Abstract
Oxidative and carbonyl stress is increased in lungs of smokers and patients with chronic obstructive pulmonary disease (COPD), as well as in cigarette smoke (CS)-exposed rodent lungs. We previously showed that sirtuin1 (SIRT1), an antiaging protein, is reduced in lungs of CS-exposed mice and patients with COPD and that SIRT1 attenuates CS-induced lung inflammation and injury. It is not clear whether SIRT1 protects against CS-induced lung oxidative stress. Therefore, we determined the effect of SIRT1 on lung oxidative stress and antioxidants in response to CS exposure using loss- and gain-of-function approaches, as well as a pharmacological SIRT1 activation by SRT1720. We found that CS exposure increased protein oxidation and lipid peroxidation in lungs of wild-type (WT) mice, which was further augmented in SIRT1-deficient mice. Furthermore, both SIRT1 genetic overexpression and SRT1720 treatment significantly decreased oxidative stress induced by CS exposure. FOXO3 deletion augmented lipid peroxidation products but reduced antioxidants in response to CS exposure, which was not affected by SRT1720. Interestingly, SRT1720 treatment exhibited a similar effect on lipid peroxidation and antioxidants (i.e., manganese superoxide dismutase, heme oxygenase-1, and NADPH quinone oxidoreductase-1) in WT and nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-deficient mice in response to CS exposure. This indicates that SIRT1 protects against CS-induced oxidative stress, which is mediated by FOXO3, but is independent of Nrf2. Overall, these findings reveal a novel function of SIRT1, which is to reduce CS-induced oxidative stress, and this may contribute to its protective effects against lung inflammation and subsequent development of COPD.
Keywords: oxidative stress, lipid peroxidation, antioxidants, chronic obstructive pulmonary disease, FOXO3, nuclear erythroid-related factor 2
cigarette smoke (cs) induces inflammation and oxidative stress, leading to chronic inflammatory pulmonary diseases, including chronic obstructive pulmonary disease (COPD) (37, 55, 57, 69). CS contains an estimated 1 × 1016 oxidants/free radicals per puff and thousands of different chemical compounds, including reactive aldehydes (11). CS also induces endogenous production of reactive oxygen species (ROS) from the inflammatory cells infiltrating in lungs. This causes the formation of further reactive and unstable free radicals, such as superoxide anion, nitric oxide, and hydroxyl radicals, thereby leading to uncontrolled tissue destruction and inflammatory responses. We have previously shown that leukocytes isolated from patients with COPD generate elevated levels of superoxide anions, which were associated with increased systemic inflammatory responses (44, 49, 50, 55). 4-Hydroxy-2-nonenal (4-HNE), a highly reactive and diffusible end-product of lipid peroxidation, is significantly increased in lungs of patients with COPD and in mouse lungs with emphysema (14–16, 57, 65). Furthermore, increased oxidative stress shows a strong correlation with disease severity (23, 24, 57). Antioxidant depletion is one of the mechanisms for CS-induced oxidative stress (36). For example, the endogenous antioxidant defense system is impaired in CS-exposed mouse lungs and in several lung diseases, including COPD (31, 37, 53, 61, 65). This may be due to polymorphisms, epigenetic changes, or mutations of genes encoding antioxidant enzymes, such as superoxide dismutase (SOD), glutathione (GSH) S-transferase, glutamate cysteine ligase, and nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (18, 26, 31, 38, 63, 71). Thus the redressing of oxidant and antioxidant imbalance would be a promising avenue to intervene in the progression of CS-related lung diseases including COPD.
Sirtuin1 (SIRT1) is a type III histone/protein deacetylase, which plays an important role in inflammation, stress resistance, and cellular senescence/aging (22, 51, 70). We and others have shown that the level and activity of SIRT1 are reduced in cells in vitro and in mouse lungs in vivo exposed to CS as well as in lungs of patients with COPD (21, 45, 58, 66). This is partly due to SIRT1 posttranslational modifications including phosphorylation and carbonylation by CS-induced oxidative/carbonyl stress, leading to proteasomal degradation (7, 58, 73). Previous studies have shown that SIRT1 regulates the resistance to oxidative stress (2, 29). However, it is unknown whether SIRT1 reduces CS-induced oxidative stress in lungs. Both FOXO3 and Nrf2 are transcription factors, which have been shown to modulate oxidative stress (20, 33, 35, 40). Indeed, the levels and activities of both FOXO3 and Nrf2 were decreased in response to CS (20, 33, 39, 66). We therefore hypothesized that SIRT1 protects against CS-induced lung oxidative stress through FOXO3 and Nrf2. To test this hypothesis, SIRT1 heterozygous knockout (SIRT1+/−) and SIRT1 overexpressing/transgenic (SIRT1 Tg) as well as their wild-type (WT) littermates were exposed to CS, and lung oxidative stress and various antioxidant genes/enzymes were measured. Furthermore, a specific SIRT1 activator, SRT1720, was administered to FOXO3 and Nrf2 knockout mice to study their roles in SIRT1 regulation of CS-induced lung oxidative stress.
MATERIALS AND METHODS
Ethics statement.
All experiments for animal studies were performed in accordance with the standards established by the United States Animal Welfare Act, as set forth by the National Institutes of Health guidelines. The University Committee on Animal Research Committee of the University of Rochester approved the research protocol for mouse studies.
Mice.
The generation of SIRT1+/− and SIRT1 Tg mice was previously described with their background WT mice being the 129/SvJ and C57BL/6J × 129/SvJ strains, respectively (5, 42). SIRT1+/− mice were used in this study because SIRT1 homozygous knockout mice have a low perinatal survival rate (42). Lung SIRT1 protein levels were decreased in SIRT1+/− mice, whereas it was increased in SIRT1 Tg mice compared with their WT littermates (66). Heterozygous FOXO3 (FOXO3+/−) mouse (FVB;129S6-Foxo3atm1.1Rdp) were obtained from the Mutant Mouse Regional Resource Centers, University of California at Davis (Davis, CA; stock number 016132-UCD) (8). The homozygous FOXO3 knockout (FOXO3−/−) mice and their WT littermates were generated and used in the experiments (20, 66). The Nrf2 knockout (Nrf2−/−) mouse strain (C57BL/6J background) used in this study was described earlier (1, 25) and was generously supplied by Prof. Masayuki Yamamoto, University of Tsukuba, Japan via the RIKEN BioResource Center, Tsukuba, Japan. These mice were housed in the vivarium facility at the University of Rochester with a 12-h:12-h light/dark cycle (light on at 6:00 am).
CS exposure.
Eight- to ten-week-old mice were used for CS exposure as described previously (66, 67). Research grade cigarettes (3R4F, University of Kentucky) were used to generate smoke, and mice were exposed to CS according to the Federal Trade Commission protocol (1 puff/min of 2-s duration and 35-ml volume) with a Baumgartner-Jaeger CSM2072i automatic CS-generating machine (CH Technologies). The smoke concentration was set at a value of ∼300 mg/m3 total particulate matter (TPM) by adjusting the flow rate of the diluted medical air, and the level of carbon monoxide in the chamber was ∼350 ppm (66). Mice received two 1-h exposures (1 h apart) daily for three consecutive days and were killed at 24 h after last exposure. Control mice were exposed to filtered air in an identical chamber according to the same protocol described for CS exposure. For 6 mo of CS exposure, 3R4F cigarettes were used to generate a mixture of sidestream smoke (89%) and mainstream smoke (11%) by a Teague smoking machine (Model TE-10; Teague Enterprises, Davis, CA) at a concentration of ∼100 mg/m3 TPM to avoid the possible toxicity to mice at a high concentration of long-term CS exposure (65, 66, 68). Each burning cigarette was inhaled for 2 s, once every minute for a total of eight puffs, at a flow rate of 1.05 l/min, to provide a standard puff of 35 cm3. Mice received 5 h of inhalation per day, 5 days/wk for 6 mo, and were killed at 24 h after last CS exposure.
SRT1720 treatment.
SRT1720 [100 mg/kg, >95% pure by C-13 nuclear magnetic resonance and liquid chromatography–mass spectrometry (LC-MS), synthesized from Life Chemicals] was administered through oral gavage 1 h before CS exposure daily for 3 days (43, 66). In a separate experiment, SRT1720 (100 mg/kg) was orally administered daily for 3 wk after 6 mo of CS exposure.
Western blots and oxyblots.
For Western blots, protein samples were separated on a SDS-PAGE, and separated proteins were electroblotted onto nitrocellulose membranes. The membranes were blocked for 1 h at room temperature with 5% BSA and then probed with a 1:1,000 diluted antibodies of anti-SIRT1 (Cell Signaling, Beverly, MA) at 4°C overnight. After three washing steps (10 min each), the levels of protein were detected using the secondary anti-rabbit antibody [1:5,000 dilution in 2.5% BSA in PBS containing 0.1% Tween 20 (vol/vol) 20 for 1 h] linked to horseradish peroxidase (Dako, Carpenteria, CA), and bound complexes were detected using ECL method with the ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA). Equal loading of the samples was determined by quantitation of proteins and by reprobing membranes for GAPDH. Protein oxidation was determined using the OxyBlot protein oxidation detection kit following the manufacturer's instruction (S7150; Millipore, Billerica, MA) (3). Protein carbonyl groups were derivatized to their 2,4-dinitrophenylhydrazones by reaction with 2,4-dinitrophenylhydrazine (DNPH) for 15 min in 6% (wt/vol) SDS. The samples designated for the negative controls were added by derivatization-control solution instead of DNPH solution. β-Mercaptoethanol was then added to the sample mixture at a final concentration of 5% (vol/vol). The samples were subjected to SDS-PAGE gels, and proteins were then transferred to a PVDF membrane. The membrane was then probed with anti-DNP antibody for 1 h at room temperature followed by incubation with a horseradish peroxidase second antibody conjugate directed against the primary antibody. Protein loaded was confirmed by the Ponceau S staining, and the results were quantified by densitometry.
LC-MS/MS analysis for protein oxidation.
Immunoprecipitated 4-HNE samples resolved by SDS-PAGE followed by band excision were digested with trypsin overnight (62, 68). Peptides were twice extracted with 50% acetonitrile containing 5% trifluoroacetic acid. For linear trap quadropole (LTQ) analysis, 2 μl of each sample was loaded onto a home-pulled, home-packed C18 analytical column, which was packed to 10 cm with C18 AQ 5 μm 200Å media (Michrom, Auburn, CA) using a pressure bomb. The internal diameter of the columns used was 75 μm. Peptides were eluted with the following chromatographic profile: 5% Solvent B (LC-MS grade methanol, Burdick and Jackson, Muskegon, MI) for 6 min, ramping to 20% Solvent B over 1 min then to 60% Solvent B over 75 min, washing at 95% Solvent B for 3 min, and finally returning to initial run conditions, with Solvent A as LC-MS grade water, Solvent B, and 0.1% formic acid. The flow rate was 350 nl/min. Helium was used as collision gas, with an activation Q of 0.25, activation time of 30 ms, and normalized collision energy of 35%. The processed data were exported as an xml file. Resultant .mgf and .xml files were imported into ProteinScape (Bruker Daltonics, Billerica, MA) and searched via MASCOT (MatrixScience, Boston, MS) (48). Search parameters included trypsin as an enzyme; six missed cleavages; MS tolerance of 1.5 Da; MS-MS tolerances of 0.8 Da for LTQ data; 1 for #13C, +2; +3 for charge state; instrument set to ESI-TRAP appropriately; decoy search and acceptance criteria of minimum one peptide greater than identity score; minimum score of 15; and False Discovery Rate <5%. Each spectra was performed manually then verified. Data were searched for variable modifications of oxidized methionine, aspartic acid, histidine, tryptophan, lysine, and proline. The ProteinExtractor function of ProteinScape combined search results and compiled a nonredundant list of identifications. Matched spectra were manually validated using BioTools (Bruker Daltonics), with poor matches being excluded from the final results.
Lipid peroxidation products assay in lung homogenate.
Lung tissues without lavage were perfused with cold PBS through right ventricle and were homogenized with ice-cold Tris·HCl (20 mM, pH 7.4), which was centrifuged at 3,000 revolution/min for 10 min at 4°C for collecting the supernatants. The supernatants were prepared by adding butylated hydroxytoluene (5 mM; Sigma-Aldrich, MO) to avoid further oxidation, which were used for determination of lipid peroxidation products. Lipid peroxidation products [malondialdehyde (MDA) and 4-HNE] were measured using a lipid peroxidation kit (Calbiochem, San Diego, CA) (65, 67). For MDA and 4-HNE assays, 200 μl of supernatant was added to 650 μl of 10.3 mM N-methyl-2-phenylindole in acetonitrile (vortexed), which were mixed with 150 μl of 15.4 mM methanesulfonic acid and then incubated at 45°C for 40 min. The samples were cooled on ice, and absorbance was measured at 586 nm using a spectrophotometer (Bio-Rad). The protein levels were measured using a BCA kit (Pierce, Rockford, IL).
GSH assay.
Intracellular total GSH levels in lung tissues were determined according to the method described previously (52, 65, 67). Briefly, the lungs were homogenized with 0.1 M phosphate buffer (pH 7.5) containing 5 mM EDTA, 0.1% (vol/vol) Triton X-100 and 0.6% (wt/vol) sulfosalicylic acid. The lung debris was collected by centrifugation, and the supernatant was incubated with 0.2 mg/ml of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) and 1.67 U/ml GSH reductase in phosphate buffer-EDTA for 30 s. The β-NADPH (0.2 mg/ml) was added into the reaction mixture, and the rate of DTNB reduction was measured spectrophotometrically at 405 nm. The concentration of total GSH in the supernatant was determined by comparison with the rate of DTNB reduction by known concentrations of GSH. For determining the concentration of GSH disulfide (GSSG), 0.6% sulfosalicylic acid-extracted lung homogenates were added with 2% of 2-vinylpyridine to derivatize GSH. The concentration of GSSG was determined by monitoring NADPH spectrophotometrically at 405 nm (52). Results were expressed as the nanomoles of GSH and GSSG per milligram of protein as well as GSH/GSSG ratio.
SOD activity assay.
The SOD activity in lung homogenates was measured using a commercial SOD assay kit (Enzo Lifesciences, Farmingdale, NY) according to the manufacturer's protocol. The SOD activity assay utilized the production of a water-soluble tetrazolium salt (WST-1) formazan dye from a tetrazolium salt in the presence of superoxide anions that generated from the conversion of xanthine and oxygen to uric acid and hydrogen peroxide by xanthine oxidase. SOD scavenges superoxide anion and thereby reduces the rate of formation of WST-1 formazan product. Sample results were compared against a SOD standard curve after the absorbance was read at 450 nm with a microplate reader. Potassium cyanide was added to each sample at a final concentration of 2 mM to measure the MnSOD activity. The activity was expressed as units per milligram of protein.
RNA extraction and mRNA analysis.
For RT-PCR, the lungs were submerged into the RNAlater stabilization reagent (Qiagen, Valencia, CA), and then total RNA was extracted using the RNeasy mini kit (Qiagen). Two micrograms of RNA were reversed transcribed using oligo(dT) (Promega, Madison, WI) and Maloney murine leukemia virus reverse transcriptase (Promega) according to the supplier's recommendation. The primer pairs were shown in Table 1 (10, 20). PCR was started with 5-min incubation at 94°C followed by a three-step temperature cycle: denaturation at 94°C for 30 s, annealing at 55–60°C for 30 s, and extension at 72°C for 1 to 2 min for 25 to 30 cycles with a final extension for 10 min at 72°C in a T100 thermal cycler (Bio-Rad) (10). RT-PCR was performed in total volume of 25 μl in 10× PCR buffer in the presence of 0. 2 mM dNTP (Promega), 4 μM of each primer, 1.5 mM MgCl2 (Invitrogen, Carlsbad, CA), and 7 U of Taq polymerase (Invitrogen). Samples were analyzed by gel electrophoresis on the 1.5–2% agarose gels, which were visualized by a ChemDoc MP Imaging System and quantified by densitometry using ImageJ software. To validate the expression of these antioxidant genes, quantitative PCR (qPCR) was performed with a Bio-Rad CFX96 real-time system and SYBR Green qPCR Master mix from SABioscience. The specific primer sequences were shown in Table 1 (4, 9, 41). Gene expression was normalized to 18S rRNA levels. Relative RNA abundance was quantified by the comparative 2−ΔΔCt method (21).
Table 1.
The primer sequence for RT-PCR and qPCR
| PCR | Genes | Primers |
|---|---|---|
| RT-PCR | MnSOD | Forward: 5′-AGC GGT CGT GTA AAC CTC A-3′ |
| Reverse: 5′-AGA CAT GGC TGT CAG CTT C-3′ | ||
| CuZnSOD | Forward: 5′-ATC CAC TTC GAG CAG AAG-3′ | |
| Reverse: 5′-TTC CAC CTT TGC CCA AGT-3′ | ||
| HO-1 | Forward: 5′-GAG CAG AAC CAG CCT GAA CTA-3′ | |
| Reverse: 5′-GGT ACA AGG AAG CCA TCA CCA-3′ | ||
| NQO-1 | Forward: 5′-ATT GTA CTG GCC CAT TCA GA-3′ | |
| Reverse: 5′-GGC CAT TGT TTA CTT TGA GC-3′ | ||
| Actin | Forward: 5′-GTG GGC CGC TCT AGG CAC CA-3′ | |
| Reverse: 5′-CGG TTG GCC TTA GGG TTC AGG-3′ | ||
| qPCR | MnSOD | Forward: 5′-CAC ATT AAC GCG CAG ATC ATG-3′ |
| Reverse: 5′-CCA GAG CCT CGT GGT ACT TCT C-3′ | ||
| CuZnSOD | Forward: 5′-ACC AGT GCA GGA CCT CAT TTT AA-3′ | |
| Reverse: 5′-TCT CCA ACA TGC CTC TCT TCA TC-3′ | ||
| HO-1 | Forward: 5′-GGT GAT GGC TTC CTT GTA CC-3′ | |
| Reverse: 5′-AGT GAG GCC CAT ACC AGA AG-3′ | ||
| NQO-1 | Forward: 5′-GGC ATC CTG CGT TTC TGT G-3′ | |
| Reverse: 5′-GGT TTC CAG ACG TTT CTT CCA T-3′ | ||
| 18SrRNA | Forward: 5′-CGG GTC GGG AGT GGG T-3′ | |
| Reverse: 5′-GAA ACG GCT ACC ACA TCC AAG-3′ |
qPCR, quantitative PCR; SOD, superoxide dismutase; HO-1, heme oxygenase-1; NQO-1, NADPH quinone oxidoreductase-1.
Statistical analysis.
Statistical analysis of significance was calculated using one-way ANOVA for multigroup comparisons using STATVIEW software. The results are shown as the means ± SE. P < 0.05 is considered statistically significant.
RESULTS
SIRT1 protects against CS-induced increase in protein carbonylation and lipid peroxidation products.
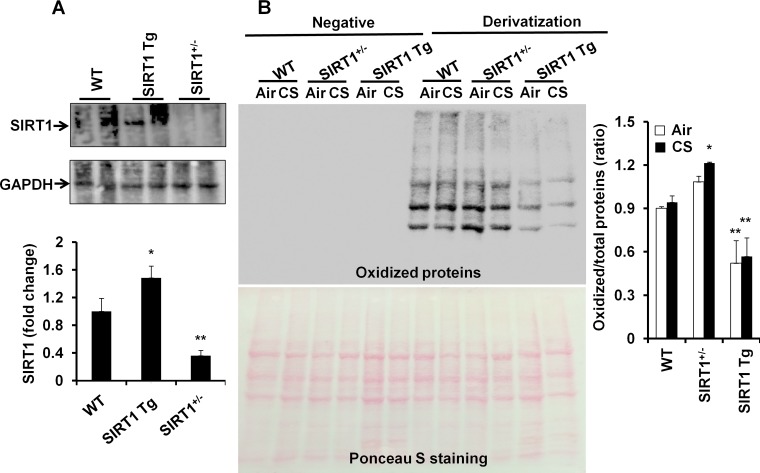
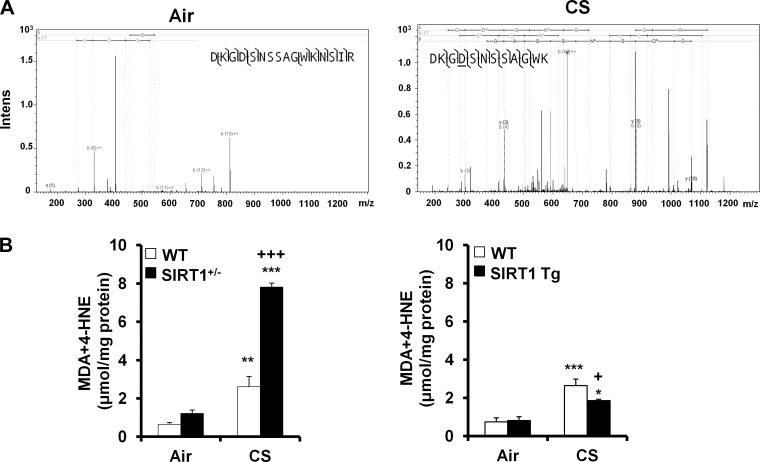
In agreement with our previous study (66), the levels of SIRT1 were increased in SIRT1 Tg mice, which was reduced in SIRT1+/− mice compared with WT mice (Fig. 1A). To determine the role of SIRT1 in regulation of protein carbonylation, the Oxyblot assay was performed for immunodetection of the carbonyl groups that were introduced into protein side chains by oxidative stress. It was found that protein oxidation was increased in SIRT1+/− mice compared with WT mice, and these responses were attenuated by SIRT1 overexpression in response to CS exposure (Fig. 1B). MS also showed that CS exposure induced the oxidation on FOXO3 protein (D198) in WT mice (Fig. 2A). It has been shown that protein carbonylation formed from lipid-derived aldehydes is more prevalent than that formed via direct oxidation of residues by carbonyl compounds contained in CS (46, 72). Thus we measured the levels of lipid peroxidation products, i.e., MDA and 4-HNE in SIRT1+/− and SIRT1 Tg mice as well as their WT littermates after 3 days of CS exposure. As shown in Fig. 2B, the levels of MDA and 4-HNE were significantly increased in lungs of WT mice exposed to CS. SIRT1 deficiency further augmented the levels of MDA and 4-HNE in mouse lungs in response to CS exposure. However, the levels of MDA and 4-HNE were significantly reduced in lungs of SIRT1 Tg mice compared with WT littermates in response to CS exposure. These findings demonstrate that SIRT1 transgenic overexpression protects against protein oxidation in mouse lungs exposed to CS.
Fig. 1.
Levels of sirtuin1 (SIRT1) and oxidized proteins in SIRT1+/−, SIRT1 transgenic (Tg) and wild-type (WT) mice. Western blot showed that the levels of SIRT1 were decreased in SIRT1+/− mice but increased in SIRT1 Tg mice compared with WT mice (A). Protein oxidation was determined by the oxyblots. Protein carbonylation was increased SIRT1+/− mice, which was reduced in SIRT1 Tg mice compared with WT mice (B). Gel pictures shown are representative of at least 4 separate mice. Data are shown as means ± SE (n = 4–8 per group). *P < 0.05, **P < 0.01 vs. WT mice. CS, cigarette smoke.
Fig. 2.
CS increased FOXO3 oxidation and lipid peroxidation products in the lungs. Representative mass spectra of oxidized residues of FOXO3 in mouse lungs exposed to air (no residue oxidation identified) and CS (Asp198 oxidation; see underlined residue “D”) (A). CS exposure increased the levels of 4-hydroxy-2-nonenal (4-HNE) and malondialdehyde (MDA) in lungs of WT littermates, which were further increased in SIRT1+/− mice. SIRT1 genetic overexpression significantly attenuated the levels of MDA plus 4-HNE in response to CS exposure (B). Data are shown as means ± SE (n = 3 to 4 per group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. the corresponding air-exposed groups; +P < 0.05, +++P < 0.001 vs. the corresponding CS-exposed WT littermates.
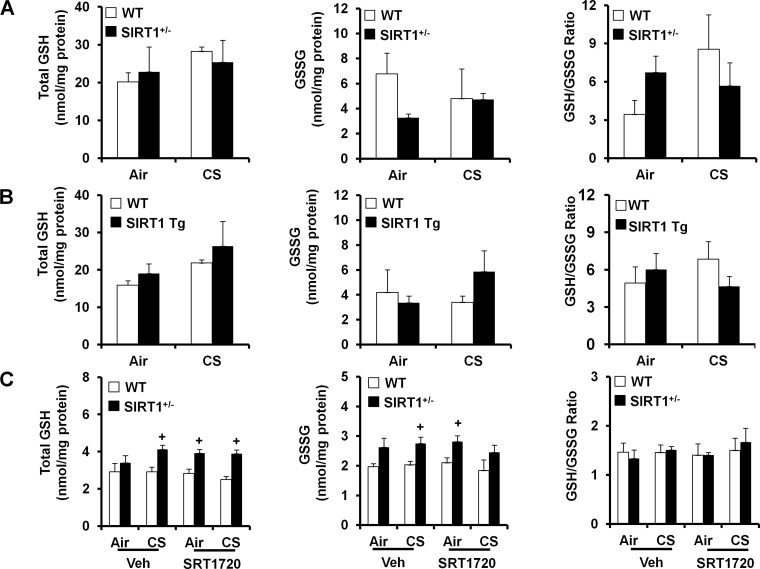
SIRT1 does not affect the level of GSH or GSSG in mouse lungs exposed to CS.
We have shown that CS depletes GSH levels, leading to oxidative stress in lungs of C57BL/6J mice (67). Thus we proposed that SIRT1 regulates GSH levels in mouse lungs in response to 3 days of CS exposure. Unexpectedly, the levels of total GSH in mouse lungs (whole homogenate) were not altered either by CS exposure or by the loss and gain of SIRT1 function, although there was a tendency of increase in total GSH levels by SIRT1 overexpression. Similarly, neither CS nor SIRT1 altered the levels of oxidized form of GSH, i.e., GSSG, or GSH/GSSG ratio in mouse lungs (Fig. 3, A and B). Similarly, SIRT1 activation by SRT1720 treatment did not alter the levels of GSH, GSSG, or GSH/GSSG ratio in either WT or SIRT1+/− mice (Fig. 3C). These results suggest the SIRT1 does not have an influence on either total GSH or GSSG levels in mouse lungs.
Fig. 3.
Lack of effect of SIRT1 on levels of glutathione (GSH), GSH disulfide (GSSG), and GSH/GSSG ratio in mouse lungs exposed to CS. SIRT1+/−, SIRT1 Tg, and their WT littermates were exposed to CS for 3 days. Neither CS exposure nor loss and gain of SIRT1 function altered the total GSH, its oxidized/disulfide form (i.e., GSSG), or GSH/GSSG ratio in mouse lungs (A and B). SRT1720 treatment did not have any effect on the levels of GSH, GSSG, or GSH/GSSG ratio in SIRT1+/− mice or WT mice (C). Data are shown as means ± SE (n = 3 to 4 per group). +P < 0.05 vs. WT mice.
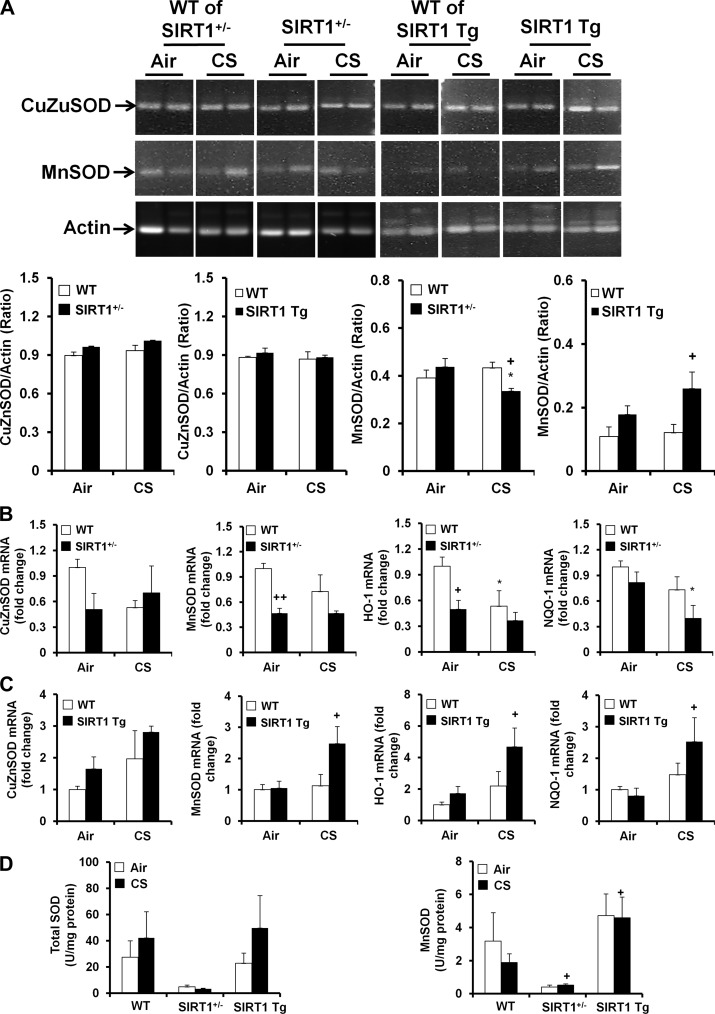
SIRT1 regulates antioxidant genes and enzymes in mouse lungs exposed to CS.
The imbalance between oxidants and antioxidants plays an important role in regulating lung oxidative stress in response to CS exposure (54). Therefore, we hypothesized that SIRT1 regulates antioxidant genes and enzymes in response to CS exposure. Firstly, the mRNA levels of CuZnSOD and MnSOD were determined by traditional RT-PCR in lungs of SIRT1+/− and SIRT1 Tg mice and their WT littermates after 3 days of CS exposure. It was found that the level of CuZnSOD mRNA was not altered either by CS exposure or by the loss and gain of SIRT1 function (Fig. 4A). Similarly, CS exposure had no effect on MnSOD mRNA in lungs of WT littermates. Interestingly, SIRT1 deficiency reduced the level of MnSOD mRNA, whereas the level of MnSOD mRNA was significantly increased in lungs of SIRT1 Tg mice compared with WT littermates exposed to CS (Fig. 4A). qPCR also showed that SIRT1 deficiency reduced the mRNA levels of MnSOD, heme oxygenase-1 (HO-1), and NADPH quinone oxidoreductase-1 (NQO-1) genes in response to air or CS exposure (Fig. 4B). SIRT1 transgenic overexpression augmented the expression of MnSOD, HO-1, and NQO-1 genes in response to CS exposure (Fig. 4C). Furthermore, the MnSOD activity was reduced in SIRT1+/− mice, which was augmented by SIRT1 overexpression compared with WT mice in response to CS exposure (Fig. 4D).
Fig. 4.
Effect of loss and gain of SIRT1 function on antioxidant genes and enzymes in mouse lungs exposed to CS. SIRT1+/−, SIRT1 Tg, and their WT littermates were exposed to CS for 3 days, and the mRNA levels of antioxidant genes were determined by RT-PCR in mouse lungs. CS exposure did not have any effect on the mRNA levels of CuZn superoxide dismutase (SOD) and MnSOD in lungs of WT mice. Neither SIRT1 deficiency nor overexpression changed the levels of CuZnSOD mRNA in mouse lungs regardless of air or CS exposure. The levels of MnSOD mRNA were reduced in SIRT1+/− mice, whereas SIRT1 genetic overexpression increased MnSOD mRNA compared with the corresponding WT littermates. Gel pictures shown are representative of at least 3 separate mice. The reassembly of noncontiguous gel lanes is demarcated by white spaces clearly (A). Real-time PCR showed that the mRNA levels of CuZnSOD, MnSOD, and NADPH quinone oxidoreductase-1 (NQO-1) were decreased in SIRT1+/− mice (B) compared with WT mice, and these effects were augmented by SIRT1 transgenic overexpression (C). MnSOD activity was decreased in SIRT1+/− mice, which was increased in SIRT1 Tg mice compared with WT mice in response to CS exposure (D). Data are shown as means ± SE (n = 3 to 6 per group). *P < 0.05 vs. the corresponding air-exposed mice; +P < 0.05, ++P < 0.01 vs. the WT mice. HO-1, heme oxygenase-1.
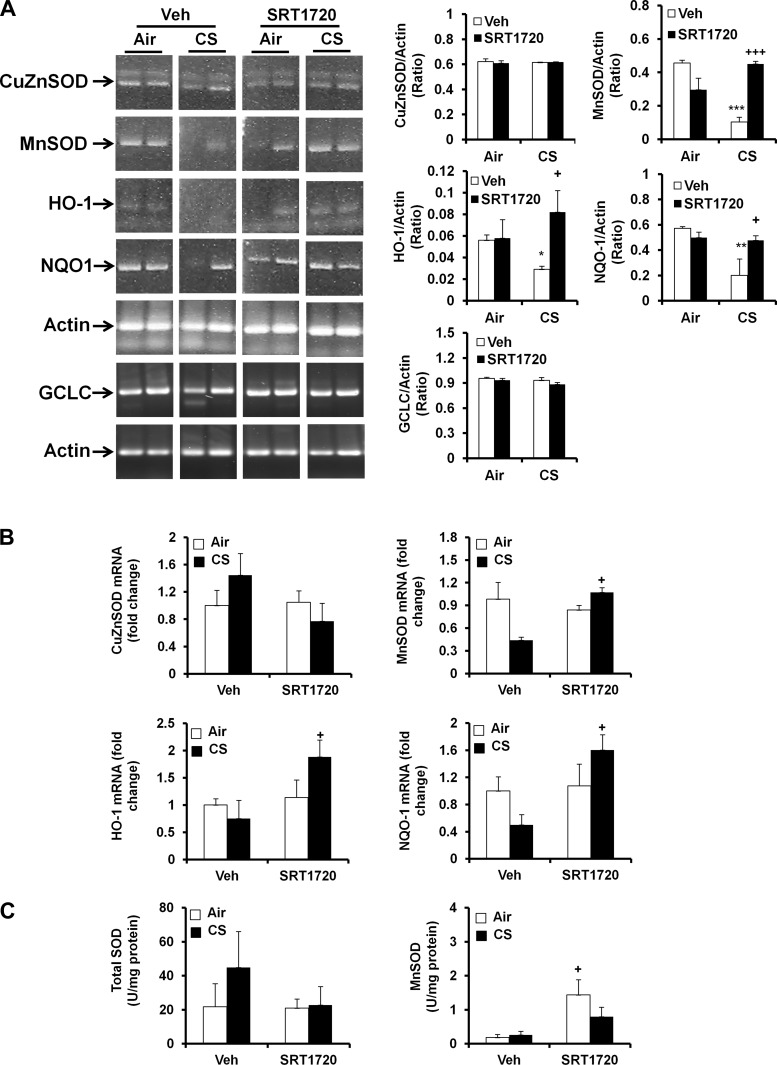
As shown in our previous study (66), SRT1720 treatment increased SIRT1 activity in mouse lungs. Hence, we administered a specific SIRT1 activator SRT1720 (100 mg/kg body wt) via oral gavage to further determine the role of SIRT1 in regulation of antioxidant genes and enzymes. The mRNA levels of CuZnSOD and glutamate-cysteine ligase catalytic subunit (GCLC) in mouse lungs were not altered by CS exposure or SRT1720 (Fig. 5A). CS exposure decreased the mRNA levels of MnSOD, HO-1, and NQO-1 in lungs of WT mice. SIRT1720 treatment significantly augmented the expression of MnSOD, HO-1, and NQO-1 genes through traditional RT-PCR in mouse lungs exposed to CS (Fig. 5A). qPCR also demonstrated that SRT1720 treatment increased the expression of MnSOD, HO-1, and NQO-1 genes in response to CS exposure (Fig. 5B). The activity assay also showed that MnSOD activity was increased by SRT1720 treatment, despite the lack of significant change of total SOD activity by SRT1720 (Fig. 5C). Altogether, SIRT1 augments the antioxidant enzyme activity and gene expression, which may account for its protection against CS-induced oxidative stress.
Fig. 5.
SRT1720 treatment increased the antioxidant genes and enzymes in WT mice in response to CS exposure. WT mice (FVB;129S6 background) were administered with SRT1720 daily before CS exposure for 3 days, and the mRNA levels of CuZnSOD, MnSOD, HO-1, NQO-1, and glutamate-cysteine ligase catalytic subunit (GCLC) were determined by RT-PCR in mouse lungs. CS exposure reduced the mRNA levels of MnSOD, HO-1, and NQO-1, which was attenuated by SRT1720 treatment. Gel pictures shown are representative of at least 3 separate mice. The reassembly of noncontiguous gel lanes is demarcated by white spaces clearly (A). Real-time PCR also showed that SRT1720 treatment increased the mRNA levels of MnSOD, HO-1, and NQO-1 genes in mouse lungs (B). SRT1720 treatment increased MnSOD activity but not total SOD activity (C). Data are shown as means ± SE (n = 3 to 10 per group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. the corresponding air-exposed mice; +P < 0.05, +++P < 0.001 vs. the corresponding vehicle (Veh)-treated mice.
Role of FOXO3 in SIRT1 regulation on lipid peroxidation and antioxidant genes/enzymes in response to CS exposure.
Our recent study has shown that FOXO3 regulates antioxidant genes in response to CS exposure (20). We proposed that SIRT1 protects against CS-induced oxidative stress through FOXO3 signal. To test this hypothesis, the FOXO3 knockout mice and their WT littermates were treated with SRT1720, and the levels of lipid peroxidation as well as antioxidant genes/enzymes were determined. As shown in Fig. 6, A–D, SRT1720 treatment significantly reduced 3 days of CS-induced increase in protein oxidation and lipid peroxidation products (i.e., MDA plus 4-HNE) in lungs of WT mice although there was no effect of SRT1720 on MDA in lungs of WT mice exposed to CS for 6 mo. Interestingly, the levels of MDA and 4-HNE were not altered by SRT1720 in FOXO3−/− mice in response to CS exposure (Fig. 6C). These data indicate that FOXO3 is required for SIRT1-mediated protection against CS-induced lipid peroxidation. qPCR demonstrated that SRT1720 treatment augmented the mRNA levels of MnSOD, NQO-1, and HO-1 in lungs of WT mice but not in FOXO3−/− mice exposed to CS (Fig. 7A). Likewise, SRT1720 treatment augmented MnSOD activity in WT mice but not in FOXO3−/− mice in response to CS exposure (Fig. 7B). These results suggest that SIRT1 protects against CS-induced oxidative stress via a FOXO3-dependent mechanism.
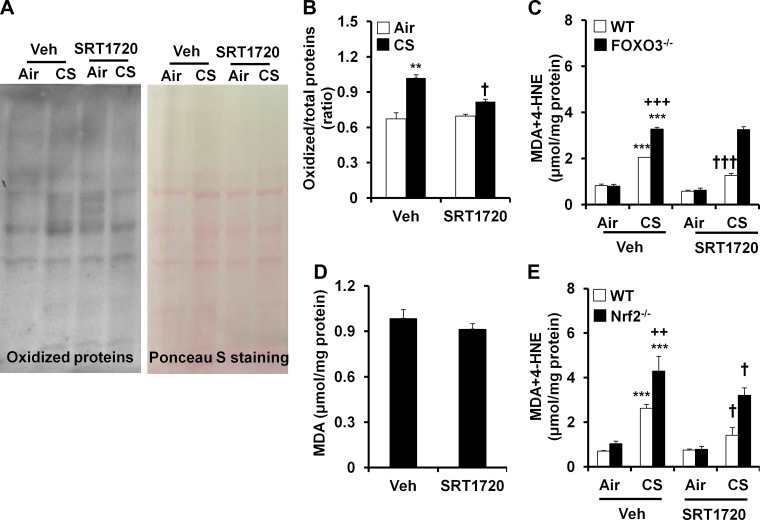
Fig. 6.
Effects of SRT1720 on protein carbonylation and lipid peroxidation in response to CS exposure. Oxyblot showed that SRT1720 treatment reduced acute CS-induced protein carbonylation in mouse lungs (A and B). Gel pictures shown are representative of at least 3 separate mice. SRT1720 treatment lowered the levels of 4-HNE plus MDA in lungs of WT mice but not in FOXO3−/− mice in response to acute CS exposure (C). SRT1720 treatment did not have any effect on MDA in WT mice exposed to CS for 6 mo (D). The levels of 4-HNE plus MDA in lungs were reduced by SRT1720 in both WT and nuclear factor (erythroid-derived 2)-like 2-deficient (Nrf2−/−) mice exposed to CS for 3 days (E). Data are shown as means ± SE (n = 3 to 6 per group). **P < 0.01, ***P < 0.001 vs. the corresponding air-exposed groups; ++P < 0.01, +++P < 0.001 vs. the corresponding CS-exposed WT littermates; †P < 0.05, †††P < 0.001 vs. the corresponding vehicle (Veh)-treated mice.
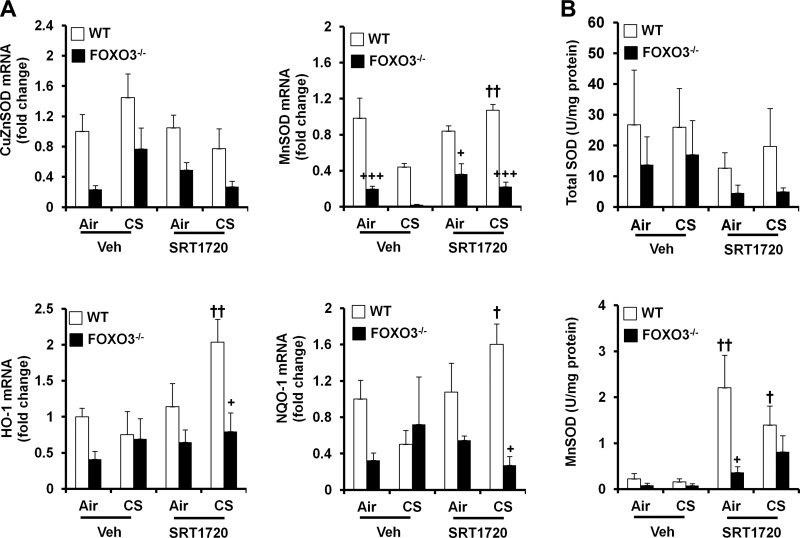
Fig. 7.
No effect of SRT1720 on antioxidant genes and enzymes in FOXO3−/− mice in response to CS exposure. FOXO3−/− mice (FVB;129S6 background) were administered with SRT1720 before CS exposure for 3 days, and the mRNA levels of CuZnSOD, MnSOD, HO-1, and NQO-1 were determined by real-time PCR in mouse lungs (A). Neither CS exposure nor SRT1720 treatment changed the mRNA levels of CuZnSOD in mouse lungs. SRT1720 treatment significantly increased the mRNA levels of MnSOD, NQO-1, and HO-1 in WT but not in FOXO3−/− mice in response to CS exposure. Similarly, SRT1720 treatment increased MnSOD activity in WT mice but not in FOXO3−/− mice (B). Data are shown as means ± SE (n = 3 to 10 per group). +P < 0.05, +++P < 0.001 vs. the WT mice; †P < 0.05, ††P < 0.01 vs. the corresponding vehicle (Veh)-treated mice.
Role of Nrf2 in SIRT1 regulation on lipid peroxidation and antioxidant genes/enzymes in response to CS exposure.
Nrf2 regulates cellular antioxidant responses to environmental stress, including smoking exposure (33, 59, 60). We hypothesize that Nrf2 is involved in the protection of SIRT1 against CS-induced oxidative stress. To test this hypothesis, Nrf2−/− mice and their WT littermates were treated with SRT1720 (100 mg/kg body wt) before 3 days of CS exposure, and the levels of lipid peroxidation as well as antioxidant genes/enzymes were determined. As shown in Fig. 6E, Nrf2 deletion augmented CS-induced increase in MDA plus 4-HNE in mouse lungs. SRT1720 treatment exhibited a similar inhibitory effect on MDA plus 4-HNE in both WT and Nrf2−/− mice. Furthermore, SRT1720 treatment increased the mRNA levels of MnSOD, HO-1, and NQO-1 genes in both Nrf2−/− and WT mice after CS exposure although there was no alteration of CuZnSOD gene by CS exposure or SRT1720 treatment in Nrf2−/− or WT mice (Fig. 8A). Total SOD activity was not changed by CS exposure, SRT1720 treatment, or Nrf2 deficiency (Fig. 8B). However, SRT1720 treatment led to augmentation of MnSOD activity in both Nrf2−/− and WT mice (Fig. 8B). These results suggest that Nrf2 is not involved in the regulation of SIRT1 in CS-induced lipid peroxidation and oxidant/antioxidant imbalance in response to CS exposure.
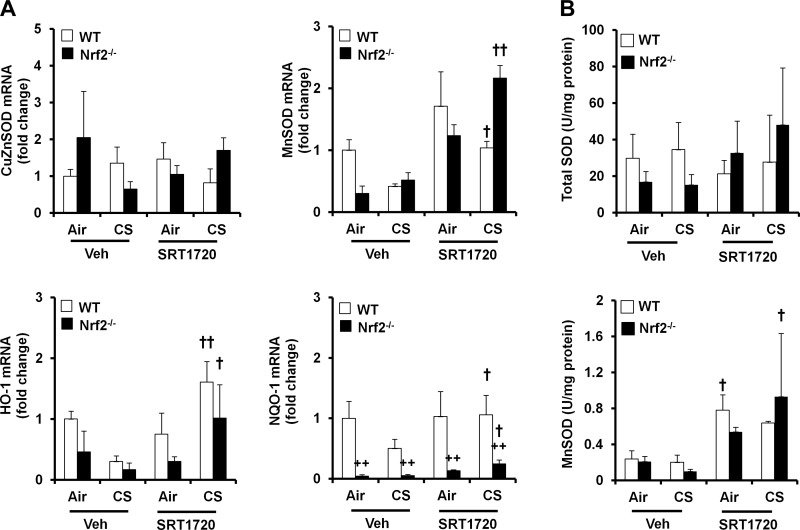
Fig. 8.
Effect of SRT1720 on antioxidant genes and enzymes in Nrf2−/− mice in response to CS exposure. Nrf2−/− mice were administered with SRT1720 before CS exposure for 3 days, and the mRNA levels of CuZnSOD, MnSOD, HO-1, and NQO-1 were determined by real-time PCR in mouse lungs. Neither CS exposure nor SRT1720 treatment changed the mRNA levels of CuZnSOD in mouse lungs. SRT1720 treatment increased the levels of MnSOD, HO-1, and NQO-1 mRNA in both Nrf2−/− and WT mice (A). Total SOD activity was not altered by either CS exposure or SRT1720 treatment, whereas SRT1720 treatment increased MnSOD activity in both Nrf2−/− and WT mice (B). Data are shown as means ± SE (n = 3 to 4 per group). ++P < 0.01 vs. the WT mice, †P < 0.05, ††P < 0.01 vs. the corresponding vehicle (Veh)-treated mice.
DISCUSSION
We and others have shown that oxidative and carbonyl stress occur in lungs of smokers and patients with COPD, as well as in CS-exposed rodent lungs (12, 32, 47, 65, 67). It has been shown that SIRT1, an antiaging protein, enhances the resistance to oxidative stress (2). It remains unknown whether SIRT1 protects against CS-induced lung oxidative stress. Therefore, we determined the effect of loss and gain of SIRT1 function on oxidative stress and antioxidant gene expression in mouse lungs exposed to CS. We found that CS exposure increased the oxidation of global and specific proteins as well as the lipid peroxidation products in lungs of WT mice, which was further augmented in SIRT1-deficient mice. Furthermore, both SIRT1 genetic overexpression and pharmacological activation by SRT1720 significantly decreased protein oxidation and lipid peroxidation products induced by acute CS exposure. We noticed that SRT1720 treatment did not have any effect on lipid peroxidation (i.e., MDA) in mouse lungs exposed to CS for 6 mo. This may be due to the relative abundance of SIRT1 in mouse lungs after chronic CS exposure compared with that in acute CS-exposed mouse lungs (66). Altogether, these findings suggest that SIRT1 is an important regulator in protecting against CS-induced oxidative stress. This may be one of the mechanisms for the protection of SIRT1 against CS-induced pulmonary dysfunction and emphysema (66).
GSH is the most abundant cellular thiol antioxidant in the maintenance of intracellular redox balance and in the detoxification reaction. It is interesting to note that the level of GSH was not altered either by CS exposure or the loss and gain of SIRT1 function in whole lung homogenate. This is supported by a study showing that no change of lung GSH was observed in C57BL/6J mice after 5 h of CS exposure (17). Previously, we have shown that CS exposure (3 days) decreased the content of GSH in lungs of C57BL/6J and A/J mice but not in AKR/J, CD-1, or 129/SvJ mice (67). These discrepancies may be due to the different mouse strains and CS exposure durations, as the background of SIRT1+/− and SIRT1 Tg mice were corresponding 129/SvJ and C57BL/6Jx129/SvJ, and 3 days of CS exposure was performed in the present study. GCLC is a rate-limiting enzyme in GSH biosynthesis (34, 56). The levels of GCLC were not changed either by CS exposure, SIRT1 deficiency, or overexpression. This may contribute to the findings that no effect of CS exposure or SIRT1 genetic manipulation on lung GSH was observed. In addition to GSH, other antioxidants are also important in preventing oxidative damage. For example, NQO-1 is an antioxidant that catalyzes an obligate two-electron reduction of various substrates including quinones, quinoneimines, and nitroaromatics (13), whereas MnSOD catalyzes the conversion of two molecules of superoxide anion into hydrogen peroxide that is further oxidized to water. HO-1 degrades heme and thereby protects cells from oxidative challenge. We found that both SIRT1 genetic overexpression and pharmacological activation increased the expression of antioxidant genes, including MnSOD, HO-1, and NQO-1. This is in agreement with the findings that SIRT1 activation increased the levels of HO-1 in glomerular mesangial cells (19). These findings suggest that SIRT1 redresses CS-induced oxidant/antioxidant imbalance. This may contribute to the protection of SIRT1 against CS-induced lung inflammation and cellular senescence (21, 66) because an increased oxidative stress is an important contributing factor in triggering inflammatory response and cellular senescence (16, 22, 50, 64).
We recently showed that FOXO3, a transcription factor, regulates antioxidant gene expression in mouse lungs exposed to CS, and genetic disruption of FOXO3 reduced the expression of antioxidant genes (20). We and others have also shown that SIRT1 deacetylates FOXO3, thereby affecting its activity and level (6, 20, 66). Interestingly, the FOXO3 protein was also subjected to the oxidation, which may present another mechanism for altered FOXO3-dependent gene transcription in response to CS exposure (20). Thus we determined whether SIRT1 protects against CS-induced oxidative stress through FOXO3 regulation. As expected, FOXO3 gene deletion increased the levels of lipid peroxidation production but reduced the levels of antioxidants including MnSOD, HO-1, and NQO-1 in mouse lung in response to CS exposure. Furthermore, SIRT1 activator SRT1720 reduced the levels of MDA plus 4-HNE in lungs of WT mice, but not in FOXO3−/− mice, in response to CS exposure. In addition, SRT1720 treatment augmented the expression of antioxidant genes, i.e., MnSOD, HO-1, and NQO-1 in WT mice, but not in FOXO3−/− mice. These findings suggest that FOXO3 mediates the protection of SIRT1 against CS-induced oxidative stress. It has been shown that FOXO3 binds to the MnSOD promoter region encompassing FOXO-binding element, thereby regulating MnSOD transcription (35). Therefore, further experiments are needed to determine whether and how SIRT1 regulates FOXO3-dependent transcriptional mechanisms on the promoters of antioxidant genes, including MnSOD, HO-1, and NQO-1.
There are two contradictory reports showing that SIRT1 can positively and negatively regulate Nrf2-dependent gene transcription (19, 28). However, it remains unknown whether Nrf2 is involved in the protection of SIRT1 against CS-induced oxidative stress. We found that Nrf2 deletion did not influence the ability of SRT1720 to reduce CS-induced increase in MDA plus 4-HNE in mouse lungs. Moreover, SRT1720 treatment augmented the expression of MnSOD, HO-1, and NQO-1 genes in both WT and Nrf2−/− mice in response to CS exposure. This indicates that Nrf2 does not participate in the regulation of SIRT1 in CS-induced oxidative stress. Nrf2 reduces oxidative and electrophilic stress by inducing the expression of antioxidant and phase II detoxification genes that include NQO-1 and HO-1 (25, 30). It is interesting to note that HO-1 gene expression was not changed in Nrf2−/− mice compared with WT mice. These findings are consistent with the study that Nrf2 is not the transcription factor responsible for CS-induced alteration of these antioxidant response element-related genes (e.g., NQO-1 and HO-1) (27). Nevertheless, further studies are required to determine the effect of SIRT1 on Nrf2 transcriptional activity and its recruitment on promoters of these antioxidant genes in response to CS exposure. It is also unknown whether the different mouse strains influence the sensitivity to CS-induced oxidative stress after SRT1720 treatment, as the background strains for FOXO3 and Nrf2 knockout mice are different, which needs further investigation.
In summary, our findings demonstrate that SIRT1 protects against CS-induced oxidative stress and increases the expression of antioxidant genes and enzymes in mouse lungs. FOXO3 knockout mice showed increased lipid peroxidation products as well as reduced antioxidant genes and enzymes, which cannot be attenuated by SRT1720, suggesting the involvement of FOXO3 in SIRT1-mediated regulation of CS-induced oxidative stress. Interestingly, SRT1720 treatment exhibited a similar effect on lipid peroxidation and antioxidant genes/enzymes between WT and Nrf2-deficient mice in response to CS exposure. This indicates that SIRT1 protects against CS-induced oxidative stress independent of Nrf2. Therefore, SIRT1 reduced CS-induced oxidative stress, which may contribute to its protection against lung inflammation, cellular senescence, and subsequent development of COPD.
GRANTS
This study was supported by NIH 1R01HL092842, 2R01 HL085613, 1R01HL097751 (to I. Rahman), American Lung Association RG-266456-N (to H. Yao), University of Rochester CTSI 5UL1RR024160 incubator project (to P. Sime and R. Phipps), University of Rochester Drug Discovery Pilot Award, and NIEHS Environmental Health Science Center grant P30-ES01247.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.Y. and I.R. conception and design of research; H.Y., I.K.S., T.A., C.L., J.G., and A.E.F. performed experiments; H.Y., and A.E.F. analyzed data; H.Y., I.K.S., T.A., C.L., A.E.F., and I.R. interpreted results of experiments; H.Y. prepared figures; H.Y. and I.R. drafted manuscript; H.Y., I.K.S., T.A., C.L., J.G., A.E.F., R.P.P., P.J.S., M.W.M., L.P.G., and I.R. edited and revised manuscript; H.Y., I.K.S., T.A., C.L., J.G., A.E.F., R.P.P., P.J.S., M.W.M., L.P.G., and I.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Suzanne E. Cook and Michelle Friedman for technical assistance and Miss Anne C. Skuse for editing the manuscript.
REFERENCES
- 1.Adenuga D, Caito S, Yao H, Sundar IK, Hwang JW, Chung S, Rahman I. Nrf2 deficiency influences susceptibility to steroid resistance via HDAC2 reduction. Biochem Biophys Res Commun 403: 452–456, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100: 1512–1521, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Anjem A, Imlay JA. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J Biol Chem 287: 15544–15556, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolisetty S, Traylor AM, Kim J, Joseph R, Ricart K, Landar A, Agarwal A. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol 21: 1702–1712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6: 759–767, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Caito S, Rajendrasozhan S, Cook S, Chung S, Yao H, Friedman AE, Brookes PS, Rahman I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J 24: 3145–3159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301: 215–218, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Chartoumpekis DV, Ziros PG, Zaravinos A, Iskrenova RP, Psyrogiannis AI, Kyriazopoulou VE, Sykiotis GP, Habeos IG. Hepatic gene expression profiling in Nrf2 knockout mice after long-term high-fat diet-induced obesity. Oxid Med Cell Longev 2013: 340731, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 26: 175–182, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 64: 111–126, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deslee G, Woods JC, Moore C, Conradi SH, Gierada DS, Atkinson JJ, Battaile JT, Liu L, Patterson GA, Adair-Kirk TL, Holtzman MJ, Pierce RA. Oxidative damage to nucleic acids in severe emphysema. Chest 135: 965–974, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinkova-Kostova AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 501: 116–123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doorn JA, Petersen DR. Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxy-2-nonenal and 4-oxo-2-nonenal. Chem Res Toxicol 15: 1445–1450, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11: 81–128, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Facchinetti F, Amadei F, Geppetti P, Tarantini F, Di Serio C, Dragotto A, Gigli PM, Catinella S, Civelli M, Patacchini R. Alpha,beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am J Respir Cell Mol Biol 37: 617–623, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gould NS, Min E, Gauthier S, Martin RJ, Day BJ. Lung glutathione adaptive responses to cigarette smoke exposure. Respir Res 12: 133, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He JQ, Ruan J, Connett JE, Anthonisen NR, Pare PD, Sandford AJ. Antioxidant gene polymorphisms and susceptibility to a rapid decline in lung function in smokers. Am J Respir Crit Care Med 166: 323–328, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Huang K, Huang J, Xie X, Wang S, Chen C, Shen X, Liu P, Huang H. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-beta1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med 65: 528–540, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Hwang JW, Rajendrasozhan S, Yao H, Chung S, Sundar IK, Huyck HL, Pryhuber GS, Kinnula VL, Rahman I. FOXO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J Immunol 187: 987–998, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang JW, Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J 28: 176–194, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang JW, Yao H, Caito S, Sundar IK, Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med 61C: 95–110, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichinose M, Sugiura H, Yamagata S, Koarai A, Shirato K. Increase in reactive nitrogen species production in chronic obstructive pulmonary disease airways. Am J Respir Crit Care Med 162: 701–706, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Igishi T, Hitsuda Y, Kato K, Sako T, Burioka N, Yasuda K, Sano H, Shigeoka Y, Nakanishi H, Shimizu E. Elevated urinary 8-hydroxydeoxyguanosine, a biomarker of oxidative stress, and lack of association with antioxidant vitamins in chronic obstructive pulmonary disease. Respirology 8: 455–460, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Juul K, Tybjaerg-Hansen A, Marklund S, Lange P, Nordestgaard BG. Genetically increased antioxidative protection and decreased chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173: 858–864, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kathiria AS, Butcher MA, Hansen JM, Theiss AL. Nrf2 is not required for epithelial prohibitin-dependent attenuation of experimental colitis. Am J Physiol Gastrointest Liver Physiol 304: G885–G896, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai Y, Garduno L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem 286: 7629–7640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan RS, Fonseca-Kelly Z, Callinan C, Zuo L, Sachdeva MM, Shindler KS. SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front Cell Neurosci 6: 63, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res 690: 12–23, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Kinnula VL. Focus on antioxidant enzymes and antioxidant strategies in smoking related airway diseases. Thorax 60: 693–700, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest 144: 266–273, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 294: L478–L488, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res 7: 132, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419: 316–321, 2002 [DOI] [PubMed] [Google Scholar]
- 36.MacNee W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 50–60, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Macnee W, Rahman I. Oxidants and antioxidants as therapeutic targets in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 160: S58–S65, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Mak JC, Ho SP, Yu WC, Choo KL, Chu CM, Yew WW, Lam WK, Chan-Yeung M. Polymorphisms and functional activity in superoxide dismutase and catalase genes in smokers with COPD. Eur Respir J 30: 684–690, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med 178: 592–604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest 117: 2133–2144, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest 120: 4207–4219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol 23: 38–54, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450: 712–716, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med 159: 473–479, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Nakamaru Y, Vuppusetty C, Wada H, Milne JC, Ito M, Rossios C, Elliot M, Hogg J, Kharitonov S, Goto H, Bemis JE, Elliott P, Barnes PJ, Ito K. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J 23: 2810–2819, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Niki E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors 34: 171–180, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Pastukh VM, Zhang L, Ruchko MV, Gorodnya O, Bardwell GC, Tuder RM, Gillespie MN. Oxidative DNA damage in lung tissue from patients with COPD is clustered in functionally significant sequences. Int J Chron Obstruct Pulmon Dis 6: 209–217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20: 3551–3567, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanisms. Cell Biochem Biophys 43: 167–188, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 28: 219–242, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Rahman I, Kinnula VL, Gorbunova V, Yao H. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev Med 54, Suppl: S20–S28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1: 3159–3165, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 16: 534–554, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Rahman I, MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic Biol Med 21: 669–681, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 154: 1055–1060, 1996 [DOI] [PubMed] [Google Scholar]
- 56.Rahman I, Smith CA, Lawson MF, Harrison DJ, MacNee W. Induction of gamma-glutamylcysteine synthetase by cigarette smoke is associated with AP-1 in human alveolar epithelial cells. FEBS Lett 396: 21–25, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166: 490–495, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177: 861–870, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114: 1248–1259, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rangasamy T, Misra V, Zhen L, Tankersley CG, Tuder RM, Biswal S. Cigarette smoke-induced emphysema in A/J mice is associated with pulmonary oxidative stress, apoptosis of lung cells, and global alterations in gene expression. Am J Physiol Lung Cell Mol Physiol 296: L888–L900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am J Respir Crit Care Med 156: 341–357, 1997 [DOI] [PubMed] [Google Scholar]
- 62.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858, 1996 [DOI] [PubMed] [Google Scholar]
- 63.Siedlinski M, Postma DS, van Diemen CC, Blokstra A, Smit HA, Boezen HM. Lung function loss, smoking, vitamin C intake, and polymorphisms of the glutamate-cysteine ligase genes. Am J Respir Crit Care Med 178: 13–19, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Velarde MC, Flynn JM, Day NU, Melov S, Campisi J. Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging (Albany NY) 4: 3–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao H, Arunachalam G, Hwang JW, Chung S, Sundar IK, Kinnula VL, Crapo JD, Rahman I. Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM. Proc Natl Acad Sci USA 107: 15571–15576, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Ronty M, Kinnula VL, Rahman I. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest 122: 2032–2045, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 294: L1174–L1186, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Yao H, Hwang JW, Sundar IK, Friedman AE, McBurney MW, Guarente LP, Gu W, Kinnula VL, Rahman I. SIRT1 redresses tissue inhibitor of matrix metalloproteinase-1/matrix metalloproteinase-9 imbalance in the development of mouse emphysema and human COPD. Am J Physiol Lung Cell Mol Physiol 305: L615–L624, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yao H, Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol Appl Pharmacol 254: 72–85, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yao H, Rahman I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol 84: 1332–1339, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Young RP, Hopkins R, Black PN, Eddy C, Wu L, Gamble GD, Mills GD, Garrett JE, Eaton TE, Rees MI. Functional variants of antioxidant genes in smokers with COPD and in those with normal lung function. Thorax 61: 394–399, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuan Q, Zhu X, Sayre LM. Chemical nature of stochastic generation of protein-based carbonyls: metal-catalyzed oxidation versus modification by products of lipid oxidation. Chem Res Toxicol 20: 129–139, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Zee RS, Yoo CB, Pimentel DR, Perlman DH, Burgoyne JR, Hou X, McComb ME, Costello CE, Cohen RA, Bachschmid MM. Redox regulation of sirtuin-1 by S-glutathiolation. Antioxid Redox Signal 13: 1023–1032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]