Abstract
Diabetes is a lifelong condition requiring ongoing medical care and patient self-management. Exogenous insulin therapy is essential in type 1 diabetes and becomes a necessity in patients with longstanding type 2 diabetes who fail to achieve optimal control with lifestyle modification, oral agents, and glucagon-like peptide 1-based therapy. One of the risks that hinders insulin use is hypoglycemia. Optimal insulin therapy should therefore minimize the risk of hypoglycemia while improving glycemic control. Insulin degludec (IDeg) is a novel basal insulin that, following subcutaneous injection, assembles into a depot of soluble multihexamer chains. These subsequently release IDeg monomers that are absorbed at a slow and steady rate into the circulation, with the terminal half-life of IDeg being ~25 hours. Thus, it requires only once-daily dosing unlike other basal insulin preparations that often require twice-daily dosing. Despite its long half-life, once-daily IDeg does not cause accumulation of insulin in the circulation after reaching steady state. IDeg once a day will produce a steady-state profile with a lower peak:trough ratio than other basal insulins. In clinical trials, this profile translates into a lower frequency of nocturnal hypoglycemia compared with insulin glargine, as well as an ability to allow some flexibility in dose timing without compromising efficacy and safety. Indeed, a study that tested the extremes of dosing intervals of 8 and 40 hours showed no detriment in either glycemic control or hypoglycemic frequency versus insulin glargine given at the same time each day. While extreme flexibility in dose timing is not recommended, these findings are reassuring. This may be particularly beneficial to elderly patients, patients with learning difficulties, or others who have to rely on health-care professionals for their daily insulin injections. Further studies are required to confirm whether this might benefit adherence to treatment, reduce long-term hypoglycemia or reduce diabetes-related complications.
Keywords: basal insulin, diabetes, hypoglycemia, safety
Introduction
As the prevalence of diabetes continues to increase on a global level, research is moving toward the development of new treatments that can improve overall management of the condition, including new insulins with novel pharmacologic profiles. One such is insulin degludec (Tresiba®; Novo Nordisk, Bagsværd, Denmark), a recently developed basal insulin. When injected subcutaneously, insulin degludec produces an ultra-long pharmacokinetic (PK) absorption profile through a unique pharmacological mechanism,1 which translates into a very long duration of action that has been shown to exceed 42 hours in most patients.2 Previously available basal-insulin products have shorter durations of action that sometimes require twice-daily dosing, although the basal-insulin analogs glargine (Lantus®; Sanofi SA, Paris, France) and detemir (Levemir®; Novo Nordisk) are often considered to have durations of action close to 24 hours and are typically started with a once-daily dosing schedule.3,4 Insulin degludec is also given once daily, but the prospect of dosing a basal insulin at a frequency that is much shorter than its duration of action raises questions about how it will affect the risk of hypoglycemia, how it should be titrated to reach an optimal steady state, and whether there will be practical differences to consider when using the new insulin rather than previous products. This review therefore revisits some of the fundamental principles of diabetes management with insulin therapy, and the impact on dosing requirements and patient safety consequent to an insulin with an ultra-long duration of action.
Diabetes
Diabetes is a chronic illness requiring ongoing medical care and patient self-management. In healthy individuals, insulin is essential for the cells in the liver, skeletal muscle, and fat tissue to absorb glucose from the blood, where it is metabolized to produce energy, or stored. By controlling blood glucose levels, insulin prevents hyperglycemia, which, chronically, can cause micro- and macrovascular morbidity. Diabetes occurs when pancreatic beta cells fail to produce insulin (type 1 diabetes)5 or produce insufficient insulin to overcome insulin resistance in target tissues (type 2 diabetes).6
In type 1 diabetes, the immune system destroys the insulin-producing beta cells. In the absence of insulin, glucose cannot enter the cells of target tissues, so protein and fat are broken down as an alternative energy source, potentially leading to ketoacidosis. Type 1 diabetes, which usually develops before the age of 40, and often during teenage years, is less common than type 2 diabetes, accounting for ~10% of diabetes diagnoses in the USA and Europe.7
Type 2 diabetes occurs when muscle, liver, and adipose cells become less responsive to insulin (insulin resistance) and the beta cells fail to produce enough insulin to compensate. Type 2 diabetes accounts for ~90% of adult cases in the USA and Europe,7 and is characterized by gradual but persistent disease progression due to declining beta-cell function. It is often associated with obesity, poor diet, and physical inactivity. Type 2 diabetes may be controlled by lifestyle changes including diet and exercise and oral glucose-lowering treatments or incretin-based therapies,8 but over time, treatment intensification, including exogenous insulin therapy may be required.
Role of insulin therapy
Type 1 diabetes necessitates lifelong therapy with insulin, with dose adjustments based on self-monitoring of blood glucose levels. Long-term management requires a multidisciplinary approach that includes physicians, nurses, dietitians, pharmacists, and mental-health professionals with a specific interest in diabetes care.8 In patients with type 1 diabetes, the American Diabetes Association recommends the use of multiple-dose insulin injections or continuous subcutaneous insulin infusion to recreate the physiological plasma insulin kinetic profile.8 This comprises a continuous basal level of insulin secretion supplemented by peaks of secretion that take place in response to food ingestion.9 Therefore, prandial insulin therapy must be matched to carbohydrate intake, pre-meal blood glucose, and anticipated activity.
The objective in treating type 2 diabetes is to achieve and maintain glycemic control and to change interventions when therapeutic goals are not being met.8,10 Management of hyperglycemia in individuals with type 2 diabetes involves a cascade of interventions beginning from diagnosis, and often commencing with metformin in combination with lifestyle changes.8,10 The hemoglobin A1c (glycosylated hemoglobin, HbA1c) test, based on the attachment of glucose to hemoglobin, reflects an individual’s blood glucose levels over the previous 2–3 months. Where HbA1c targets are not achieved, treatment intensification is based on the addition of another blood glucose-lowering drug from a different class. In the past, insulin was considered the final treatment option for individuals with type 2 diabetes, but current treatment guidelines emphasize individualized therapy and recommend consideration of insulin, in particular basal insulin, as a component of treatment from much earlier in the disease process.8,10
Insulin was first developed as an impure preparation sourced from animals, but considerable changes have subsequently been made to insulin products to improve their efficacy, safety, and tolerability profile.11 In particular, analog insulin products have been developed that have onsets and durations of action that more closely mimic the kinetics of physiologic insulin secretion.11–13 Bolus, or prandial, insulin analogs are designed to mimic the physiologic response to meal-time carbohydrate absorption, while basal insulin analogs are designed to provide a lower and more constant circulating insulin level throughout the day to suppress excess hepatic glucose production.11
The most commonly used products in the basal insulin field currently are neutral protamine Hagedorn (NPH) insulin, insulin glargine, and insulin detemir. Insulin glargine and insulin detemir offer increased duration of action and reduced peaks and variability in their action profiles compared with NPH insulin,4,12 resulting in a reduced risk of hypoglycemia, particularly during the night when the influence of a basal insulin on blood glucose is less confounded by other factors.3,11,13 However, these basal insulin analogs still have suboptimal PK and pharmacodynamic (PD) properties; they need to be injected at the same time each day, potentially limiting adherence.14
Hypoglycemia: the primary adverse effect of insulin therapy
In healthy people, low blood glucose is counteracted by the secretion of glucohomeostatic regulatory hormones such as catecholamines, glucagon, cortisol, and growth hormones.15 In patients with type 1 diabetes, however, this response is defective, while in patients with type 2 diabetes the counter-regulatory function becomes progressively ineffective.16–20 Consequently, patients with diabetes are vulnerable to iatrogenic hypoglycemia, which occurs when the glucose-lowering action of therapies exceeds the physiological need, causing glucose levels to fall too far.
Hypoglycemia is the most common adverse effect of insulin therapy; a meta-analysis of six studies in patients with type 2 diabetes including 903,510 participants and 1.0–5.6 years of follow-up reported that, during the follow-up period, 0.6%–5.8% patients experienced severe hypoglycemia, for which third-party intervention was required.21
“Neuroglycopenia,” a shortage of glucose in the brain, is the most severe consequence of hypoglycemia, affecting the function of neurons and altering brain function and behavior.22 Prolonged or recurrent neuroglycopenia can result in loss of consciousness, damage to the brain, and eventual death.
Standard classifications of hypoglycemia include confirmed, severe, and nocturnal hypoglycemic events. “Severe hypoglycemia” is defined as an event for which an individual requires the assistance of another person and cannot be treated with oral carbohydrates due to confusion or unconsciousness.23 Hypoglycemia frequently occurs during sleep, and episodes of nocturnal hypoglycemia range from asymptomatic to severe.23 Due to a lack of counter-regulatory response, patients with type 1 diabetes experience a higher frequency of hypoglycemic events than those with type 2 diabetes.24
Consequences of hypoglycemia
Mild hypoglycemia can cause unpleasant and distressing adrenergic symptoms frequently leading to emergency hospitalization or extensive use of medical resources, while severe hypoglycemia can result in neurological (and, potentially, cardiovascular) sequela.16,19,22 Even mild hypoglycemic episodes may, understandably, cause the patient anxiety and ultimately lead them to reduce their insulin dose both in the short- and long-term.14,25,26 A recent survey of patients and physicians reported a high level of insulin omission/nonadherence: 33.2% of patients reported insulin omission/nonadherence, with a mean of 3.3 nonadherent days in the previous month.14 The fear of hypoglycemia can also delay patients and health-care providers from initiating or intensifying insulin therapy. In the same survey, physicians indicated that they would treat diabetes more aggressively if there were no concern regarding hypoglycemia,14 suggesting that insulin associated with a lower hypoglycemia risk would be used more effectively. This could potentially lead to improvements in blood-glucose control and hence reductions in complications.
Severe hypoglycemia has a major impact on quality of life, the costs of direct health care, as well as indirect costs mainly related to reduced productivity, absenteeism from work, and, occasionally, early retirement. For example, in a large cohort study, 536,581 people with type 2 diabetes with ~1.21 million person-years of follow-up, the cost of hypoglycemic events between 2004 and 2008 was US$52 million.27
Factors affecting the safety of insulin therapy
Non-pharmacologic factors
Factors such as temperature, degree of resuspension, and accuracy of dose can markedly alter the physiological response to an insulin injection, as can inadvertent intramuscular injection.28 Pen devices may be more accurate than vials and syringes, and continuous subcutaneous insulin infusion pumps, when coupled with continuous glucose monitoring, can allow minute-by-minute adjustment of insulin dose and thereby offer a further reduction in the risk of hypoglycemia along with modest improvements in HbA1c compared with multiple daily injections.29 Host risk factors for hypoglycemia include young and old age, emotional stress, and depression.30–32 Longer-term factors, such as weight change, concomitant medications or diseases, liver or renal dysfunction, and dementia33 can also have a marked impact on the PK/PD profiles of insulin-injection therapy.34,35
Pharmacologic factors
The ultimate aim for the design of a basal insulin is to recreate the flat and continuous kinetic profile of physiological insulin secretion, with minimum day-to-day intra-patient variability. NPH insulin, insulin glargine, and insulin detemir, however, all display suboptimal absorption kinetics that result in a non-flat (peaked) profile that translates into an increased risk of hypoglycemia. Clamp studies of NPH insulin show not only a pronounced peak in the blood glucose-lowering effect, but also high variability in the PD profiles that arise from identical doses given at identical times to an individual patient.12,36 The PD profiles of insulin glargine and insulin detemir both show a reduced peak effect with less within-patient variability;12 however, these still cannot be considered to represent the ideal basal-insulin profile.4,37
Insulin degludec is a new-generation, soluble basal insulin with an ultra-long, peak-less PK profile attributed to subcutaneous multihexamer formation and slow release of insulin degludec monomers.1 Insulin degludec is currently approved for use in Europe, Japan, India, and Mexico; approval in the USA is conditional on a satisfactory outcome in a cardiovascular safety trial. In clinical trials, insulin degludec offered similar efficacy, but a lower risk of nocturnal hypoglycemia, compared with insulin glargine.38–41
The pharmacology of insulin degludec
In pancreatic beta cells, endogenous insulin naturally forms hexamers in the presence of zinc. When secreted, these dissociate rapidly into the biologically active monomers. The concept behind much basal insulin development has been to exploit the property of insulin self-association as a mechanism for delaying its absorption from a subcutaneous depot. Insulin monomers absorb rapidly into the circulation across capillary membranes, but the larger hexamers absorb more slowly while even larger complexes are retained in the depot. Insulin degludec was therefore designed to self-associate into even larger complexes than hexamers. Insulin degludec is a desB30 insulin acylated at the LysineB29 (LysB29) residue with a γ-glutamate linker and 16-carbon fatty diacyl side chain (Figure 1).1 Its ability to form a multihexamer structure is key to prolonging the absorption rate.
Figure 1.
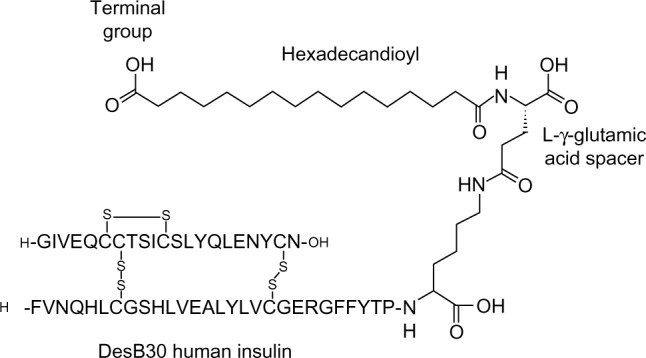
Schematic representation of insulin degludec. DesB30 human insulin is acylated at the ε-amino group of LysineB29 with hexadecanoic acid via a γ-L-glutamic acid linker.
Note: Reproduced from Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29(8):2104–2114. Copyright © 2012, the authors.1
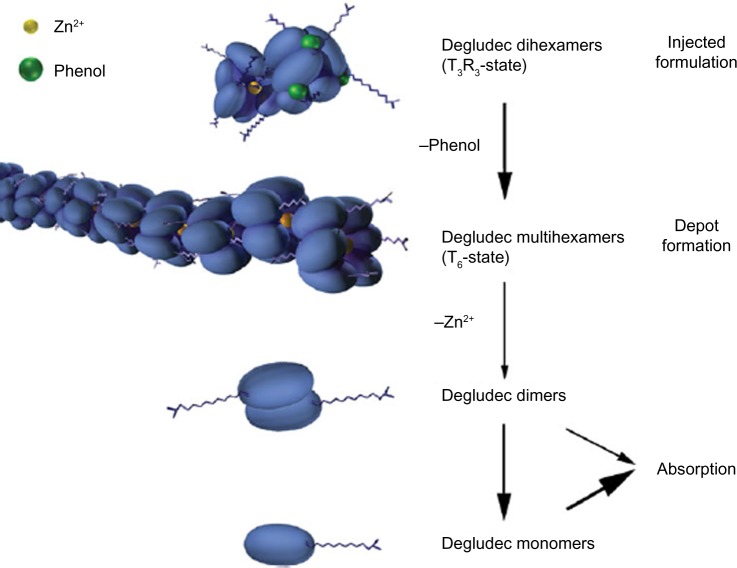
In the pharmaceutical formulation (ie, in the presence of phenol), the insulin degludec hexamer adopts a “T3R3” conformation in which one end of the hexamer is open. This allows for interaction between an internal zinc ion in one hexamer and the side chain of another hexamer, forming a stable dihexamer. After injection, the phenol diffuses, causing the dihexamers to become open at both ends, enabling the formation of multihexamers (Figure 2).1 The gradual diffusion of zinc from the ends of the multihexamers subsequently causes the dissociation of the terminal hexamers into monomers, which are released and absorbed at a slow and steady rate. This results in a slow and gradual delivery of insulin degludec from the subcutaneous injection site into the circulation.
Figure 2.
Schematic representation of the hypothesis for the mode of retarded absorption of insulin degludec.1 Insulin degludec is injected subcutaneously as a zinc phenol formulation containing insulin degludec dihexamers in the T3R3 conformation. Rapid loss of phenol changes the degludec hexamers to T6 configuration and multihexamer chains form. With slow diffusion of zinc, these chains break down into dimers, which quickly dissociate into readily absorbed monomers.
Note: Reproduced from Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29(8): 2104–2114. Copyright © 2012, the authors.1
PK/PD profile
Several studies investigating the pharmacological profile of insulin degludec were conducted both in patients with type 1 and type 2 diabetes. The mean terminal half-life of insulin degludec exceeds 25 hours in patients with either type 1 or type 2 diabetes, with a duration of action exceeding 42 hours in most patients with type 1 diabetes.2,42,43 Due to its mechanism of protraction and the constant absorption rate, insulin degludec reaches clinical steady state with once-daily dosing in 2–3 days, producing a remarkably flat and stable PD profile.44
Further, there is relatively little within-subject variability in the glucose-lowering action from day to day or hour to hour, with overall intra-patient variability shown to be significantly (four-times) lower compared with insulin glargine in patients with type 1 diabetes.45 The PK profile of insulin degludec is not altered in specific population types including the elderly (aged ≥65 years), children, adolescents, or those with renal or hepatic impairment.46–49
Will an insulin dosed more frequently than its half-life result in insulin stacking?
“Insulin stacking” can be defined as insulin accumulating in the blood to inappropriately high levels as a result of absorption from repeated doses. This scenario is most commonly seen with rapidly absorbed mealtime insulins when a “corrective” additional dose is given if it is perceived that the original dose has not been sufficient to limit a postprandial rise in blood glucose. The result can be an overcompensation leading to a hypoglycemic event.50–52
In the case of a basal insulin with a very long action profile, it may seem logical to question whether daily dosing would lead to excessive accumulation in the circulation, thereby increasing the risk, severity, or duration of hypoglycemic events. However, the goal of a basal insulin is to achieve steady state, whereby the rate of insulin absorption into the circulation equals the rate of uptake and elimination in target tissues over a 24-hour period. Ideally, this would be achieved with circulating insulin levels remaining as constant as possible (ie, a low peak:trough ratio). In the case of insulin, the plasma half-life is short, with elimination predominantly via receptor internalization, so the PD profile essentially reflects the dynamic rate of absorption.
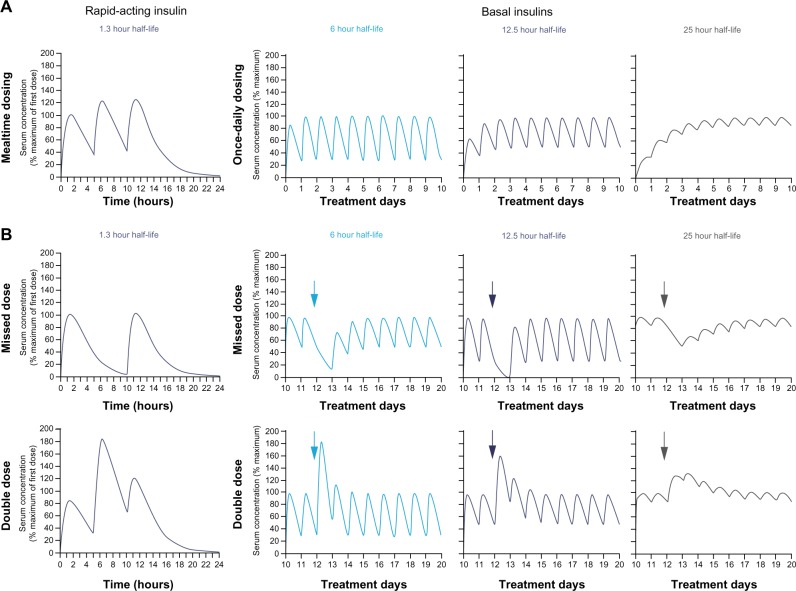
Studies with once-daily administration of insulin degludec have clearly shown that concentrations increase over the initial days of treatment until they reach steady-state levels, after which no further accumulation occurs with a stable dose.44 In fact, the longer the elimination half-life is relative to the dosing interval, the lower the peak:trough ratio will be at steady-state (Figure 3).52 Moreover, if the elimination half-life is long relative to the dosing interval, the impact of missed or excessively high doses is buffered when the insulin is at steady state.
Figure 3.
Hypothetical examples of profiles of insulins with various half-lives.52
Notes: (A) Accumulation from first dose to steady state and (B) perturbations following various types of common dosing errors as indicated by arrows, when introduced at steady-state. When interpreting the effects of double-dosing (bottom row), it is important to note that different pharmacokinetic scales have been used for the rapid-acting and basal insulin curves. Fluctuations in insulin concentration (and therefore glucose-lowering action) are greatest, and dosing errors have the most acute effects, with basal insulin having a shorter half-life (eg, 6 hours) and duration of action. Fluctuations are dampened and dosing errors have less acute effects with insulin formulations having a longer half-life/duration of action. The half-lives for basal insulin shown in the figure correspond approximately to those of neutral protamine Hagedorn insulin (6 hours), insulin glargine (12.5 hours), and insulin degludec (25 hours). Reprinted from Endocrine Practice. Heise T, Meneghini LF. Insulin stacking versus therapeutic accumulation: understanding the differences. Endocr Pract. 2013;20(1):75–83. Copyright 2013, with permission from the American Association of Clinical Endocrinologists.52
As hypoglycemia is most likely to occur during the times when insulin is at peak levels, it follows that, with appropriate dosing, the steady-state profile of an ultra-long-acting insulin with a low peak:trough ratio should carry a lower risk of hypoglycemia than a shorter-acting basal insulin given at the same dose frequency. This anticipated risk reduction should be aided by a low intra-patient variability in insulin absorption. The risk of hypoglycemia associated with closely repeated dosing of rapid-acting insulins reflects the fact that the absorption rate is much faster, hence the elimination rate would eventually balance at a steady state with a much greater plasma insulin concentration.
Efficacy and safety results from clinical trials
The results from the insulin degludec Phase III clinical trials (the BEGIN program) suggest that the PK/PD properties of insulin degludec do translate into a reduced risk of hypoglycemia versus other basal insulins at an equivalent level of blood-glucose control. Reduced rates of nocturnal hypoglycemia were reported in studies comparing insulin degludec with insulin glargine in both basal-only and basal-bolus regimens in patients with either type 1 or type 2 diabetes (Table 1).38,39,41,53–63
Table 1.
Hypoglycemia rates in clinical trials of insulin degludec
| ClinicalTrials.gov registration number | Study design | Patient population | Randomized trial treatment (full analysis set) | Initial insulin dose | Overall confirmed hypoglycemia (IDeg vs IGlar) episodes/PYE (rate ratio [95% CI]) | Nocturnal confirmed hypoglycemia (IDeg vs IGlar) episodes/PYE (rate ratio [95% CI]) |
|---|---|---|---|---|---|---|
| NCT00982644 (BEGIN ONCE LONG)41,57 | 52-week, open-label, randomized, treat-to-target, non-inferiority trial | Insulin-naïve adults with type 2 diabetes inadequately controlled with oral antidiabetic drugs | 3:1 randomization ratio IDeg OD (n=773) IGlar OD (n=257) |
Ten units | 1.52 vs 1.85 episodes/PYE (0.82 [0.64–1.04], NS) | 0.25 vs 0.39 episodes/PYE (0.64 [0.42–0.98], P=0.038) |
| NCT00972283 (BEGIN BB)38,58 | 52-week, open-label, randomized, treat-to-target, non-inferiority trial | Adults with uncontrolled type 2 diabetes treated with any insulin regimen for ≥3 months | 3:1 randomization ratio IDeg OD (n=744) IGlar OD (n=248) |
Starting dose in insulin-naïve patients: ten units Patients receiving prior OD basal insulin switched on a 1:1 basis, whereas patients on a pre-study BID basal insulin regimen were switched to lower starting doses of IGlar and IDeg. For IGlar, the starting dose was 20%–30% lower than the pre-study total daily insulin dose according to approved product labeling; for IDeg, the starting dose was determined by the investigator on an individual patient basis |
11.09 vs 13.63 episodes/PYE (0.82 [0.69–0.99], P=0.036) | 1.39 vs 1.84 episodes/PYE (0.75 [0.58–0.99], P=0.040) |
| NCT00982228 (BEGIN BB T1 LONG)39,59 | 52-week, open-label, randomized, treat-to-target, non-inferiority trial | Adults with uncontrolled type 1 diabetes treated with basal–bolus insulin for ≥1 year | 3:1 randomization ratio IDeg OD + IAsp (n=472) IGlar OD + IAsp (n=157) |
Patients receiving prior OD basal insulin switched on a 1:1 basis, whereas patients on a pre-study BID basal insulin regimen were switched to lower starting doses of IGlar and 1:1 for IDeg. For IGlar, the starting dose was 20%–30% lower than the pre-study total daily insulin dose according to approved product labeling | 42.54 vs 40.18 episodes/PYE (1.07 [0.89–1.28], NS) | 4.41 vs 5.86 episodes/PYE (0.75 [0.59–0.96], P=0.021) |
| NCT01006291 (BEGIN FLEX)53,60 | 26-week, open-label, randomized, treat-to-target, non-inferiority trial | Insulin-naïve adults with type 2 diabetes inadequately controlled with oral antidiabetic drugs Adults with uncontrolled type 2 diabetes treated with any insulin regimen for ≥3 months |
1:1:1 randomization ratio IDeg flexible OD (n=229) IDeg fixed OD (n=228) IGlar OD (n=230) |
Starting dose in insulin-naïve patients: 10 units Patients receiving prior OD basal insulin switched on a 1:1 basis, whereas patients on a pre-study BID basal insulin regimen were switched to lower starting doses of IGlar and IDeg. For IGlar, the starting dose was 20%–30% lower than the pre-study total daily insulin dose according to approved product labeling; for IDeg, the starting dose was determined by the investigator on an individual patient basis |
3.6 (IDeg Flex) vs 3.6 (IDeg Fixed) vs 3.5 (IGlar) episodes/PYE (IDeg Flex vs IGlar: 1.03 [0.75–1.40], NS; IDeg Flex vs IDeg Fixed: 1.10 [0.79–1.52], NS) | 0.6 (IDeg Flex) vs 0.6 (IDeg Fixed) vs 0.8 (IGlar) episodes/PYE (IDeg Flex vs IGlar: 0.77 [0.44–1.35], NS; IDeg Flex vs IDeg Fixed: 1.18 [0.66–2.12], NS) |
| NCT01079234 (BEGIN FLEX T1)54,61 | 26-week, open-label, randomized, treat-to-target, non-inferiority trial | Adults with uncontrolled type 1 diabetes treated with basal-bolus insulin for ≥1 year | 1:1:1 randomization ratio IDeg flexible OD (n=164) IDeg fixed OD (n=165) IGlar OD (n=164) |
Patients receiving prior OD basal insulin switched on a 1:1 basis, whereas patients on a pre-study BID basal insulin regimen were switched to lower starting doses of IGlar and IDeg. For IGlar, the starting dose was 20%–30% lower than the pre- study total daily insulin dose according to approved product labeling; for IDeg, the starting dose was determined by the investigator on an individual patient basis | 82.4 (IDeg Flex) vs 88.3 (IDeg Fixed) vs 79.7 (IGlar) episodes/PYE (IDeg Flex vs IGlar: 1.03 [0.85–1.26], NS; IDeg Flex vs IDeg Fixed: 0.92 [0.76–1.12], NS) |
6.2 (IDeg Flex) vs 9.6 (IDeg Fixed) vs 10.0 (IGlar) episodes/PYE (IDeg Flex vs IGlar: 0.60 [0.44–0.82], P=0.001; IDeg Flex vs IDeg Fixed: 0.63 [0.46–0.86], P=0.003) |
| NCT01059799 (BEGIN ONCE ASIA)55,62 | 26-week, open-label, randomized, Pan-Asian, treat-to-target, non-inferiority trial | Insulin-naïve adults with type 2 diabetes inadequately controlled with oral antidiabetic drugs | 2:1 randomization ratio IDeg OD (n=289) IGlar OD (n=146) |
Ten units | 3.0 vs 3.7 episodes/PYE (0.82 [0.60–1.11], P=0.20) |
0.8 vs 1.2 episodes/PYE (0.62 [0.38–1.04], P=0.07) |
| NCT01068665 (BEGIN LOW VOLUME)56,63 | 26-week, open-label, randomized, treat-to-target, non-inferiority trial | Insulin-naïve adults with type 2 diabetes inadequately controlled with oral antidiabetic drugs | 1:1 randomization ratio IDeg U200 OD (n=228) IGlar U100 OD (n=229) |
Ten units | 1.22 vs 1.42 episodes/PYE (0.86 [0.58–1.28], NS) |
0.18 vs 0.28 episodes/PYE (0.64 [0.30–1.37], NS) |
Notes: In the BEGIN studies, “confirmed hypoglycemia” was defined as episodes in which the plasma glucose value was <3.1 mmol/L (irrespective of symptoms) or severe (requiring assistance). “Nocturnal hypoglycemia” was defined as episodes occurring between 0001 and 0559 hours (inclusive).
Abbreviations: BID, twice daily; CI, confidence interval; IAsp, insulin aspart; IDeg, insulin degludec; IDeg Flex, insulin degludec administered at varying times day to day; IDeg fixed, insulin degludec administered at a fixed time every day; IGlar, insulin glargine; NS, nonsignificant; OD, once daily; PYE, patient years of exposure; U200, 200 units/mL formulation; U100, 100 units/mL formulation; vs, versus.
The hypoglycemia data comparing insulin degludec with insulin glargine from the BEGIN program were analyzed further in a meta-analysis across the entire treatment period as well as the maintenance period (stable glycemia and insulin dose from 16 weeks onward).40 In patients with type 2 diabetes, significantly lower rates of overall confirmed and nocturnal confirmed hypoglycemic episodes were reported with insulin degludec than with insulin glargine over the entire treatment period (risk ratio [95% confidence interval {CI}] 0.83 [0.74–0.94] and 0.68 [0.57–0.82], respectively). In patients with type 1 diabetes, there was a 17% risk reduction (nonsignificant) in nocturnal confirmed hypoglycemia across the entire treatment period, but the rate of nocturnal confirmed hypoglycemic episodes became significantly lower with insulin degludec than with insulin glargine during the maintenance period (risk ratio [95% CI], 0.75 [0.60–0.94]). Indeed, the relative risk reductions in hypoglycemia associated with insulin degludec were more pronounced during the maintenance period in all populations.
An additional meta-analysis of three basal-only insulin trials from the BEGIN program looked at the duration of the hypoglycemia experienced by insulin-naïve patients with type 2 diabetes treated with either insulin degludec or insulin glargine.64 The results indicated that there were no statistically significant differences between either treatment arm in terms of time to recognize (7.2 minutes for both), duration (26.4 versus [vs] 28.2 minutes, insulin degludec vs insulin glargine), recovery time (34.2 vs 32.4 minutes, insulin degludec vs insulin glargine), or impact on daily activities.64
Other adverse events associated with insulin degludec included injection-site reactions (common; ≥1/100 to <1/10), peripheral edema and lipodystrophy (both uncommon; ≥1/1,000 to <1/100).65 Cardiovascular safety data for insulin degludec will be reported in due course. A large-scale safety outcomes study was recommended by a recent US Food and Drug Administration advisory board in response to indications of a possible increased risk versus comparators – albeit based on small numbers in a composite endpoint for adverse cardiac events and in clinical trials not specifically designed for assessing cardiovascular risk.
Dosing implications arising from the PK/PD profile of insulin degludec
To explore whether insulin degludec could be dosed less frequently than once daily, two 26-week, randomized, open-label, non-inferiority trials compared the efficacy of insulin degludec administered by injection before breakfast (3TWAM) or with the evening meal (3TWPM) three times weekly with that of insulin glargine administered once daily in adults with type 2 diabetes.66 Non-inferiority for HbA1c (with the pre-specified definition for non-inferiority being that the 95% confidence intervals for the difference in HbA1c should not exceed 0.4%) was not confirmed in either trial (estimated treatment difference [95% CI] 0.34% [0.18–0.51] [insulin degludec 3TWAM vs insulin glargine once daily]; 0.26% [0.11–0.41] [insulin degludec 3TWPM vs insulin glargine once daily]). Consequently, the once-daily dosing regimen was selected for further clinical development.
Beyond reducing the risk of hypoglycemia, a further implication of the ultra-long PK profile of insulin degludec dosed once daily is that day-to-day differences in dose timing should have a relatively minor effect on the overall kinetic profile, as indicated by the relative impact of major regimen perturbations in Figure 3. Two studies compared insulin degludec in a “forced-flexible” dosing regimen of alternating intervals of 8 and 40 hours between dosing compared with a fixed once-daily dosing of insulin degludec, and with once-daily insulin glargine (as per label).53,54 In a 26-week, open-label, treat-to-target, non-inferiority trial, patients with type 1 diabetes received either insulin degludec given in a forced-flexible schedule (8 and 40 hours between doses), or insulin degludec or insulin glargine given at the same time daily.54 In the 26-week extension, all patients receiving insulin degludec were transferred to a free-flexible (Free-Flex) regimen, which allowed any-time-of-day dosing (provided the intervals between doses remained no shorter than 8 and no longer than 40 hours), and compared with patients continued on insulin glargine. Overall confirmed hypoglycemia was similar at week 52 in patients receiving Free-Flex insulin degludec and insulin glargine, but nocturnal confirmed hypoglycemia was significantly reduced in patients receiving Free-Flex insulin degludec compared with insulin glargine (−25% [estimated rate ratio {95% CI} 0.75 {0.58–0.97}; P=0.026], full analysis set; −27% [0.73 {0.54–0.98}; P=0.035], extension trial set).
The forced-flexible regimen was also tested in a 26-week trial in patients with type 2 diabetes, where the rate of both confirmed and nocturnal confirmed hypoglycemia did not differ between patients receiving forced-flexible insulin degludec and those receiving insulin glargine (rate ratio [95% CI] 1.03 [0.75–1.40], nonsignificant [NS] [overall confirmed hypoglycemia]; 0.77 [0.44–1.35], NS [nocturnal confirmed hypoglycemia]), or between forced-flexible and same-time insulin degludec (rate ratio [95% CI] 1.10 [0.79–1.52], NS [overall confirmed hypoglycemia]; 1.18 [0.66–2.12], NS [nocturnal confirmed hypoglycemia]).53
These two trials suggest that the ultra-long duration of action of insulin degludec allows variation of daily administration times within an 8–40 hour window without compromising efficacy or patient safety. The degludec summary of product characteristics states that a minimum of 8 hours between injections should always be ensured in practice.65
A further consideration in terms of dosing and patient safety concerns accidental or deliberate overdose.67 Figure 3 illustrates that an overdose of insulin degludec can be expected to result in a slower rise in insulin level to a lower peak compared with a shorter-acting basal insulin, allowing more time for intervention. However, the elevation of serum insulin concentration will endure for longer, which might imply that a longer period of monitoring and possible repeated interventions to raise blood-glucose levels could be required. If a dose is forgotten, the dose should be taken on discovery and usual once-daily dosing should then be resumed.
Another dosing implication concerns the rate and increment of dose titration. The insulin degludec summary of product characteristics advises that patients with either type 1 or type 2 diabetes can switch unit-to-unit to insulin degludec from other once-daily basal insulins, with individual adjustments.65 It is noteworthy, however, that in studies in patients with type 1 diabetes, the rate of hypoglycemia was higher in the titration period in patients receiving insulin degludec,39,54 potentially undermining a later benefit. This is possibly because patients switching from a previous basal insulin were always switched unit-for-unit to insulin degludec, whereas patients switching from twice-daily basal insulin to once-daily insulin glargine had a dose reduction. Additionally, ambitious fasting blood-glucose targets and titration algorithms were used. We propose that initial titration of an ultra-long acting insulin might be better done with lower dose increments over a more extended titration phase than used in the trials. Although dose reduction is not suggested during transition from once-daily basal insulin to insulin degludec, a dose at least 20% less than the daily dose of basal insulin should be considered for patients with type 1 diabetes coming from a twice-daily basal insulin regimen or a twice-daily pre-mixed insulin therapy, or whose HbA1c is already less than 8% (64 mmol/mol). Dose and timing of the prandial insulin or the doses of the concomitant oral antidiabetic drugs may need to be adjusted. Further dose adjustment should be individualized. Close glucose monitoring is recommended during the transfer and in the following weeks.
Insulin degludec is manufactured at a standard concentration of 100 U/mL, but additionally in a high-concentration formulation (200 U/mL). This has been shown to be bioequivalent to the 100 U/mL formulation and, unusually for insulin, the higher concentration does not result in an altered PK/PD profile.68 Insulin degludec forms soluble multihexamers on subcutaneous injection, resulting in a depot from which monomers are slowly and continuously absorbed, and it is speculated that the monomer release rate may be the rate-limiting factor and be unaffected by depot concentration or surface area.1,42,45 Patients requiring a large unit dose can administer insulin degludec in a smaller volume using the 200 U/mL formulation and in some cases avoid needing to split the dose between two injections. A 26-week randomized, controlled trial in insulin-naïve patients with type 2 diabetes reported similar HbA1c reductions with insulin degludec U200 (200 units/mL formulation) compared with insulin glargine U100 (100 units/mL formulation), with significantly better fasting blood-glucose reductions and a low rate of hypoglycemia.56
Which patients could benefit from a basal insulin with an ultra- long duration of action?
Results from the Global Attitudes of Patients and Physicians in Insulin Therapy survey have revealed several factors that govern why a patient omits an insulin dose.14 The top six reasons given by patients were: too busy, traveling, skipped a meal, stress, embarrassing to inject in public, and challenging to take it at the same time every day. These data suggest that in addition to the lifestyle concerns of most individuals, where a flexible option may on occasion be useful, there are subsets of people for whom a flexible insulin may be frequently required. In particular, this could include individuals who travel regularly and often face the challenge of different time zones. Shift workers may also greatly benefit from the freedom to change their dosing schedule from week to week based on their diary. Further studies are required to evaluate the impact of insulin degludec on glycemic control and risk reduction of hypoglycemia in these patient groups.
Elderly patients with diabetes are often more prone to hypoglycemic events due to many factors.69,70 Being in a care home or hospital or having a visiting nurse may mean that insulin administration is the responsibility of a carer, and the patient has no control over the regularity or time of their insulin or, possibly, their meals. Therefore, an insulin that, for example, does not commit a visiting nurse to a very specific time could be a great benefit to them and the patient. A preplanned meta-analysis of seven BEGIN trials showed a numerically lower risk of overall confirmed hypoglycemia over the total treatment period in the pooled elderly patient population (type 1 and type 2 diabetes) receiving insulin degludec compared with insulin glargine (estimated risk ratio [95% CI], 0.82 [0.66–1.00]; NS) and statistically significantly lower risk of nocturnal confirmed hypoglycemia (estimated risk ratio [95% CI], 0.65 [0.46–0.93]; P<0.05).71 This demonstrates that the hypoglycemic benefits of insulin degludec are retained in elderly patients.
Conclusion
Iatrogenic hypoglycemia is a major barrier to glycemic control. As a consequence, it has a significant impact on individual well-being and health outcomes. Insulin degludec is a new basal insulin with an ultra-long duration of action and a mechanism of protraction that induces a flat and stable action profile with low variability. Insulin degludec has a long terminal half-life (>25 hours in patients with either type 1 or type 2 diabetes) and reaches steady state in 2–3 days. Clinical data suggest that this pharmacologic profile translates into a reduced risk of nocturnal hypoglycemia compared with other basal insulins at equivalent levels of HbA1c. The results of clinical studies using a flexible dosing regimen suggest that, while a “once a day, at the same time” regimen is recommended, efficacy and tolerability are not compromised by flexible dosing, and this may be of particular value in certain circumstances such as travel and shift working and for those people with extremely busy lives or who are in care or unable to administer their own insulin. Further studies will be required in specific patient populations to confirm the possible beneficial effect of insulin degludec on adherence to treatment and hypoglycemic risk reduction, and to identify the optimum protocols for dose titration.
Acknowledgments
The authors are grateful to Watermeadow Medical for writing and editorial assistance, funded by Novo Nordisk.
Footnotes
Disclosure
The authors take full responsibility for the content of this manuscript. Dr Aye has no conflicts of interest to disclose; Professor Atkin has received research income from, and has been a speaker for, all of the major pharmaceutical companies.
References
- 1.Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29(8):2104–2114. doi: 10.1007/s11095-012-0739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurtzhals P, Heise T, Strauss HM, et al. Multi-hexamer formation is the underlying mechanism behind the ultra-long glucose-lowering effect of insulin degludec. Diabetes. 2011;60(Suppl 1A):42-LB. (abstract) [Google Scholar]
- 3.Devries JH, Nattrass M, Pieber TR. Refining basal insulin therapy: what have we learned in the age of analogues? Diabetes Metab Res Rev. 2007;23(6):441–454. doi: 10.1002/dmrr.762. [DOI] [PubMed] [Google Scholar]
- 4.Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;9(5):648–659. doi: 10.1111/j.1463-1326.2007.00756.x. [DOI] [PubMed] [Google Scholar]
- 5.Ding L, Gysemans C, Mathieu C. β-Cell differentiation and regeneration in type 1 diabetes. Diabetes Obes Metab. 2013;15(Suppl 3):98–104. doi: 10.1111/dom.12164. [DOI] [PubMed] [Google Scholar]
- 6.Fineberg SK. Application of newer concepts in diabetes. J Am Geriatr Soc. 1966;14(5):463–471. doi: 10.1111/j.1532-5415.1966.tb03072.x. [DOI] [PubMed] [Google Scholar]
- 7.National Diabetes Information Clearinghouse (NDIC) Diabetes overview [web page on the Internet] Bethesda, MD: NDIC; 2008. [Accessed January 26, 2014]. NIH Publication No 09–3873. Available from: http://diabetes.niddk.nih.gov/dm/pubs/overview/ [Google Scholar]
- 8.American Diabetes Association Standards of medical care in diabetes – 2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polonsky KS, Given BD, Hirsch LJ, et al. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med. 1988;318(19):1231–1239. doi: 10.1056/NEJM198805123181903. [DOI] [PubMed] [Google Scholar]
- 10.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garber AJ. Restaging insulin therapy for patients with type 2 diabetes. Diabetes Obes Metab. 2009;11(Suppl 5):1–5. doi: 10.1111/j.1463-1326.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- 12.Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614–1620. doi: 10.2337/diabetes.53.6.1614. [DOI] [PubMed] [Google Scholar]
- 13.Simon AC, DeVries JH. The future of basal insulin supplementation. Diabetes Technol Ther. 2011;13(Suppl 1):S103–S108. doi: 10.1089/dia.2010.0251. [DOI] [PubMed] [Google Scholar]
- 14.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29(5):682–689. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garber AJ, Cryer PE, Santiago JV, Haymond MW, Pagliara AS, Kipnis DM. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest. 1976;58(1):7–15. doi: 10.1172/JCI108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 17.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia. 2002;45(7):937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 18.Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51(3):724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 19.Graveling AJ, Frier BM. Hypoglycaemia: an overview. Prim Care Diabetes. 2009;3(3):131–139. doi: 10.1016/j.pcd.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Frier BM, Fisher M. Impaired hypoglycaemia awareness. In: Frier BM, Fisher M, editors. Hypoglycaemia in Clinical Diabetes. Chichester: Wiley; 1999. pp. 111–146. [Google Scholar]
- 21.Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533. doi: 10.1136/bmj.f4533. [DOI] [PubMed] [Google Scholar]
- 22.Briscoe VJ, Davis SN. Hypoglycemia in type 1 and type 2 diabetes: physiology, pathophysiology, and management. Clin Diabetes. 2006;24(3):115–121. [Google Scholar]
- 23.Workgroup on Hypoglycemia, American Diabetes Association Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28(5):1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly LA, Morris AD, Frier BM, et al. DARTS/MEMO Collaboration Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabet Med. 2005;22(6):749–755. doi: 10.1111/j.1464-5491.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- 25.Leiter LA, Yale JF, Chiasson JL, Harris SB, Kleinstiver P, Saurio L. Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemia management. Can J Diabetes. 2005;29(3):186–192. [Google Scholar]
- 26.Tahrani A, Barnett A, Brod M, Rana M, Peyrot M. GAPP2™: Global survey finds three quarters of patients experience hypoglycaemia on insulin analogue causing dose irregularities and increased blood glucose monitoring. Diabetologia. 2012;55(Suppl 1):S98–S99. [Google Scholar]
- 27.Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care. 2011;17(10):673–680. [PubMed] [Google Scholar]
- 28.American Diabetes Association Insulin administration. Diabetes Care. 2004;27(90001):106S–107S. [Google Scholar]
- 29.Pickup JC. Management of diabetes mellitus: is the pump mightier than the pen? Nat Rev Endocrinol. 2012;8(7):425–433. doi: 10.1038/nrendo.2012.28. [DOI] [PubMed] [Google Scholar]
- 30.Bot M, Pouwer F, de Jonge P, Tack CJ, Geelhoed-Duijvestijn PH, Snoek FJ. Differential associations between depressive symptoms and glycaemic control in outpatients with diabetes. Diabet Med. 2013;30(3):e115–e122. doi: 10.1111/dme.12082. [DOI] [PubMed] [Google Scholar]
- 31.Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142(Suppl):S8–S21. doi: 10.1016/S0165-0327(12)70004-6. [DOI] [PubMed] [Google Scholar]
- 32.Katon WJ, Young BA, Russo J, et al. Association of depression with increased risk of severe hypoglycemic episodes in patients with diabetes. Ann Fam Med. 2013;11(3):245–250. doi: 10.1370/afm.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaffe K, Falvey CM, Hamilton N, et al. Health ABC Study Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med. 2013;173(14):1300–1306. doi: 10.1001/jamainternmed.2013.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis TM, Brown SG, Jacobs IG, Bulsara M, Bruce DG, Davis WA. Determinants of severe hypoglycemia complicating type 2 diabetes: the Fremantle diabetes study. J Clin Endocrinol Metab. 2010;95(5):2240–2247. doi: 10.1210/jc.2009-2828. [DOI] [PubMed] [Google Scholar]
- 35.Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in Type 2 diabetes. Diabet Med. 2008;25(3):245–254. doi: 10.1111/j.1464-5491.2007.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholtz HE, Pretorius SG, Wessels DH, Becker RH. Pharmacokinetic and glucodynamic variability: assessment of insulin glargine, NPH insulin and insulin ultralente in healthy volunteers using a euglycaemic clamp technique. Diabetologia. 2005;48(10):1988–1995. doi: 10.1007/s00125-005-1916-y. [DOI] [PubMed] [Google Scholar]
- 37.Garber AJ. Will the next generation of basal insulins offer clinical advantages? Diabetes Obes Metab. 2013 Oct 4; doi: 10.1111/dom.12219. Epub. [DOI] [PubMed] [Google Scholar]
- 38.Garber AJ, King AB, Del Prato S, et al. NN1250-3582 (BEGIN BB T2D) Trial Investigators Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1498–1507. doi: 10.1016/S0140-6736(12)60205-0. [DOI] [PubMed] [Google Scholar]
- 39.Heller S, Buse J, Fisher M, et al. BEGIN Basal-Bolus Type 1 Trial Investigators Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1489–1497. doi: 10.1016/S0140-6736(12)60204-9. [DOI] [PubMed] [Google Scholar]
- 40.Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15(2):175–184. doi: 10.1111/dom.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zinman B, Philis-Tsimikas A, Cariou B, et al. NN1250-3579 (BEGIN Once Long) Trial Investigators Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long) Diabetes Care. 2012;35(12):2464–2471. doi: 10.2337/dc12-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heise T, Nosek L, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14(10):944–950. doi: 10.1111/j.1463-1326.2012.01638.x. [DOI] [PubMed] [Google Scholar]
- 43.Heise T, Hövelmann U, Nosek L, Bøttcher S, Granhall C, Haahr H. Insulin degludec has a two-fold longer half-life and a more consistent pharmacokinetic profile than insulin glargine. Diabetes. 2011;60(Suppl 1A):37-LB. (abstract) [Google Scholar]
- 44.Heise T, Nosek L, Coester HV, et al. Steady state is reached within two to three days of once-daily administration of ultra-long-acting insulin degludec. Diabetes. 2012;61(Suppl 1):A259. (abstract) [Google Scholar]
- 45.Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14(9):859–864. doi: 10.1111/j.1463-1326.2012.01627.x. [DOI] [PubMed] [Google Scholar]
- 46.Kupčová V, Arold G, Roepstorff C, Højbjerre M, Klim S, Haahr HL. Insulin degludec: pharmacokinetic properties in subjects with hepatic impairment. Clin Drug Investig. 2014;34(2):127–133. doi: 10.1007/s40261-013-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiss I, Arold G, Roepstorff C, Bøttcher SG, Klim S, Haahr H. Insulin degludec: pharmacokinetics in patients with renal impairment. Clin Pharmacokinet. 2013;53(2):175–183. doi: 10.1007/s40262-013-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korsatko S, Deller S, Mader JK, et al. Ultra-Long Pharmacokinetic Properties of Insulin Degludec are Comparable in Elderly Subjects and Younger Adults with Type 1 Diabetes Mellitus. Drugs Aging. 2013;31(1):47–53. doi: 10.1007/s40266-013-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danne T, Bieter T, Blaesig S, et al. Ultra-long pharmacokinetic properties of insulin degludec in adults with type 1 diabetes is preserved in children and adolescents after single-dose administration. Diabetologia. 2011;54(Suppl 1):S425. [Google Scholar]
- 50.Hirsch IB. Insulin analogues. N Engl J Med. 2005;352(2):174–183. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- 51.DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289(17):2254–2264. doi: 10.1001/jama.289.17.2254. [DOI] [PubMed] [Google Scholar]
- 52.Heise T, Meneghini LF. Insulin stacking versus therapeutic accumulation: understanding the differences. Endocr Pract. 2013;20(1):75–83. doi: 10.4158/EP13090.RA. [DOI] [PubMed] [Google Scholar]
- 53.Meneghini L, Atkin SL, Gough SC, et al. NN1250-3668 (BEGIN FLEX) Trial Investigators The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care. 2013;36(4):858–864. doi: 10.2337/dc12-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathieu C, Hollander P, Miranda-Palma B, et al. NN1250-3770 (BEGIN: Flex T1) Trial Investigators Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab. 2013;98(3):1154–1162. doi: 10.1210/jc.2012-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Onishi Y, Iwamoto Y, Yoo SJ, Clauson P, Tamer SC, Park S. Insulin degludec compared with insulin glargine in insulin-naïve patients with type 2 diabetes: A 26-week, randomized, controlled, Pan-Asian, treat-to-target trial. J Diabetes Investig. 2013;4(6):605–612. doi: 10.1111/jdi.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gough SC, Bhargava A, Jain R, Mersebach H, Rasmussen S, Bergenstal RM. Low-volume insulin degludec 200 units/mL once daily improves glycemic control similarly to insulin glargine with a low risk of hypoglycemia in insulin-naive patients with type 2 diabetes: a 26-week, randomized, controlled, multinational, treat-to-target trial: the BEGIN LOW VOLUME trial. Diabetes Care. 2013;36(9):2536–2542. doi: 10.2337/dc12-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novo Nordisk . ClinicalTrials.gov [website on the Internet] Bethseda, MD: US National Library of Medicine; 2008. [Accessed January 26, 2014]. Comparison of NN1250 Versus Insulin Glargine in Subjects With Type 2 Diabetes (BEGIN™) [updated September 17, 2013]. Available from: http://clinicaltrials.gov/show/NCT00982644. NLM identifier: NCT00982644. [Google Scholar]
- 58.Novo Nordisk . ClinicalTrials.gov [website on the Internet] Bethseda, MD: US National Library of Medicine; 2009. [Accessed January 26, 2014]. Comparison of NN1250 with insulin glargine plus insulin aspart with/without metformin and with/without pioglitazone in type 2 diabetes (BEGIN™) [updated November 14, 2013]. Available from: http://clinicaltrials.gov/ct2/show/NCT00972283. NLM identifier: NCT00972283. [Google Scholar]
- 59.Novo Nordisk . ClinicalTrials.gov [website on the Internet] Bethseda, MD: US National Library of Medicine; 2009. [Accessed January 26, 2014]. Comparison of NN1250 plus insulin aspart with insulin glargine plus insulin aspart in type 1 diabetes (BEGIN™) [updated November 28, 2013]. Available from: http://clinicaltrials.gov/show/NCT00982228. NLM identifier: NCT00982228. [Google Scholar]
- 60.Novo Nordisk . ClinicalTrials.gov [website on the Internet] Bethseda, MD: US National Library of Medicine; 2009. [Accessed January 26, 2014]. Comparison of NN1250 with insulin glargine in type 2 diabetes (BEGIN™) [updated November 26, 2013]. Available from: http://clinicaltrials.gov/show/NCT01006291. NLM identifier: NCT01006291. [Google Scholar]
- 61.Novo Nordisk . ClinicalTrials.gov [website on the Internet] Bethseda, MD: US National Library of Medicine; 2010. [Accessed January 26, 2014]. Comparison of NN1250 with insulin glargine in type 1 diabetes (BEGIN™) [updated November 20, 2013]. Available from: http://clinicaltrials.gov/show/NCT01079234. NLM identifier: NCT01079234. [Google Scholar]
- 62.Novo Nordisk . ClinicalTrials.gov [website on the Internet] Bethseda, MD: US National Library of Medicine; 2010. [Accessed January 26 2014]. Comparison of NN1250 versus insulin glargine in subjects with type 2 diabetes (BEGIN™) [updated November 21, 2013]. Available from: http://clinicaltrials.gov/show/NCT01059799. NLM identifier: NCT01059799. [Google Scholar]
- 63.Novo Nordisk . ClinicalTrials.gov [website on the Internet] Bethseda, MD: US National Library of Medicine; 2010. [Accessed January 26, 2014]. Comparison of NN1250 with insulin glargine in subjects with type 2 diabetes (BEGIN™) [updated November 26, 2013]. Available from: http://clinicaltrials.gov/show/NCT01068665. NLM identifier: NCT01068665. [Google Scholar]
- 64.Harris SB, Vora J, Christensen T, Kapur R, Brod M. Duration and impact of hypoglycemic events with insulin degludec and insulin glargine – a meta-analysis. Can J Diabetes. 2013;37(Suppl 4):S54. [Google Scholar]
- 65.Tresiba (degludec) [summary of product characteristics] Bagsværd: Novo Nordisk; 2013. [Accessed January 26, 2014]. Available from: http://ec.europa.eu/health/documents/community-register/2013/20130121124987/anx_124987_en.pdf. [Google Scholar]
- 66.Zinman B, DeVries JH, Bode B, et al. NN1250-3724 (BEGIN:EASY AM), NN1250-3718 (BEGIN:EASY PM) Trial Investigators Efficacy and safety of insulin degludec three times a week versus insulin glargine once a day in insulin-naive patients with type 2 diabetes: results of two phase 3, 26 week, randomised, open-label, treat-to-target, non-inferiority trials. Lancet Diabetes Endocrinol. 2013;1(2):123–131. doi: 10.1016/S2213-8587(13)70013-5. [DOI] [PubMed] [Google Scholar]
- 67.Sharpe L. Improving safety of insulin administration: A pilot audit of hospital staff knowledge. J Diabetes Nurs. 2012;16(1):8–16. [Google Scholar]
- 68.Korsatko S, Deller S, Koehler G, et al. A comparison of the steady-state pharmacokinetic and pharmacodynamic profiles of 100 and 200 U/mL formulations of ultra-long-acting insulin degludec. Clin Drug Investig. 2013;33(7):515–521. doi: 10.1007/s40261-013-0096-7. [DOI] [PubMed] [Google Scholar]
- 69.Chelliah A, Burge MR. Hypoglycaemia in elderly patients with diabetes mellitus: causes and strategies for prevention. Drugs Aging. 2004;21(8):511–530. doi: 10.2165/00002512-200421080-00003. [DOI] [PubMed] [Google Scholar]
- 70.Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997;157(15):1681–1686. [PubMed] [Google Scholar]
- 71.Sorli C, Warren M, Oyer D, Mersebach H, Johansen T, Gough SC. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30(12):1009–1018. doi: 10.1007/s40266-013-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]