Abstract
Background
Chemerin is a novel adipokine that plays a role in inflammation and atherosclerosis. Although there are several correlations between hypertension and the inflammatory system, there is still insufficient information about the relationship between blood pressure variability and inflammatory markers. In this study, we aimed to determine whether chemerin levels are elevated in non-dipper patients compared with dippers and healthy controls.
Material/Methods
This study was composed of a group of 90 patients: 60 hypertensive patients and 30 healthy control subjects (12 males, mean age 53.2±15.4 years). Ambulatory blood pressure monitoring devices (ABPM) were connected to all patients. Using data from the ABPM, hypertensive patients were divided into 2 groups: 30 dipper patients (12 males, mean age 52.5±15.1 years) and 30 non-dipper patients (11 males, mean age 54.6±13.0 years). Complete blood count and biochemistry were measured by standard methods and plasma chemerin concentrations were quantified by ELISA.
Results
Non-dipper patients demonstrated higher chemerin levels compared to dippers and normotensives (219.7±16.3 vs. 182.4±21.4 ng/ml; 219.7±16.3 vs. 85.4±38.1 ng/ml, respectively, p<0.001 for both comparisons). A receiver operating characteristic curve analysis revealed that the optimal cut-off value for chemerin to predict a non-dipping pattern was 201.4, with 90% sensitivity and 90% specificity. There was a positive correlation between chemerin levels and all ambulatory blood pressure values in all hypertensive patients.
Conclusions
Chemerin, which plays a role in inflammation and atherosclerosis, was higher in non-dippers compared to dippers and normotensives. Additionally, chemerin shows positive correlations with blood pressure.
MeSH Keywords: Adipokines, Hypertension, Inflammation
Background
Hypertension remains a major public health problem despite continuous research and development in cardiovascular medicine [1,2]. Hypertension is one of the leading causes of cardiovascular mortality [3]. Besides being one of the most important risk factors of ischemic heart disease, hypertension has other major adverse effects on target organs, including the heart, kidneys, and eyes.
Blood pressure (BP) and heart rate have long been known to show circadian changes depending on sleep and wakefulness. Ambulatory blood pressure monitoring allows for the evaluation of daily rhythms of blood pressure and a determination of various parameters demonstrated to be associated with adverse cardiovascular prognoses. In many studies, a non-dipper condition, which is one of the most important abnormalities in circadian BP rhythm, was associated with target organ damage in both hypertensive (HT) and normotensive subjects [4]. Patients demonstrating a drop of 10% or more in blood pressure values when measured during the night as compared to daytime values were defined as “dippers”, whereas patients demonstrating a drop of less than 10% were defined as “non-dippers” [5]. In prospective studies, lack of nighttime blood pressure drop, or a blood pressure higher at night than during the day, were identified as independent risk factors for cardiovascular disease [6–9].
Adipose tissue has been identified as an important organ affecting energy regulation and metabolism. It acts through bioactive mediators called adipokines, among which there are many hormones and cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, angiotensinogen, adiponectin, leptin, and resistin [10–12]. Chemerin is an adipokine and is also known as tazarotene-induced gene 2 protein (TIG2) or a retinoid acid receptor responder 2 (RARRES2). However, chemerin has been defined as a new adipokine that plays a role in acquired and natural immunity [13,14]. Chemerin is secreted as a ligand for “orphan” G protein-coupled receptor chemokine-like receptor (CMKLR) 1, chemokine (C-C motif) receptor-like (CCRL) 2, and G protein-coupled receptor (GPR) 1 [13].
Chronic inflammation has been demonstrated to be a risk factor for the development of arterial hypertension. Inflammation markers such as C-reactive protein (CRP), TNF-α, and IL-6, were shown to increase in patients with essential hypertension. Therefore, the chemerin/CMKLR1 signal, the secretion of which is controlled by inflammatory cytokines, was found to contribute to pathogenesis of hypertension [15–17].
In our study, we aimed to determine whether chemerin levels are elevated in non-dipper patients, compared with dippers and healthy controls. We also tried to determine if chemerin levels are related to circadian blood pressure patterns in essential hypertensive patients.
Material and Methods
Study population
This study included a group of 90 patients: 60 hypertensive patients and 30 healthy control subjects (18 females, 12 males, mean age =53.2±15.4 years). Ambulatory BP monitoring devices (ABPM) were connected to all patients. Through the use of data from the ABPMs, hypertensive patients were divided into 2 groups: 30 dipper patients (18 females, 12 males, mean age =52.5±15.1 years) and 30 non-dipper patients (19 females, 11 males, mean age =54.6±13.0 years). The ABPM also confirmed that the subjects in the control group were normotensive and have a dipper profile.
Hypertension was defined as systolic BP (SBP) ≥140 mmHg or a diastolic BP (DBP) ≥90 mmHg and/or using anti-hypertensive drug therapy [18]. After the diagnosis of hypertension, ambulatory blood pressure monitoring (ABPM) was performed for all patients. Exclusion criteria included secondary hypertension, chronic heart failure, diabetes mellitus, renal or hepatic dysfunction, clinical evidence of cancer, systemic inflammatory disease, chronic or acute infectious disease, auto-immune disease, serious valvular heart disease, cardiomyopathy, atrial fibrillation, hematological disease, and known coronary artery or cerebrovascular disease.
Clinical BP measurements were conducted in the morning using a sphygmomanometer, and the average of 3 measurements was used. Prior to BP measurements, the subject was allowed to rest for a minimum of 5 minutes. Blood pressure was measured when the subject had not consumed tea/coffee within the last hour and had not smoked within the last 30 min. BP was measured while the subject’s arm was supported at the heart level in a sitting position. The measurement was made on both arms and the higher value was used.
The patients’ clinical and demographic characteristics were noted and included age, sex, smoking habits, and antihypertensive drug use. Additionally, fasting blood glucose levels, creatinine levels, and fasting serum lipid status, including total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels were recorded. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2).
This study complied with the Declaration of Helsinki, was approved by the local ethics committee, and each patient gave written informed consent.
Ambulatory blood pressure monitoring and dipping status
Ambulatory BP monitoring studies were carried out using a Tracker NIBP2 (Del Mar Reynolds Medical Ltd, Hertford, UK) monitoring device. Blood pressure was measured and recorded for 24 h with 15-min daytime intervals and 30-min nighttime intervals using this device, which performs oscillometric measurements. The cuff of the ABPM device was placed on the non-dominant arm if the BP difference between the 2 arms during clinical BP measurement was less than 10 mmHg. However, if the BP difference was greater than 10 mmHg, the cuff was placed on the arm that had the higher reading. The recordings were analyzed with interactive software. The subjects were given information about the procedure and asked to perform daily routine activities, avoid excessive activities, and hold their arms still at heart level during measurements.
From the hourly averages of ambulatory BP recordings, daytime, nighttime, and 24-h averages of SBP, DBP, and mean BP were calculated for each patient. Patients with a BP drop of 10% or more during nighttime were accepted as dipper hypertensives, whereas patients with a BP drop of less than 10% were accepted as non-dipper hypertensives, according to the criteria of Verdecchia et al. [9].
Collection of blood samples and biochemical analyses
Blood samples were taken in the morning after a 30-min rest following a 12-h fasting period. Samples were centrifuged (Shimadzu UV160A, S. No: 28006648, Japan) at 3000 rpm for 10 min and the serum was stored at −80°C. Serum glucose, creatinine, total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride concentrations were analyzed by a Roche Hitachi Cobas 8000 device using Roche Diagnostics GmbH kits. Hemoglobin and leukocyte concentrations were determined by using a Siemens Advia 2120i Hematology System device. The concentrations of chemerin in the serum were measured using commercially available ELISA kits (Human Chemerin Elisa kit, BioVendor-Laboratorni Medicina AS, Cat No.RD191136200R, Brno, Czech Republic). The enzymatic reactions were quantified in an automatic microplate photometer and the chemerin levels were expressed as ng/ml. The mean interassay coefficient of variation (CV) percent and intraassay CV percent for chemerin were 8.3% and 5.1%, respectively. All assays were conducted according to the manufacturer’s instructions. The samples that showed higher concentrations were diluted and measured in duplicate.
Statistical analysis
Statistical analyses were conducted using the Statistical Package for the Social Sciences for Windows 21.0 (SPSS, Chicago, IL). Descriptive statistics are given as mean, standard deviation, frequency, and percentage. The Kolmogorov-Smirnov test and graphical methods were used to test the normality of continuous variables. One-way analysis of variance (ANOVA) (for data with normal distribution) or Kruskal-Wallis test (for data without normal distribution) was used for the comparison of the 3 groups with regard to baseline clinical characteristics. Tukey HSD was used as the post hoc test after ANOVA and built-in pairwise comparison test of the software was used after Kruskal-Wallis test. For the comparison of dippers and non-dippers with regard to blood pressure measurements, either Student’s t test for independent variables or Mann-Whitney U test was used, depending on the normality of data. Comparisons of categorical values were carried out by the chi-squared test. Correlations between blood pressure and chemerin levels were tested using Spearman correlation analysis because chemerin data did not have normal distribution. Additionally, we performed a receiver operating characteristic (ROC) analysis to identify the most sensitive chemerin cut-off level for identifying patients with non-dipper hypertension. A probability value <0.05 was considered the minimum level of statistical significance. A 2-sided p-value was considered for all comparisons.
Results
Demographic characteristics, laboratory data, and medication usage of patient and control groups are given in Table 1. There was no significant difference between the groups in terms of age, sex, creatinine, fasting glucose, fasting lipid status, hemoglobin levels, white blood cell, body mass index, smoking, or antihypertensive drug use.
Table 1.
Baseline clinical characteristics of the study population.
| Control (n=30) | Dipper (n=30) | Non-dipper (n=30) | P for overall difference | |
|---|---|---|---|---|
| Age (years) | 53.2±15.4 | 52.5±15.1 | 54.6±13.0 | 0.858 |
| Men, n (%) | 12 (40) | 12 (40) | 11 (36.7) | 0.954 |
| Body mass index (kg/m2) | 28.8±4.6 | 28.8±4.5 | 28.9±4.3 | 0.993 |
| Smokers | 10 (33.3) | 7 (23.3) | 8 (26.7) | 0.679 |
| Clinical SBP (mmHg) | 126.3±8.8 | 144.5±15.9 | 148.2±21.8 | <0.001 |
| Clinical DBP (mmHg) | 75.5±9.1 | 83.4±12.3 | 83.7±13.3 | 0.012 |
| Total cholesterol (mg/dl) | 206.2±36.4 | 198.6±48.7 | 193.2±41.9 | 0.303 |
| Low-density lipoprotein (mg/dl) | 127.2±35.3 | 123.2±38.3 | 114.9±31.7 | 0.489 |
| High-density lipoprotein (mg/dl) | 48.8±10.1 | 44.8±10.6 | 44.7±11.7 | 0.258 |
| Triglycerides (mg/dl) | 161.2±67.0 | 144.7±57.2 | 132.8±68.5 | 0.120 |
| Creatinine (mg/dl) | 0.84±0.20 | 0.90±0.30 | 0.83±0.25 | 0.704 |
| Fasting glucose (mg/dl) | 102.0±13.7 | 101.3±15.4 | 98.9±10.6 | 0.766 |
| Hemoglobin (gr/dl) | 14.3±1.4 | 13.9±1.3 | 13.7±1.4 | 0.176 |
| White blood cell count (103/mm3) | 7.40±1.49 | 7.49±1.38 | 7.22±1.60 | 0.781 |
| Chemerin (ng/ml) | 85.4±38.1 | 182.4±21.4 | 219.7±16.3 | <0.001 |
| Medical treatments | ||||
| ACE inh. use (%) | 14 (46.7) | 12 (40.0) | 0.602 | |
| ARB use (%) | 7 (23.3) | 10 (33.3) | 0.390 | |
| Beta-blocker use (%) | 6 (20.0) | 10 (33.3) | 0.243 | |
| Ca-channel blocker use (%) | 8 (26.7) | 8 (26.7) | 1.000 | |
| Diuretic use (%) | 13 (43.3) | 12 (40.0) | 0.793 | |
SBP – systolic blood pressure; DBP – diastolic blood pressure; ACE inh. – angiotensin-converting-enzyme inhibitor; ARB – angiotensin receptor blocker.
Table 2 shows the comparisons of clinical blood pressure and chemerin levels of the groups at baseline. As expected, clinical BP values were significantly higher in dipper and non-dipper patients compared to normotensive patients (SBP 144.5±15.9 and 148.2±21.8 vs. 126.3±8.8 mmHg, p<0.001 for both; DBP 83.4±12.3 and 83.7±13.3 vs. 75.5±9.1 mmHg, p=0.029 and 0.022, respectively). However, clinical BP values were similar between dipper and non-dipper patients (Table 2).
Table 2.
Comparison of clinical blood pressure and chemerin levels of the groups at baseline.
| Control (n=30) | Dipper (n=30) | P, dipper vs. control | Non-dipper (n=30) | P, non-dipper vs. control | P, non-dipper vs. dipper | |
|---|---|---|---|---|---|---|
| Clinical SBP (mmHg) | 126.3±8.8 | 144.5±15.9 | <0.001 | 148.2±21.8 | <0.001 | 1.000 |
| Clinical DBP (mmHg) | 75.5±9.1 | 83.4±12.3 | 0.029 | 83.7±13.3 | 0.022 | 0.995 |
| Chemerin (ng/ml) | 85.4±38.1 | 182.4±21.4 | <0.001 | 219.7±16.3 | <0.001 | <0.001 |
SBP – systolic blood pressure; DBP – diastolic blood pressure.
Nocturnal SBP, DBP, and mean BP were significantly higher in non-dippers compared with dippers (Table 3). While daytime BP measurements were similar between non-dippers and dippers, there was a significant difference between these groups during nighttime measurements (nighttime SBP 132.4±20.1 vs. 114.2±11.2 mmHg, p<0.001; nighttime DBP 73.9±12.6 vs. 64.8±7.7 mmHg, p=0.001).
Table 3.
Blood pressure values of dipper and non-dipper groups.
| Dipper (n=30) | Non-dipper (n=30) | p | |
|---|---|---|---|
| Daytime SBP (mmHg) | 129.3±16.8 | 134.7±19.8 | 0.412 |
| Daytime DBP (mmHg) | 75.8±11.2 | 76.1±12.1 | 0.930 |
| Daytime average BP (mmHg) | 87.7±12.4 | 89.8±13.4 | 0.518 |
| Nighttime SBP (mmHg) | 114.2±11.2 | 132.4±20.1 | <0.001 |
| Nighttime DBP (mmHg) | 64.8±7.7 | 73.9±12.6 | 0.001 |
| Nighttime average BP (mmHg) | 75.6±8.4 | 87.3±13.6 | <0.001 |
| 24-h SBP (mmHg) | 125.6±14.7 | 134.4±19.4 | 0.051 |
| 24-h DBP (mmHg) | 73.1±9.7 | 75.7±11.8 | 0.367 |
| 24-h average BP (mmHg) | 84.8±10.7 | 89.2±13.0 | 0.154 |
SBP – systolic blood pressure; DBP – diastolic blood pressure; BP – blood pressure.
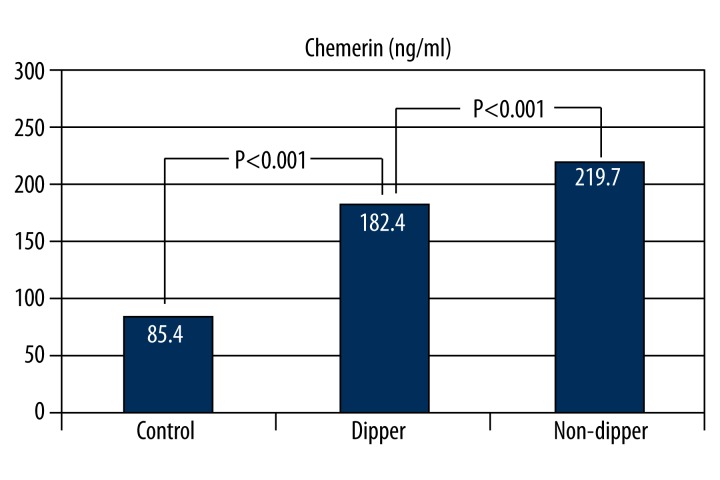
Chemerin levels were significantly higher in non-dipper patients compared to dippers and normotensives. As reported in Table 2 and Figure 1, non-dipper patients demonstrated higher levels of chemerin compared to dippers and normotensives (219.7±16.3 vs. 182.4±21.4 ng/ml; 219.7±16.3 vs. 85.4±38.1 ng/ml, p<0.001 for both, respectively).
Figure 1.
Comparison of chemerin levels in non-dippers compared with dippers and controls.
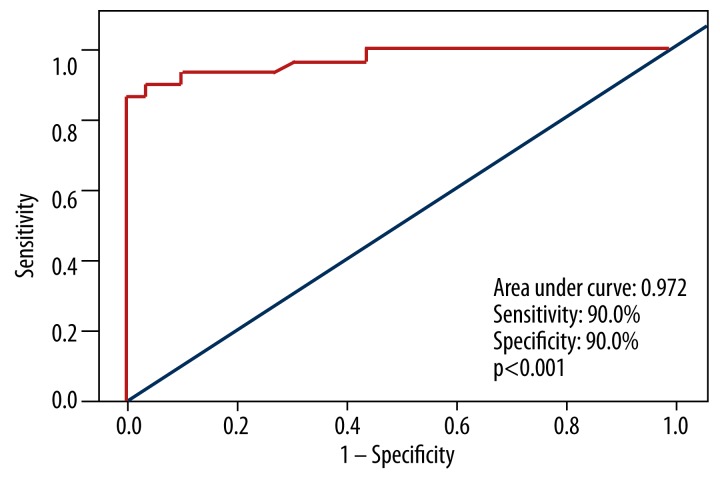
In hypertensive patients, ROC curves discovered the correlation between the non-dipping status and chemerin, for which ROC analysis showed an optimum cut-off of 201.4 (area under the curve 0.972, p<0.001). According to the cut-off, we calculated the sensitivity and specificity as 90% and 90%, respectively (Figure 2).
Figure 2.
Receiver operating characteristic (ROC) curve of chemerin for predicting non-dipping patterns in hypertensive groups.
In dipper and non-dipper hypertensive patients, we found a positive correlation between chemerin and all ambulatory BP values (daytime DBP and SBP, nighttime SBP and DBP, and 24-h SBP and DBP) (Table 4).
Table 4.
Correlation between chemerin levels and blood pressure levels in hypertensive patients.
| Chemerin | ||||
|---|---|---|---|---|
| Dipper | Non-dipper | |||
| r | p | r | p | |
| Daytime SBP | 0.649 | <0.001 | 0.789 | <0.001 |
| Daytime DBP | 0.583 | 0.001 | 0.509 | 0.004 |
| Daytime average BP | 0.637 | <0.001 | 0.704 | <0.001 |
| Nighttime SBP | 0.418 | 0.022 | 0.704 | <0.001 |
| Nighttime DBP | 0.415 | 0.016 | 0.662 | <0.001 |
| Nighttime average BP | 0.460 | 0.011 | 0.510 | 0.004 |
| 24-h SBP | 0.643 | <0.001 | 0.784 | <0.001 |
| 24-h DBP | 0.598 | <0.001 | 0.474 | 0.008 |
| 24-h average BP | 0.643 | <0.001 | 0.667 | <0.001 |
SBP – systolic blood pressure; DBP – diastolic blood pressure; BP – blood pressure.
There was no significant difference for antihypertensive medications taken by the dipper and non-dipper groups. Normotensives did not take any medicine (Table 1).
Discussion
As a result of our literature search, we found no study demonstrating the correlation between dipper and non-dipper groups and plasma chemerin levels. The objective of our study was to compare plasma chemerin levels in a non-dipper hypertensive (HT) group, in which endothelial dysfunction, inflammation, and hence, target organ damage are known to occur more, with those in a dipper HT group. In our study, plasma chemerin levels in the non-dipper HT group were statistically higher than those in the dipper HT and normotensive groups.
The pathophysiology of hypertension includes endothelial damage and dysfunction, inflammatory activation, insulin resistance, platelet activation, and changes causing predisposition to prothrombotic conditions in the coagulation cascade. More than just BP values, hypertension is considered a complex cardiovascular disease [19]. Pathophysiologically, inflammation has been associated with hypertension because it causes both arterial stiffness and endothelial dysfunction. In many studies, well understood pro-inflammatory markers, such as high-sensitivity C-reactive protein (hsCRP), have been shown to be increased in HT patients, even after correcting for other potential factors. Moreover, high hsCRP values were demonstrated to be a predictor of HT development in prehypertensives and normotensives [16,17]. Medications, including statin, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers, which are commonly used and positively affect the prognosis in HT patients, are known to have anti-inflammatory effects. Inflammatory conditions with hypertension appear to be a new treatment target for future pharmaceutical development. Therefore, early identification of these inflammatory factors and elucidation of their mechanisms are essential [15–16,20].
Adipose tissue is not only a stationary energy storage tissue, but also functions as an endocrine organ, and produces various bioactive substances, including adipokines, chemokines and, free fatty acids, before secreting them into the blood stream. Through the local and systemic effects of these bioactive molecules, adipose tissue plays an important role in carbohydrate and lipid metabolism, homeostasis, insulin resistance, diabetes, atherosclerosis, endothelial dysfunction, inflammation, and cardiovascular function [13,14,21]. Chemerin was recently isolated and is considered to be a new member of the adipocytokine family [13,14]. Circulating chemerin levels are higher in patients with metabolic syndrome, obesity, gestational diabetes mellitus, type 2 diabetes mellitus with hypertension, rheumatoid arthritis, and chronic pancreatitis [22–26].
Most experimental evidence demonstrates that chemerin/CMKLR1 in adipose tissue plays a major role in inflammation. Serum chemerin levels are also correlated with tumor necrosis factor (TNF)-alpha, interleukin (IL)-6, C-reactive protein, resistin, and leptin levels [27,28]. Moreover, CMKLR1 was shown to be expressed in vascular endothelial cells and expression levels are regulated by inflammatory cytokines [29]. All of these studies show that chemerin participates in the pathogenesis of hypertension [11].
In numerous studies, ambulatory BP has been linked to hypertension-mediated organ damage. Ambulatory BP has a greater correlation with cardiovascular events and predicts cardiovascular risks at a higher level than can examination office BP values; this holds true for both treated and untreated HT patients [6–9]. Although it is now known there are several correlations between hypertension and the inflammatory system, there is still insufficient information about the relationship between BP variability and markers of inflammation.
Non-dipper HT patients also tend to have increased inflammatory activity. Non-dipper hypertension is associated with greater inflammation and poor prognoses. Various inflammatory markers, such as hsCRP, gamma-glutamyl transferase, and uric acid, have been associated with non-dipper hypertension in several studies [30–33].
Gu et al. [11] conducted a case-control study evaluating the correlation between chemerin and hypertension to determine the role of chemerin in hypertension. Consistent with our study results, plasma chemerin levels were significantly increased in the essential hypertension group when compared to the normotensive group. The same study discovered that chemerin concentrations show a positive and significant correlation with SBP in HT subjects. They also found that chemerin positively correlates with some markers of inflammation (hsCRP, TNF-α, and IL-6), regardless of age, sex, and metabolic characteristics. In their multivariate logistic regression analysis, the authors showed that chemerin is independently associated with hypertension. Yang et al. [22] reported that chemerin plasma levels were significantly increased in HT patients with type 2 diabetes mellitus compared with non-hypertensive patients with type 2 diabetes mellitus.
Serum chemerin levels weakly correlate with coronary plaque burden and the number of non-calcified plaques in humans; however, this significance disappears after accounting for cardiovascular risk factors [27]. In an autopsy study of 41 cases, chemerin expression in periaortic and pericoronary adipose tissue was positively correlated with aortic and coronary atherosclerosis, suggesting that chemerin may have paracrine effects [34]. Hah et al. [35] reported that higher levels of chemerin were observed in subjects with multiple stenotic coronary arteries compared to those with only 1 stenotic coronary artery. Hypertension is closely associated with atherosclerosis and chemerin may play a role in the pathogenesis of atherosclerosis. It must still be established whether chemerin levels correlate with markers of atherosclerosis, such as cardiovascular ankle index or intimal-medial thickness. Further studies are required to elucidate the role of chemerin in atherosclerosis and cardiovascular disease [36].
In our study, high chemerin levels are related to the non-dipper pattern in hypertensive patients. It was shown in previous studies that non-dipper pattern is related to the target organ damage and worse clinical outcomes [4,6]. Therefore, measurement of chemerin levels may be clinically valuable. Since there is a potential relationship between chemerin and inflammation, decreasing inflammation by lowering chemerin levels may improve blood pressure control and prevent complications of hypertension, but this hypothesis should be evaluated in large prospective clinical trials.
Limitations of the study
Our sample size was small, thus the statistical power of the study was limited. Other inflammation markers were not studied.
Conclusions
Chemerin, which plays a role in inflammation and atherosclerosis, was found to be higher in non-dippers compared to dippers and normotensives. Chemerin levels also show a positive correlation with blood pressure. These results are important because they show the likely role of inflammation in the pathogenesis of hypertension.
Footnotes
Source of support: Departmental sources
References
- 1.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005:3617–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Suri MF, Kirmani JF, Divani AA. Prevalence and trends of prehypertension and hypertension in United States: National Health and Nutrition Examination Surveys 1976 to 2000. Med Sci Monit. 2005;11(9):CR403–9. [PubMed] [Google Scholar]
- 3.Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996:27571–76. [PubMed] [Google Scholar]
- 4.Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002:2183–89. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien E, Sheridan J, O’malley K. Dippers and non-dippers. Lancet. 1988:97. doi: 10.1016/s0140-6736(88)92867-x. [DOI] [PubMed] [Google Scholar]
- 6.Verdeccchia P, Schillaci G, Guerrieri M, et al. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990:828–36. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 7.Ohkubo T, Imai Y, Tsuji I, et al. Relation between Nocturnal Decline in Blood Pressure and Mortality: the Ohasama Study. Am J Hypertens. 1997;10(11):1201–7. doi: 10.1016/s0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 8.Verdecchia P, Schillaci G, Borgioni C, et al. Altered circadian blood pressure profile and prognosis. Blood Press Monit. 1997;2(6):347–52. [PubMed] [Google Scholar]
- 9.Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure: an independent predictor of prognosis in essential hypertension. Hypertension. 1994:293–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 10.Wittamer V, Bondue B, Guillabert A, et al. Neutrophil-mediated maturation of chemerin: a link between innate and adaptive immunity. J Immunol. 2005:1787–93. doi: 10.4049/jimmunol.175.1.487. [DOI] [PubMed] [Google Scholar]
- 11.Gu P, Jiang W, Lu B, Shi Z. Chemerin is associated with inflammatory markers and metabolic syndrome phenotypes in hypertension patients. Clin Exp Hypertens. 2013 doi: 10.3109/10641963.2013.827697. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Cui J, Zhang C. Emerging role of adipokines as mediators in atherosclerosis. World J Cardiol. 2010:70–76. doi: 10.4330/wjc.v2.i11.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010:260–67. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Zabel BA, Allen SJ, Kulig P, et al. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem. 2005;280(41):34661–66. doi: 10.1074/jbc.M504868200. [DOI] [PubMed] [Google Scholar]
- 15.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 16.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005:149–54. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 17.Sesso HD, Buring JE, Rifai N, et al. C-reactive protein and the risk of developing hypertension. JAMA. 2003:29945–51. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee. The seventh report of the Joint National Committee on detection, evaluation, and treatment of high blood pressure (JNC 7) Hypertension. 2003:4206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 19.Giles TD. Assessment of global risk: a foundation for a new, better definition of hypertension. J Clin Hypertens. 2006;8(s8):5–14. doi: 10.1111/j.1524-6175.2006.05835.x. [DOI] [PubMed] [Google Scholar]
- 20.Boos CJ, Lip GY. Is hypertension an inflammatory process? Curr Pharm Des. 2006:1623–35. doi: 10.2174/138161206776843313. [DOI] [PubMed] [Google Scholar]
- 21.Stępień M, Stępień A, Wlazeł RN, et al. Obesity indices and adipokines in non-diabetic obese patients with early stages of chronic kidney disease. Med Sci Monit. 2013:1063–72. doi: 10.12659/MSM.889390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang M, Yang G, Dong J, et al. Elevated plasma levels of chemerin in newly diagnosed type 2 diabetes mellitus with hypertension. J Investig Med. 2010:583–86. doi: 10.231/JIM.0b013e3181ec5db2. [DOI] [PubMed] [Google Scholar]
- 23.Pfau D, Stepan H, Kratzsch J, et al. Circulating levels of the adipokine chemerin in gestational diabetes mellitus. Horm Res Paediatr. 2010:76–61. doi: 10.1159/000282114. [DOI] [PubMed] [Google Scholar]
- 24.Bozaoglu K, Bolton K, McMillan J, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007:14687–94. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 25.Adrych K, Stojek M, Smoczynski M, et al. Increased serum chemerin concentration in patients with chronic pancreatitis. Dig Liver Dis. 2012;44(5):393–97. doi: 10.1016/j.dld.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Ha YJ, Kang EJ, Song JS, et al. Plasma chemerin levels in rheumatoid arthritis are correlated with disease activity rather than obesity. Joint Bone Spine. 2014;81(2):189–90. doi: 10.1016/j.jbspin.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Lehrke M, Becker A, Greif M, et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol. 2009:1639–44. doi: 10.1530/EJE-09-0380. [DOI] [PubMed] [Google Scholar]
- 28.Weigert J, Neumeier M, Wanninger J, et al. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol. 2010:742–48. doi: 10.1111/j.1365-2265.2009.03664.x. [DOI] [PubMed] [Google Scholar]
- 29.Kaur J, Adya R, Tan BK, et al. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010:39762–68. doi: 10.1016/j.bbrc.2009.12.150. [DOI] [PubMed] [Google Scholar]
- 30.Inanc T, Kaya MG, Yarlioglues M, et al. The mean platelet volume in patients with non-dipper hypertension compared to dippers and normotensives. Blood Press. 2010:11–85. doi: 10.3109/08037050903516284. [DOI] [PubMed] [Google Scholar]
- 31.Kaya MG, Yarlioglues M, Gunebakmaz O, et al. Platelet activation and inflammatory response in patients with non-dipper hypertension. Atherosclerosis. 2010:2078–82. doi: 10.1016/j.atherosclerosis.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Ermis N, Yagmur J, Acikgoz N, et al. Serum gamma-glutamyl transferase (GGT) levels and inflammatory activity in patients with non-dipper hypertension. Clin Exp Hypertens. 2012;34(5):311–15. doi: 10.3109/10641963.2011.577485. [DOI] [PubMed] [Google Scholar]
- 33.Erden M, Kocaman SA, Poyraz F, et al. Incremental effects of serum uric acid levels, autonomic dysfunction, and low-grade inflammation on nocturnal blood pressure in untreated hypertensive patients and normotensive individuals. Turk Kardiyol Dern Ars. 2011:331–39. doi: 10.5543/tkda.2011.01545. [DOI] [PubMed] [Google Scholar]
- 34.Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010:115–30. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- 35.Hah YJ, Kim NK, Kim MK, et al. Relationship between chemerin levels and cardiometabolic parameters and degree of coronary stenosis in Korean patients with coronary artery disease. Diabetes Metab J. 2011:348–54. doi: 10.4093/dmj.2011.35.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhee EJ. Chemerin: A novel link between inflammation and atherosclerosis? Diabetes Metab J. 2011;35(3):216–18. doi: 10.4093/dmj.2011.35.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]