Abstract
Gentamicin is an aminoglycoside antibiotic used to treat gram-negative bacterial infections. Treatment with this antibiotic carries the potential for adverse side effects, including ototoxicity and nephrotoxicity. Ototoxic effects are at least in part a consequence of oxidative stress, and various antioxidants have been used to attenuate gentamicin-induced hair cell death and hearing loss. Here, a combination of nutrients previously shown to reduce oxidative stress in the hair cells and attenuate hearing loss after other insults was evaluated for potential protection against gentamicin-induced ototoxicity. Guinea pigs were maintained on a nutritionally complete standard laboratory animal diet or a diet supplemented with β-carotene, vitamins C and E, and magnesium. Three diets with iterative increases in nutrient levels were screened; the final diet selected for study use was one that produced statistically reliable increases in plasma levels of vitamins C and E and magnesium. In two separate studies, significant decreases in gentamicin-induced hearing loss at frequencies including 12 kHz and below were observed, with less benefit at the higher frequencies. Consistent with the functional protection, robust protection of both the inner and outer hair cell populations was observed, with protection largely in the upper half of the cochlea. Protection was independently assessed in two different laboratories, using two different strains of guinea pigs. Additional in vitro tests did not reveal any decrease in antimicrobial activity with nutrient additives. Currently, there are no FDA-approved treatments for the prevention of gentamicin-induced ototoxicity. The current data provide a rationale for continued investigations regarding translation to human patients.
Keywords: gentamicin, ototoxicity, otoprotection, hearing loss, hair cell death, β-carotene, vitamin C, vitamin E, magnesium
INTRODUCTION
Many of the aminoglycoside antibiotics are ototoxic, causing damage to cells in the inner ears. Drugs such as neomycin, tobramycin, kanamycin, streptomycin, amikacin, and gentamicin can affect hearing, balance, or both (Clark 1977; Xie et al. 2011; Ahmed et al. 2012). Despite potential ototoxicity and nephrotoxicity, aminoglycosides remain a drug of choice for use against gram-negative bacteria (see Xie et al. 2011; Mulheran et al. 2001; Hanberger et al. 2013; Edson and Terrell 1999). Aminoglycoside antibiotics are also widely used to treat multidrug-resistant tuberculosis (MDR-TB). In developing countries, the care of HIV-positive patients with MDR-TB is an enormous healthcare burden (Gao et al. 2013; Metcalfe et al. 2013; Varghese et al. 2013); ototoxicity is a greater risk factor in HIV-positive patients with MDR-TB than HIV-negative patients with MDR-TB (Harris et al. 2012).
Estimates regarding prevalence of permanent hearing loss after gentamicin range from 2 to 3 % (Best et al. 2011; Colding et al. 1989; Echeverria et al. 1978; Moffat and Ramsden 1977) to 17 % (Mulherin et al. 1991) or even 25 % (Prins et al. 1993) of treated patients. Multiple factors influence observed prevalence [for recent review, see (Frymark et al. 2010)]. Hearing loss can develop slowly, sometimes arising weeks after aminoglycoside treatment ends (see, for example, Stebbins et al. 1979), thus testing during or immediately after the series of gentamicin treatments ends could miss hearing loss in some patients. Variable estimates on ototoxicity may also be related to patient history. For example, specific mitochondrial defects that are more prevalent in some Asian populations produce extreme sensitivity to aminoglycoside ototoxicity (Usami et al. 1998; Zhao et al. 2004; for review see Bindu and Reddy 2008). Additionally, previous aminoglycoside treatment has been suggested to effect risk of ototoxicity from subsequent administration based on cumulative drug dose (Chen et al. 2013). The nutritional health of the patient may also influence vulnerability, with reduced protein intake increasing aminoglycoside ototoxicity in animal subjects, as a function of reduced endogenous glutathione (GSH) levels (Lautermann et al. 1995). Finally, the tests selected for use in monitoring patient auditory function will influence prevalence, as some tests are more sensitive to change. Because the basal cochlea is affected first, hearing loss prevalence increases when extended high frequency (EHF) threshold changes are considered (Singh Chauhan et al. 2011; Fausti et al. 1992). The use of otoacoustic emission tests also increases prevalence, as these tests reveal subtle changes in outer hair cell (OHC) function (Constantinescu et al. 2009; Stavroulaki et al. 1999). Increased awareness of the clinical issue of drug-induced ototoxicity has led to the demand for better screening for hearing loss risk factors, better disclosure of ototoxicity as a potential side effect, and better audiological monitoring (Das-Purkayastha and Rutka 2011; American Academy of Audiology 2009).
New antibiotics with fewer adverse side effects are in the pipeline (Matt et al. 2012; Nudelman et al. 2009) and could ultimately reduce the need for otoprotective agents. However, the aminoglycoside antibiotic medications available now are ototoxic, these newer-generation and presumably more costly drugs will be less accessible in developing countries, and thus protective agents are needed. Novel therapeutics that can be co-administered to reduce ototoxic drug side effects, without compromising antimicrobial efficiency, would be a significant advance (Rybak and Kelly 2003), particularly if these therapeutics are low cost. Development of otoprotective agents is well-grounded in work on the mechanisms of gentamicin-induced pathology. The key role of free radicals in aminoglycoside-induced hearing loss has been well documented (Xie et al. 2011; Schacht et al. 2012). The OHC population is a primary target, as OHC loss has been observed in human patient temporal bones, particularly in the basal turn (Kusunoki et al. 2004; Backus et al. 1987; Keene et al. 1982; Johnsson et al. 1981). Basal turn OHC loss is consistent with the high frequency deficits detected in audiometric tests. Progressive drug-induced deficits from base to apex are attributed to intrinsic differences in metabolic activity and metabolic demand in hair cells (higher in base) and lower GSH production in the base (Sha et al. 2001a), as well as basal turn OHCs being more vulnerable to the effects of gentamicin on OHC calcium channels (Tan et al. 2001). Basal OHCs accumulate ROS faster than more apical OHCs, and cells in the first row of OHCs accumulate ROS more quickly than cells in the second or third row of OHCs (Choung et al. 2009).
Since Schacht (1986) first proposed the important contribution of free radical formation and oxidative stress to aminoglycoside-induced hearing loss, the role of free radical formation in drug-induced OHC loss has been clearly defined. Among the earliest observations of free radical formation in OHCs after aminoglycoside treatment was in vitro formation of hydrogen peroxide and hydroxyl radicals in OHCs treated with gentamicin or kanamycin (Clerici et al. 1996); superoxide and hydrogen peroxide radicals were later shown to decrease auditory function in vivo (Clerici and Yang 1996). Production of ROS after gentamicin has been confirmed in other in vitro models (Basappa et al. 2010; Sha and Schacht 1999a; Bas et al. 2012), and ROS formation in the inner ear has been shown in tissues harvested from mice treated in vivo with kanamycin (Jiang et al. 2005). ROS production may be secondary to actions of gentamicin as an iron chelator, as it appears that the iron-gentamicin complex drives free radical formation (Priuska and Schacht 1995). The second major category of free radicals is reactive nitrogen species (RNS). RNS immunolabeling was not detected in the organ of Corti (OHCs, supporting cells) after kanamycin (see Jiang et al. 2005) or gentamicin (Heinrich et al. 2008), although increased nitric oxide was observed in the lateral wall (Heinrich et al. 2008). There are endogenous antioxidant systems, including GSH, that protect the inner ear against oxidative stress (for detailed review, see Le Prell and Bao 2011). It appears an endogenous antioxidant defense is initially mounted, and ultimately overwhelmed, as endogenous antioxidant stores are depleted after aminoglycoside therapy. Superoxide dismutase (SOD), catalase, GSH, and glutathione peroxidase (GPx) were all reduced in cochleas harvested from guinea pigs treated with amikacin (Klemens et al. 2003) as well as tissue cultures treated with gentamicin (Bas et al. 2012). Consistent with endogenous antioxidant defense, transgenic mice that overexpress SOD1 are more resistant to kanamycin-induced ototoxicity than wild-type mice (Sha et al. 2001b).
A variety of investigations into antioxidant defense against gentamicin insult and other aminoglycosides have been encouraging (for reviews, see Xie et al. 2011; Rybak and Kelly 2003; Rybak and Whitworth 2005; Abi-Hachem et al. 2010; Poirrier et al. 2010; Campbell and Le Prell 2012). There are now a variety of drugs and other substances that have been shown to effectively attenuate cell death and hearing loss after gentamicin insult, although complete protection has been elusive (for summary, see Table 1). In the first human interventions, aspirin reduced gentamicin-induced hearing loss in human patients in China (Sha et al. 2006), an effect that has since been confirmed in a second patient population in Saudi Arabia (Behnoud et al. 2009). However, gastric events were an adverse side effect for some patients. The ideal antioxidant strategy will be inexpensive, safe, readily available, and will have few side effects. In this study, we evaluated the potential for protection against gentamicin-induced hearing loss using a combination of antioxidant agents including β-carotene, vitamins C and E, and magnesium, delivered as an oral dietary supplement. These nutrients are of interest as vitamins C and E have shown benefit with respect to reducing gentamicin-induced free radical formation and renal toxicity (Devbhuti et al. 2009, 2011; Kadkhodaee et al. 2005, 2007; Mal et al. 2012; Patel Manali et al. 2011; Saleem et al. 2012; Stojiljkovic et al. 2012; Varzi et al. 2007; Wahed et al. 2008; Kavutcu et al. 1996), and some benefits have been shown with respect to reducing gentamicin ototoxicity using vitamin E (Fetoni et al. 2003, 2004). Several pieces of data suggest the potential for benefit using magnesium (Mg) supplements as well. First, gentamicin treatment increases urinary excretion of Mg, in addition to sodium, potassium, and calcium; Mg monitoring during gentamicin is recommended (Sassen et al. 2006; Atsmon and Dolev 2005). Second, Mg attenuates gentamicin-induced hair cell death in zebrafish (as well as neomycin-induced hair cell death; see Coffin et al. 2009). Here, vitamins C and E were used in combination with Mg as well as β-carotene. The combination has effectively attenuated hearing loss after noise exposure, which is another insult that induces oxidative stress in cells in the inner ear (Le Prell et al. 2007a, 2011a, b; Tamir et al. 2010).
TABLE 1.
Antioxidant protection against gentamicin ototoxicity, assessed in vivo, is inconsistent across agents and across reports. All values reported in this table were extracted from published figures as cited; in some cases, threshold shift was calculated by subtracting baseline threshold from post-gentamicin thresholds
| Species | Gentamicin (GM) insult | Protective agent (drug and dosing) | Average threshold shift in control subjects | Average threshold shift in treated subjects | |
|---|---|---|---|---|---|
| Garetz et al. (1994)a | Pigmented guinea pig | 100 mg/kg/days × 14 days | 0.6 ml of 0.3 M glutathione (GSH) (oral gavage) | 42 dB at 2 kHz 60 dB at 8 kHz 70 dB at 18 kHz |
25 dB at 2 kHz 38 dB at 8 kHz* 40 dB at 18 kHz* |
| Sha and Schacht (1999b) | Pigmented guinea pig | 120 mg/kg/days × 19 days | 100 mg/kg sodium salicylate, s.c., BID | 38 dB at 3 kHz 42 dB at 9 kHz 62 dB at 18 kHz |
15 dB at 3 kHz* 20 dB at 9 kHz* 20 dB at 18 kHz |
| Lopez-Gonzalez et al. (2000) | Wistar rats | 160 mg/kg/days × 5 days | 10 mg/L melatonin in drinking water | DPOAE amplitude decreased by 21.5 dB at 6 kHz | DPOAE amplitude decreased by 3 dB at 6 kHz |
| 250 μg/day melatonin s.c. | DPOAE amplitude decreased by 5 dB at 6 kHz | ||||
| 250 μg/day melatonin i.m. | DPOAE amplitude decreased by 6.5 dB at 6 kHz | ||||
| Sha and Schacht (2000) | Pigmented guinea pig | 120 mg/kg/days × 19 day | 200 mg/kg d-methionine QD s.c. | 38 dB at 3 kHz 47 dB at 9 kHz 45 dB at 18 kHz |
8 dB at 3 kHz* 29 dB at 9 kHz* 32 dB at 18 kHz |
| 200 mg/kg d-methionine BID s.c./i.p. | 38 dB at 3 kHz 40 dB at 9 kHz 40 dB at 18 kHz |
20 dB at 3 kHz 28 dB at 9 kHz* 20 dB at 18 kHz* |
|||
| Ylikoski et al. (2002) | Albino guinea pig | 120 mg/kg/days × 14 days | 1 mg/kg/day Cephalon (CEP-1347, s.c.) × 29 days, starting 1 day pre-GM | 62 dB at 2 kHz 75 dB at 4 kHz 78 dB at 8 kHz 80 dB at 16 kHz 60 dB at 32 kHz |
4 dB at 2 kHz* 30 dB at 4 kHz* 48 dB at 8 kHz* 53 dB at 16 kHz* 50 dB at 32 kHz* |
| Fetoni et al. (2003) | Albino guinea pig | 100 mg/kg/days × 14 days | 100 mg/kg vitamin E (dl-a-tocopherol) i.m. | 25 dB at 2 kHz 30 dB at 4 kHz 50 dB at 8 kHz 48 dB at 12 kHz 56 dB at 16 kHz |
10 dB at 2 kHz* 15 dB at 4 kHz* 24 dB at 8 kHz* 28 dB at 12 kHz* 27 dB at 16 kHz* |
| Fetoni et al. (2004) | Albino guinea pig | 100 mg/kg/days × 14 days | 100 mg/kg vitamin E (dl-a-tocopherol) i.m. | 35 dB at 2 kHz 38 dB at 4 kHz 55 dB at 8 kHz 56 dB at 12 kHz 66 dB at 16 kHz |
10 dB at 2 kHz* 14 dB at 4 kHz* 27 dB at 8 kHz* 30 dB at 12 kHz* 34 dB at 16 kHz* |
| Okuda et al. (2005) | Albino guinea pig | 12 mg/ml gentamicin delivered intra-cochlear at 0.5 μl/h × 14 days | 250 μM z-VAD-FMK (general caspase inhibitor) delivered intra-cochlear at 0.5 μl/h × 14 days | 37 dB at 8 kHz | 17 dB at 8 kHz* |
| 250 μM z-LEHD-FMK (caspase 9 inhibitor) delivered intracochlear at 0.5 μl/h × 14 days | 21 dB at 8 kHz* | ||||
| Nordang and Anniko (2005) | Sprague–Dawley rats | 20 μl of 480 mg/ml GM on days 0, 1 and 3, injected through tympanic membrane | 1 mg of l-NAME with GM | 5 dB at 12 kHz 15 dB at 16 kHz 35 dB at 20 kHz 40 dB at 35 kHz |
0 dB at 12 kHz 0 dB at 16 kHz −2 dB at 20 kHz −5 dB at 35 kHz |
| Sha et al. (2006) | Human | 80–160 mg/day × 5–7 days, twice daily | 3 g/day aspirin × 14 days, divided into 3 doses/day | 13 % had ≥15 dB shift at both 6 and 8 kHz | 3 % had ≥15 dB shift at both 6 and 8 kHz* |
| Kharkheli et al. (2007) | Human | 80 mg 3 times/day × 7 days | 2,800 mg Vitamin E, divided into 3 doses/day (1,200 mg, 800 mg, 800 mg) | 3.40 % had ≥20 dB shift two adjacent frequencies ; 10.3 % had ≥15 dB shift two adjacent frequencies; 13.8 % had ≥10 dB shift two adjacent frequencies |
13.0 % had ≥20 dB shift two adjacent frequencies ; 8.7 % had ≥15 dB shift two adjacent frequencies; 10.4 % had ≥10 dB shift two adjacent frequencies |
| Ye et al. (2009)b | Pigmented guinea pig | 120 mg/kg/day × 17 days | 0.3 ml/kg melatonin i.m. | DPOAE amplitude decreased by: 6 dB at 3 kHz 10 dB at 4 kHz 13 dB at 6 kHz 11 dB at 8 kHz |
DPOAE amplitude decreased by: 2 dB at 3 kHz* 4 dB at 4 kHz* 5 dB at 6 kHz* 4 dB at 8 kHz* |
| Pfannenstiel et al. (2009) | C57BL/6 mice | 1 μl of 40 μg/μl gentamicin sulfate applied to RWM | Adenovector-expressing human bcl-2 (Ad.11D.bcl-2) delivered to posterior semicircular canal |
21 dB at 4 kHz 13 dB at 8 kHz 18 dB at 16 kHz 24 dB at 32 kHz |
−1 dB at 4 kHz 3 dB at 8 kHz −2 dB at 16 kHz 2 dB at 32 kHz* |
| Behnoud et al. (2009) | Human | 240 mg/day × 7 days (administered as 80 mg every 8 h) | 1.5 g/day Aspirin | 1.1 dB at 250 Hz* 1.6 dB at 500 Hz* 1.7 dB at 1000 Hz* 5.1 dB at 2000 Hz* 9.3 dB at 4000 Hz* 13.3 dB at 8000 Hz* 37 % had ≥15 dB shift at 4 kHz; 20 % had ≥15 dB shift at 8 kHz |
0.7 dB at 250 Hz 0.9 dB at 500 Hz 1.9 dB at 1000 Hz 2.7 dB at 2000 Hz 5.0 dB at 4000 Hz* 6.0 dB at 8000 Hz* 3 % had ≥15 dB shift at 4 kHz; 3 % had ≥15 dB shift at 8 kHz |
| Choung et al. (2011) | Sprague–Dawley rats | 160 mg/kg × 5 days (i.p.) | 500 mg/kg/day Korean Red Ginseng × 12 days (starting 7 days pre-GM) | 9 dB at 16 kHz 9 dB at 32 kHz |
5 dB at 16 kHz 5 dB at 32 kHz* |
| Bas et al. (2012) | Wistar rats | 10 mg in 200 μl saline in mini-pump, or 10 mg in 40 μl saline on RWM using gelfoam | Dexamethasone | (Click Stimuli) Mini-pump: 30 dB Gelfoam: 31 dB |
Mini-pump: 5 dB* Gelfoam: 0 dB * |
| Melatonin | Mini-pump: 10 dB* Gelfoam: 5 dB* |
||||
| Tacrolimus | Mini-pump: 0 dB* Gelfoam: 0 dB* |
||||
| Uzun et al. (2012) | Wistar rats | 120 mg/kg × 16 days (i.p.) | 4 % garlic diet (pulverized whole clove) | 61 dB at 10 kHz | 27 dB at 10 kHz* |
| Fetoni et al. (2012) | Albino guinea pig | 100 mg/kg × 14 days (i.p.) | Q-ter, 100 mg/kg (i.p.), 1 h pre-gentamicin | 20 dB at 2 kHz 27 dB at 4 kHz 35 dB at 6 kHz 36 dB at 8 kHz 47 dB at 12 kHz 45 dB at 16 kHz 39 dB at 20 kHz |
10 dB at 2 kHz* 20 dB at 4 kHz* 24 dB at 6 kHz* 26 dB at 8 kHz* 26 dB at 12 kHz* 27 dB at 16 kHz* 25 dB at 20 kHz* |
| Jankauskas et al. (2012) | Outbred white rats | 160 mg/kg × 6 days (i.p.) | SkQR1, 100 nmol/kg 3 h pre-gentamicin | Preyer reflex, average score = 2.5 Score based on sound level where reflex first observed: 0 = reflex absent (deaf) 1 = high level (severe hearing reduction); 2 = moderate level (moderate hearing decrease) 3 = low level (hearing intact) |
Preyer reflex, average score = 2.9* |
| Tian et al. (2013) | Sprague–Dawley rats | 500 mg/ml × 20 μl, intratympanic | 500 mg/kg/day Korean Red Ginseng × 21 day (starting 7 days pre-GM) | 32 dB at 16 kHz 29 dB at 32 kHz |
22 dB at 16 kHz* 20 dB at 32 kHz* |
| Ojano-Dirain et al. (2013) | Albino guinea pig | 130 mg/kg × 10 days (s.c.) | MitoQ, 0.03 g/L in drinking water for duration of study (~85 days) | 36 dB at 4 kHz 45 dB at 8 kHz 57 dB at 16 kHz |
21 dB at 4 kHz* 30 dB at 8 kHz* 48 dB at 16 kHz* |
aSurvival rate—7 of 13 gentamicin controls, 6 of 12 glutathione plus gentamicin animals
bSurvival rate—13 of 18 gentamicin controls, 13 of 15 glutathione plus gentamicin animals
*p < 0.05, significant reduction in threshold shift for between group comparisons as reported by authors with the exception of Behnoud et al. (2009) who reported the statistical reliability of threshold changes within groups. Aspirin-treated human patients had statistically significant threshold shifts at fewer frequencies than those receiving placebo
A critical step for any combination is clearly establishing that there are no reductions in the antimicrobial activity of the antibiotic drug agent; the desired death of bacteria may require induction of oxidative stress (Kohanski et al. 2007). There has been no reduction in antibacterial activity of gentamicin during co-treatment with GSH (Garetz et al. 1994) and no effect of aspirin on gentamicin efficacy in a human trial (Sha et al. 2006). Also promising was that there was no reduction in gentamicin serum levels during co-treatment with d-methionine (Sha and Schacht 2000). Data on antibiotic efficacy were also collected here.
MATERIALS AND METHODS
Subject Group #1—Dietary Treatment, Plasma Sampling, and Analysis
Subjects
A preliminary pharmacokinetic study was conducted during development of the custom-formulated nutrient-enhanced diet. Serum levels of active agents were measured in 16 pigmented male guinea pigs (250–300 g) obtained from an approved laboratory animal supplier (Elm Hill, Chelmsford, MA, USA) and housed in the Animal Care Facility at the University of Michigan. All animals were given at least 48 h to acclimate to the environment and recover from transportation-related stress prior to surgical placement of a chronic vascular access port connected to a cannula inserted into the jugular vein, as in Le Prell et al. (2011a). Dietary manipulation was initiated following recovery from the surgical procedure. This experimental protocol was reviewed and approved by the University of Michigan Institutional Animal Care and Use Committee, and all procedures conformed to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Dietary Manipulation
Subjects were assigned to a nutritionally complete control diet (TD.2040, n = 4) or one of three iteratively supplemented diets (TD.07626, n = 2; TD.08435, n = 4; TD.08623, n = 6; see Table 2 for nutrient content). Blood samples were collected from the control animals after 46–47 days on the TD.2040 diet. For the two animals maintained on TD.07626, blood samples were collected prior to and 48 days after dietary manipulation began (n = 1) or prior to and 7, 14, and 48 days after dietary manipulation began (n = 1). For the four animals maintained on TD.08435, blood samples were collected prior to and 1, 4, and 6 days after dietary manipulation (n = 1) or prior to and 1, 4, 6, 11, and 18 days after dietary manipulation began (n = 3). For the six animals maintained on TD.08623, blood samples were collected prior to and 20 days after dietary manipulation for four of the six subjects. In the other two subjects, blood samples were limited to the 20-day dietary time point, with no premanipulation sample collected. Blood sampling time points during preliminary diet development were variable, in part due to the use of a vascular access port (VAP) in some animals, which did not yield a sample from each attempt. Subjects without VAPs only received an end-of-study assessment.
TABLE 2.
Custom diet formulations. All amounts are mg additive per kilogram of pelleted chow
| TD.2040 (Teklad Global Guinea Pig Diet) “control” | TD.07626a | TD.08435b | TD.08623c | |
|---|---|---|---|---|
| Vitamin Ad | 16.30 IU/G | 15.96 IU/G | 15.95 IU/G | 15.35 IU/G |
| Retinold | 4.90 mg/kg | 4.80 mg/kg | 4.8 mg/kg | 4.62 mg/kg |
| Beta-carotene (mg/kg chow) | 36.30 mg/kg | 125.54 mg/kg | 485.53 mg/kg | 1,034.19 mg/kg |
| Vitamin C: ascorbic acid (mg/kg chow) | 1,050.00 mg/kg | 10,448.04 mg/kg | 10,447.67 mg/kg | 20,989.10 mg/kg |
| Vitamin E: alpha-tocopherol (mg/kg chow) | 115.80 mg/kg | 4,513.38 mg/kg | 4,513.34 mg/kg | 7,109.08 mg/kg |
| Magnesium (mg/kg chow) | 0.33 % (3,300 mg/kg) | 10,230 mg/kg | 10,230 mg/kg | 33,110 mg/kg |
dBecause the custom diets contain TD.2040 Diet plus additives, the total amount of vitamin A, retinol, and any other nutrient in the base diet decrease based on the fraction of the TD.2040 diet within the custom diet. All nutrient values in this table are adjusted based on the fraction of the diet made up of base diet, with the supplement added to the adjusted base value
aManufactured using 979.09 g/kg TD.2040 Global Guinea Pig Diet plus 0.09 g/kg beta-carotene supplied by King Chemical (20 %), 9.42 g/kg vitamin C (l-ascorbyl-2-polyphosphate, 35 %), 4.4 g/kg citamin E (dl-alpha tocopherol acetate, 500 IU/g), and 7.0 g/kg magnesium tribasic
bManufactured using 978.73 g/kg TD.2040 Global Guinea Pig Diet plus 0.45 g/kg beta-carotene supplied by King Chemical (20 %), 9.42 g/kg vitamin C (l-ascorbyl-2-polyphosphate, 35 %), 4.4 g/kg vitamin E (dl-alpha tocopherol acetate, 500 IU/g), and 7.0 g/kg magnesium tribasic
cManufactured using 942 g/kg TD.2040 Global Guinea Pig Diet plus 1.0 g/kg beta-carotene supplied by King Chemical (20 %), 20.0 g/kg vitamin C (l-ascorbyl-2-polyphosphate, 35 %), 7.0 g/kg vitamin E (dl-alpha tocopherol acetate, 500 IU/g), and 30.0 g/kg magnesium tribasic
Blood Samples
Blood samples (approximately 0.5 mL) were collected from the jugular vein cannula via an implanted vascular access port (Access Technologies; n = 10) or from intracardiac puncture under deep anesthesia prior to euthanasia (n = 6) and serum was harvested. Following sample collection, an equal amount of sodium chloride was administered through the jugular vein cannula to replace the volume of blood collected. The catheter was then locked using Taurolidine citrate solution™ (Access Technologies). The sample was centrifuged and serum was separated and placed into three microcentrifuge tubes for assay, one for vitamins A and E, a second for the magnesium assay, and a third for vitamin C. For stability, the serum for ascorbate analysis was mixed with a preservation buffer containing 10 % meta-phosphoric acid and 2 mg/ml dithiothreitol (DTT). All samples were stored at approximately −20 °C, with protection from light. The serum levels of the actives were measured by the Diagnostic Center for Population and Animal Health at Michigan State University (analysis of vitamins E and A was via HPLC, ascorbic acid analyzed using colorimetric method from Teitz Clinical Chemistry Read On Spectrophotometer, and magnesium assayed via Automated Beckman Coulter AU).
Subject Group #2—10-Day Dietary Pretreatment
Animals
A total of 12 male pigmented guinea pigs (250–300 g) from an approved laboratory animal supplier (Elm Hill) were used. Guinea pigs were housed in the Animal Care Facility at the University of Michigan, and were acclimated as described above. Subjects were screened for normal hearing sensitivity at 12 and 32 kHz in the left ear using the sound-evoked auditory brainstem response (ABR). After confirming normal hearing, subjects were randomly assigned to a nutritionally complete control diet (TD.2040) or the supplemented diet (TD.08623). Ten days after the onset of dietary manipulation, a 16-day regimen of once-daily gentamicin injections began. ABR tests were repeated 14 days after the final gentamicin injection. This experimental protocol was reviewed and approved by the University of Michigan Institutional Animal Care and Use Committee, and all procedures conformed to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Experimental Groups
Animals were assigned to receive a nutritionally complete standard laboratory animal diet (control; TD.2040) or a nutrient-supplemented version of the TD.2040 diet (ACEMg; TD.08623). As per Table 2, the ACEMg diet had 1.0 g/kg β-carotene, 20 g/kg vitamin C (l-ascorbyl-2-polyphosphate), 7 g/kg vitamin E (500 IU/g dl-alpha tocopheryl acetate), and 30 g/kg magnesium citrate (tribasic), added to the basic formulation. The β-carotene was purchased in the form of a stabilized bead product (20 % BC TAB; Kingchem, Allendale, NJ, USA). The dietary treatment was initiated 10 days prior to the start of the gentamicin injections, continued during the 16-day period during which the once-daily gentamicin injections were administered, and was maintained throughout the 2-week post-gentamicin follow-up window.
Electrophysiological Testing: Auditory Brainstem Response
Prior to ABR testing, animals were anesthetized intramuscularly with ketamine (58.8 mg/kg), xylazine (2.4 mg/kg), and acepromazine (1.2 mg/kg) and placed on a water-circulating heating pad to maintain body temperature. Additional anesthetic (ketamine and xylazine) was administered if needed to maintain anesthesia depth sufficient to insure immobilization and relaxation. ABRs were recorded in an electrically and acoustically shielded chamber (Acoustic Systems, Austin, TX, USA). Tucker Davis Technologies (TDT) System III hardware and SigGen/BioSig software (TDT, Alachua, FL, USA) were used to present the stimulus and record responses. Stimulus levels were calibrated using SigCal software (TDT) and a microphone (Bruel & Kjaer type 4136 microphone, type 2619 preamp, type 2804 power supply) that was placed approximately 1 mm from the vinyl tubing used for stimulus delivery. The microphone and the tubing were coupled inside a plastic tube selected to approximate the guinea pig ear canal.
Neural activity in response to brief tone bursts were measured using sterile needle electrodes inserted subcutaneously posterior to each pinna and at the vertex of the skull. Tones were delivered through an EC1 driver (TDT, aluminum enclosure made in-house), with the speculum placed just inside the tragus. Each tone burst was 15 ms in duration, with 1 ms rise/fall times, presented 10 per second. Up to 1,024 responses were averaged for each stimulus level. Responses were collected for stimulus levels in 10-dB steps at higher stimulus levels, with additional 5-dB steps near the threshold. Thresholds were interpolated between the lowest stimulus level where a response was observed, and 5 dB lower, where no response was observed. Animals were injected with warmed saline (10 ml/kg) after initial ABR. The final ABR data were collected 2 weeks after cessation of gentamicin injections.
Aminoglycoside Insult
Gentamicin injections (140 mg/kg/day, s.c.) were delivered once daily for 16 days. Injections started after 10 days on the assigned study diet.
Histological Examinations
Immediately after the final ABR measurement, the animals were deeply anesthetized as described above, injected intracardiac with 1 ml of xylazine and then decapitated. The cochleae were immediately removed and transferred into 4 % paraformaldehyde. Under a dissecting microscope, the bone nearest the apex and the round and oval windows were opened, followed by gentle local perfusion of fixative from the apex. The tissue was kept in fixative for approximately 18–24 h, then the bony capsule and the lateral wall tissues were removed, and the modiolar core carefully removed from the temporal bone. Following permeabilization with Triton X-100 (0.3 %, 30 min), F-actin in the organ of Corti was labeled with rhodamine-conjugated phalloidin (1 %, 60–120 min) to outline hair cells and their stereocilia (Raphael and Altschuler 1991). After washing the tissues with PBS, the organ of Corti was dissected and surface preparations were mounted on glass slides. The tissues were observed under fluorescence microscopy, and the number of missing inner (IHC) and outer (OHC) hair cells were counted from the apex to the base in 0.19 mm segments (as described in Yamashita et al. 2004). Counting was begun approximately 0.76–1.14 mm from the apex, thus omitting the initial irregular most apical part of the cochlear spiral. Percentages of hair cell loss in each 0.19 mm length of tissue were plotted along the cochlear length.
Subject Group #3—28-Day Dietary Pretreatment
Animals
A total of 19 albino male guinea pigs (250–300 g) from an approved laboratory animal supplier (Charles River, Wilmington, MA, USA) were used. Guinea pigs were housed in the Animal Care Facility at the University of Florida. Following arrival, all animals were given at least 48 h to acclimate to the environment and recover from transportation-related stress. Subjects were screened for normal hearing sensitivity at 2, 4, 8, 16, and 24 kHz in the right and the left ear using the sound-evoked ABR. After confirming normal hearing, subjects were assigned to control diet (TD.2040) or supplemented diet (TD.08623). ABR tests were repeated after 28 days on the study diet; on the 14th day of gentamicin injections; and 2, 4, 6, and 9 weeks post-gentamicin. This experimental protocol was reviewed and approved by the University of Florida Institutional Animal Care and Use Committee, and all procedures conformed to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Experimental Groups
Animals were assigned to receive the nutritionally complete standard laboratory animal diet (control; TD.2040) or the nutrient-supplemented version of the TD.2040 diet (ACEMg; TD.08623). The TD.2040 and TD.08623 diets were identical to the diets described above; however, the dietary treatments were initiated 28 days prior to the start of the gentamicin injections, continued during the 14-day period during which the once-daily gentamicin injections were administered and were maintained throughout the 9-week post-gentamicin follow-up window. The 28-day pretreatment duration was selected based on the protection against NIHL in mice that were maintained on an enhanced diet for 28 days prior to noise (Le Prell et al. 2011b).
Electrophysiological Testing: Auditory Brainstem Response
Prior to ABR testing, animals were anesthetized with ketamine (40 mg/kg, s.c.) and xylazine (8–10 mg/kg, s.c.) and placed on a water-circulating heating pad to maintain body temperature. During ABR tests, neural activity in response to brief tone pips was measured using sterile, 27-gauge electrodes inserted subcutaneously posterior to each pinna and at the vertex of the skull. ABRs were recorded in a double-walled sound attenuating chamber (IAC Acoustics, Bronx, NY, USA). TDT System III hardware and SigGen/BioSig software were used to present the stimulus and record responses. Tones were delivered through an EC1 driver (TDT, aluminum enclosure made in-house), with the speculum placed just inside the tragus. Stimulus levels were calibrated using SigCal software (TDT) and a microphone placed approximately 1 mm from the vinyl tubing inside a plastic tube approximating the guinea pig ear canal as described above. Tone levels were decreased from 90 to 0 dB SPL in 10-dB increments. Each pip was 10 ms in duration, and tones were repeated at a rate of 17/s until 1,026 responses were acquired. Animals were injected with warmed saline (10 ml/kg) at the conclusion of any procedure requiring anesthesia, with the exception of the final 9-week post-gentamicin ABR test. At the completion of the 9-week post-gentamicin ABR test, animals received an overdose of sodium pentobarbital, and cochlear tissues were harvested.
Aminoglycoside Insult
Gentamicin injections (140 mg/kg/day) were delivered subcutaneously once daily for 14 days, with the site of injection rotated daily. Injections started after 28 days on the assigned study diet.
Histological Examinations
Immediately after the final ABR measurement, the animals were deeply anesthetized using an overdose of sodium pentobarbitol (2 ml i.p.), then decapitated. The cochleae were immediately removed, transferred into 4 % methanol-free formaldehyde, for dissection and gentle local perfusion, with the tissues then kept in fixative for 2–3 h. All additional dissection and processing followed the procedures described above.
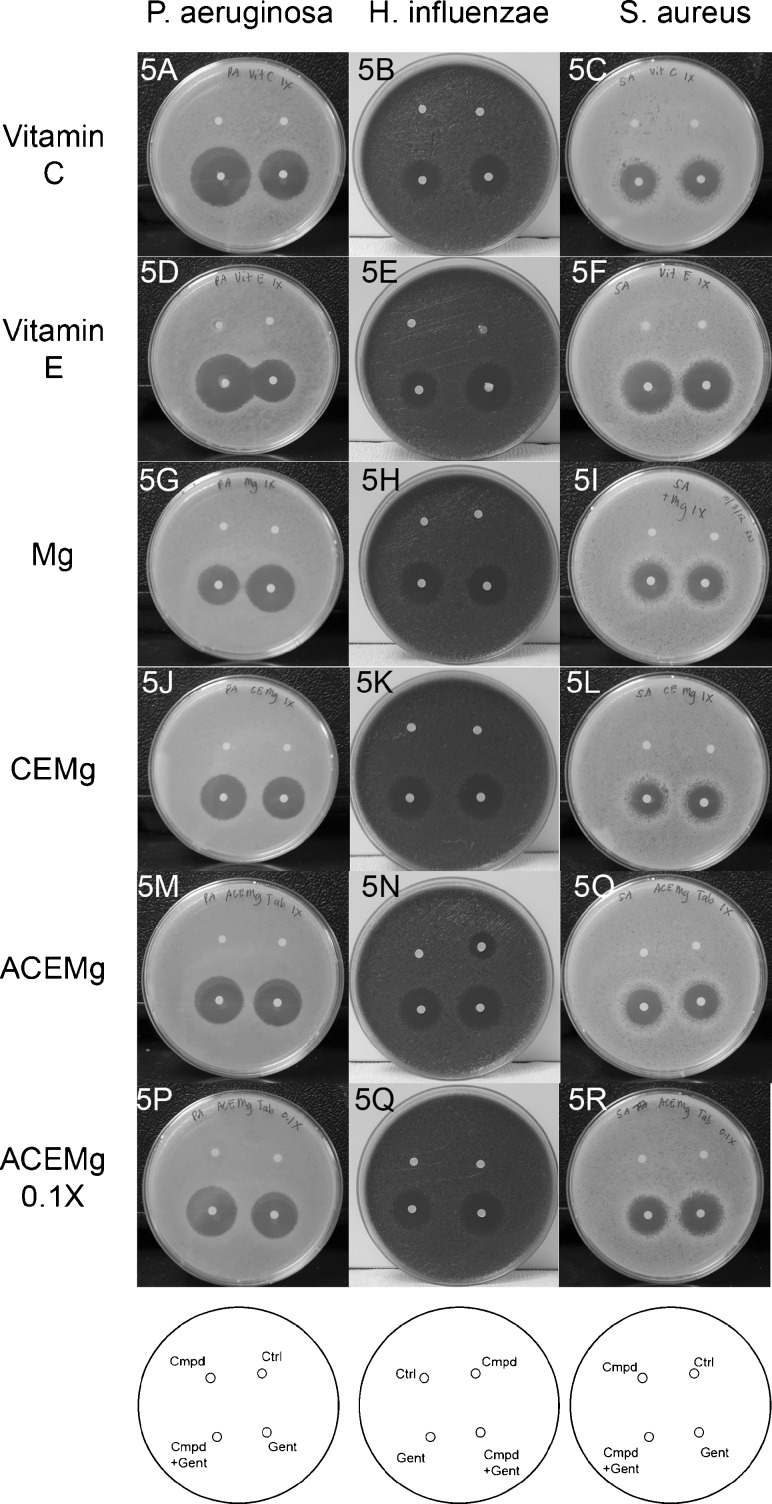
In Vitro Assessment of Antimicrobial Efficacy
The antimicrobial efficacy of gentamicin against a strain of Pseudomonas aeruginosa (ATCC 19142), Staphylococcus aureus (ATCC 29213), and Haemophilus influenza (ATCC 62094) was assessed using the Kirby-Bauer disk-diffusion method. Briefly, a cryopreserved strain of P. aeruginosa, S. aureus, and H. influenzae were quad-streaked onto appropriate agar plates and incubated overnight at 37 °C. For P. aeruginosa and S. aureus, three to five isolated colonies were selected, suspended in growth media, and incubated overnight with constant agitation at 37 °C. The bacteria were transferred to fresh media and grown to early log phase (optical density of about 0.2–640 nm). The turbidity of the log phase bacteria was adjusted to 0.5 McFarland standard. For H. influenzae, the direct colony suspension method was used, where colonies are taken directly from 18-h chocolate agar plates in preparing the bacterial suspension. Using aseptic technique, 100 μL of the log-phase bacteria was streaked into the plates to form bacterial lawn. The plates were allowed to dry for 5 min. Using a flame-sterilized forceps, the filter paper disks containing the specific agents with and without 10 μg gentamicin (diluted from a 5 mM stock solution) is then placed on the surface of the agar. Single agents added to the dish included ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA), vitamin E (over-the-counter capsule, Nature Made), and magnesium sulfate (Sigma-Aldrich); a combination of vitamins C and E and Mg were also tested. Finally, a proprietary tablet containing β-carotene, vitamins C and E, and magnesium was ground up and used both “as is” and also in a solution diluted tenfold. Plates were incubated overnight at 37 °C and the zone of inhibition was assessed. Each treatment was run in triplicate, and an observer masked to treatment condition picked a representative image.
STATISTICAL ANALYSIS
For subjects in which threshold shift was assessed at only one time (pigmented guinea pigs), statistical comparisons were completed using a two-way analysis of variance (ANOVA) with treatment and frequency as independent variables; the dependent variable was threshold shift. For subjects in which threshold shift was assessed at more than one time (albino guinea pigs), statistical comparisons were completed using two-way repeated measures ANOVA with treatment as an independent variable and time treated as a repeated independent variable; the dependent variable was threshold shift. Within each frequency, we evaluated the potential for a statistically significant main effect of treatment (standard diet versus supplemented diet), a statistically significant main effect of time (did gentamicin-induced threshold shift vary as a function of test time, with tests on days 0, 44, 58, 72, 86, and 107 post-gentamicin), and we assessed whether there was a statistically significant interaction effect for treatment × time. Given significant interaction effects at most frequencies, interpretation was based on pair-wise comparisons of the control vs treated within test times, with corrections for multiple comparisons. All statistical analysis was completed using the statistical functions in SigmaPlot. The comparisons of nutrient levels in plasma samples were completed using t tests (β-carotene, vitamin E, and Mg) and the nonparametric equivalent, the Mann–Whitney U test (for vitamin C, which failed to meet the normality assumptions required for use of a t test) at the 20-day time point.
RESULTS
Plasma Levels: β-carotene
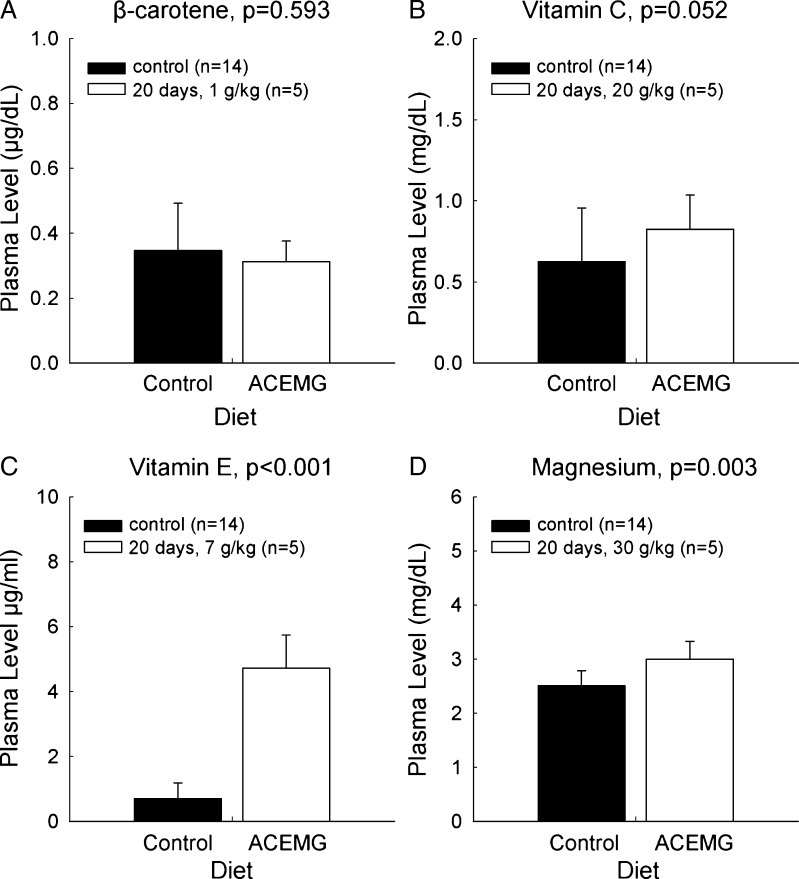
The control diet included 36.3 mg of β-carotene per kilogram of chow. The level of β-carotene was increased by 90 mg per kilogram of chow (TD.0726), 450 mg per kilogram of chow (TD.08435), and 1,000 mg per kilogram of chow (TD.08623) The level of β-carotene in plasma was below the level of detection in all samples (data not shown) from all guinea pigs, a finding that is identical to that of Le Prell et al. (2011a), who fed guinea pigs an oral suspension of 2.1 mg/kg β-carotene. Since β-carotene can be converted into vitamin A, samples were also evaluated for the presence of vitamin A. Vitamin A was present at baseline but there was no increase in plasma concentrations in either the sequential measurements within dietary conditions or when the levels measured prior to dietary manipulation were compared to the levels measured after 20 days of 1,000 mg/kg chow fortification (Fig. 1a; t = 0.545, df = 18; p = 0.593).
FIG. 1.
Plasma concentrations of β-carotene (A), vitamin C (B), vitamin E (C), and magnesium (D) while maintained on a nutritionally complete control diet, and after 20 days of maintenance on a custom diet (TD.08623) with supplemented levels of the above nutrients. There was no reliable change in the plasma concentration of β-carotene. There were statistically reliable increases in the levels of the other active agents.
Plasma Levels: Vitamin C
The control diet included 1.05 g of ascorbic acid per kilogram of chow. The amount of vitamin C additive was 9.42 g/kg chow in the first diets (TD.0726 and TD.08435) and 20 g/kg chow in the final diet (TD.08623). The level of vitamin C in serum increased over the first 14 days in the six animals maintained on the lower-level additive and statistically reliable differences in vitamin C levels were revealed when premanipulation levels were compared to the levels measured after 20 days on the higher level supplement (Fig. 1b; t = 71.500; p = 0.052).
Plasma Levels: Vitamin E
The control diet included 115.8 mg of alpha-tocopherol per kilogram of chow. The amount of vitamin E additive was 4.4 g/kg chow in the first diets (TD.0726 and TD.08435) and 7 g/kg chow in the final diet (TD.08623). The level of vitamin E in serum increased over the first 14 days in the six animals maintained on the lower-level additive, and statistically reliable differences in vitamin E levels were revealed when premanipulation levels were compared to the levels measured after 20 days on the higher-level supplement (Fig. 1C; t = −12.207; df = 18; p < 0.001).
Plasma Levels: Magnesium
The control diet included 0.33 % magnesium (3.3 g of magnesium per kilogram chow). The amount of magnesium additive was 7 g/kg chow in the first diets (TD.0726 and TD.08435) and 30 g/kg chow in the final diet (TD.08623). The level of magnesium in plasma increased over the first 14 days in the six animals maintained on the lower level additive, and statistically reliable differences in magnesium levels were revealed when premanipulation levels were compared to the levels measured after 20 days on the higher level supplement (Fig. 1D; t = −3.443, df = 18; p = 0.003).
Threshold Shift
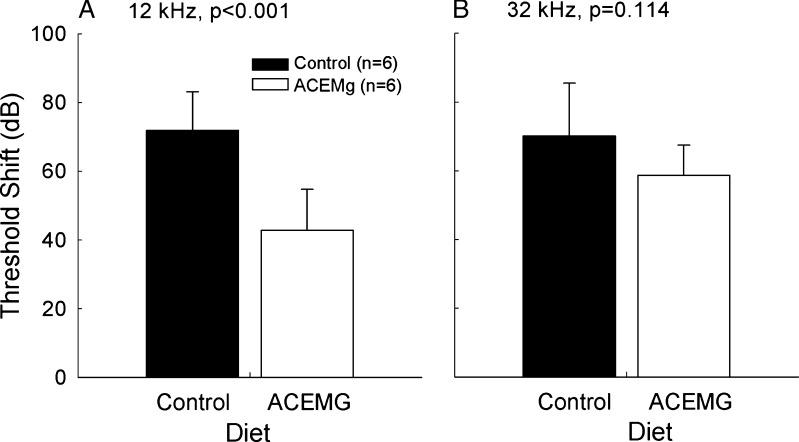
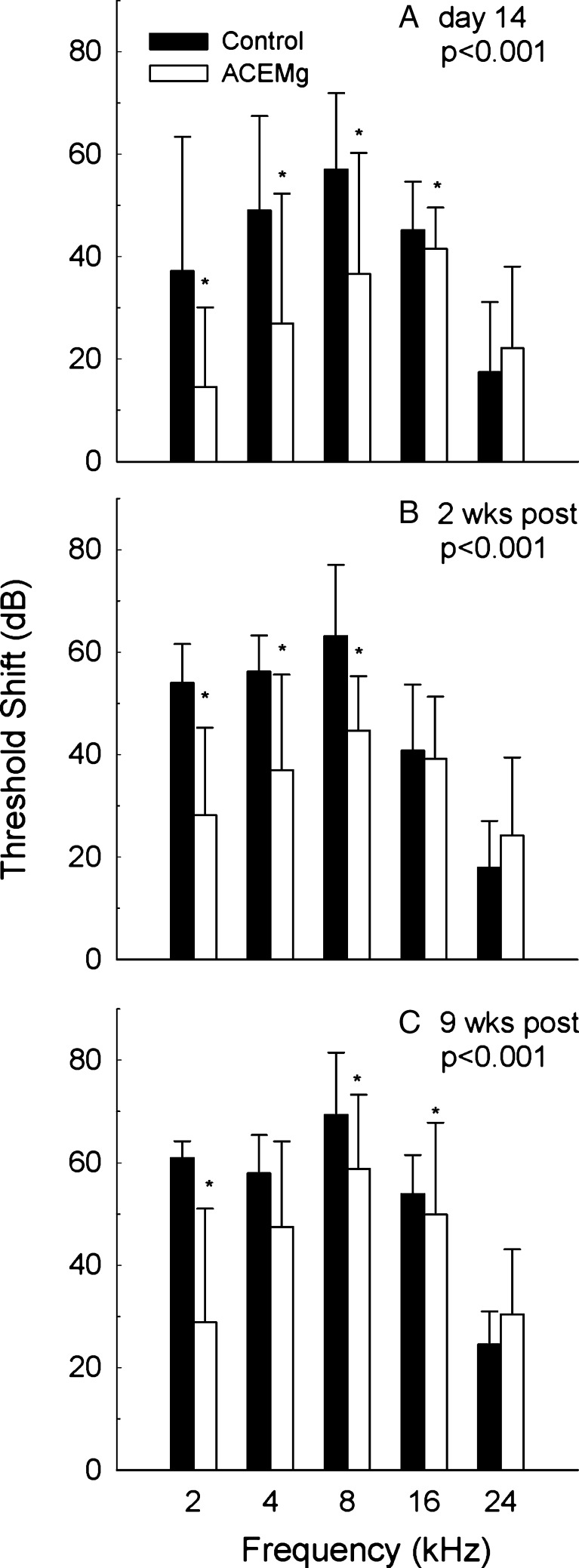
Significant gentamicin-induced hearing loss was observed in both subject cohorts (see Figs. 2 and 3). The effects of aminoglycoside antibiotics on hearing are well known to begin at higher frequencies, and, in the first subject cohort (the pigmented animals tested at the University of Michigan), threshold tests were limited to 12 and 32 kHz. There was a statistically significant main effect of diet (F = 16.957, df = 1,23; p < 0.001). Pairwise comparisons, corrected for multiple pair-wise comparisons using the Student-Newman-Keuls method, revealed a statistically reliable group difference as a function of diet at 12 kHz (Fig. 2A: q = 5.897, p < 0.001). However, there was not a statistically reliable reduction in threshold shift at the highest frequency, 32 kHz (Fig. 2B; q = 11.50, p = 0.114). The second cohort of subjects (albino guinea pigs tested at the University of Florida) was tested at frequencies ranging from 2 to 24 kHz. Tests conducted on the 14th day of gentamicin administration (Fig. 3A; F = 24.887, df = 1,64; p < 0.001), 2 weeks post-gentamicin (Fig. 3B; F = 15.662, df = 1,69; p < 0.001), 4 weeks post-gentamicin (F = 16.509, df = 1,69; p < 0.001), 6 weeks post-gentamicin (F = 23.293, df = 1,69; p < 0.001), and 9 weeks post-gentamicin (F = 33.720, df = 1,69; p < 0.001) all revealed robust, statistically reliable, reductions in drug-induced threshold shift. Pairwise comparisons, corrected using Student-Newman-Keuls method, revealed statistically significant differences at 2, 4, and 8 kHz at most test times (see Table 3). However, the statistical reliability of the differences at 16 kHz was not consistently significant at the alpha = 0.05 level, and there was no evidence for protection at the higher test frequencies (16 and 24 kHz). Functional protection at lower frequencies was maintained throughout the study duration (Fig. 3C).
FIG. 2.
In pigmented guinea pigs, tested at the University of Michigan, functional outcomes were reliably improved at 12 kHz (A), but not at 32 kHz (B). Thresholds were assessed 2 weeks after the final gentamicin injection.
FIG. 3.
In albino guinea pigs, tested at the University of Florida, functional outcomes were reliably improved at 2, 4, and 8 kHz on the 14th d of gentamicin (A), as well as 2 weeks post-gentamicin (B). However, there was no evidence of protection at 16 or 24 kHz. At the final test time, 9 weeks after the final gentamicin injections (C), there was a significant main effect of treatment; statistically reliable differences were observed at 2 and 8 kHz, but the reliability of the 4 kHz difference observed at earlier test times was reduced.
Table 3.
Threshold shift outcomes from two-ANOVA within frequencies; treatment and time treated as independent variables
| 2 kHz (F = 56.177, df = 1,83; p < 0.001) | 4 kHz (F = 17.456, df = 1,83; p < 0.001) | 8 kHz (F = 45.196, df = 1,83; p < 0.001) | 16 kHz (F = 17.277, df = 1,83; p < 0.001) | 24 kHz (F = 1.332, df = 1,83, p = 0.252) | |
|---|---|---|---|---|---|
| Pre (baseline 1 vs baseline 2) | q = 0.467, p = 0.742 | q = 0.309, p = 0.828 | q = 1.344, p = 0.345 | q = 0.560, p = 0.694 | – |
| Day 14 of gentamicin | q = 3.658, p = 0.012 | q = 4.118, p = 0.005 | q = 6.665, p < 0.001 | q = 5.183, p < 0.001 | – |
| 2 Weeks post | q = 4.687, p = 0.002 | q = 3.118, p = 0.031 | q = 3.956, p = 0.001 | q = 0.546, p = 0.700 | – |
| 4 Weeks post | q = 6.114, p < 0.001 | q = 1.748, p = 0.220 | q = 4.549, p = 0.002 | q = 2.350, p = 0.101 | – |
| 6 Weeks post | q = 5.638, p < 0.001 | q = 3.068, p = 0.033 | q = 5.017, p < 0.001 | q = 2.422, p = 0.091 | – |
| 9 Weeks post | q = 6.215, p < 0.001 | q = 1.979, p = 0.166 | q = 4.171, p = 0.004 | q = 3.169, p = 0.028 | – |
Hair Cell Counts
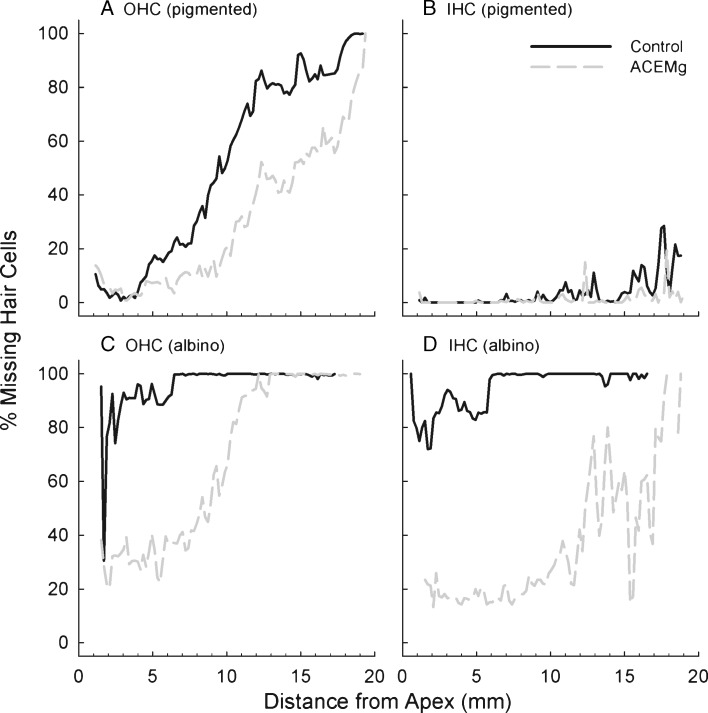
Significant gentamicin-induced ototoxicity, measured in the form of drug-induced hair cell death, was observed in both the pigmented guinea pigs treated with 140 mg/kg/day × 16 day and the albino guinea pigs treated with 140 mg/kg/day × 14 day (Fig. 4). However, there was significantly greater hair cell loss in the albino animals than in the pigmented animals, even with the shorter gentamicin regimen for the albino animals. Albino guinea pigs are more vulnerable to the effects of aminoglycoside antibiotics (Conlee et al. 1989, 1991, 1995) and thus greater hair cell loss in cohort 3 (albino) than in cohort 2 (pigmented) is not surprising.
Fig. 4.
In pigmented guinea pigs, outer hair cell (OHC) survival was improved by dietary manipulation (A), but there was little inner hair cell (IHC) loss in either dietary group (B). In albino guinea pigs, outer hair cell (OHC) survival was improved by dietary manipulation (C), and inner hair cell (IHC) survival was also enhanced (4).
In the pigmented guinea pig controls, there was significant OHC loss, particularly in the basal half of the cochlea; OHC loss was reduced when animals were maintained on the nutrient enhanced TD.08623 diet from 10 days prior to the start of the gentamicin injections (Fig. 4A). There was very little inner hair cell loss in any of the pigmented guinea pig subjects maintained on either diet (Fig. 4B). In the albino guinea pig controls, there was virtually complete loss of both OHCs (Fig. 4C) and IHCs (Fig. 4D), across the entire length of the cochlear spiral. In spite of this strain-related increase in sensitivity, it was clear that both the OHC (Fig. 4C) and IHC (Fig. 4D) populations were protected against gentamicin insult when animals were maintained on the nutrient enhanced TD.08623 diet from 28 days prior to the start of the gentamicin injections. Protection was more complete for the IHC population than the OHC population, and the best protection was observed in the apical half of the cochlea. Taken together, maintenance on the TD.08623 reduced hair cell death, with protection observed in both the 10- and 28-day pretreatment groups, and with the amount of protection varying with the overall level of insult; i.e., protection was less complete in the more vulnerable albino guinea pigs than in the pigmented subjects.
These data suggest that with better survival of the hair cells in the region located around 14 mm from the apex (corresponding to the 12 kHz frequency, as described by Greenwood 1990), there is a corresponding improvement in functional outcome (Fig. 2A).
Gentamicin Antimicrobial Efficacy
For tests assessing gentamicin antimicrobial efficacy against P. aeruginosa and H. influenza, which are both Gram negative, the ascorbic acid and vitamin E appeared to improve gentamicin antimicrobial efficacy (see Fig. 5A, B, D, E); this effect was not observed for S. aureus, which is Gram positive (see Fig. 5C, F). In contrast, the magnesium sulfate may have had some slight inhibition of gentamicin efficacy against all three bacteria, with the most dramatic effect observed in P. aeruginosa (see Fig. 5G, H, I). While that outcome would suggest potential concerns for a magnesium-based therapy, neither the CEMg combination (see Fig. 5J, K, L) nor the ACEMg tablet appeared to compromise gentamicin efficacy against the three bacteria (see Fig. 5M, N, O, P, Q, R).
FIG. 5.
Kirby-Bauer test assessing zone of inhibition of bacterial growth by gentamicin (“Gent”), compounds of interest (“Cmpd”), including vitamin C, vitamin E, magnesium, and combinations of these three agents alone (CEMg) or with the addition of β-carotene (ACEMg), the compound of interest plus gentamicin (“Cmpd+Gent”), and a control (“Ctrl”) with no agent added to the disk. The diagram at the bottom of each panel indicated the placement of each condition within the bacterial test dishes; the order was reversed for H. influenzae (B, E, H, K, N, and Q), relative to P. aeruginosa (A, D, G, J, M, and P) and S. aureus (C, F, I, L, O, and R).
DISCUSSION
The data from these studies demonstrated that oral dietary manipulation of nutrient levels produced reliable increases in serum concentration of three of the active agents (vitamins C and E and Mg), and that these increased serum levels resulted in a significant reduction in drug-induced threshold shift as measured at times extending to 9 weeks post-gentamicin. Outcomes were demonstrated in two strains of guinea pigs (pigmented and albino) and in two different laboratories, with masked analysis of treatment condition during threshold assessment and hair cell quantification. Here, the β-carotene, vitamins C and E, and Mg, were all delivered orally, as a food supplement, in the form of a custom dietary formulation. Animals maintained on a high-level nutrient supplement had less gentamicin-induced hearing loss at lower frequencies (12 kHz and below) and less hair cell loss, particularly in the upper half of the cochlea.
Plasma levels of the nutrients were assessed for three different sequentially increasing formulations; a diet that increased the levels of vitamins C, E, and Mg was selected for in vivo testing in animals that received gentamicin insult. It is possible that increasing the nutrient levels further might improve the degree of protection, as there are data suggesting a dose–response curve exists for mice exposed to noise insult, with higher dietary nutrient content providing better protection against noise insult (Le Prell et al. 2011b). However, in addition to the caspase-dependent apoptotic cell death pathways that are initiated by toxic accumulation of free radicals, there are other caspase-independent cell death pathways activated by aminoglycosides (for recent review, see Steyger 2011). For example, translocation of endonuclease G from the mitochondria to the nucleus (Jiang et al. 2006a) and disruption of phosphoinositide signaling (Jiang et al. 2006b) were observed in kanamycin-treated mice. Thus, it is possible that antioxidant strategies may never be wholly effective with respect to complete prevention of ototoxicity, which could explain why antioxidant agents have provided incomplete protection in several studies. Protection against gentamicin-induced ototoxicity has been incomplete for two different d-methionine based dose paradigms (once-daily and twice-daily d-methionine treatments, see Sha and Schacht 2000), an alpha-tocopherol-based dosing paradigm (Fetoni et al. 2004), and a salicylate-based study (Sha and Schacht 1999b) in addition to other studies as summarized in Table 1.
There are some cases in which treatment benefit for antioxidant agents have been assessed after local drug delivery, directly into the inner ear. One paradigm was based on delivery of a viral vector expressing human bcl-2 into the inner ear of the mouse (Pfannenstiel et al. 2009). This was very effective in preventing gentamicin-induced hearing loss, but there are technical and safety challenges to be resolved with respect to gene therapy in the human inner ear (Fukui and Raphael 2013; Rivolta 2013; Xia and Yin 2013). Intracochlear delivery does not necessarily improve outcomes. When the nitric oxide (NO) inhibitor nitro-l-arginine methyl ester (l-NAME) was delivered via repeat injections through the tympanic membrane, l-NAME reduced gentamicin-induced hearing loss in the high-frequency range, but gave no protection in the middle or low frequencies (Nordang and Anniko 2005). A surgical approach to the cochlea to allow either gelfoam to be placed on the round or a drug delivery cannula to be inserted through the wall of the cochlea may have allowed agents including dexamethasone, melatonin, and tacrolimus to provide greater protection, but ABR thresholds were assessed using clicks, making frequency specific conclusions more difficult (Bas et al. 2012). Therapeutic interventions that require surgical intervention are unlikely to have widespread clinical utility and they will be difficult to adopt in developing countries, where aminoglycoside use is greatest. Thus, antioxidant strategies based on systemic delivery of agents that reduce the effects of ototoxicity of life-saving drugs will be one of the safest and most cost-effective approaches to protection of hearing function.
Although we have provided a frank account of the key limitation noted to date for antioxidant therapies; i.e., incomplete protection, it must be noted that animal models typically use gentamicin doses that are much higher, and thus much more toxic, than those used clinically in patients. Manufacturer-recommended gentamicin doses for conventional dosing in patients with normal renal function are 3–5 mg/kg/day, divided into three equal daily doses [see Chapter 4, The Aminoglycoside Antibiotics, in 97; pp. 97–206] and extended-interval doses are slightly increased (4–7 mg/kg/day for gentamicin; see Bauer 2008). Other dosing paradigms, such as once or twice daily regimens, are also used clinically. Regardless, human doses used clinically do not begin to approach the higher level of insult used here (140 mg/kg per day) and in other animal-based studies. It is possible that no physiological amount of antioxidants, scavengers, or chelators could be sufficient to prevent cell death with that dosing model. We would not expect aminoglycoside dose and duration in humans to produce the extreme pathology as in our animal model (which follows common animal models used by others); thus, it may be possible to obtain more complete protection in human patients receiving conventional therapeutic dosing with aminoglycoside agents.
Given the massive trauma observed in gentamicin-only controls, it is indeed compelling that protection was observed. These data clearly confirm the potential for a dietary nutrient-based antioxidant strategy to attenuate gentamicin-induced cell death and hearing loss. These initial data set the stage for additional studies needed to answer questions that will be important for the translation of these findings to clinical populations. For example, additional data could be helpful in determining whether modified dosing protocols improve benefit, and also for determining if all four active agents are required to confer protection. The lack of change in β-carotene levels clearly draws into question the extent to which this specific nutrient contributes to the observed protection. Presumably, β-carotene either did not reach the guinea pig blood stream in significant quantities, or it was rapidly absorbed from the blood stream into surrounding tissues—two possibilities previously suggested when a 2.1 mg/kg/day oral dose failed to result in detectable levels in guinea pig plasma samples (Le Prell et al. 2011a). The metabolism of carotenoids by humans remains incompletely understood for a variety of reasons, including not only technical and methodological challenges, but also interactions across dietary antioxidants, interactions between carotenoids, fats, and fiber, and the lack of a single good animal model in which absorption and metabolism are similar to that in human subjects (Kim and Kim 2011; Maiani et al. 2009). It is, however, quite clear that β-carotene supplements increase plasma levels of β-carotene (for recent review, see Maiani et al. 2009) and that conversion of carotenoids to retinol and/or retinoic acid is contingent on retinol deficiency. When adequate retinol stores exist, carotenoids circulate in plasma in their carotenoid form, directly preventing toxicity from carotenoid sources (Solomons and Orozco 2003). This unanswered question will require future investigation using single agents, as well as combined agents.
Additional mechanistic studies will also be important. Here, we assessed plasma levels of the nutrients (following protocols used by Le Prell et al. 2011a). Beta carotene was not been measured in detectable levels in plasma samples from guinea pigs and may not be available in the inner ear. In contrast, there is a clear relationship between dietary Mg and Mg levels measured in cochlear perilymph (Scheibe et al. 1999, 2002; Xiong et al. 2013), with perilymph levels being significantly reduced relative to plasma levels. The increased plasma levels measured here are consistent with plasma levels measured in other studies, in which increased perilymph concentrations were also detected (Scheibe et al. 2002). At this time, we have not found any reports documenting the measurement of beta-carotene, vitamins A, C, or E, in perilymph. Based on protection of auditory function against various insults with each of these additional agents (although typically with longer term dosing; for review, see Le Prell and Bao 2011), it would be surprising if these vitamins were not biologically available in the inner ear fluids. However, this prediction has not been empirically tested. In addition, while we assume that the functional protection achieved here using nutrient manipulation was mediated largely by the known antioxidant properties of these active agents, we did not explicitly assess oxidative stress. Lipid hydroperoxides, malondialdehyde, and isoprostanes may provide biomarkers for changes in in vivo oxidative stress in other mechanism-based studies (Evans and Halliwell 1999).
One of the more important mechanistic questions is whether protection conferred here was perhaps driven by vitamin E, as Fetoni et al. (2003, 2004) have demonstrated reductions in gentamicin-induced toxicity using alpha-tocopheral. In those studies, there was approximately 20 dB of protection across frequencies, from 2 to 16 kHz, in albino guinea pigs that were treated with 100 mg/kg/day gentamicin, with or without 100 mg/kg alpha-tocopherol i.m. (see Table 1). In that study, protection was assessed on days 14–18 of treatments, which is consistent with our day 14 tests (which were shown in Fig. 3A). The dietary administration of the agents used here achieved approximately equivalent protection at 2–8 kHz, even with the higher gentamicin doses used in our study. The dietary combination provided less compelling benefit at 16 kHz, in contrast to the results achieved by Fetoni et al.; direct comparisons are difficult, however, given design differences including different gentamicin doses and also different methods of delivery. Given evidence of protection here, critical next steps must include single agent and combinatorial approaches to determine which agents are necessary and sufficient, whether there is a synergistic benefit to multiple agents, and also mechanistic studies to confirm the pathways through which protection is mediated.
In summary, there is good reason for enthusiasm regarding antioxidant agents. The data from two clinical trials, using aspirin as an investigational agent, clearly confirm the promise of antioxidant agents for reducing drug-induced hearing loss in human patients that require these agents for their life-saving therapeutic benefit (Sha et al. 2006; Behnoud et al. 2009). The data presented here document preservation of hair cells, and protection of auditory thresholds, in two different guinea pig strains, tested in two different laboratories, using an oral nutrient supplement. Finally, there was no reduction in antibacterial activity of gentamicin against three different bacteria. The data presented here build on a compelling literature documenting the role of oxidative stress in acquired hearing loss, and demonstrate the benefit of free radical scavengers. There is an important role of free radical formation in hearing loss after cisplatin (Cheng et al. 2005; Dehne et al. 2001; Devarajan et al. 2002) and noise (for reviews, see Le Prell and Bao 2011; Abi-Hachem et al. 2010; Poirrier et al. 2010; Henderson et al. 2006; Le Prell et al. 2007b). Oxidative stress has also been implicated in age-related hearing loss (ARHL; Seidman 2000; Le and Keithley 2007; Jiang et al. 2007). Human clinical tests assessing the translation of different antioxidant agents are a critical next step to reduce the humanitarian and health care costs associated with each of these insults.
SUMMARY AND CONCLUSIONS
There is a significant component of gentamicin-induced cell death in the inner ear that is driven by toxic accumulation of free radicals. Endogenous antioxidant systems provide some defense, but are ultimately overwhelmed with ongoing aminoglycoside treatment. Agents that enhance endogenous defense, or serve to directly scavenge toxic free radicals, enhance hair cell survival and preserve hearing function. Complete protection using systemic antioxidant therapy has been elusive. Nonetheless, significant protection is better than no protection, and multiple studies have revealed benefits in the lower frequency regions, which are particularly critical for human speech discrimination and understanding. Safety studies in humans are needed to assure that antimicrobial efficacy is not comprised and efficacy studies are needed to determine effective dosing for human patients. With that evidence in hand, it may be possible to improve patient care and health outcomes and preserved auditory function.
Acknowledgments
Portions of this research were presented at the 36th Midwinter Meeting of the Association for Research in Otolaryngology (Le Prell et al. 2012) and in undergraduate Honor’s Theses submitted to the College of Public Health and Health Professions by E. Rudnick and M. Nelson. Support for this research was provided by the Ruth and Lynn Townsend Professorship and the Center for Communication Disorders at the University of Michigan, the College of Public Health and Health Professions and the Hearing Research Center at the University of Florida, and NIDCD P30 grant DC005188. The authors thank Glenn Green, M.D. and Patrick Antonelli, M.D., for helpful discussions of human clinical dosing. The authors thank Justin Murray, Jessica Santos, Jennifer Benson, Kimberly Wearne, and Angela Vandoli for technical assistance. All authors have read the journal’s policy on disclosure of potential conflicts of interest and all authors have disclosed any financial or personal relationship with organizations that could potentially be perceived as influencing the described research.
Disclosure
Dr. Miller is the sole inventor on US Patent 8,338,397, “Composition and method of treating side effects from antibiotic treatment,” which is assigned to the University of Michigan.
Abbreviations
- ABR
Auditory brainstem response
- ACEMg
Vitamins A, C, E, and magnesium, delivered in combination
- ANOVA
Analysis of variance
- C
Celsius
- EHF
Extended high frequency
- FDA
US Food and Drug Administration
- GPx
Glutathione peroxidase
- GSH
Glutathione
- HIV
Human immune-deficiency virus
- kHz
Kilohertz
- MDR-TB
Multidrug-resistant tuberculosis
- Mg
Magnesium
- mg
Milligram
- kg
Kilogram
- OHC
Outer hair cell
- i.p.
Interperitoneal
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- SOD
Superoxide dismutase
- VAP
Vascular access port
References
- Abi-Hachem RN, Zine A, Van De Water TR. The injured cochlea as a target for inflammatory processes, initiation of cell death pathways and application of related otoprotectives strategies. Recent Patents on CNS Drug Discovery. 2010;5:147–163. doi: 10.2174/157488910791213121. [DOI] [PubMed] [Google Scholar]
- Ahmed RM, Hannigan IP, MacDougall HG, Chan RC, Halmagyi GM. Gentamicin ototoxicity: a 23-year selected case series of 103 patients. Med J Aust. 2012;196:701–704. doi: 10.5694/mja11.10850. [DOI] [PubMed] [Google Scholar]
- American Academy of Audiology (2009) Position statement and clinical practice guidelines: ototoxicity monitoring. http://www.audiology.org/resources/documentlibrary/Pages/OtotoxicityMonitoring.aspx. Accessed 30 Oct 2012
- Atsmon J, Dolev E. Drug-induced hypomagnesaemia: scope and management. Drug Saf. 2005;28:763–788. doi: 10.2165/00002018-200528090-00003. [DOI] [PubMed] [Google Scholar]
- Backus RM, De Groot JC, Tange RA, Huizing EH. Pathological findings in the human auditory system following long-standing gentamicin ototoxicity. Arch Otorhinolaryngol. 1987;244:69–73. doi: 10.1007/BF00458549. [DOI] [PubMed] [Google Scholar]
- Bas E, Van De Water TR, Gupta C, et al. Efficacy of three drugs for protecting against gentamicin-induced hair cell and hearing losses. Br J Pharmacol. 2012;166(6):1888–1904. doi: 10.1111/j.1476-5381.2012.01890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basappa J, Turcan S, Vetter DE. Corticotropin-releasing factor-2 activation prevents gentamicin-induced oxidative stress in cells derived from the inner ear. J Neurosci Res. 2010;88:2976–2990. doi: 10.1002/jnr.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer L. Applied clinical pharmacokinetics. New York: McGraw-Hill Medical; 2008. [Google Scholar]
- Behnoud F, Davoudpur K, Goodarzi MT. Can aspirin protect or at least attenuate gentamicin ototoxicity in humans? Saudi Med J. 2009;30:1165–1169. [PubMed] [Google Scholar]
- Best EJ, Gazarian M, Cohn R, Wilkinson M, Palasanthiran P. Once-daily gentamicin in infants and children: a prospective cohort study evaluating safety and the role of therapeutic drug monitoring in minimizing toxicity. Ped Inf Dis J. 2011;30(10):827–832. doi: 10.1097/INF.0b013e31821e405d. [DOI] [PubMed] [Google Scholar]
- Bindu LH, Reddy PP. Genetics of aminoglycoside-induced and prelingual non-syndromic mitochondrial hearing impairment: a review. Int J Audiol. 2008;47:702–707. doi: 10.1080/14992020802215862. [DOI] [PubMed] [Google Scholar]
- Campbell KCM, Le Prell CG. Potential therapeutic agents. Semin Hear. 2012;33:97–113. [Google Scholar]
- Chen KS, Bach A, Shoup A, Winick NJ. Hearing loss and vestibular dysfunction among children with cancer after receiving aminoglycosides. Ped Blood Cancer. 2013;60(11):1772–1777. doi: 10.1002/pbc.24631. [DOI] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13:343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- Choung YH, Taura A, Pak K, Choi SJ, Masuda M, Ryan AF. Generation of highly-reactive oxygen species is closely related to hair cell damage in rat organ of corti treated with gentamicin. Neuroscience. 2009;161:214–226. doi: 10.1016/j.neuroscience.2009.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choung YH, Kim SW, Tian C, et al. Korean red ginseng prevents gentamicin-induced hearing loss in rats. Laryngoscope. 2011;121:1294–1302. doi: 10.1002/lary.21756. [DOI] [PubMed] [Google Scholar]
- Clark CH. Toxicity of aminoglycoside antibiotics. Mod Vet Pract. 1977;58:594–598. [PubMed] [Google Scholar]
- Clerici WJ, Yang L. Direct effects of intraperilymphatic reactive oxygen species generation on cochlear function. Hear Res. 1996;101:14–22. doi: 10.1016/s0378-5955(96)00126-8. [DOI] [PubMed] [Google Scholar]
- Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98:116–124. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- Coffin AB, Reinhart KE, Owens KN, Raible DW, Rubel EW. Extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Hear Res. 2009;253:42–51. doi: 10.1016/j.heares.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colding H, Andersen EA, Prytz S, Wulffsberg H, Andersen GE. Auditory function after continuous infusion of gentamicin to high-risk newborns. Acta Paediatr Scand. 1989;78:840–843. doi: 10.1111/j.1651-2227.1989.tb11160.x. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Gill SS, McCandless PT, Creel DJ. Differential susceptibility to gentamicin ototoxicity between albino and pigmented guinea pigs. Hear Res. 1989;41:43–51. doi: 10.1016/0378-5955(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Jensen RP, Parks TN, Creel DJ. Turn-specific and pigment-dependent differences in the stria vascularis of normal and gentamicin-treated albino and pigmented guinea pigs. Hear Res. 1991;55:57–69. doi: 10.1016/0378-5955(91)90092-n. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Bennett ML, Creel DJ. Differential effects of gentamicin on the distribution of cochlear function in albino and pigmented guinea pigs. Acta Otolaryngol (Stockh) 1995;115:367–374. doi: 10.3109/00016489509139331. [DOI] [PubMed] [Google Scholar]
- Constantinescu RM, Georgescu M, Pascu A, et al. Otoacoustic emissions analysers for monitoring aminoglycosides ototoxicity. Rom J Intern Med. 2009;47:273–278. [PubMed] [Google Scholar]
- Das-Purkayastha PK, Rutka JA. How should awareness of ototoxicity change the way medicine is practiced? Semin Hear. 2011;32:236–247. [Google Scholar]
- Dehne N, Lautermann J, Petrat F, Rauen U, de Groot H. Cisplatin ototoxicity: involvement of iron and enhanced formation of superoxide anion radicals. Toxicol Appl Pharmacol. 2001;174:27–34. doi: 10.1006/taap.2001.9171. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Savoca M, Castaneda MP, et al. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hear Res. 2002;174:45–54. doi: 10.1016/s0378-5955(02)00634-2. [DOI] [PubMed] [Google Scholar]
- Devbhuti P, Saha A, Sengupta C. Gentamicin induced lipid peroxidation and its control with ascorbic acid. Acta Pol Pharm. 2009;66:363–369. [PubMed] [Google Scholar]
- Devbhuti P, Sikdar D, Saha A, Sengupta C. Protective effect of ascorbic acid on netilmicin-induced lipid profile and peroxidation parameters in rabbit blood plasma. Acta Pol Pharm. 2011;68:15–22. [PubMed] [Google Scholar]
- Echeverria P, Fina D, Norton S, Smith AL. Ototoxicity of gentamicin: clinical experience in a children’s hospital. Chemotherapy. 1978;24:267–271. doi: 10.1159/000237791. [DOI] [PubMed] [Google Scholar]
- Edson RS, Terrell CL. The aminoglycosides. Mayo Clin Proc. 1999;74:519–528. doi: 10.4065/74.5.519. [DOI] [PubMed] [Google Scholar]
- Evans P, Halliwell B. Free radicals and hearing. Cause, consequence, and criteria. Ann N Y Acad Sci. 1999;884:19–40. doi: 10.1111/j.1749-6632.1999.tb08633.x. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Henry JA, Schaffer HI, Olson DJ, Frey RH, McDonald WJ. High-frequency audiometric monitoring for early detection of aminoglycoside ototoxicity. J Infect Dis. 1992;165:1026–1032. doi: 10.1093/infdis/165.6.1026. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Sergi B, Scarano E, Paludetti G, Ferraresi A, Troiani D. Protective effects of alpha-tocopherol against gentamicin-induced oto-vestibulo toxicity: an experimental study. Acta Otolaryngol (Stockh) 2003;123:192–197. doi: 10.1080/00016480310001484. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Sergi B, Ferraresi A, Paludetti G, Troiani D. Alpha-tocopherol protective effects on gentamicin ototoxicity: an experimental study. Int J Audiol. 2004;43:166–171. doi: 10.1080/14992020400050023. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Eramo SL, Rolesi R, Troiani D, Paludetti G. Antioxidant treatment with coenzyme q-ter in prevention of gentamycin ototoxicity in an animal model. Acta Otorhinolaryngol Ital. 2012;32:103–110. [PMC free article] [PubMed] [Google Scholar]
- Frymark T, Leech H, Mullen R, Schooling T, Venediktov R, Wang B. (2010) Evidence-based systematic review: drug-induced hearing loss—gentamicin. American Speech-Language-Hearing Association (ASHA), National Center for Evidence-Based Practice in Communication Disorders 1–20
- Fukui H, Raphael Y. Gene therapy for the inner ear. Hear Res. 2013;297:99–105. doi: 10.1016/j.heares.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Zheng P, Fu H. Prevalence of TB/HIV co-infection in countries except china: a systematic review and meta-analysis. PLoS ONE. 2013;8:e64915. doi: 10.1371/journal.pone.0064915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garetz SL, Altschuler RA, Schacht J. Attenuation of gentamicin ototoxicity by glutathione in the guinea pig in vivo. Hear Res. 1994;77:81–87. doi: 10.1016/0378-5955(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Greenwood DD. A cochlear frequency-position function for several species—29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Hanberger H, Edlund C, Furebring M, et al. Rational use of aminoglycosides—review and recommendations by the Swedish Reference Group for Antibiotics (SRGA) Scand J Infect Dis. 2013;45:161–175. doi: 10.3109/00365548.2012.747694. [DOI] [PubMed] [Google Scholar]
- Harris T, Bardien S, Schaaf HS, Petersen L, De Jong G, Fagan JJ. Aminoglycoside-induced hearing loss in HIV-positive and HIV-negative multidrug-resistant tuberculosis patients. S Afr Med J. 2012;102:363–366. doi: 10.7196/samj.4964. [DOI] [PubMed] [Google Scholar]
- Heinrich UR, Helling K, Sifferath M, et al. Gentamicin increases nitric oxide production and induces hearing loss in guinea pigs. Laryngoscope. 2008;118:1438–1442. doi: 10.1097/MLG.0b013e3181739bd9. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- Jankauskas SS, Plotnikov EY, Morosanova MA, et al. Mitochondria-targeted antioxidant SkQR1 ameliorates gentamycin-induced renal failure and hearing loss. Biochemistry. 2012;77:666–670. doi: 10.1134/S0006297912060144. [DOI] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Schacht J (2005) NF-kappaB pathway protects cochlear hair cells from aminoglycoside-induced ototoxicity. J Neurosci Res 79:644–651 [DOI] [PubMed]
- Jiang H, Sha SH, Forge A, Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ. 2006;13:20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Schacht J. RAC/rho pathway regulates actin depolymerization induced by aminoglycoside antibiotics. J Neurosci Res. 2006;83:1544–1551. doi: 10.1002/jnr.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol Aging. 2007;28:1605–1612. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson LG, Hawkins JE, Jr, Kingsley TC, Black FO, Matz GJ. Aminoglycoside-induced cochlear pathology in man. Acta Otolaryngol Suppl. 1981;383:1–19. [PubMed] [Google Scholar]
- Kadkhodaee M, Khastar H, Faghihi M, Ghaznavi R, Zahmatkesh M. Effects of co-supplementation of vitamins E and C on gentamicin-induced nephrotoxicity in rat. Exp Physiol. 2005;90:571–576. doi: 10.1113/expphysiol.2004.029728. [DOI] [PubMed] [Google Scholar]
- Kadkhodaee M, Khastar H, Arab HA, Ghaznavi R, Zahmatkesh M, Mahdavi-Mazdeh M. Antioxidant vitamins preserve superoxide dismutase activities in gentamicin-induced nephrotoxicity. Transplant Proc. 2007;39:864–865. doi: 10.1016/j.transproceed.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Kavutcu M, Canbolat O, Ozturk S, et al. Reduced enzymatic antioxidant defense mechanism in kidney tissues from gentamicin-treated guinea pigs: effects of vitamins E and C. Nephron. 1996;72:269–274. doi: 10.1159/000188853. [DOI] [PubMed] [Google Scholar]
- Keene M, Hawke M, Barber HO, Farkashidy J. Histopathological findings in clinical gentamicin ototoxicity. Arch Otolaryngol. 1982;108:65–70. doi: 10.1001/archotol.1982.00790500001001. [DOI] [PubMed] [Google Scholar]
- Kharkheli E, Kevanishvili Z, Maglakelidze T, Davitashvili O, Schacht J. Does vitamin E prevent gentamicin-induced ototoxicity? Georgian Med News. 2007;146:14–7. [PubMed] [Google Scholar]
- Kim J, Kim Y. Animal models in carotenoids research and lung cancer prevention. Transl Oncol. 2011;4:271–281. doi: 10.1593/tlo.11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemens JJ, Meech RP, Hughes LF, Somani S, Campbell KC. Antioxidant enzyme levels inversely covary with hearing loss after amikacin treatment. J Am Acad Audiol. 2003;14:134–143. [PubMed] [Google Scholar]
- Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- Kusunoki T, Cureoglu S, Schachern PA, et al. Effects of gentamicin on sensorineural elements of the cochlea in human temporal bones. Am J Otolaryngol. 2004;25:313–317. doi: 10.1016/j.amjoto.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Lautermann J, McLaren J, Schacht J. Glutathione protection against gentamicin ototoxicity depends on nutritional status. Hear Res. 1995;86:15–24. doi: 10.1016/0378-5955(95)00049-a. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Bao J (2011) Prevention of noise-induced hearing loss: potential therapeutic agents, in noise-induced hearing loss: scientific advances. In: Le Prell CG, Henderson D, Fay RR, Popper AN (eds) Springer Handbook of Auditory Research. Springer, New York, pp 285–338
- Le Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic Biol Med. 2007;42:1454–1463. doi: 10.1016/j.freeradbiomed.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Yamashita D, Minami S, Yamasoba T, Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. 2007;226:22–43. doi: 10.1016/j.heares.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Dolan DF, Bennett DC, Boxer PA. Nutrient treatment and achieved plasma levels: reduction of noise-induced hearing loss at multiple post-noise test times. Transl Res. 2011;158:54–70. doi: 10.1016/j.trsl.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Gagnon PM, Bennett DC, Ohlemiller KK. Nutrient-enhanced diet reduces noise-induced damage to the inner ear and hearing loss. Transl Res. 2011;158:38–53. doi: 10.1016/j.trsl.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Rudnick EW, Nelson MA, Prieskorn DM, DeRemer S, Miller JM. Dietary nutrients attenuate gentamicin ototoxicity: reduced hearing loss and increased hair cell survival in guinea pigs maintained on supplemented diet. Abstr Assoc Res Otolaryngol. 2012;35:58. [Google Scholar]
- Le T, Keithley EM. Effects of antioxidants on the aging inner ear. Hear Res. 2007;226:194–202. doi: 10.1016/j.heares.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lopez-Gonzalez MA, Guerrero JM, Torronteras R, Osuna C, Delgado F. Ototoxicity caused by aminoglycosides is ameliorated by melatonin without interfering with the antibiotic capacity of the drugs. J Pineal Res. 2000;28:26–33. doi: 10.1034/j.1600-079x.2000.280104.x. [DOI] [PubMed] [Google Scholar]
- Maiani G, Caston MJ, Catasta G, et al. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. 2009;53(Suppl 2):S194–S218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- Mal P, Dutta S, Bandyopadhyay D, Dutta K, Basu A, Bishayi B. Gentamicin in combination with ascorbic acid regulates the severity of Staphylococcus aureus infection-induced septic arthritis in mice. Scand J Immunol. 2012;76:528–540. doi: 10.1111/j.1365-3083.2012.02766.x. [DOI] [PubMed] [Google Scholar]
- Matt T, Ng CL, Lang K, et al. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. Proc Natl Acad Sci U S A. 2012;109:10984–10989. doi: 10.1073/pnas.1204073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe JZ, Porco TC, Westenhouse J, et al. Tuberculosis and HIV co-infection, California, USA, 1993–2008. Emerg Infect Dis. 2013;19:400–406. doi: 10.3201/eid1903.121521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat DA, Ramsden RT. Profound bilateral sensorineural hearing loss during gentamicin therapy. J Laryngol Otol. 1977;91:511–516. doi: 10.1017/s0022215100083985. [DOI] [PubMed] [Google Scholar]
- Mulheran M, Degg C, Burr S, Morgan DW, Stableforth DE. Occurrence and risk of cochleotoxicity in cystic fibrosis patients receiving repeated high-dose aminoglycoside therapy. Antimicrob Agents Chemother. 2001;45:2502–2509. doi: 10.1128/AAC.45.9.2502-2509.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulherin D, Fahy J, Grant W, Keogan M, Kavanagh B, FitzGerald M. Aminoglycoside induced ototoxicity in patients with cystic fibrosis. Ir J Med Sci. 1991;160:173–175. doi: 10.1007/BF02961666. [DOI] [PubMed] [Google Scholar]
- Nordang L, Anniko M. Nitro-l-arginine methyl ester: a potential protector against gentamicin ototoxicity. Acta Otolaryngol. 2005;125:1033–1038. doi: 10.1080/00016480510038022. [DOI] [PubMed] [Google Scholar]
- Nudelman I, Rebibo-Sabbah A, Cherniavsky M, et al. Development of novel aminoglycoside (NB54) with reduced toxicity and enhanced suppression of disease-causing premature stop mutations. J Med Chem. 2009;52:2836–2845. doi: 10.1021/jm801640k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojano-Dirain CP, Antonelli PJ, Le Prell CG (2014) Mitochondria-targeted antioxidant mitoq reduces gentamicin-induced ototoxicity. Otol Neurotol 35(3):533–539. doi:10.1097/MAO.0000000000000192 [DOI] [PubMed]
- Okuda T, Sugahara K, Takemoto T, Shimogori H, Yamashita H. Inhibition of caspases alleviates gentamicin-induced cochlear damage in guinea pigs. Auris Nasus Larynx. 2005;32:33–37. doi: 10.1016/j.anl.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Patel Manali B, Deshpande S, Shah G. Evaluation of efficacy of vitamin E and n-acetyl cysteine in gentamicin-induced nephrotoxicity in rats. Ren Fail. 2011;33:341–347. doi: 10.3109/0886022X.2011.560987. [DOI] [PubMed] [Google Scholar]
- Pfannenstiel SC, Praetorius M, Plinkert PK, Brough DE, Staecker H. Bcl-2 gene therapy prevents aminoglycoside-induced degeneration of auditory and vestibular hair cells. Audiol Neuro Otol. 2009;14:254–266. doi: 10.1159/000192953. [DOI] [PubMed] [Google Scholar]
- Poirrier AL, Pincemail J, Van Den Ackerveken P, Lefebvre PP, Malgrange B. Oxidative stress in the cochlea: an update. Curr Med Chem. 2010;17:3591–3604. doi: 10.2174/092986710792927895. [DOI] [PubMed] [Google Scholar]
- Prins JM, Buller HR, Kuijper EJ, Tange RA, Speelman P. Once versus thrice daily gentamicin in patients with serious infections. Lancet. 1993;341:335–339. doi: 10.1016/0140-6736(93)90137-6. [DOI] [PubMed] [Google Scholar]
- Priuska EM, Schacht J. Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochem Pharmacol. 1995;50:1749–1752. doi: 10.1016/0006-2952(95)02160-4. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil Cytoskeleton. 1991;18:215–227. doi: 10.1002/cm.970180307. [DOI] [PubMed] [Google Scholar]
- Rivolta MN. New strategies for the restoration of hearing loss: challenges and opportunities. Br Med Bull. 2013;105:69–84. doi: 10.1093/bmb/lds035. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Kelly T. Ototoxicity: bioprotective mechanisms. Curr Opin Otolaryngol Head Neck Surg. 2003;11:328–333. doi: 10.1097/00020840-200310000-00004. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA. Ototoxicity: therapeutic opportunities. Drug Discov Today. 2005;10:1313–1321. doi: 10.1016/S1359-6446(05)03552-X. [DOI] [PubMed] [Google Scholar]
- Saleem U, Ahmad B, Rehman K, Mahmood S, Alam M, Erum A. Nephro-protective effect of vitamin C and Nigella sativa oil on gentamicin associated nephrotoxicity in rabbits. Pak J Pharm Sci. 2012;25:727–730. [PubMed] [Google Scholar]
- Sassen MC, Kim SW, Kwon TH, et al. Dysregulation of renal sodium transporters in gentamicin-treated rats. Kidney Int. 2006;70:1026–1037. doi: 10.1038/sj.ki.5001654. [DOI] [PubMed] [Google Scholar]
- Schacht J. Molecular mechanisms of drug-induced hearing loss. Hear Res. 1986;22:297–304. doi: 10.1016/0378-5955(86)90105-x. [DOI] [PubMed] [Google Scholar]
- Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec. 2012;295(11):1837–1850. doi: 10.1002/ar.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe F, Haupt H, Ising H. Total magnesium concentrations of perilymph, cerebrospinal fluid and blood in guinea pigs fed different magnesium-containing diets. Eur Arch Otorhinolaryngol. 1999;256:215–219. doi: 10.1007/s004050050144. [DOI] [PubMed] [Google Scholar]
- Scheibe F, Haupt H, Ising H, Cherny L. Therapeutic effect of parenteral magnesium on noise-induced hearing loss in the guinea pig. Magnes Res. 2002;15:27–36. [PubMed] [Google Scholar]
- Seidman MD. Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope. 2000;110:727–738. doi: 10.1097/00005537-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Sha SH, Schacht J. Stimulation of free radical formation by aminoglycoside antibiotics. Hear Res. 1999;128:112–118. doi: 10.1016/s0378-5955(98)00200-7. [DOI] [PubMed] [Google Scholar]
- Sha SH, Schacht J. Salicylate attenuates gentamicin-induced ototoxicity. Lab Investig. 1999;79:807–813. [PubMed] [Google Scholar]
- Sha SH, Schacht J. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: d-methionine is a potential protectant. Hear Res. 2000;142:34–40. doi: 10.1016/s0378-5955(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155:1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Sha SH, Zajic G, Epstein CJ, Schacht J. Overexpression of copper/zinc-superoxide dismutase protects from kanamycin-induced hearing loss. Audiol Neuro Otol. 2001;6:117–123. doi: 10.1159/000046818. [DOI] [PubMed] [Google Scholar]
- Sha SH, Qiu J-H, Schacht J. Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med. 2006;354:1856–1857. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]
- Singh Chauhan R, Saxena RK, Varshey S. The role of ultrahigh-frequency audiometry in the early detection of systemic drug-induced hearing loss. Ear Nose Throat J. 2011;90:218–222. doi: 10.1177/014556131109000506. [DOI] [PubMed] [Google Scholar]
- Solomons NW, Orozco M (2003) Alleviation of vitamin A deficiency with palm fruit and its products. Asia Pac J Clin Nutr 12:373–384 [PubMed]
- Stavroulaki P, Apostolopoulos N, Dinopoulou D, Vossinakis I, Tsakanikos M, Douniadakis D. Otoacoustic emissions—an approach for monitoring aminoglycoside induced ototoxicity in children. Int J Pediatr Otorhinolaryngol. 1999;50:177–184. doi: 10.1016/s0165-5876(99)00247-5. [DOI] [PubMed] [Google Scholar]
- Stebbins WC, Hawkins JE, Jr, Johnson LG, Moody DB. Hearing thresholds with outer and inner hair cell loss. Am J Otolaryngol. 1979;1:15–27. doi: 10.1016/s0196-0709(79)80004-6. [DOI] [PubMed] [Google Scholar]
- Steyger PS. Mechanisms involved in ototoxicity. Semin Hear. 2011;32:217–228. doi: 10.1055/s-0031-1286616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojiljkovic N, Stoiljkovic M, Randjelovic P, Veljkovic S, Mihailovic D. Cytoprotective effect of vitamin C against gentamicin-induced acute kidney injury in rats. Exp Toxicol Pathol. 2012;64:69–74. doi: 10.1016/j.etp.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Tamir S, Adelman C, Weinberger JM, Sohmer H. Uniform comparison of several drugs which provide protection from noise induced hearing loss. J Occup Med Toxicol. 2010;5:26–32. doi: 10.1186/1745-6673-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CT, Lee SY, Yao CJ, Liu SH, Lin-Shiau SY. Effects of gentamicin and ph on [ca2+]i in apical and basal outer hair cells from guinea pigs. Hear Res. 2001;154:81–87. doi: 10.1016/s0378-5955(01)00222-2. [DOI] [PubMed] [Google Scholar]
- Tian CJ, Kim SW, Kim YJ, et al. Red ginseng protects against gentamicin-induced balance dysfunction and hearing loss in rats through antiapoptotic functions of ginsenoside rb1. Food Chem Toxicol. 2013;60:369–376. doi: 10.1016/j.fct.2013.07.069. [DOI] [PubMed] [Google Scholar]
- Usami S, Abe S, Shinkawa H, Kimberling WJ. Sensorineural hearing loss caused by mitochondrial DNA mutations: special reference to the a1555g mutation. J Commun Disord. 1998;31:423–434. doi: 10.1016/s0021-9924(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Uzun L, Balbaloglu E, Akinci H. Garlic-supplemented diet attenuates gentamicin-induced ototoxicity: an experimental study. Ann Otol Rhinol Laryngol. 2012;121:139–143. doi: 10.1177/000348941212100211. [DOI] [PubMed] [Google Scholar]
- Varghese GM, Janardhanan J, Ralph R, Abraham OC. The twin epidemics of tuberculosis and HIV. Curr Infect Dis Rep. 2013;15:77–84. doi: 10.1007/s11908-012-0311-3. [DOI] [PubMed] [Google Scholar]
- Varzi HN, Esmailzadeh S, Morovvati H, Avizeh R, Shahriari A, Givi ME. Effect of silymarin and vitamin e on gentamicin-induced nephrotoxicity in dogs. J Vet Pharmacol Ther. 2007;30:477–481. doi: 10.1111/j.1365-2885.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- Wahed MA, Islam MA, Khondakar P, Haque MA. Effect of micronutrients on morbidity and duration of hospital stay in childhood pneumonia. Mymensingh Med J. 2008;17:S77–S83. [PubMed] [Google Scholar]
- Xia L, Yin S. Local gene transfection in the cochlea (review) Mol Med Rep. 2013;8:3–10. doi: 10.3892/mmr.2013.1496. [DOI] [PubMed] [Google Scholar]
- Xie J, Talaska AE, Schacht J. New developments in aminoglycoside therapy and ototoxicity. Hear Res. 2011;281:28–37. doi: 10.1016/j.heares.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M, Wang J, Yang C, Lai H. The cochlea magnesium content is negatively correlated with hearing loss induced by impulse noise. Am J Otolaryngol. 2013;34(3):209–215. doi: 10.1016/j.amjoto.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang H, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201–209. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- Ye LF, Tao ZZ, Hua QQ, et al. Protective effect of melatonin against gentamicin ototoxicity. J Laryngol Otol. 2009;123:598–602. doi: 10.1017/S002221510800385X. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U. Blockade of c-jun n-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear Res. 2002;163:71–81. doi: 10.1016/s0378-5955(01)00380-x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Li R, Wang Q, et al. Maternally inherited aminoglycoside-induced and nonsyndromic deafness is associated with the novel c1494t mutation in the mitochondrial 12s rRNA gene in a large Chinese family. Am J Hum Genet. 2004;74:139–152. doi: 10.1086/381133. [DOI] [PMC free article] [PubMed] [Google Scholar]