy into frequencies both above and below the selected tone frequency, a phenomena called “spectral splatter” (e.g., see Fig. 1 of Gorga et al. 1988). Thus, the CRave,onset,L (pCAP) response is due, in part, to synchronous excitation of neurons in these nearby frequencies, as well as at the frequency of the sinusoid (which, during the onset, is not at full amplitude). During the onset, the sound is wider in frequency and lower in amplitude at any one frequency, than during the steady-state part of the sound. Thus, for the same tone-pip sound level, CRave,onset,L(pCAP) responses are from lower level sounds than are CRave,mid (pANOW) responses. Overall, the time sequences of response reduction by TTX in Figure 6 are all consistent with the interpretation that responses to lower level sounds are affected by TTX before responses to higher level sounds from the same region.
FIG. 6.
Comparison of the effects of TTX injections on CRave,onset,L (pCAP) and CRave,mid (ANOW) to low-frequency sounds. Data are normalized to pre-injection measures between −8 and 0 min. Colored traces Same CRave,mid (ANOW) data as in Figures. 4 and 5. Black and gray traces Mean CRave,onset,L (pCAP) to low-frequency sounds. Vertical bars are standard error of the means. CRave,mid (ANOW) declined later than the simultaneously recorded CRave,onset,L (pCAP). The data support the interpretation that responses to lower-level sounds are affected by TTX before responses to higher-level sounds (see text for more detail).
The Stability of a Variety of Nonneural Measures Indicates that Cochlear Functions Are Little Changed by TTX
While the CR from low-frequency tones measured at the round window was greatly affected by TTX injections, a variety of other measurements were not affected by TTX injections (e.g., Fig. 7). Two-tailed t tests from measurements at before (i.e., −8 min for EP and −10 for distortion product otoacoustic emission (DPOAE)) and 32 min after injection start showed no significant change in endocochlear potential measured from the third cochlear turn in four animals (t(6) = 0.89, P = 0.41) and in DPOAEs (t(6) = 0.02, P = 0.98). In some ears, responses to tones were recorded from inside scala media of the third cochlear turn. Of particular interest is CRdif,mid which can be thought of as a proxy for CM. In Figure 8C, D, CRdif,mid (pCM) measurements from inside scala media are compared to CRdif,mid (pCM) measurements from the round window. Note that pre-injection CRdif,mid (pCM) amplitudes from inside scala media were much larger than from the round window: meanscala media 50 dB SPL = 478 ± 901 μV from 15 measurements in three ears (five pre-injection measures from three ears) and meanround window 50 dB SPL = 16 ± 3 μV from 20 measurements in four ears. The large scala media/round window difference is consistent with Salt et al. (2013). The consistency during TTX administration of CRdif,mid (pCM) from inside the scala media (Fig. 8C, D) indicates that this potential is little changed by TTX blocking neural responses; therefore, this CRdif,,mid is true CM. Overall, the constancy during TTX administration of endocochlear potential, DPOAEs, and CRdif,,mid from inside the scala media demonstrates that basic cochlear functions, including hair cell function, remain normal during apical TTX injections.
FIG. 7.
Endocochlear potential (red, averaged from four animals) and distortion product otoacoustic emissions (DPOAE, black, from one animal) were little changed during apical TTX injections. Vertical bars are standard errors of the mean. For the DPOAE measurements, both primary tones were 70 dB SPL. These data demonstrate that endocochlear potential and hair cell function were unaffected by apical TTX injections.
FIG. 8.
Changes in recorded potentials during TTX injections normalized to pre-injection measurements. Dashed lines and solid lines in all panels were measured from the window from the four animals in Figures 4, 5, and 6. Dotted lines in C and D are from inside scala media of the third cochlear turn from four additional animals. All measurements in the first row were from 50 dB SPL. All round window measurements in the second row (CRdif,mid and ANOW) were from 65 dB SPL, while scala media CM was from 60 dB SPL. Vertical bars are standard errors of the mean. CRdif,mid measurements from scala media were unaffected by TTX, demonstrating that these measurements contain negligible neural components, originate from hair cells, and represent true CM responses. In contrast, CRdif,mid from the round window was markedly affected by TTX, demonstrating that a substantial component of this originated from neural responses, which is why throughout this report we refer to CRdif,mid from the round window as putative CM (pCM).
An interesting point shown by the data in Figure 8 is that while CRdif,mid (pCM) measured in scala media (which is a good measure of CM) stays constant during TTX injections, CRdif,mid (pCM) measured from the round window does not. The TTX block of neural action potentials produced a 40 to 80 % reduction in the amplitude of round window CRdif,mid (pCM). This means that CRdif,mid (pCM) measured from the round window is due, in large part, to neural responses. These results show that the round window CR includes a neural response that can be larger than the CM component that remains after TTX treatment. These results demonstrate that the round window a.c. response to low-frequency tones cannot be regarded as originating predominantly from outer hair cells in the cochlear base, a conclusion that has been reached before (e.g., Dallos 1973, p. 33, Fig. 2.6) and is now quantified here.
CRave,mid from Low-Level Sounds Is ANOW, but CRave,mid from High-Level Sounds Has Different Origins
We showed in Figures 4, 5, 6, and 7 that CRave,mid (ANOW) responses to sound levels up to 65 dB SPL were largely reduced within 20 min of starting an apical TTX injection. This demonstrated the apical neural origin of CRave,mid and justified calling this response “ANOW,” where the “AN” refers to the auditory nerve as its origin. With higher sound levels, however, a different picture emerges. Normalized CRave,mid (pANOW) amplitudes from sound levels 50–90 dB SPL during apical TTX injections are shown in Figure 9. Reduction of CRave,mid (pANOW) amplitudes from 50 dB SPL tones occurred within approximately 20 min of the injection start, consistent with the experiments shown in Figures 4, 5, 6, and 7. In contrast, mean CRave,mid (pANOW) responses from 90 dB SPL were not abolished by TTX and remained above 50 % of the pre-injection amplitudes indicating that more than 50 % of these responses were not neural. Results from a repeated-measures two-way ANOVA identified that these trends were significantly different (F(2, 2) = 14.53, P = 1.5 × 10−6), and a Tukey’s HSD test identified that trends from 50 dB SL differed from the others. These results indicate that CRave,mid (pANOW) from low-frequency high-level sounds originates, at least in part, from sources that are not neural action potentials. This suggests that ANOW is not a suitable name for CRave,mid (pANOW) at high sound levels.
FIG. 9.
Amplitudes of CRave,mid (pANOW) to 500 Hz tone bursts during apical TTX injections averaged from three animals. Measurements were normalized to those acquired during the ∼8 min of pre-injection recordings. These measures were from different animals but are consistent with the round-window measurements shown in Figures 4, 5, 6, and 7. TTX injections produced marked reduction of responses to 50 dB SPL but not to 90 dB SPL. Vertical bars are standard errors of the mean.
If CRave,mid (pANOW) from high-level sound does not have a predominantly neural origin, then what generates this response? One possibility is that the CRave,mid (pANOW) response at 90 dB SPL is generated by nonlinearity in the hair cell current responses to high-level sounds, as illustrated in Figure 10. CM measurement from inside the scala media (Fig. 10, top) and a simple Boltzmann function representing a saturating mechano-electric transducer with a non-zero operating point (OP; Fig. 10, bottom) show that a second harmonic (i.e., a response oscillating at twice the probe frequency) can be generated by hair cells in response to high-level stimuli. A sigmoidal saturating nonlinear function (Fig. 10E) that is commonly used as a model for in vivo hair cell transduction (e.g., Salt et al. 2009; Lichtenhan 2012) is justified by direct measurements of hair cell transduction (e.g., Hudspeth and Corey 1977). The CM measurements from inside the scala media2 saturate and become asymmetric in response to high-level sounds (Fig. 10B, C). This asymmetry is represented in the frequency domain by increases of energy at 2f1 (Sirjani et al. 2004). Averaging the responses to sounds of opposite polarity (Fig. 10D) cancels the primary, but does not cancel the 2f1 component. The result is a waveform oscillating at twice the stimulus frequency that has hair-cell current origins, not neural origins. This analysis indicates that CM distortion can be an important origin of CRave,midfrom high-level sound.
FIG. 10.
Asymmetric response waveforms from scala media with high-level sounds (B and C) and from simulations (F and G) when averaged result in an uncancelled second harmonic (D and H). Upper: B and C show zero-meaned responses from the basal turn of scala media in response to 90 dB SPL, 500 Hz tones of opposite polarity. Such responses follow the frequency of the probe tone and the asymmetry becomes more apparent when one of them is plotted on the y-axis versus an appropriately operating point-shifted sinusoid on the x-axis (A, which shows multiple response cycles from one polarity). The average of B and C yields a waveform that oscillates at twice the frequency (D). This result is caused by the asymmetry of the response waveforms, seen as more saturation at negative potentials compared to positive in B and C. Lower: E is a Boltzmann input–output function (for equations, see Salt et al. 2013) with parameters derived from fitting the measured response.
The input to the function for panels A and E is the sum of an applied sinusoid and the OP (indicated by the green diamond symbol, the position on the transducer curve at zero crossings of the input sinusoid). The region traversed by the 90-dB SPL stimulus is shown in panel E as a blue line. Output waveforms (blue lines in panels F and G) show more saturation at negative potentials due to the negative operating point. The average of F and G yields a waveform that oscillates at twice the frequency (panel H). In contrast, waveforms calculated with a zero operating point would be symmetric about the x-axis and would not yield a waveform at twice the frequency. The red lines in panels E, F, and G are calculated responses to 70 dB SPL tones. These non-saturated responses from the linear part of the transduction curve are more symmetric and produce negligible residual component when opposite polarities are averaged (panel H).
Another possible source of CRave,mid (pANOW) from high-level sounds is excitation of the low-frequency tails of tuning curves from high characteristic frequency auditory nerve fibers. Such responses would be expected to be blocked by TTX. The data in Figure 9 extend to 32 min after the onset of the injection at which time all fibers with characteristic frequencies below about 16 kHz would be blocked (Fig. 3). Guinea pig auditory nerve fibers with characteristic frequencies 16 kHz and below have thresholds at 500 Hz ranging from near 70 to over 90 dB SPL; those with higher characteristic frequencies have thresholds at 500 Hz mostly higher than 90 dB SPL (Harrison and Evans 1977). Thus, at 32 min after the injection, all of the fibers that might respond to 70 dB SPL and almost all of the fibers that might respond to 90 dB SPL would have been blocked. However, at 32 min after the injection, substantial CRave,mid (pANOW) remained (Fig. 9). Neural responses from high characteristic frequency auditory nerve fibers seem unlikely to account for this remaining CRave,mid (pANOW) response.
CR phase measurements provide additional data on the origin of CRave,mid (pANOW) from high-level sounds. Figure 11 shows CRave,mid (pANOW) phases at pre-injection times and at long post-TTX injection times. Pre-injection CRave,mid (pANOW) phases from 50 and 70 dB SPL are similar, but those from 90 dB SPL are quite different. While this data set is limited to a few sound levels, the phase dichotomy is consistent with the hypothesis that voltages from two different origins dominate the responses at low- and high-sound levels. Based on the previous analysis, the CRave,mid (pANOW) responses to 50 and 70 dB SPL are ANOWs dominated by the second harmonic from overlapped neural responses, whereas the CRave,mid (pANOW) responses to 90 dB SPL are dominated by something else. Once TTX injections were complete, the phase data from 50 dB SPL were due to noise and irrelevant because the ANOW response was abolished. After TTS, the phase data from 70 and 90 dB SPL were tightly grouped which is consistent with them being derived from the same origin. The simplest explanation is that this phase is from the CM second harmonic distortion with negligible contribution from neural excitation. Presumably, the neural component that was present pre-TTX and dominant at 70 dB SPL was blocked by TTX so that only the CM second harmonic distortion remains in the injection-complete responses.
FIG. 11.
The effect of TTX injections on the phase of CRave,mid (pANOW) to 500 Hz tone bursts. Shown are ∼10 min of data before TTX injections began and ∼10 min of data collected near the conclusion of the experiment when the ears were loaded with TTX. These data are from the same experiments as Figure 9. We hypothesize that the CRave,mid (pANOW) measurements are grouped because each group was dominated by second harmonic components from a single origin, presumably neural responses or CM distortion. The vertical bars are standard errors of the mean, offset in time for visualization.
Understanding Why CRave,onset,H (pCAP) Thresholds Plateaued During TTX Application
A final issue to consider is why the CRave,onset,H (pCAP) thresholds plateaued during apical TTX injections (Fig. 3) and did not continuously shift, i.e., why CRave,onset,H (pCAP), presumed to be a neural response, was not abolished by TTX. Examination of the CRave,onset,H (pCAP) waveform morphology provides some insight. Figure 12 shows results from an experiment in which response waveforms were collected during threshold measurements. The CRave,onset,H (pCAP) thresholds in response to 8 kHz (solid black line in top panel) were shifted by only 20–30 dB in this animal. The bottom panel of Figure 12 shows the lowest above-threshold waveforms collected at different times during the injection. The normal N1P1 morphology seen during injection times −10 to 30 min began to progressively morph at around 40 min into a negative potential with a peak onset latency shorter than the original response. In responses to high-level tones, the waveforms present after treatment with TTX have been studied previously and are thought to originate from excitatory post-synaptic potentials (EPSPs), not neural action potentials (Dolan et al. 1989; Henry and Price 1994)3. The use of a low high-pass filter setting is essential to studying these measurements; otherwise, the high-pass filtering makes the monophasic waveform in the bottom of Figure 2 appear to be diphasic. Our results extend those of previous studies by showing that this putative EPSP also contributes to CR onset responses at relatively low-sound levels (around 50 dB SPL in this case) and is not found solely in responses to high-level sounds that additionally evoke a measurable summating potential. The fact that the monophasic waveform at the bottom of Figure 12 was not abolished by TTX, along with the results from the previous studies, indicate that the post-TTX CRave,onset,H(pCAP) responses in Figure 12, bottom, is not a CAP response and may arise from EPSPs or the current flow related to EPSPs.
FIG. 12.
TTX-induced changes to CRave,onset,H (pCAP) thresholds and waveforms. Upper panel: CRave,onset,H (pCAP) threshold changes that are comparable to those in Figure 3, though slower due to a slightly delayed changing of the injection rate relative to other experiments reported here: injection rate increased to 100 nl/min at 13.80 min and to 200 nl/min at 32.23 min. The arrows are a guide to when waveforms of associated color in the lower panel were acquired and are not meant to precisely quantify the sound level used to evoke the waveforms. Lower panel: CRave,onset,H (pCAP) waveforms to 8 kHz tone bursts at various times during an apical TTX injection experiment. Each of these responses was from a level 5 dB higher than the threshold. As time progressed and TTX flowed from the apex to base, the typical N1P1 (i.e., CAP) waveform morphed gradually into a negative potential with an earlier peak response latency (indicated by the vertical line). Such post-TTX CRave,onset,H (pCAP) responses are not from nerve action potentials and are hypothesized to be from EPSPs.
DISCUSSION
The slow delivery of pharmaceuticals into the cochlear apex is a novel approach that allows CR origins along the length of the cochlea to be identified. Drug delivery into the cochlear base would not be suitable for this purpose because solutions injected in the base spread only slowly towards the apex, predominantly by diffusion (Chen et al. 2005). The rate of spread of drug in our apical injections is not limited by the rate of diffusion but is governed by the rate of fluid flow down the cochlea toward the cochlear aqueduct in the scala tympani, which is controlled by the injection rate. This allows the entire length of the cochlea to be treated with drug in real time, permitting cochlear frequency place-specific origins contributing to the CR to be dissected and compared. Sealing an injection pipette into the base and making a fluid release hole in the cochlear apex would not be a suitable alternative for several reasons. An unsealed hole in the apex would produce an artifactual “fast-wave” response (Cooper and Rhode 1996) that would render our low-frequency measurements invalid. Also, a perforation would permit cerebral spinal fluid to flow along the scala tympani at ∼1 μL/min, thus diluting and displacing applied drug solutions (Salt and Stopp 1979).
ANOW Is a Neural Measure that Originates Near the Cochlear Apex
The primary finding from this study is that the CRave,mid (i.e., ANOW) to 353, 500, and 707 Hz tone bursts of moderate level (50–65 dB SPL) declined soon after apical TTX injections began. In contrast, the decline of the high-frequency response, CRave,onset,H (pCAP), occurred well after injections began and after ANOW had declined. The delayed decline of CRave,onset,H (pCAP) was true for both CRave,onset,H (pCAP) amplitudes to moderate-level 8 kHz tone bursts and for CRave,onset,H (pCAP) threshold shifts to 2, 4, 8, and 16 kHz (Fig. 3). CAPs have a well-documented origin near the peak of the traveling wave in the basal half of the cochlea (see Lichtenhan 2012 for a review). Thus, the finding that CRave,mid (i.e., ANOW) declines soon after the onset of TTX injections into the apex confirms that ANOW from moderate-level, low-frequency sounds originates from the cochlear apex.
Low-frequency cochlear function has been proven difficult to be assessed by ABR, CAP, and otoacoustic emissions. Measuring ANOW is a solution to this difficulty because at sound levels below 70 dB SPL, it arises from phase-locked neural firing that can be separated from other components of the CR (hair cell current responses) by averaging responses to sounds with alternating polarity. The CM inverts when sound polarity is inverted. In contrast, the half-wave rectified, phase-locked neural firing that occurs primarily during one phase of the sound does not invert and effectively shifts one half cycle in time when polarity is inverted. Averaging CRs from alternating polarity sounds cancels the CM and overlaps the phase-locked neural firing. Our demonstration that ANOW to low- and moderate-level, low-frequency sounds comes from the cochlear apex shows that ANOW provides a valid objective tool to assess neuronal function to low-frequency sounds. Identifying the origin of ANOW is a necessary foundation for realizing the many possible applications of ANOW, several of which are described in Lichtenhan et al. (2013).
CRave,mid (ANOW) from low- and moderate-level, low-frequency sounds provides a metric to quantify neural thresholds in the cochlear apex, but the same measure from high-level sounds (70 dB SPL or greater) does not. CRave,mid (pANOW) from high-level sounds appears to originate from both phase-locked neural firing and from hair cell current distortion (i.e., asymmetry) that cannot be separated by signal processing. As sound level is increased, the contribution of distortion in hair cell currents to CRave,mid (pANOW) increases. CRave,mid (pANOW) response phase is consistent with this interpretation. CRave,mid phase from low-level sounds appears to originate from one origin, and CRave,mid (pANOW) phase from high levels appears to originate from a different origin. The origins of CRave,mid (pANOW) to low and high sound levels can be determined when one origin is removed, as was done with TTX in our invasive experiments. TTX removes the neural origin and abolished the CRave,mid (pANOW) response to 50 dB SPL sounds which means that these responses originated from neural sources. Because of their similar phases, we identify the CRave,mid (pANOW) response to 70 dB SPL sounds as being dominated by the same source. Similarly, since the CRave,mid (pANOW) response to 90 dB SPL sounds is not abolished, this response must have a substantial nonneural component.
The 70-dB limit of valid ANOW measurements applies to guinea pigs. To determine the highest level at which ANOW measurements can be made in other species (e.g., humans), a useful contribution would be development of a noninvasive technique that quantifies the extent to which hair cell current and phase-locked neural firing contribute to high-level CRave,mid (pANOW) oscillations at twice the probe frequency. Forward masking may be helpful in doing this by driving postsynaptic neural responses into short-term adaptation (e.g., Harris and Dallos 1979) and thereby disabling the contribution of neural responses to the gross measurements.
One hypothesis is that CRave,mid (pANOW) from high-level (70 dB SPL or greater), low-frequency sounds originates from the excitation of low-frequency tails of cochlear neurons tuned to high characteristic frequencies. For this hypothesis to be supported, CRave,mid (pANOW) amplitude from high-level sounds should have been reduced at times greater than 20 min when TTX reached the base and blocked action potentials from high characteristic frequency auditory nerve fibers, i.e., at the same time that TTX produced shifts of CRave,onset,H (pCAP) thresholds to high-frequency sounds and declines in CRave,onset,H (pCAP) amplitudes to 8 kHz tones. We saw no clear evidence indicating that any CRave,mid (pANOW) originated from excitation of the low-frequency, tuning curve tails of high characteristic frequency auditory nerve fibers. However, we cannot rule out that such excitation occurred because a small contribution of this kind could have been masked by the distortion of hair cell currents.
On the Frequency Place Specificity of the TTX Injection Technique
The rapid time course of the effects of apical TTX injections on CRave,mid (pANOW) provided compelling evidence for the apical origins of ANOW. The work reported here was designed to determine if ANOW does, or does not, originate in the cochlear apex versus from the cochlear base. The experimental approach was not optimized to separate the time course of effects on ANOW evoked from nearby frequencies. It was expected that TTX effects would occur in a strict low-to-high frequency sequence, but that was not always the case. In addition, the time separation between abolishing responses from frequencies an octave apart was less at low frequencies than for frequencies 2 kHz and above. Some possible reasons for these phenomena are as follows:
Guinea pig cochlear frequency place maps (Greenwood 1990; Tsuji and Liberman 1997) show a distance of <2 mm for the octave span of 353 to 707 Hz. The small cross-sectional area of apical scala tympani predicts that <2 min time would be needed for solutions to flow a 2-mm distance. Our injection protocols were designed for a relatively slow 2-min data collection interval to differentiate between apical and basal ANOW origins, not between ANOW origins from closely spaced frequencies within the apex.
The auditory nerve is exposed to apical perilymph in the general apical region where our injections are made (Tinling and Chole 1994). The bone separating cochlear fluids from the modiolous is porous, and thus, ANOW could have been reduced by flow directly into the spiral ganglion which may have affected fibers out of sequence.
The anatomy of the helicotrema is complex and is not as clearly defined as in the tube-like boundaries of scala tympani in the rest of the cochlea (see Fig. 1 of Salt et al. 2009). Thus, when injections commenced, drug levels may have increased in a more complex spatial pattern at the apex before progressing systematically toward the cochlear base.
Another finding to be explained is that in the data of Figure 6, TTX affected responses from low-level sounds before those from higher level sounds. An explanation might be that tuning curves at low frequencies are very wide and relatively symmetric. The higher the sound level, the more distant the characteristic frequency of the auditory nerve fibers that are excited. Responses from characteristic frequencies more apical than the tone frequency would be abolished by TTX before responses from characteristic frequencies near the tone frequency, and responses from characteristic frequencies more basal than the tone frequency would be abolished later. So at higher sound levels, TTX-induced response reductions might start earlier, but because the excited region goes the furthest basal for high sound levels, TTX will abolish the responses to high sound levels last. Another possible factor is related to the synchrony of the neural responses elicited at different sound levels and how synchrony is affected by TTX. The inner hair cell (IHC)-to-auditory nerve synapse is unusual in that more intense stimuli (i.e., bigger inner hair cell depolarizations) increase the rate of auditory nerve EPSPs, but produce little change in the wide EPSP amplitude distribution (Goutman and Glowatzki 2007). Higher EPSP rates produce more synchrony in the resulting auditory nerve firing (Buran et al. 2010) which will increase the amplitudes of both CRave,mid (pANOW) and CRave,onset,L (pCAP) responses. TTX, at concentrations that block some sodium channels but not enough to prevent action potentials, will reduce the sodium current and decrease the synchrony in the resulting action potentials (Santos-Sacchi 1993). Thus, the decreased synchrony from low levels of TTX, plus the decreased synchrony from low-level sounds, may reduce both CRave,mid (pANOW) and CRave,onset,L (pCAP) to be below threshold for responses from low-level sounds before it reduces the responses from high-level sounds.
Previous Reports on the Cochlear Response from the Round Window
Effects of TTX on CR measured from near the round window have been reported previously. Henry (1995) found that applying TTX to the gerbil round window resulted in a smoother CRave. He et al. (2012) also applied TTX to the gerbil round window and found a reduction in the amplitude of CR recorded from the round window. These findings are consistent with abolition of phase-locked neural firing from contributing to the CRs. Our results extend these previous reports by using measurement of systematic changes as TTX flowed from apex to base to identify the cochlear apex as the origin of the neural responses to low-level, low-frequency sounds. The work presented here provides the first quantification of the neural contribution to the round window CR and shows that neural responses can contribute upwards of 50 % of such recordings. Additionally, our results extend these previous reports by demonstrating that TTX does not change the endocochlear potential or DPOAEs.
Basic and Clinical Implications of Our Findings
A neural response that contributes more than 50 % to the CR at the round window has interesting implications that may influence basic science work, as some reports have assumed that the round window a.c. potential from low-frequency tones originates from outer hair cell currents in the cochlear base, i.e., CM (e.g., Patuzzi et al. 1989). While Patuzzi et al. (1989) used high-level tones to mitigate neural responses, our results demonstrate that in guinea pigs, the CR recorded from low- to moderate-level tones at the round window does not necessarily, or even mostly, originate from hair cell currents. Cheatham et al. (2011) assumed that their round window recordings from low-frequency sound were only from CM, but these recordings were from the mouse, a species in which the lowest single auditory nerve fiber characteristic frequency is ∼3 kHz and there are no neural responses at frequencies below 1 kHz (Taberner and Liberman 2005). Thus, the Cheatham et al. interpretation is correct for the mouse, but it should not be assumed valid for species with low-frequency hearing such as guinea pigs or humans. An alternative approach to remove the contribution of phase-locked neural firing when CM measurements are desired is to record from the round window using infrasonic frequencies that do not evoke neural excitation (Salt and Lichtenhan 2011; Lichtenhan and Salt 2013). Other alternatives are to record the CM with differential electrodes or from inside the scala media where hair cell contributions are substantially larger than, or perhaps electrically isolated from, neural components (Fig. 8).
A neural response contributing more than 50 % to a gross measure has interesting implications that may influence clinical work, as thought-to-be CM to low-frequency tones can potentially be used for diagnostic purposes, for example (a) diagnosing abnormal endolymph volume states (e.g., Sirjani et al. 2004), (b) understanding cochlear homeostasis (e.g., Patuzzi 2011), (c) diagnosing auditory neuropathy (e.g., Starr et al. 1996) and auditory synaptopathy (e.g., Moser et al. 2013), (d) when used in conjunction with derived-band masking protocols to identify the source and location of functional cells that remain in ears with hearing loss, (e) understanding activation of the efferent system (reviewed by Guinan 1996a, b, 2010) which may have diagnostic for learning disabilities (reviewed by Guinan 2010), (f) understanding social and public health problems reported by those living near wind turbines (Salt et al. 2013), and (g) other purposes reviewed by Lichtenhan et al. (2011)4. Our findings may influence the interpretation of measurements used for the purposes described here.
Terminology Issues
Our findings demonstrate that, under specific conditions, the CRave,mid (pANOW) and CRave,onset,H (pCAP) can include nonneural action potential components (CM and EPSP, respectively) and that CRdif,mid (pCM) may include a non-hair cell component (phase-locked neural firing). Conditions when terminology that attributes such potentials to a single source is, and is not, appropriate are summarized below:
With high-level stimulation (≥70 dB SPL), cochlear transducers saturate and can have operating points that result in an asymmetric hair cell current-originated CM waveform with a prominent 2f1 component. In such conditions, averaging responses to high-level tones of opposite polarities does not cancel the asymmetric 2f1 component and it remains in the CRave waveform (Figs. 9 and 10). Under these conditions, CRave may contain some ANOW, but it also contains a prominent nonneural component, and therefore should not be termed ANOW. The term ANOW is appropriate for measurements made to low- and medium-level (<70 dB SPL), low-frequency sounds in time periods away from CRave,onset (pCAP).
The a.c. waveform at the stimulus frequency seen in CRdif recorded at the round window from low-level, low-frequency sounds can contain a significant neural component (Fig. 8 and Dallos 1973, p. 33, Fig. 2.6) and should not be termed a CM. Since CRdif contains a large neural component, the tone responses from which CRdif was calculated must also have large neural components. Thus, CR measurements to single, low-frequency tones should not be termed CM.
Algorithms for determining CAP amplitude can give misleading results in ears treated with TTX or other neural blockers, as shown by the 8-kHz waveforms of Figure 12. After blocking by TTX, the origin of the negative peak in Figure 12, bottom, is likely to be EPSPs, or perhaps hair cell currents related to vesicle release, but is certainly not from neural action potentials. Under such conditions, CRave,onset,H and CRave,onset,L (pCAPs) do not originate from action potentials and should not be termed CAPs. In such cases, a measurement procedure-based terminology like CRave,onset,L is a more appropriate terminology than CAP. Be that as it may, perhaps a bigger issue than terminology in these cases is the possible miss-identification of neural threshold. For the data of Figure 12, most CAP-detecting algorithms (including the one we used in making Fig. 12A) would consider the response at the bottom of Figure 12B to be a CAP, although actually, the response is not CAP but something else.
Decreased Response Latency in Damaged Ears
Decreased CAP and auditory brainstem response latencies have been reported in ears with permanent sensorineural hearing loss (e.g., Eggermont 1979, Strelcyk et al. 2009). Decreased CAP latency has also been reported in human ears with temporary noise-induced hearing loss (Lichtenhan and Chertoff 2008). One explanation for decreased latencies is that hearing loss leads to broadening of cochlear filters which leads to shorter group delays. Another is that as level is increased, the EPSP rate is increased which increases the probability that a short-latency EPSP will be the first to excite a fiber (Buran et al. 2010). While these compelling hypotheses may indeed be causes of decreased response latencies in some forms of sensorineural hearing loss, our finding of decreased CRave,onset,H (pCAP) latency (Fig. 12) that results from putative EPSPs (Dolan et al. 1989; Henry and Price 1994) gives another possible reason for finding decreased CRave,onset,H (pCAP) latencies in patients with hearing loss.
Interestingly, full abolition of CRave,mid (pANOW) amplitudes during apical TTX injections indicates that such hypothesized EPSPs were not measured from the round window in response to low-frequency sounds (Figs. 4, 5, 6, and 7). This does not mean that low-characteristic frequency neurons do not exhibit phase-locked EPSPs, indeed they do (e.g., Goutman 2012). Rather, the abolition of low-frequency CRave,mid (pANOW) suggests that apical sensory cells do not generate EPSPs that are large enough or have current flow that is in the proper direction to be measured by a round window electrode.
CONCLUSIONS
TTX injected into the cochlear apex systematically blocked neural action potentials as solution flowed from the apex to the base. ANOWs from low-frequency tones were reduced well before CAP responses from 2 to 8 kHz tones were reduced, confirming that ANOW is a neural response that originates near the cochlear apex. Endocochlear potentials, CM when recorded in scala media, and DPOAEs were unchanged by TTX which indicates that the effects of TTX in the cochlea are specific to blocking neural action potentials.
Acknowledgments
We thank Doctors Ann E. Hickox, Mark A. Rutherford, Christopher A. Shera, and Mrs. Uzma S. Wilson for helpful discussion on this research. Two anonymous reviewers and Associate Editor Dr. Nigel Cooper provided productive criticisms that improved this report. This work was supported by grants R03 DC012844 (J.T.L.), R01 DC000235 (J.J.G.), and R01 DC001368 (A.N.S.) from the National Institutes of Health, National Institute on Deafness and Other Communication Disorders.
Footnotes
Mean ± standard error of the mean
CRdif,mid (pCM) measurements in scala media are not reduced by TTX, consistent with their being of hair-cell current origin (i.e., they are CM, see Fig. 8, middle column).
TTX has been classically used to abolish spike activity and leaves EPSPs unaffected (e.g., see Hille 2001 for review).
It remains to be seen to what extent noise-evoked CM (e.g., Chertoff et al. 2003) is influenced by phase-locked neural firing from the cochlear apex.
References
- Buran BN, Strenzke N, Neef A, Gundelfinger ED, Moser T, Liberman MC. Onset coding is degraded in auditory nerve fibers from mutant mice lacking synaptic ribbons. J Neurosci. 2010;30:7587–7597. doi: 10.1523/JNEUROSCI.0389-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham MA, Naik K, Dallos P. Using the cochlear microphonic as a tool to evaluate cochlear function in mouse models of hearing. J Assoc Res Otolaryngol. 2011;12(1):113–125. doi: 10.1007/s10162-010-0240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Kujawa SG, McKenna MJ, Fiering JO, Mescher MJ, Borenstein JT, Swan EE, Sewell WF. Inner ear drug delivery via a reciprocating perfusion system in the guinea pig. J Control Release. 2005;110(1):1–19. doi: 10.1016/j.jconrel.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertoff ME, Yi X, Lichtenhan JT. Influence of hearing sensitivity on mechano-electric transduction. J Acoust Soc Am. 2003;114(6):3251–3263. doi: 10.1121/1.1625932. [DOI] [PubMed] [Google Scholar]
- Cooper NP, Rhode WS. Fast travelling waves, slow travelling waves and their interactions in experimental studies of apical cochlear mechanics. Audit Neurosci. 1996;2:289–299. [Google Scholar]
- Dallos P. The auditory periphery. Biophysics and physiology. New York: Academic; 1973. [Google Scholar]
- Dolan DF, Xi L, Nuttall AL. Characterization of an EPSP-like potential recorded remotely from the round window. J Acoust Soc Am. 1989;86(6):2167–2171. doi: 10.1121/1.398477. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Narrow-band AP latencies in normal and recruiting human ears. J Acoust Soc Am. 1979;65(2):463–470. doi: 10.1121/1.382345. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Kaminski JR, Beauchaine KA, Jestead W. Auditory brainstem response to tone bursts in normally hearing subjects. J Speech Hear Res. 1988;30:87–97. doi: 10.1044/jshr.3101.87. [DOI] [PubMed] [Google Scholar]
- Goutman JD. Transmitter release from cochlear hair cells is phase locked to cyclic stimuli of different intensities and frequencies. J Neurosci. 2012;32(47):17025–17036. doi: 10.1523/JNEUROSCI.0457-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman JD, Glowatzki E. Time course and calcium dependence of transmitter release at a single ribbon synapse. Proc Natl Acad Sci U S A. 2007;104:16341–16346. doi: 10.1073/pnas.0705756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DD. A cochlear frequency-position function for several species—29 years later. J Acoust Soc Am. 1990;87(6):2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr . The physiology of olivocochlear efferents. In: Dallos PJ, Popper AN, Fay RR, editors. The cochlea. New York: Springer; 1996. pp. 435–502. [Google Scholar]
- Guinan JJ., Jr . Physiology of the medial and lateral olivocochlear systems. In: Ryugo D, Fay RR, Popper AN, editors. Auditory and vestibular efferents. New York: Springer; 1996. pp. 39–82. [Google Scholar]
- Guinan JJ., Jr Cochlear efferent innervation and function. Curr Opin Otolaryngol Head Neck Surg. 2010;18(5):447–453. doi: 10.1097/MOO.0b013e32833e05d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DM, Dallos P (1979) Forward masking of auditory nerve fiver responses. J Neurophysiol 42(4):1083–1107 [DOI] [PubMed]
- Harrison RV, Evans EF. The effects of hair cell loss (restricted to outer hair cells) on the threshold and tuning properties of cochlear fibres in the guinea pig. In: Portmann M, Aran JM, editors. Inner ear biology. Paris: INSERM; 1977. pp. 105–124. [Google Scholar]
- He W, Porsov E, Kemp D, Nuttall AL, Ren T (2012) The group delay and suppression pattern of the cochlear microphonic potential recorded at the round window. PLos One 7(3):e34356 [DOI] [PMC free article] [PubMed]
- Henry KR. Auditory nerve neurophonic recorded from the round window of the Mongolian gerbil. Hear Res. 1995;90(1–2):176–184. doi: 10.1016/0378-5955(95)00162-6. [DOI] [PubMed] [Google Scholar]
- Henry KR, Price JM. Amplitude enhancement is seen in the cochlear nerve but not at, or before, the afferent synapse. Hear Res. 1994;79(1–2):190–196. doi: 10.1016/0378-5955(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. 3. Massachusetts: Sinauer Associates, Inc; 2001. [Google Scholar]
- Hudspeth AJ, Corey DP (1977) Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A 74(6):2407–2411 [DOI] [PMC free article] [PubMed]
- Lichtenhan JT. Effects of low-frequency biasing on otoacoustic and neural measures suggest that stimulus-frequency otoacoustic emissions originate near the peak region of the traveling wave. J Assoc Res Otolaryngol. 2012;13:17–28. doi: 10.1007/s10162-011-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenhan JT, Chertoff ME (2008) Temporary hearing loss influences post-stimulus time histogram and single neuron action potential estimates from human compound action potentials. J Acoust Soc Am 123(4):2200–2212 [DOI] [PMC free article] [PubMed]
- Lichtenhan JT, Salt AN (2013) Amplitude modulation of audible sounds by non-audible sounds: understanding the effects of wind turbine noise. International Congress on Acoustics, hosted by the Acoustical Society of America and the Canadian Acoustical Association. Montréal, Canada. ASA Proceedings of Meetings
- Lichtenhan JT, Brown DJ, McLean WJ, Chertoff ME, Salt AN (2011) A source of cochlear distortion and its utility for differential diagnosis of sensorineural hearing loss. Audiology Today. Nov/Dec
- Lichtenhan JT, Cooper NP, Guinan JJ., Jr A new auditory threshold estimation technique for low frequencies: proof of concept. Ear Hear. 2013;34(1):42–51. doi: 10.1097/AUD.0b013e31825f9bd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Predoehl F, Starr A. Review of hair cell synapse defects in sensorineural hearing impairment. Otol Neurotol. 2013;34(6):995–1004. doi: 10.1097/MAO.0b013e3182814d4a. [DOI] [PubMed] [Google Scholar]
- Patuzzi R. Ion flow in stria vascularis and the production and regulation of cochlear endolymph and the endolymphatic potential. Hear Res. 2011;277(1–2):4–19. doi: 10.1016/j.heares.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Patuzzi RB, Yates GK, Johnstone BM. The origin of the low-frequency microphonic in the first cochlear turn of guinea-pig. Hear Res. 1989;39(1–2):177–188. doi: 10.1016/0378-5955(89)90089-0. [DOI] [PubMed] [Google Scholar]
- Salt AN, Lichtenhan JT (2011) Responses of the inner ear to infrasound. Proceedings of the Fourth International Meeting on Wind Turbine Noise. Rome, Italy. April 12–14
- Salt AN, Stopp PE. The effect of cerebrospinal fluid pressure on perilymphatic flow in the opened cochlea. Acta Otolaryngol. 1979;88:198–202. doi: 10.3109/00016487909137160. [DOI] [PubMed] [Google Scholar]
- Salt AN, Hale SA, Plonkte SK. Perilymph sampling from the cochlear apex: a reliable method to obtain higher purity perilymph samples from scala tympani. J Neurosci Methods. 2006;153(1):121–129. doi: 10.1016/j.jneumeth.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Brown DJ, Hartsock JJ, Plontke SK. Displacements of the organ of Corti by gel injections into the cochlear apex. Hear Res. 2009;250(1–2):63–75. doi: 10.1016/j.heares.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, King EB, Hartsock JJ, Gill RM, O’Leary SJ. Marker entry into vestibular perilymph via the stapes following applications to the round window niche of guinea pigs. Hear Res. 2012;283(1–2):14–23. doi: 10.1016/j.heares.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Lichtenhan JT, Gill RM, Hartsock JJ. Large endolymphatic potentials from low-frequency and infrasonic tones in the guinea pig. J Acoust Soc Am. 2013;133(3):1561–1571. doi: 10.1121/1.4789005. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Voltage-dependent ionic conductances of type I spiral ganglion cells from the guinea pig inner ear. J Neurosci. 1993;13:3599–3611. doi: 10.1523/JNEUROSCI.13-08-03599.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirjani DB, Salt AN, Gill RM, Hale SA. The influence of transducer operating point on distortion generation in the cochlea. J Acoust Soc Am. 2004;115(3):1219–1229. doi: 10.1121/1.1647479. [DOI] [PubMed] [Google Scholar]
- Spoor A, Eggermont JJ. Electrocochleography as a method for objective audiogram determination. In: Hirsch SK, Eldredge DH, Hirsch IJ, Silverman SR, editors. Hearing and Davis: essays honoring Halowell Davis. Saint Louis, MO: Washington University Press; 1976. pp. 411–418. [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119:741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Strelcyk O, Christoforidis D, Dau T. Relation between derived-band auditory brainstem response latencies and behavioral frequency selectivity. J Acoust Soc Am. 2009;126(4):1878–1888. doi: 10.1121/1.3203310. [DOI] [PubMed] [Google Scholar]
- Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol. 2005;93:557–569. doi: 10.1152/jn.00574.2004. [DOI] [PubMed] [Google Scholar]
- Tinling SP, Chole RA. Apical cochlear nerve exposed to perilymph in the gerbil and rat. Hear Res. 1994;73(2):203–208. doi: 10.1016/0378-5955(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Tsuji J, Liberman MC. Intracellular labeling of auditory nerve fibers in guinea pig: central and peripheral projections. J Comp Neurol. 1997;381(2):188–202. doi: 10.1002/(SICI)1096-9861(19970505)381:2<188::AID-CNE6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]