Abstract
Endolymphatic hydrops (ELH) is a disorder of the inner ear that causes tinnitus, vertigo, and hearing loss. An elevated ratio of the summating potential (SP) to the action potential (AP) measured by electrocochleography has long been considered to be the electrophysiological correlate of ELH-related clinical conditions, such as Meniere’s disease, but in vivo confirmation and correlation between an elevated SP/AP ratio and ELH has not yet been possible. Confirming this relationship will be important to show that elevated SP/AP ratio is indeed diagnostic of ELH. Here, we sought to confirm that an elevated SP/AP ratio is associated with ELH and test the hypothesis that severity of ELH and hearing loss would also correlate with the SP/AP ratio in vivo using the PhexHyp-Duk/Y mouse model of postnatal ELH. In addition, we describe a minimally invasive approach for electrocochleography in mice. Auditory brainstem responses and electrocochleography data were collected from controls and PhexHyp-Duk/Y mutants at postnatal day 21 and the mice (all male) were euthanized immediately for cochlear histology. Our results show that (1) the SP/AP ratio was significantly elevated in mice with histological ELH compared to controls, (2) the SP/AP ratio was not correlated with the severity of histological ELH or hearing loss, and (3) the severity of hearing loss correlated with the severity of histological ELH. Our study demonstrates that an elevated SP/AP ratio is diagnostic of ELH and that the severity of hearing loss is a better predictor of the severity of ELH than is the SP/AP ratio.
Keywords: Phex mouse, electrocochleography, endolymphatic hydrops
INTRODUCTION
Endolymphatic hydrops (ELH) is a disorder of the inner ear that causes tinnitus, vertigo, and hearing loss. It is caused by a dysregulation of the amount of endolymph in the cochlea, leading the Reissner’s membrane (RM) to bow toward the scala tympani (Fig. 1). Electrocochleography (ECochG) is one of the most commonly used electrophysiological measures in the clinical diagnosis of ELH-related clinical conditions, such as Meniere’s disease (MD) (Ge and Shea 2002; Semaan and Megerian 2011). ELH is considered a pathologic correlate of MD, but ELH can also occur secondarily to injury and other diseases (Arenberg et al. 1970; Belal and Ylikoski 1980; Fraysse et al. 1980; Paparella 1984; Schuknecht 1976). ECochG is a recording of the evoked potential signals that mostly originate from the basal turn of the cochlea (Durrant et al. 1998). When recorded using surface electrodes, those evoked potentials generate a waveform with two components: a summating potential (SP) and an action potential (AP) (Ferraro 2010). The SP component reflects the hair cell response to sound stimuli; the AP component is a recording of the electric potential generated by the collective firing of the auditory nerve fibers.
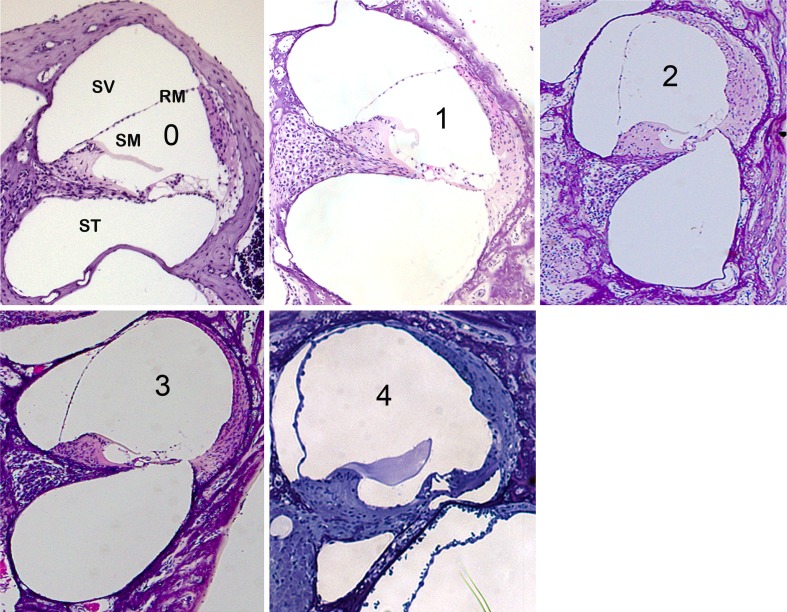
FIG. 1.
Grading system used to analyze Phex H-D /Y mice after ECochG. Cochleae of P21 mice stained with hematoxylin and eosin, illustrating the range of severity of ELH possible at this stage. The magnification is ×10 for all panels. Here, grades 1–3, but not grade 4, were observed. Grade 4 is rare, but possible at P21; to illustrate this, a sample of grade 4 ELH obtained from other experiments (unpublished observations) is included here. ST scala tympani, SM scala media, SV scala vestibuli, RM Reissners’ membrane.
Schmidt et al. (1974) first described the use of ECochG in measuring the SP in individuals diagnosed with MD; they detected an increased SP amplitude and presumed that this was in part related to ELH. An elevated SP/AP ratio has since been considered essential to the diagnosis of ELH and is currently used clinically in the evaluation of patients with suspected ELH. Although a correlation between an elevated SP/AP ratio and ELH is supported by clinical applications and the medical literature (Dauman et al. 1986; Ferraro et al. 1985; Gibson et al. 1977; Goin et al. 1982; Kumagami et al. 1982; Mori et al. 1987), confirmation of this in vivo has not been possible. Several diagnostic modalities have been used to indirectly support the presence and/or downstream sequelae of ELH in humans; for example, ECochG and cervical and ocular vestibular-evoked myogenic potentials (Sheykholeslami et al. 2009) have been widely used in clinical settings. However, the correlation between ECochG findings and the histological presence and/or severity of ELH has not yet been assessed in vivo in humans with ELH-related conditions. In general, there have been few human studies of ELH-related clinical conditions such as MD due to the limited availability of patients for clinical studies compounded by the lack of noninvasive techniques to study cochlear pathology. With the current advances in magnetic resonance imaging and high-resolution imaging of the cochlea, it will become possible to conduct such studies on humans in the future (Gürkov et al. 2011; Pyykkö et al. 2013; Yoshida et al. 2013). Until then, we have to rely on animal models that mirror the development of ELH and inner ear dysfunction associated with MD.
Previous studies looking at the association between ELH and the SP/AP thus far have used animal models in which ELH is induced by surgical obliteration of the endolymphatic duct (van Deelen et al. 1987; van Deelen and Smoorenburg 1986; Ferraro et al. 1985; Horner 1993; Bixenstine et al. 2008; Hott et al. 2003; Megerian 2005; Horner and Cazals 1988), a mechanism that deviates from the spontaneous development of ELH that usually occurs in MD. Furthermore, in previous animal studies (van Deelen et al. 1987; Horner 1993), recordings were conducted over time, precluding real-time analysis of the SP/AP ratio’s relationship with histologic ELH, and none of these previous studies attempted to correlate the SP/AP ratio to ELH severity. Our report is significant because we address these issues using a suitable animal model. Here, we use as a model a spontaneous mouse mutant that develops ELH and inner ear dysfunction postnatally without occlusion of the endolymphatic duct: male PhexH-D/Y mice. These carry the PhexHyp-Duk allele, a mutant allele of the X-linked phosphate-regulating gene Phex (Lorenz-Depiereux et al. 2004) that contains an intragenic deletion in the Phex gene resulting in loss of the functional Phex protein. Around weaning age (postnatal days (P)21–25), the PhexH-D/Y mice typically develop ELH associated with hearing loss (Megerian et al. 2008). Hearing in PhexH-D/Y mice displays higher thresholds at low frequencies (2, 4, and 8 kHz) compared to thresholds at higher frequencies (16 and 32 kHz) at ages P21–P40. Some of the mutants exhibited fluctuation in their auditory brainstem response (ABR) thresholds across frequencies (Megerian et al. 2008). Although the causal mechanism underlying the inner ear phenotype in PhexH-D/Y mice remains to be elucidated, here, we exploit this phenotype at P21 to shed light on the relationship between the SP/AP ratio, histologic ELH, and hearing loss.
METHODS
Mice
The PhexH-D allele arose from a spontaneous mutation on the BALB/cAnBomUrd (BALB/cUrd) background (Lorenz-Depiereux et al. 2004). Male mice carrying the PhexH-D allele in the BALB/cUrd background were obtained by breeding BALB/cUrd +/PhexH-D carrier females with BALB/cUrd wild-type (+/Y) mice. “Control” mice used in this study refers to mice carrying the +/Y genotype. Compared to carrier females, hemizygous males carrying the mutant allele (PhexH-D/Y) show an inner ear phenotype more consistently at weaning age (P21–25; Megerian et al. 2008). Mutant male mice were identified among unaffected littermates using the genotyping protocol previously described (Megerian et al. 2008). Unaffected male (wild-type (+/Y)) mice were used as controls.
Each ear was analyzed separately because each of the mutant’s ears has been shown to develop hearing loss independently (Megerian et al. 2008). ECochG and ABR were recorded at P21; mice were immediately killed for histological analysis of their cochleae.
The Animal Care and Use Committee of CWRU approved the care and use of the mice in this study under protocol number 2011-0166.
Electrophysiological Testing
ABR recording was conducted as previously described (Melki et al. 2010). Briefly, mice aged P21 were anesthetized using an intraperitoneal injection of ketamine, xylazine, and acepromazine at doses of 40, 5, and 1 mg/kg, respectively. Testing was carried out in a soundproof chamber and body temperature was maintained at 37–38 °C by placing the mice on a homoeothermic heating pad (Harvard Apparatus, Holliston, MA).
ABR and ECochG recordings were carried out on each ear separately using a BioSig program (Tucker Davis Technology). Platinum subdermal needle electrodes were inserted as follows. The recording electrode was inserted percutaneously along the zygomatic bone to the tympanic bulla without penetrating the middle ear to avoid injuring the ossicular chain. The reference electrode was placed on the contralateral mandible and the ground electrode was placed on the vertex. With this approach, the electrode is inserted as far as possible so it is several millimeters away from the round window. We refer to this approach as “minimally invasive electrocochleography in mice,” or MIEM. The main difference between a standard ABR recording and MIEM is the placing of the electrodes as close as possible to the round window.
The ABR thresholds were obtained for each animal by reducing the stimulus intensity from a 120-dB peak equivalent sound pressure level (dB peSPL) in 10-dB steps until the lowest intensity that evoked a reproducible ABR pattern was reached. For ECochG, the stimulus used was a 0.1-ms click at 120 dB peSPL, with alternating polarity so that the cochlear microphonic potential was canceled, revealing the SP (Ferraro and City 2000). The PhexH-D/Y mice were tested at P21, an age that coincides with the maturity of cochlear function in wild-type mice and a time at which ELH has been observed in PhexH-D/Y mutants (Megerian et al. 2008). A high-pass filter of 100 Hz and low-pass filter of 3,000 Hz were used. For both ABR and ECochG, a gain factor of 20 was used on the amplifier.
Histology
After the ECochG recording and while anesthetized, each mouse was euthanized. The cochlea was dissected, placed in 4 % paraformaldehyde for 24 h, placed in EDTA for 144 h at room temperature, and then embedded in paraffin. The paraffin blocks were cut into 5-μm sections and stained with hematoxylin and eosin. The slides were analyzed using a Leica microscope for ELH grading. A grading scale from 0 to 4 was used (Fig. 1): grade 0, no ELH, RM in the wild-type position; grade 1, slight distention of the RM; grade 2, significant distention of the RM, with a portion of the RM in contact with the lining of the scala vestibuli situated immediately proximal to the stria vascularis proper; grade 3, significant distention of the RM, with more than half of the RM in contact with the luminal surface (“roof”) of the scala vestibuli; grade 4, ELH occupies more than 75 % of the scala vestibuli. This system is a modification of the grading system of Hott et al. (2003).
Data Analysis
The numerical values were collected, entered into Excel (Microsoft, Redmond, WA), and imported into GraphPad Prism (GraphPad, San Diego, CA) for analysis. Both parametric and non-parametric statistics were used as appropriate. The ABR threshold for each mouse was determined by reducing the stimulus intensity from 120 dB peSPL in 10-dB steps until the lowest intensity that evoked a reproducible ABR pattern was reached. Hearing loss was determined from the ABR thresholds. Because the thresholds are categorical (10-dB steps), we chose a non-parametric test, the Mann–Whitney, to be conservative in our statistical analysis of the hearing loss in the PhexH-D/Y compared to the controls. To compare the mean SP/AP ratio between the PhexH-D/Y and the controls, we performed statistical analysis using the independent samples one-tailed t test assuming unequal variances. The Spearman rank test (simple linear regression) was used to assess the correlation between SP/AP ratio, ELH grade, and hearing threshold in the mutants and controls. The criterion for statistical significance was set at P ≤ 0.05, one-tailed.
A receiver operating characteristics (ROC) curve was constructed from the data to evaluate the validity of ECochG for correct diagnosis of ELH (Bewick et al. 2004). A ROC curve is a plot of the true positive rate versus the false positive rate, also known as “sensitivity versus 1 − specificity.” If the resulting area under the curve is greater than 0.5 and the difference is statistically significant (P < 0.05), the test correctly diagnoses a disease in more than 50 % of patients. The P value is calculated using a Z test (Liu and Li 2005). A perfect test has an area of 1, meaning 100 % sensitivity and specificity (Bewick et al. 2004).
RESULTS
ELH at P21 in PhexH-D/Y Mice
The cochlea and auditory function in mice mature around P21. ELH was first observed in PhexH-D/Y mice around P21, and loss of hearing is profound in most PhexH-D/Y mice around P30 (Megerian et al. 2008). However, it is not known whether these mutants show significant variation in the degree (or grade) of ELH at a single time point or whether they have more stable/predictable degrees of distention of the RM. We focused on mice at P21 in this study. Images from the mid-basal turn of the cochlea of PhexH-D/Y mice at P21 showed that the position of the RM varied from a near-normal position to severe distention. This showed (for the first time, to our knowledge) that the severity of ELH in PhexH-D/Y mice varies from one ear to another at a single time point, whether you compare between the two ears of one mouse or between mice. On the basis of these results, we used a grading system (modified from Hott et al. 2003) from 0 to 3, with grade 0 being no ELH and grade 3 being the significant distention of the RM observed in the cohort of mutants studied in this report (Fig. 1). In other studies, we have occasionally noticed extreme distention of the RM in PhexH-D/Y mice at P21 (our unpublished observations). This extreme distention was assigned grade 4; a sample is shown for reference in Figure 1. The results demonstrate that distention of the RM and, hence, the severity of ELH vary between mutants at P21. Therefore, the variability of ELH in the PhexH-D/Y mouse model at one time point (P21) provided a good platform to study the correlation between the severity of ELH, hearing loss, and the SP/AP ratio.
Recording SP and AP Signals in Mice Using a Minimally Invasive Approach
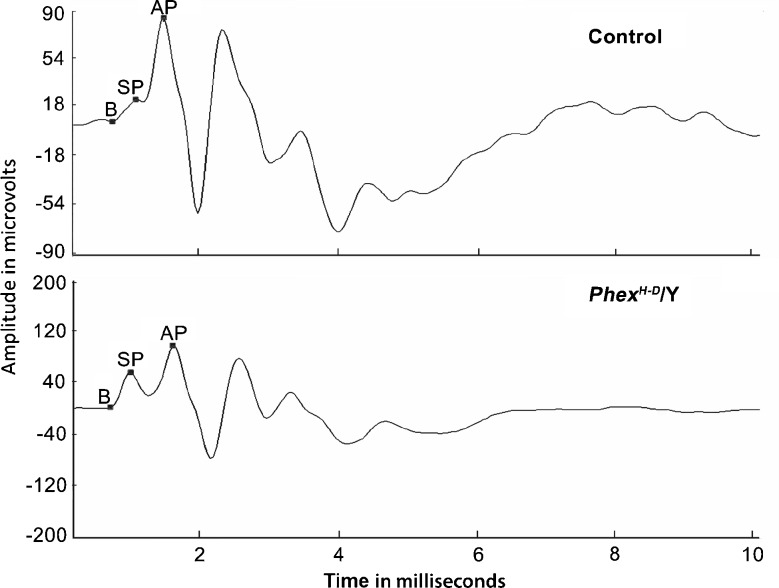
ECochG is the method of choice for determining the SP/AP ratio. A small number of control and PhexH-D/Y mice were tested at P21 to determine whether MIEM would work. The results show that the signal is very clear and allows easy visualization of the SP and AP. The sample ECochG tracing obtained is shown as a snapshot from the Biosig3 software (Fig. 2). The markers used to generate the SP/AP ratio are shown. The amplitudes of the SP and AP were measured in relation to the baseline of the tracing, which was chosen to be at the foot of the SP curve.
FIG. 2.
Sample ECochG waveform from control and Phex H-D /Y mice. The baseline (B), summating potential (SP), and action potential (AP) are indicated for each genotype. In this sample, the SP/AP ratios are 0.2 and 0.5 for the wild-type and mutant mice, respectively.
ELH, Hearing Loss and SP/AP Ratio in PhexH-D/Y Mice at P21
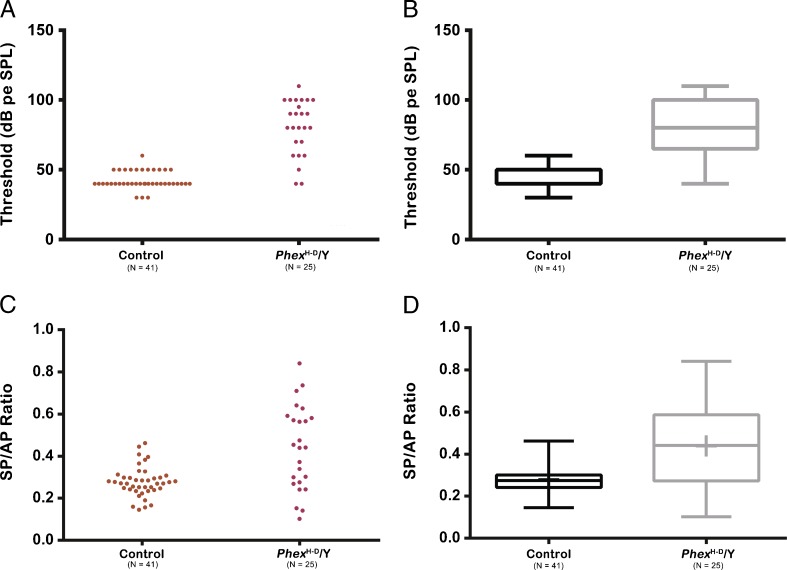
Forty-one control ears and 25 PhexH-D/Y ears were subjected to ABR and ECochG recordings at P21. We started out recording only the right ear of each mouse and then recorded from both ears from each mouse to reduce the number of animals used, hence the odd number of ears for control and PhexH-D/Y genotypes. Most of the mutants showed decreased hearing thresholds in both ears to various extents (Fig. 3A). Three PhexH-D/Y ears had normal or near-normal hearing thresholds (<50 dB threshold), and all of the other ears had thresholds of 60 dB or higher, with a mean threshold of 78.3 dB peSPL (95% confidence interval (CI) = 69.97–87.70). The control mice had a mean threshold of 43.41 dB peSPL (95% CI = 41.1–45.7). This difference is statistically significant (Mann–Whitney: P < 0.0002). More than half of the PhexH-D/Y mice showed elevated SP/AP ratios when compared to controls, and this difference was statistically significant (t(27.62) = 3.874, P = 0.0003; Fig. 3B). The mean SP/AP ratio for the PhexH-D/Y mice was 0.44 (95% CI = 0.36–0.52), and the mean SP/AP ratio for control mice was 0.28 (95% CI = 0.25–0.3). For the histological analysis, we examined 21 of the 25 PhexH-D/Y ears because four were found unsuitable for the analysis owing to fixation artifacts. All 21 PhexH-D/Y ears examined at P21 showed ELH, but the degree of ELH varied: 23.8 % were grade 1, 66.6 % were grade 2, and 9.5 % were grade 3.
FIG. 3.
Hearing threshold and SP/AP ratio of control and Phex H-D /Y (mutant) mice at P21. The figure represents the data in the form of a scatter plot (A or C) and as a box and whisker plot (B or D). Boxes represent the 25th–75th percentile, the horizontal bar within the box represents the median, and the outermost bars represent the extremes of the data. A, B Elevation of hearing thresholds in Phex H-D /Y ears compared to the control. C, D Elevation of the SP/AP ratio of the Phex H-D /Y ears compared to the control.
Correlations Among ELH, the SP/AP Ratio, and Hearing Threshold
The Spearman test was used to examine the relationships among ELH, the SP/AP ratio, and hearing threshold. No correlation was discovered between the severity of ELH and the SP/AP ratio (r = −0.042; Spearman rank-order test, P = 0.42; Fig. 4A) or between the hearing threshold and the SP/AP ratio (r = −0.22; Spearman rank-order test, P = 0.15; Fig. 4B)]. However, a correlation was found between hearing thresholds and the severity of ELH, with a higher grade corresponding to decreased hearing sensitivity (r = 0.7; Spearman rank-order test, P < 0.0002; Fig. 4C).
FIG. 4.
Relationship between ELH grade and SP/AP ratio. A There was no correlation between ELH and the SP/AP ratio. B There was no correlation between the hearing threshold and the SP/AP ratio. C There is a positive correlation between ELH and the SP/AP ratio. This figure was plotted using the same data as in A and B, but the display in this figure does not reveal all of the data points because some of them overlap.
Sensitivity and Specificity
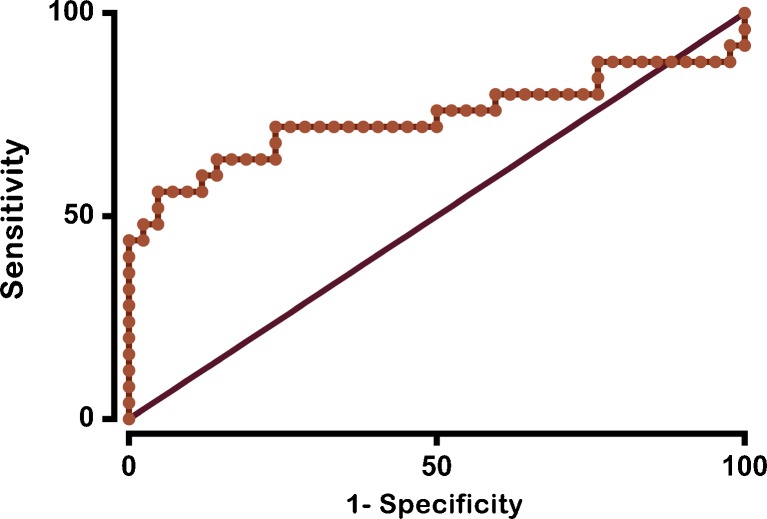
A ROC curve was constructed from the data collected (Fig. 5). The area under the curve is 0.74 (95% CI = [0.59–0.88, P = 0.001). For an SP/AP ratio of 0.4, ECochG is 58 % sensitive and 93 % specific. This also translated into a positive predictive value of 83.33 % and a negative predictive value of 78 %. The false positive rate was 16 % and the false negative rate was 42 %. When the threshold for positivity is changed to an SP/AP ratio of 0.47, specificity is 100 %, but sensitivity drops to 40 %.
FIG. 5.
ROC curve for ECochG. Sensitivity is plotted against 1 − specificity. An area under the curve that is significantly greater than 50 % indicates that a test can discriminate between two states, in this case, a normal wild-type mouse and a mutant Phex H-D /Y mouse with ELH. The data points on the curve represent the sensitivity and specificity for each SP/AP ratio.
DISCUSSION
Using a mouse mutant that develops ELH after birth, we confirm that an elevated SP/AP ratio is diagnostic of ELH. However, contrary to our hypothesis, the SP/AP ratio did not show a linear relationship with degree of ELH or hearing loss. On the other hand, the severity of hearing loss correlated with the severity of ELH. Our results provide in vivo confirmation of the long-standing assertion in the medical literature that an elevated SP/AP ratio is diagnostic of ELH and also shed new light on the relationships among SP/AP ratio, ELH, and hearing loss in vivo.
ECochG Recording in the Mouse Model of ELH
ECochG is the method of choice for determining the SP/AP ratio. ECochG recordings have been performed on various rodents, such as guinea pigs, rats, and chinchillas (Campbell et al. 1993; van Deelen et al. 1987; van Deelen and Smoorenburg 1986). The techniques used are usually invasive, involving perforation of the tympanic membrane and surgical implantation of a cochlear electrode in the round window niche (van Deelen et al. 1987). This approach damages the ear and precludes longitudinal studies. However, in one study using chinchilla, the electrode was placed on the surface of the bulla (Campbell et al. 1993) by percutaneous techniques that obviate the perforation of the tympanic membrane. We have described MIEM, a simple approach to perform ECochG in mice. In MIEM, the placement of the recording electrodes is very similar to that used to record ABRs and therefore does not violate the integrity of the tympanic membrane or the bulla. ABR and ECochG are recorded using essentially the same setup. The main difference is that we focused on placing the electrodes as close as possible to the round window without entering the external ear or violating the eardrum percutaneously. To our knowledge, this is the first time this technique has been used in mice to record ECochG in a hydropic model. The recording displayed here (Fig. 2) shows that the SP and AP signals can be visualized and quantified in mice using this minimally invasive approach. Most importantly, because this technique to record ECochG does not violate the tympanic membrane or bulla, this technique should make it possible to conduct ECochGs for longitudinal studies.
The sensitivity and specificity of the ECochG recordings in mice are consistent with those reported in the human literature for MD patients (Ge and Shea 2002). In humans, an SP/AP ratio of 0.4 or greater was associated with ELH (Ge and Shea 2002). According to the ROC curve generated from our current study, a ratio of 0.4 in mice seems to yield similar sensitivity and specificity to that reported in humans (Sass 1998). Although an SP/AP ratio ≥0.4 lacked sensitivity for ELH, it gave high specificity. This is further bolstered by strong positive and negative predictive values of 83.3 and 78 %, respectively. Despite the absence of a correlation between the severity of ELH and the SP/AP ratio, the ROC curve suggests that a higher SP/AP ratio is a more specific (but less sensitive) measurement of ELH. Therefore, we believe that our results support the utility of ECochG in human patients.
Our report is significant for four reasons. First, we describe MIEM, a minimally invasive approach to perform ECochG in mice. This methodological advance would make it easier to perform ECochG and allow for longitudinal evaluation of SP/AP in mouse models. Second, all of the previous studies looking at the association between ELH and the SP/AP thus far have used animal models in which ELH is surgically induced; we used a mouse model in which ELH is genetically induced (Megerian et al. 2008). However, consistent with other reports (Al-momani et al. 2009; Dauman et al. 1986; van Deelen et al. 1987; van Deelen and Smoorenburg 1986; Durrant et al. 1998; Ferraro 2010; Ferraro et al. 1994, 1985; Ferraro and City 2000; Ferraro and Ferguson 1989), our study confirms that ELH is associated with an increased SP/AP ratio. Second, the timing of the histological analysis immediately after recording the SP/AP ratio allows a real-time snapshot of the current state of the cochlea and bolsters the significance of the electrophysiological changes observed in our experimental model. In other animal studies using surgically induced ELH model (van Deelen et al. 1987; Horner 1993), recordings were conducted over time, precluding real-time analysis of the SP/AP ratio’s relationship with ELH. Third, none of these previous studies attempted to correlate the SP/AP ratio to ELH severity.
ELH and Elevated SP/AP Ratio
The presence of elevated SP/AP ratios is diagnostic of the presence of hydrops, but not predictive of the severity of ELH in PhexH-D/Y mice. To our knowledge, this is the first report of this relationship. Our previous study showed no significant loss of hair cells up to P90 in PhexH-D/Y mice (Melki et al. 2010). Because the present study was conducted at P21, there is no reason to believe that hair cell degeneration contributes to the phenotype. Consistent with this expectation, we did not notice any hair cell loss when grading the ELH for this report. Therefore, the abnormal SP/AP ratio in the PhexH-D/Y mice cannot be attributed to a loss or abnormal ratio of inner versus outer hair cells at P21.
There are various possible explanations for the elevation of the SP/AP ratio in ELH. The SP is a DC current produced by the hair cells in response to auditory stimuli; these cells are mostly inner hair cells (IHCs), according to Durrant et al. (1998). The AP is the result of the collective firing of auditory neurons. ELH is postulated to displace the basilar membrane toward the scala tympani and, by doing so, to change the operating potential of the outer hair cells (OHCs) to be closer to that of the IHCs and, therefore, to add their SP potential to the total SP. Because there are three times as many OHCs as IHCs, the SP is increased and the AP is either unchanged or diminished. However, the lack of correlation between ELH severity and the SP/AP ratio could be suggestive of the underlying pathology of MD at the hair cell and spiral ganglion neuron level. Hair cells show a nonlinear response to mechanical stimuli (Dallos et al. 1996). Therefore, it is possible that ELH-induced pressure displaces the OHCs from one point to another and that the amplitude of that displacement has no correlation with the subsequent electric response.
ELH and Hearing Loss
One of the big questions in MD and other conditions associated with ELH is whether the severity of ELH is correlated with hearing loss. The analysis of PhexH-D/Y mice presented here provides direct histological evidence that ELH and hearing loss are closely related. In humans, no direct histological evidence exists to verify that ELH and hearing loss are correlated. Recent studies in MD patients report circumstantial evidence that suggests the presence of ELH based on the presence of hearing loss and the diagnosis of MD. Using MRI technology, Seo et al. (2012) attempted to correlate visualized hydrops with ECochG or hearing loss in patients with MD. They showed that 3D-FLAIR MRI enabled visualization of ELH in patients with definite MD, but found significant differences in ECochG between patients in whom MRI showed cochlear hydrops compared to those in whom it did not. In 26 patients that were strongly suspected to have MD, elevated SP/AP ratios were reported in 15 of 21 patients with positive findings by cochlear MRI, and one elevated SP/AP ratio was reported among five patients in whom cochlear hydrops was not visualized (Seo et al. 2012). Gürkov et al. (2011) reported a significant correlation between the degree of MRI-visualized hydrops and average hearing level, but found no correlation between hydrops and the SP/AP ratio. Orchik et al. (1993) reported that a positive ECochG (an elevated SP/AP ratio) in patients was associated with hearing loss, and they interpreted this as representing a longer duration of MD in these particular patients. In fact, only 66 % of their patients with MD and no hearing loss had a positive ECochG, but more than 80 % of MD patients who had hearing loss of 25 dB or higher had a positive ECochG. This is circumstantial evidence that more severe ELH is associated with more hearing loss. However, there is no mention of the numerical correlation of the SP/AP ratio to the ABR threshold, and these findings cannot be verified by histological measurements of ELH (Orchik et al. 1993). In the ELH mouse model study reported here, hearing loss was associated with more severe ELH (shown by histology). This finding is consistent with previous studies in guinea pig models, where surgically induced hydrops was found to correlate with hearing loss (Bixenstine et al. 2008; Hott et al. 2003; Megerian 2005). However, no relationship was found between the SP/AP ratio and hearing loss in our study.
Limitations of the Study and Future Directions
Using click stimulus to record ECochG is standard practice in human and animal studies, and a large body of literature is available for comparison (Campbell et al. 1993; van Deelen et al. 1987; van Deelen and Smoorenburg 1986; Ge and Shea 2002; Horner and Cazals 1988). Therefore, we used click stimulus to record ECochG in the mouse model of ELH. One of the limitations here is that this study used only click stimuli as opposed to pure tone stimuli to perform ECochG, and hence our results lack tone specificity. Current clinical reports indicate more consistent findings with low-frequency tone bursts (Hornibrook et al. 2012; Iseli and Gibson 2010; Zhang 2012). It is possible that tone bursts might yield more reliable findings. This could be an interesting avenue to explore because Bixenstine et al. (2008) and others showed differential spiral ganglion neuron degeneration between the low-frequency (apex) and high-frequency (base) regions of the cochlea in guinea pig models of ELH. Another limitation is the snapshot approach taken in this study (focused on a single time point). Although the PhexH-D/Y ears showed a wide range of disease in terms of ELH severity, SP/AP ratio, and hearing loss at P21, a single time point study does not capture the phenotype associated with inherently fluctuating nature of MD or other ELH-linked conditions. This limitation can be overcome by carrying out longitudinal studies in the animal model. In this regard, our report showcases the safety and reliability of the MIEM approach and suggests that it can be used for longitudinal studies.
CONCLUSIONS
This is the first study to elucidate the relationships among an elevated SP/AP ratio, the degree of ELH, and hearing loss using a mouse model. We demonstrate that an elevated SP/AP ratio is diagnostic of ELH and that the increasing severity of hearing loss is a better predictor of the severity of ELH than is an increasing SP/AP ratio.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (R01DC009246 to Q.Y.Z; R01DC01816 to K.N.A.) and funds from the Maniglia Endowed Chair, University Hospitals Case Medical Center (to K.N.A) and Pogue Endowed Chair (To C.A.M). We thank Heping Yu for her assistance in histological processing and animal breeding.
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
S.J.M. and Y.L. are co-first authors.
C.A.M and K.N.A are co-senior authors.
Contributor Information
Sami J Melki, Email: sami.melki@case.edu.
Yiping Li, Email: Yiping.li@case.edu.
Maroun T Semaan, Email: maroun.semaan@uhhospitals.org.
Qing Yin Zheng, Email: qyz@case.edu.
Cliff A. Megerian, Email: cliff.megerian@uhhospitals.org
Kumar N Alagramam, Phone: +1-216-8447261, Email: kna3@case.edu.
References
- Al-momani MO, Ferraro JA, Gajewski BJ, Ator G. Improved sensitivity of electrocochleography in the diagnosis of Meniere’s disease. Int J Audiol. 2009;48:811–819. doi: 10.3109/14992020903019338. [DOI] [PubMed] [Google Scholar]
- Arenberg IK, Marovitz WF, Shambaugh GE (1970) The role of the endolymphatic sac in the pathogenesis of endolymphatic hydrops in man. Acta Otolaryngol Suppl 275:1–49 [PubMed]
- Belal A, Ylikoski J (1980) Pathologic significance of Meniere’s symptom complex. A histopathologic and electron microscopic study. Am J Otolaryngol 1:275–84 [DOI] [PubMed]
- Bewick V, Cheek L, Ball J. Statistics review 13: receiver operating characteristic curves. Crit Care. 2004;8:508–512. doi: 10.1186/cc3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixenstine PJ, Maniglia MP, Vasanji A, et al. Spiral ganglion degeneration patterns in endolymphatic hydrops. Laryngoscope. 2008;118:1217–1223. doi: 10.1097/MLG.0b013e31816ba9cd. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Faloon KM, Rybak LP. Noninvasive electrodes for electrocochleography in the chinchilla. Arch Otolaryngol Head Neck Surg. 1993;119:767–771. doi: 10.1001/archotol.1993.01880190063013. [DOI] [PubMed] [Google Scholar]
- Dallos P, Popper AN, Fay RR (1996) The cochlea. doi:10.1007/978-1-4612-0757-3
- Dauman R, Aran JM, Portmann M. Summating potential and water balance in Meniere’s disease. Ann Otol Rhinol Laryngol. 1986;95:389–395. doi: 10.1177/000348948609500413. [DOI] [PubMed] [Google Scholar]
- Durrant JD, Wang J, Ding DL, Salvi RJ. Are inner or outer hair cells the source of summating potentials recorded from the round window? J Acoust Soc Am. 1998;104:370–377. doi: 10.1121/1.423293. [DOI] [PubMed] [Google Scholar]
- Ferraro JA. Electrocochleography: a review of recording approaches, clinical applications, and new findings in adults and children. J Am Acad Audiol. 2010;21:145–152. doi: 10.3766/jaaa.21.3.2. [DOI] [PubMed] [Google Scholar]
- Ferraro JA, City K (2000) Clinical electrocochleography: overview of theories, techniques and applications
- Ferraro JA, Ferguson R. Tympanic ECochG and conventional ABR: a combined approach for the identification of wave I and the I–V interwave interval. Ear Hear. 1989;10:161–166. doi: 10.1097/00003446-198906000-00004. [DOI] [PubMed] [Google Scholar]
- Ferraro JA, Arenberg IK, Hassanein RS. Electrocochleography and symptoms of inner ear dysfunction. Arch Otolaryngol. 1985;111:71–74. doi: 10.1001/archotol.1985.00800040035001. [DOI] [PubMed] [Google Scholar]
- Ferraro JA, Thedinger BS, Mediavilla SJ, Blackwell WL. Human summating potential to tone bursts: observations on tympanic membrane versus promontory recordings in the same patients. J Am Acad Audiol. 1994;5:24–29. [PubMed] [Google Scholar]
- Fraysse BG, Alonso A, House WF (1980) Menière’s disease and endolymphatic hydrops: clinical-histopathological correlations. Ann Otol Rhinol Laryngol Suppl 89:2–22 [DOI] [PubMed]
- Ge X, Shea JJ. Transtympanic electrocochleography: a 10-year experience. Otol Neurotol. 2002;23:799–805. doi: 10.1097/00129492-200209000-00032. [DOI] [PubMed] [Google Scholar]
- Gibson WP, Moffat DA, Ramsden RT. Clinical electrocochleography in the diagnosis and management of Meneère’s disorder. Audiology. 1977;16:389–401. doi: 10.3109/00206097709071852. [DOI] [PubMed] [Google Scholar]
- Goin DW, Staller SJ, Asher DL, Mischke RE. Summating potential in Meniere’s disease. Laryngoscope. 1982;92:1383–1389. doi: 10.1288/00005537-198212000-00008. [DOI] [PubMed] [Google Scholar]
- Gürkov R, Flatz W, Louza J, et al. In vivo visualization of endolyphatic hydrops in patients with Meniere’s disease: correlation with audiovestibular function. Eur Arch Otorhinolaryngol. 2011;268:1743–1748. doi: 10.1007/s00405-011-1573-3. [DOI] [PubMed] [Google Scholar]
- Horner KC. Functional changes associated with experimentally induced endolymphatic hydrops. Hear Res. 1993;68:1–18. doi: 10.1016/0378-5955(93)90059-A. [DOI] [PubMed] [Google Scholar]
- Horner KC, Cazals Y. Independent fluctuations of the round-window summating potential and compound action potential following the surgical induction of endolymphatic hydrops in the guinea pig. Int J Audiol. 1988;27:147–155. doi: 10.3109/00206098809081585. [DOI] [PubMed] [Google Scholar]
- Hornibrook J, Kalin C, Lin E, et al. Transtympanic electrocochleography for the diagnosis of Ménière’s disease. Int J Otolaryngol. 2012;2012:852714. doi: 10.1155/2012/852714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hott ME, Graham M, Bonassar LJ, Megerian CA. Correlation between hearing loss and scala media area in guinea pigs with long-standing endolymphatic hydrops. Otol Neurotol. 2003;24:64–72. doi: 10.1097/00129492-200301000-00014. [DOI] [PubMed] [Google Scholar]
- Iseli C, Gibson W. A comparison of three methods of using transtympanic electrocochleography for the diagnosis of Meniere’s disease: click summating potential measurements, tone burst summating potential amplitude measurements, and biasing of the summating potential using a low frequency tone. Acta Otolaryngol. 2010;130:95–101. doi: 10.3109/00016480902858899. [DOI] [PubMed] [Google Scholar]
- Kumagami H, Nishida H, Baba M. Electrocochleographic study of Ménière’s disease. Arch Otolaryngol. 1982;108:284–288. doi: 10.1001/archotol.1982.00790530020006. [DOI] [PubMed] [Google Scholar]
- Liu H, Li G. Testing statistical significance of the area under a receiving operating characteristics curve for repeated measures design with bootstrapping. J Data Sci. 2005;3:257–278. [Google Scholar]
- Lorenz-Depiereux B, Guido VE, Johnson KR, et al. New intragenic deletions in the Phex gene clarify X-linked hypophosphatemia-related abnormalities in mice. Mamm Genome. 2004;15:151–161. doi: 10.1007/s00335-003-2310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megerian CA. Diameter of the cochlear nerve in endolymphatic hydrops: implications for the etiology of hearing loss in Ménière’s disease. Laryngoscope. 2005;115:1525–1535. doi: 10.1097/01.mlg.0000167804.82950.9e. [DOI] [PubMed] [Google Scholar]
- Megerian CA, Semaan MT, Aftab S, et al. A mouse model with postnatal endolymphatic hydrops and hearing loss. Hear Res. 2008;237:90–105. doi: 10.1016/j.heares.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki SJ, Heddon CM, Frankel JK, et al. Pharmacological protection of hearing loss in the mouse model of endolymphatic hydrops. Laryngoscope. 2010;120:1637–1645. doi: 10.1002/lary.21018. [DOI] [PubMed] [Google Scholar]
- Mori N, Asai H, Sakagami M, Matsunaga T. Comparison of summating potential in Menière’s disease between trans- and extratympanic electrocochleography. Audiology. 1987;26:348–355. doi: 10.3109/00206098709081562. [DOI] [PubMed] [Google Scholar]
- Orchik DJ, Shea JJ, Jr, Ge X. Transtympanic electrocochleography in Meniere’s disease using clicks and tone-bursts. Am J Otol. 1993;14:290–294. [PubMed] [Google Scholar]
- Paparella MM (1984) Pathogenesis of Meniere’s disease and Meniere’s syndrome. Acta Otolaryngol Suppl 406:10–25 [DOI] [PubMed]
- Pyykkö I, Nakashima T, Yoshida T, et al. Meniere’s disease: a reappraisal supported by a variable latency of symptoms and the MRI visualisation of endolymphatic hydrops. BMJ Open. 2013 doi: 10.1136/bmjopen-2012-001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass K. Sensitivity and specificity of transtympanic electrocochleography in Meniere’s disease. Acta Otolaryngol. 1998;118:150–156. doi: 10.1080/00016489850154838. [DOI] [PubMed] [Google Scholar]
- Schmidt PH, Eggermont JJ, Odenthal DW. Study of Menière’s disease by electrocochleography. Acta Otolaryngol Suppl. 1974;316:75–84. doi: 10.1080/16512251.1974.11675748. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF (1976) Pathophysiology of endolymphatic hydrops. Arch Otorhinolaryngol 212:253–62 [DOI] [PubMed]
- Semaan MT, Megerian CA. Ménière’s disease: a challenging and relentless disorder. Otolaryngol Clin North Am. 2011;44:383–403. doi: 10.1016/j.otc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Seo Y, Kim J, Choi J, Lee W (2012) Visualization of endolymphatic hydrops and correlation with audio-vestibular functional testing in patients with definite Meniere’s disease. Auris Nasus Larynx [DOI] [PubMed]
- Sheykholeslami K, Megerian CA, Zheng QY. Vestibular evoked myogenic potentials in normal mice and Phex mice with spontaneous endolymphatic hydrops. Otol Neurotol. 2009;30:535–544. doi: 10.1097/MAO.0b013e31819bda13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deelen GW, Smoorenburg GF. Electrocochleography for different electrode positions in guinea pig. Acta Otolaryngol. 1986;101:207–216. doi: 10.3109/00016488609132829. [DOI] [PubMed] [Google Scholar]
- Van Deelen GW, Ruding PR, Veldman JE, et al. Electrocochleographic study of experimentally induced endolymphatic hydrops. Arch Otorhinolaryngol. 1987;244:167–173. doi: 10.1007/BF00464262. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Teranishi M, Kato M, et al. Endolymphatic hydrops in patients with tinnitus as the major symptom. Eur Arch Otorhinolaryngol. 2013 doi: 10.1007/s00405-013-2380-9. [DOI] [PubMed] [Google Scholar]
- Zhang M. Response pattern based on the amplitude of ear canal recorded cochlear microphonic waveforms across acoustic frequencies in normal hearing subjects. Trends Amplif. 2012;16:117–126. doi: 10.1177/1084713812448547. [DOI] [PMC free article] [PubMed] [Google Scholar]