Abstract
Others and we have characterized several Gβγ-dependent effectors in smooth muscle, including G protein-coupled receptor kinase 2 (GRK2), PLCβ3, and phosphatidylinositol (PI) 3-kinase-γ, and have identified various signaling targets downstream of PI 3-kinase-γ, including cSrc, integrin-linked kinase, and Rac1-Cdc42/p21-activated kinase/p38 MAP kinase. This study identified a novel mechanism whereby Gβγ acting via PI 3-kinase-γ and cSrc exerts an inhibitory influence on Gαi activity. The Gi2-coupled δ-opioid receptor agonist d-penicillamine (2,5)-enkephalin (DPDPE) activated cSrc, stimulated tyrosine phosphorylation of Gαi2, and induced regulator of G protein signaling 12 (RGS12) association; all three events were blocked by PI 3-kinase (LY294002) and cSrc (PP2) inhibitors and by expression of the COOH-terminal sequence of GRK2-(495–689), a Gβγ-scavenging peptide. Inhibition of forskolin-stimulated cAMP and muscle relaxation by DPDPE was augmented by PP2, LY294002, and a selective PI 3-kinase-γ inhibitor, AS-605420. Expression of tyrosine-deficient (Y69F, Y231F, or Y321F) Gαi2 mutant or knockdown of RGS12 blocked Gαi2 phosphorylation and Gαi2-RGS12 association and caused greater inhibition of cAMP. Parallel studies using somatostatin, cyclopentyl adenosine, or ACh to activate, respectively, Gi1-coupled somatostatin (sstr3) receptors, and Gi3-coupled adenosine A1 or muscarinic m2 receptors elicited cSrc activation, Gαi1 or Gαi3 phosphorylation, Gαi1-RGS12 or Gαi3-RGS12 association, and inhibition of cAMP. Inhibition of cAMP and muscle relaxation was greatly increased by AS-605240 and PP2. The results demonstrate that Gβγ-dependent tyrosine phosphorylation of Gαi1/2/3 by cSrc facilitated recruitment of RGS12, a Gαi-specific RGS protein with a unique phosphotyrosine-binding domain, resulting in rapid deactivation of Gαi and facilitation of smooth muscle relaxation.
Keywords: G protein activation, Gαi proteins, RGS proteins, smooth muscle, tyrosine phosphorylation
receptor-mediated G protein signaling requires the participation of the complete heterotrimer. In recent years, increasing attention has focused on the role of the Gβγ complex in effector regulation (6, 10, 12, 32). The Gβγ complex is bound to the plasma membrane via isoprenylated Gγ; it participates in G protein-receptor coupling and acts as a guanine nucleotide dissociation inhibitor, maintaining the G protein in its inactive state (10, 29). Agonist-activated receptors catalyze the exchange of GDP for GTP; binding of GTP to Gα induces a conformational change in three flexible switch regions within Gα that result in dissociation of Gβγ (7). Gα-GTP and free Gβγ subunits regulate distinct downstream effector molecules. The intrinsic hydrolysis of GTP by Gα, greatly accelerated by regulator of G protein signaling (RGS) proteins (29, 36), terminates the cycle of guanine nucleotide exchange and fosters reassociation of Gα-GDP with Gβγ.
Gβγ undergoes little or no conformational change and, unlike Gα, has no catalytic activity, acting on its effectors via protein-protein interaction (32). In the inactive state, Gβγ interacts with two structural components of Gα: the Gα NH2-terminal helix and the Gα switch II region; the latter undergoes a conformational change upon GTP binding that leads to release of Gβγ subunits, exposing a signaling surface on Gβ (7, 15). Various subsets of amino acids within this preferred binding surface on Gβ, together with complementary sites in other regions of Gβγ, are involved in binding and activation of different effectors.
Most Gβγ signaling arises from abundant Gi proteins and is susceptible to inhibition by pertussis toxin, which ADP-ribosylates Gαi, preventing guanine nucleotide exchange on Gα and dissociation of Gβγ (6, 32). Numerous Gβγ effectors, including PLCβ2 and PLCβ3, adenylyl cyclases (I-VII), N/P/Q-type Ca2+ channels, inwardly rectifying K+ channels, phosphoinositide (PI) 3-kinase-γ, and G protein-coupled receptor kinases (GRK2 and GRK3), have been identified in various cells (4, 12, 28, 32, 34). Others (9, 17, 31, 35, 37) and we (8, 19, 20–24, 39) have characterized the signaling pathways mediated by several of these Gβγ-dependent effectors, including GRK2, PLCβ3, and PI 3-kinase, in smooth muscle and have identified various signaling targets downstream of PI 3-kinase, including Rac1/cdc42, p21-activated kinase (PAK1), integrin-linked kinase (ILK), and cSrc (8, 19, 30, 39). Previous studies using freshly dispersed or cultured gastric smooth muscle cells, a model system used extensively to explore G protein-coupled receptor signaling in visceral smooth muscle, have shown that receptors coupled to Gi1 [e.g., somatostatin (sstr3)] (21), Gi2 (e.g., μ-, δ-, and κ-opioid) (23), or Gi3 (e.g., adenosine A1) (22) induce contraction by activating dual signaling pathways initiated by Gβγ (8): the first pathway involves activation of PLCβ3, stimulation of inositol 1,4,5-trisphosphate-dependent Ca2+ release, and phosphorylation of myosin light chain 20 (MLC20), resulting in a transient smooth muscle contraction. The second pathway involves sequential activation of PI 3-kinase and ILK: the latter acts as a Ca2+-independent MLC kinase and inhibits MLC phosphatase (PP1cδ), resulting in sustained MLC20 phosphorylation and smooth muscle contraction (5, 8). In the present study we examined the significance of a parallel pathway initiated by these receptors and by Gi3-coupled muscarinic m2 receptors involving sequential activation of Gβγ, PI 3-kinase-γ, and cSrc. We postulated that Gαi could be a target for tyrosine phosphorylation by cSrc, enabling tyrosine-phosphorylated Gαi to recruit RGS12, a Gαi-selective RGS protein, which uniquely contains an NH2-terminal phosphotyrosine-binding (PTB) domain (30). In this way, a signal emanating from Gβγ could accelerate inactivation of Gαi-GTP and facilitate reassociation of Gα and Gβγ into an inactive heterotrimer.
MATERIALS AND METHODS
Materials.
125I-cAMP was obtained from PerkinElmer Life Sciences (Boston, MA); collagenase CLS type II and soybean trypsin inhibitor from Worthington (Freehold, NJ); Western blotting Tris·HCl Ready Gel from Bio-Rad Laboratories (Hercules, CA); phosphotyrosine, phosphorylated (Tyr416) Src, PI 3-kinase-γ, RGS12, Gαi1, Gαi2, and Gαi3 antibodies and RGS12 small interfering RNA (siRNA) from Santa Cruz Biotechnology (Santa Cruz, CA); LY294002, PP2, and AS-605240 from Calbiochem (La Jolla, CA); Lipofectamine 2000 transfection reagent from Invitrogen (Grand Island, NY); DMEM from Fisher Scientific; and d-penicillamine (2,5)-enkephalin (DPDPE), cyclopentyl adenosine (CPA), somatostatin, ACh, 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP), and all other chemicals from Sigma (St. Louis, MO).
New Zealand white rabbits (4–5 lb body wt) were purchased from RSI Biotechnology (Clemmons, NC) and euthanized by injection of Euthasol (100 mg/kg), as approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. C57BL/6 mice (Jackson laboratories, Bar Harbor, ME) were housed in the animal facility administered by the Division of Animal Resources, Virginia Commonwealth University. All procedures were conducted in accordance with the Institutional Animal Care and Use Committee of Virginia Commonwealth University. Human tissues were obtained from the National Disease Research Interchange.
Preparation of dispersed and cultured gastric smooth muscle cells.
Smooth muscle cells from the circular muscle layer of the distal stomach were isolated by sequential enzymatic digestion of muscle strips, filtration, and centrifugation, as described previously (8, 20–22). The tissue was cut into thin slices using a Stadie-Riggs tissue slicer, and the slices were incubated at 31°C for 30 min in a smooth muscle buffer [120 mM NaCl, 4 mM KCl, 2.6 mM KH2PO4, 2.0 mM CaCl2, 0.6 mM MgCl2, 25 mM HEPES, 14 mM glucose, and 2.1% essential amino acid mixture (pH 7.4)] containing 0.1% collagenase (300 U/ml) and 0.01% (wt/vol) soybean trypsin inhibitor. The tissue was continuously gassed with 100% O2 throughout the isolation procedure. The partly digested tissues were washed twice with 50 ml of collagenase-free smooth muscle buffer, and the muscle cells were allowed to disperse spontaneously for 30 min in collagenase-free medium. The cells were harvested by filtration through 500-μm Nitex and centrifuged twice at 350 g for 10 min to eliminate broken cells and organelles. The cells were counted in a hemocytometer, and ∼95% of the cells excluded Trypan blue. Experiments were done within 2–3 h of cell dispersion.
For culture, freshly dispersed smooth muscle cells were resuspended in DMEM containing penicillin (200 U/ml), streptomycin (200 μg/ml), gentamicin (100 μg/ml), amphotericin B (2.5 μg/ml), and 10% FBS (DMEM-10). The cells were plated at 5 × 105 cells/ml and incubated at 37°C in a CO2 incubator. DMEM-10 was replaced every 3 days for 2–3 wk until confluence was attained. The smooth muscle cells in confluent primary cultures were trypsinized (0.5 mg trypsin/ml), replated at 2.5 × 105 cells/ml, and cultured under the same conditions. All experiments were done on cells in passage 1. The purity of cultured smooth muscle cells was previously determined using smooth muscle-specific γ-actin (33). Cultured smooth muscle cells were placed in serum-free medium for 24 h before each use.
Transfection of cultured smooth muscle cells with RGS12 siRNA.
Smooth muscle cells cultured in six-well plates were transiently transfected with control scrambled siRNA or RGS12 siRNA using Lipofectamine 2000 according to the manufacturer's instructions (8). Briefly, 100 pmol of siRNA in 125 μl of Opti-MEM were mixed with 5 μl of Lipofectamine 2000 in 125 μl of Opti-MEM. The mixture was incubated at room temperature for 20 min and added to wells containing 1.5 ml of DMEM with 10% FBS for 1 day. The medium was then replaced with DMEM with 10% FBS + antibiotics for 2 days. The cells were maintained for a final 24 h in DMEM without FBS before experiments were started. Successful knockdown of RGS12 protein was verified by Western blotting (8, 24, 39).
Vector and Gαi2 mutant constructs and transfection of smooth muscle cells with wild-type and mutant Gαi2.
Mutant phosphorylation site-deficient Gαi2 (Y69, Y231, or Y321) was constructed by the megaprimer method, as described previously (40). Mutants were sequenced to confirm that mutagenesis was successful. Wild-type Gαi2 and phosphorylation site-deficient Gαi2 were subcloned separately into the multiple cloning site (EcoR I) of the eukaryotic expression vector pEXV. Recombinant plasmid DNAs (2 μg each) were transiently transfected into smooth muscle cells in passage 1 by incubation with Lipofectamine Plus reagent for 48 h. The cells were cotransfected with 1 μg of pGreen Lantern-1 to monitor expression. Control cells were cotransfected with 2 μg of vector (pEXV) and 1 μg of pGreen Lantern-1 DNA. Transfection efficiency (∼75%) was monitored by the expression of green fluorescent protein using FITC filters.
Activation of cSrc.
Activation of cSrc was measured by immunoblotting using a phosphorylated (Tyr416) Src antibody. Freshly dispersed smooth muscle cells (3 × 106 cells/ml) were pretreated for 10 min with control buffer or buffer containing inhibitors of PI 3-kinase (LY294002, 1 μM) or cSrc (PP2, 1 μM) followed by addition of 1 μM DPDPE for 1 min. The cell suspension was solubilized on ice for 2 h in 20 mM Tris·HCl medium containing 1 mM DTT, 100 mM NaCl, 0.5% SDS, 1 mM PMSF, 10 μg/ml leupeptin, and 100 μg/ml aprotinin. In other experiments, cultured smooth muscle cells in passage 1 were transfected with control vector or vector containing GRK2CT-(495–689), a Gβγ-scavenging peptide. The cells were treated with DPDPE for 1 min and then solubilized as described above. The proteins were resolved by SDS-PAGE and transferred electrophoretically to polyvinylidene difluoride (PVDF) membranes. The membranes were incubated for 12 h with phosphorylated (Tyr416) Src antibody and then for 1 h with horseradish peroxidase-conjugated secondary antibody. The bands were identified by enhanced chemiluminescence.
Phosphorylation of Gαi.
Dispersed smooth muscle cells (3 × 106 cells/ml) were pretreated for 10 min with control buffer or buffer containing inhibitors of PI 3-kinase (LY294002, 1 μM) or cSrc (PP2, 1 μM) followed by addition of 1 μM DPDPE. The cell suspension was solubilized on ice for 2 h in 20 mM Tris·HCl medium containing 1 mM DTT, 100 mM NaCl, 0.5% SDS, 1 mM PMSF, 10 μg/ml leupeptin, and 100 μg/ml aprotinin. In other experiments, smooth muscle cells cultured in wells were transfected separately with control vector, vector containing GRK2CT-(495–689), or vector containing Gαi2 mutant (Y69F, Y231F, or Y321F), treated with DPDPE for 1 min, and then solubilized as described above. Gαi2 immunoprecipitates were separated by SDS-PAGE, transferred to PVDF membranes, and probed with phosphorylated tyrosine antibody. After incubation with a secondary antibody, the proteins were visualized using enhanced chemiluminescence. In some experiments, dispersed smooth muscle cells (3 × 106 cells/ml) were treated, respectively, with somatostatin (1 μM) to activate Gαi1-coupled somatostatin sstr3 receptors, ACh (1 μM) in the presence of the muscarinic m3 receptor antagonist 4-DAMP (0.1 μM) to activate Gαi3-coupled m2 receptors, or CPA (1 μM) to activate Gαi3-coupled adenosine A1 receptors. Phosphorylation of Gαi1 and Gαi3 was analyzed as described above for Gαi2.
Immunoblot analysis of Gαi-RGS12 association.
Dispersed smooth muscle cells (3 × 106 cells/ml) were pretreated for 10 min with control buffer or buffer containing inhibitors of PI 3-kinase (LY294002, 1 μM) or cSrc (PP2, 1 μM) followed by addition of 1 μM DPDPE. In other experiments, smooth muscle cells cultured in wells were transfected with control vector, vector containing GRK2CT-(495–689), or vector containing Gαi2 mutant (Y69F, Y231F, or Y321F). The cells were treated with 1 μM DPDPE for 1 min and solubilized as described above. Gαi2 immunoprecipitates were obtained from lysates of freshly dispersed or cultured muscle cells. The immunoprecipitates were separated by SDS-PAGE, transferred to PVDF membranes, and probed with RGS12 antibody. After incubation with secondary antibody, the proteins were visualized by enhanced chemiluminescence. The intensity of the protein bands was determined by densitometry.
In separate experiments, dispersed smooth muscle cells (3 × 106 cells/ml) were treated for 1 min with 1 μM somatostatin, 1 μM CPA, or 1 μM ACh + 0.1 μM 4-DAMP. Gαi1 immunoprecipitates were obtained from lysates of cells treated with somatostatin, and Gαi3 immunoprecipitates were obtained from lysates of cells treated with CPA or ACh. Immunoprecipitates were probed with RGS12 antibody as described above for analysis of Gαi-RGS12 association.
Radioimmunoassay for cAMP.
cAMP levels were measured by radioimmunoassay, as previously described (20–23). Suspensions of smooth muscle cells (2 × 106 cells/ml) were incubated for 10 min with 100 μM isobutylmethyl xanthine and 10 μM forskolin and then treated for 1 min with 1 μM DPDPE. In separate experiments, the cells were first treated for 10 min with 1 μM LY294002 or 1 μM PP2 and then for 1 min with 1 μM DPDPE. In some experiments, the cells were treated with 1 μM somatostatin, 1 μM ACh + 0.1 μM 4-DAMP, or 1 μM CPA for 1 min, with or without pretreatment with the PI 3-kinase-γ-selective inhibitor AS-605240 (25 nM) or PP2 (1 μM). In other experiments, cultured smooth muscle cells were transfected with control vector, vector containing GRK2CT-(495–689), or vector containing Gαi2 mutant. The cells were then treated with forskolin and DPDPE as described above. The reaction was terminated with 10% trichloroacetic acid. The samples were centrifuged, and the supernatant was extracted with diethyl ether and lyophilized. The samples were resuspended in sodium-acetate buffer (pH 6.2) and then acetylated with triethylamine-acetic anhydride for 10 min. cAMP was measured in duplicates, and the results are expressed as picomoles per milligram of protein.
Measurement of relaxation in dispersed smooth muscle cells.
Contraction in freshly dispersed smooth muscle cells was determined by scanning micrometry (21–24). An aliquot (0.4 ml) of cells containing ∼104 cells/ml was treated with KCl (20 mM) for 30 s, and the reaction was terminated with 1% acrolein at a final concentration of 0.1%. The mean lengths of 50 muscle cells treated with KCl were measured by scanning micrometry and compared with the mean lengths of untreated cells. The contractile response to KCl is expressed as the percent decrease in mean cell length from control cell length. Relaxation was measured as decrease in response to KCl in the presence of 10 μM forskolin. Relaxation is expressed as percent decrease in contractile response to KCl.
Statistical analysis.
Values are means ± SE of n experiments, where n represents one sample from one animal for a single experimental replicate. Results were analyzed for statistical significance using Student's t-test for paired and unpaired values. P < 0.05 was considered to be statistically significant. The GraphPad software program (San Diego, CA) was used for statistical analysis.
RESULTS
DPDPE-stimulated phosphorylation of cSrc mediated by Gβγ-dependent activation of PI 3-kinase.
Treatment of cultured smooth muscle cells with the δ-opioid receptor agonist DPDPE stimulated cSrc phosphorylation, determined using phosphorylated (Tyr416) Src antibody; phosphorylation was abolished in cells expressing the COOH-terminal sequence of GRK2 that constitutes the Gβγ binding motif (Fig. 1A). The COOH-terminal sequence of GRK2 acts as a specific Gβγ (but not Gα) antagonist by binding to Gβγ and blocking its interaction with effectors such as GRK2 (14, 16, 18, 38). Treatment of freshly dispersed smooth muscle cells with DPDPE also stimulated cSrc phosphorylation, which was abolished in smooth muscle cells pretreated with the cSrc inhibitor PP2 or the PI 3-kinase inhibitor LY294002 (Fig. 1B). The results from freshly dispersed and cultured smooth muscle cells implied that cSrc phosphorylation was mediated by Gβγ-dependent activation of PI 3-kinase.
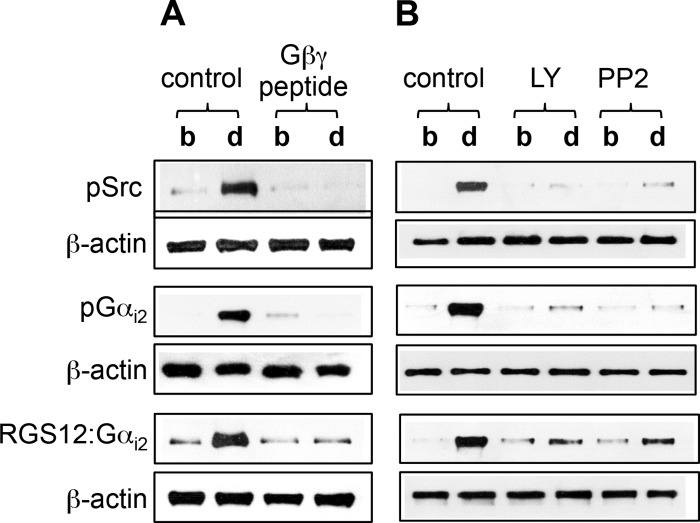
Fig. 1.
Activation of cSrc, tyrosine phosphorylation of Gαi2, and association of tyrosine-phosphorylated Gαi2 with regulator of G protein signaling 12 (RGS12) by d-penicillamine (2,5)-enkephalin (DPDPE). A: cultured smooth muscle cells transfected with control vector or vector containing Gβγ-scavenging peptide were treated with 1 μM DPDPE for 1 min. B: dispersed smooth muscle cells were pretreated for 10 min with control buffer or buffer containing inhibitors of phosphoinositide (PI) 3-kinase (LY294002, 1 μM) or cSrc (PP2, 1 μM) followed by addition of 1 μM DPDPE for 1 min. Activation of cSrc [phosphorylated Src (pSrc)] was measured using specific phosphorylated (Tyr416) Src antibody. Gαi2 immunoprecipitates were used to measure tyrosine phosphorylation [phosphorylated Gαi2 (pGαi2)] using phosphotyrosine antibody and association of Gαi2 with RGS12 (RGS12:Gαi2) using RGS12 antibody. Immunoblots are representative of 4 separate experiments. b, Basal; d, DPDPE.
DPDPE-stimulated tyrosine phosphorylation of Gαi2 mediated by Gβγ-dependent activation of PI 3-kinase and cSrc.
We considered whether Gαi2 was a possible target for tyrosine phosphorylation by cSrc. The sequence of Gαi2 contains several sites for tyrosine phosphorylation by cSrc. Treatment of cultured smooth muscle cells with DPDPE stimulated tyrosine phosphorylation of Gαi2, which was abolished in smooth muscle cells expressing Gβγ-scavenging peptide [GRK2CT-(495–689)] (Fig. 1A). Treatment of freshly dispersed smooth muscles with DPDPE also stimulated tyrosine phosphorylation of Gαi2, which was abolished in cells pretreated with LY294002 or PP2 (Fig. 1B). The results implied that tyrosine phosphorylation of Gαi2 was mediated by Gβγ-dependent sequential activation of PI 3-kinase and cSrc.
Recruitment of RGS12 by tyrosine-phosphorylated Gαi2.
We next examined whether tyrosine phosphorylation enabled Gαi2 to recruit RGS12. This multidomain RGS12 has high affinity for Gαi and contains a unique PTB domain (29, 30). Treatment of cultured smooth muscle cells with DPDPE induced RGS12-Gαi2 association, which was abolished in cells expressing Gβγ-scavenging peptide [GRK2CT-(495–689)] (Fig. 1A). Treatment of freshly dispersed smooth muscle cells with DPDPE also induced RGS12-Gαi2 association, which was abolished in cells pretreated with LY294002 or PP2 (Fig. 1B). The results implied that tyrosine phosphorylation of Gαi2 mediated by Gβγ-dependent activation of PI 3-kinase and cSrc enabled recruitment of RGS12.
Gαi2-mediated inhibition of cAMP and muscle relaxation is attenuated by Gβγ-dependent activation of PI 3-kinase-γ and cSrc.
To define the functional significance of this pathway, we determined its ability to influence inhibition of cAMP formation by DPDPE. DPDPE inhibited forskolin-stimulated cAMP in dispersed smooth muscle cells by 48 ± 4% [26.4 ± 2.5 and 13.8 ± 1.4 pmol/mg protein above basal (i.e., 2.8 ± 0.3 pmol/mg protein) for forskolin and forskolin + DPDPE, respectively, n = 5]. The inhibition was abolished in muscle cells pretreated for 60 min with 400 ng/ml of pertussis toxin [24.8 ± 3.6 and 25.2 ± 2.1 pmol/mg protein above basal (i.e., 2.4 ± 0.3 pmol/mg protein) for forskolin and forskolin + DPDPE, respectively, n = 5].
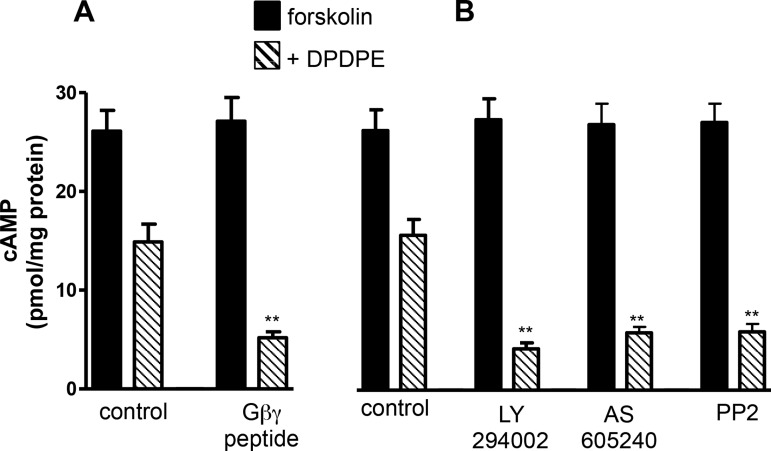
Treatment of cultured smooth muscle cells with DPDPE inhibited forskolin-stimulated cAMP formation by 45 ± 4% (n = 5); the inhibition was greatly enhanced (90 ± 3%, n = 5) in cells expressing Gβγ-scavenging peptide [GRK2CT-(495–689)] (P < 0.01; Fig. 2A). Treatment of freshly dispersed smooth muscle cells with DPDPE also inhibited forskolin-stimulated cAMP formation by 43 ± 2% (n = 5); the inhibition was greatly enhanced in cells pretreated with LY294002 (88 ± 4%, n = 5), AS-605240, a PI 3-kinase γ-selective inhibitor (86 ± 5%, n = 5), or PP2 (92 ± 5%, n = 5) (Fig. 2B). The results implied that inhibition of adenylyl cyclase activity by Gαi2 was strongly influenced (i.e., attenuated) by Gβγ-dependent activation of PI 3-kinase and cSrc. Forskolin-induced cAMP formation was not affected in the presence of LY294002 or PP2, suggesting that adenylyl cyclase activity was not affected by PI 3-kinase or cSrc.
Fig. 2.
Gαi2-induced inhibition of cAMP attenuated by Gβγ-dependent activation of PI 3-kinase and cSrc. A: cultured smooth muscle cells transfected with control vector or vector containing Gβγ-scavenging peptide were pretreated with 10 μM forskolin for 10 min and then treated with 1 μM DPDPE for 1 min. B: dispersed smooth muscle cells were pretreated for 10 min with control buffer containing 10 μM forskolin or buffer containing 10 μM forskolin in the presence or absence of inhibitors of PI 3-kinase [LY294002 (1 μM) or AS-605240 (25 nM)] or cSrc (PP2, 1 μM) followed by addition of 1 μM DPDPE for 1 min. cAMP levels were measured by radioimmunoassay. Basal levels (2.4 ± 0.21 to 2.6 ± 0.26 pmol/mg protein) of cAMP are similar in control cells and cells treated with LY294002 or PP2 and in cells expressing Gβγ-scavenging peptide. Values (means ± SE of 5 experiments) are expressed as pmol/mg protein above basal levels. **P < 0.01 compared with control.
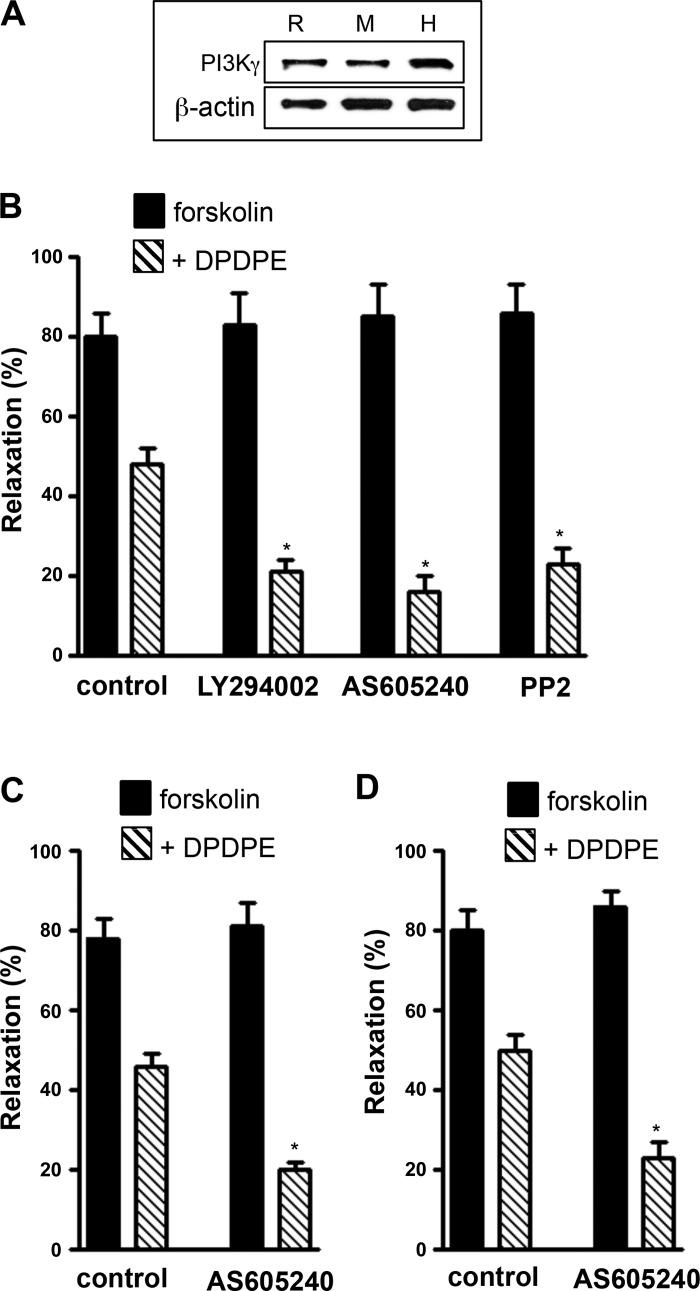
Expression of PI 3-kinase-γ was demonstrated by Western blotting in rabbit, mouse, and human gastric muscle cells (Fig. 3A). Treatment of freshly dispersed smooth muscle cells from rabbit stomach with DPDPE also inhibited forskolin-stimulated muscle relaxation by 39 ± 4% (n = 5); the inhibition was greatly enhanced in cells pretreated with LY294002 (75 ± 3%, n = 5), AS-605240, a PI 3-kinase-γ-selective inhibitor (81 ± 5%, n = 5), or PP2 (73 ± 3%, n = 5) (Fig. 3B). Similarly, treatment of freshly dispersed smooth muscle cells from mouse and human stomach with DPDPE also inhibited forskolin-stimulated muscle relaxation by 41 ± 3% and 37 ± 2% (n = 5); the inhibition was greatly enhanced in cells pretreated with AS-605240 (75 ± 4% and 73 ± 5%, n = 5; Fig. 3, C and D). The results implied that inhibition of muscle relaxation by the Gαi2-coupled δ-opioid receptor agonist DPDPE was attenuated by activation of PI 3-kinase-γ and cSrc.
Fig. 3.
Gαi2-induced inhibition of muscle relaxation attenuated by PI 3-kinase and cSrc. A: Western blot analysis of PI 3-kinase-γ expression in smooth muscle cells isolated from stomach of rabbit (R), mouse (M), and human (H). B–D: dispersed gastric smooth muscle cells from rabbit (B), mouse (C), and human (D) were pretreated for 10 min with control buffer containing 10 μM forskolin in the presence or absence of inhibitors of PI 3-kinase [LY294002 (1 μM) or AS-605240 (25 nM)] or cSrc (PP2, 1 μM) followed by addition of 1 μM DPDPE for 1 min and KCl (20 mM) for 30 s. DPDPE was added in the presence of U73122 (1 μM) to block hydrolysis of phosphoinositides by Gβγ-dependent PLCβ3. Contraction in response to KCl was measured as decrease in cell length by scanning micrometry. Relaxation was measured as decrease in contraction in response to KCl in the presence of forskolin and expressed as percent inhibition of contraction. Contraction in response to KCl was similar in gastric muscle cells isolated from rabbit (31 ± 3% decrease in cell length from control cell length of 105 ± 5 μm to 11 ± 3 μm), mouse (29 ± 2% decrease in cell length from control cell length of 112 ± 6 μm), and human (32 ± 4% decrease in cell length from control cell length of 95 ± 3 μm). Values are means ± SE of 5 experiments. *P < 0.05 compared with control.
Role of RGS12 in Gβγ-dependent modulation of Gαi2 function.
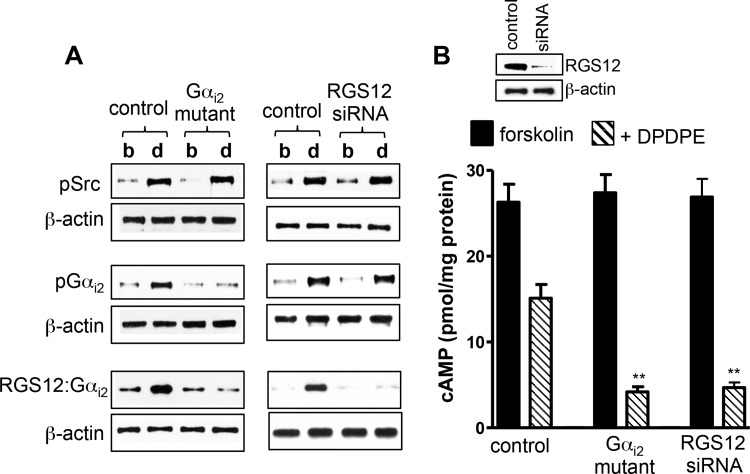
The role of tyrosine phosphorylation of Gαi2 in recruitment and activity of RGS12 was examined further by mutation of three putative tyrosine phosphorylation sites (Y69F, Y231F, and Y321F) in Gαi2 to phenylalanine. Expression of tyrosine-deficient Gαi2 mutant in cultured smooth muscle cells had no effect on cSrc activation but blocked DPDPE-induced tyrosine phosphorylation of Gαi2 and Gαi2-RGS12 association (Fig. 4A) and caused greater inhibition of forskolin-stimulated cAMP (control inhibition: 46 ± 3% vs. 91 ± 4% in cells expressing mutant Gαi2, P < 0.01, n = 5; Fig. 4B), providing evidence that tyrosine phosphorylation of Gαi2 increases Gαi2-RGS12 association and accelerates Gαi2 inactivation.
Fig. 4.
Expression of tyrosine-deficient Gαi2 mutant or RGS12 small interfering RNA (siRNA) blocks Gαi2-RGS12 association and enhances DPDPE-induced inhibition of cAMP. A: cultured smooth muscle cells transfected with control vector, vector containing Gαi2 mutant (Y69F, Y231F, or Y321F), or RGS12 siRNA were treated with 1 μM DPDPE for 1 min. Activation of cSrc (pSrc) was measured using specific phosphorylated (Tyr416) Src antibody. Gαi2 immunoprecipitates were used to measure tyrosine phosphorylation (pGαi2) using phosphotyrosine antibody and association of Gαi2 with RGS12 (RGS12-Gαi2) using RGS12 antibody. Immunoblots are representative of 4 separate experiments. b, Basal; d, DPDPE. Expression of Gαi2 mutant had no effect on cSrc activation, whereas suppression of RGS12 had no effect on cSrc activation and Gαi2 phosphorylation. B: cultured smooth muscle cells transfected with control vector, vector containing Gαi2 mutant (Y69F, Y231F, or Y321F), or RGS12 siRNA were pretreated with 10 μM forskolin for 10 min and then treated with 1 μM DPDPE for 1 min. cAMP levels were measured by radioimmunoassay. Basal levels (2.5 ± 0.25 to 2.7 ± 0.31 pmol/mg protein) of cAMP are similar in control cells and cells expressing Gαi2 mutant or RGS12 siRNA. Values (means ± SE of 5 experiments) are expressed as pmol/mg protein above basal level. **P < 0.01 compared with control.
Further evidence was obtained in smooth muscle cells expressing RGS12 siRNA. In these cells, cSrc activation and Gαi2 phosphorylation were not affected upon treatment with DPDPE, whereas Gαi2-RGS12 association was abolished (Fig. 4A) and inhibition of forskolin-stimulated cAMP was greatly enhanced (control inhibition: 47 ± 5% vs. 92 ± 3% after RGS12 knockdown, P < 0.01, n = 5; Fig. 4B), further confirming the role of RGS12 in inactivation of Gαi2.
Role of RGS12 in Gβγ-dependent modulation of Gαi1 and Gαi3 function.
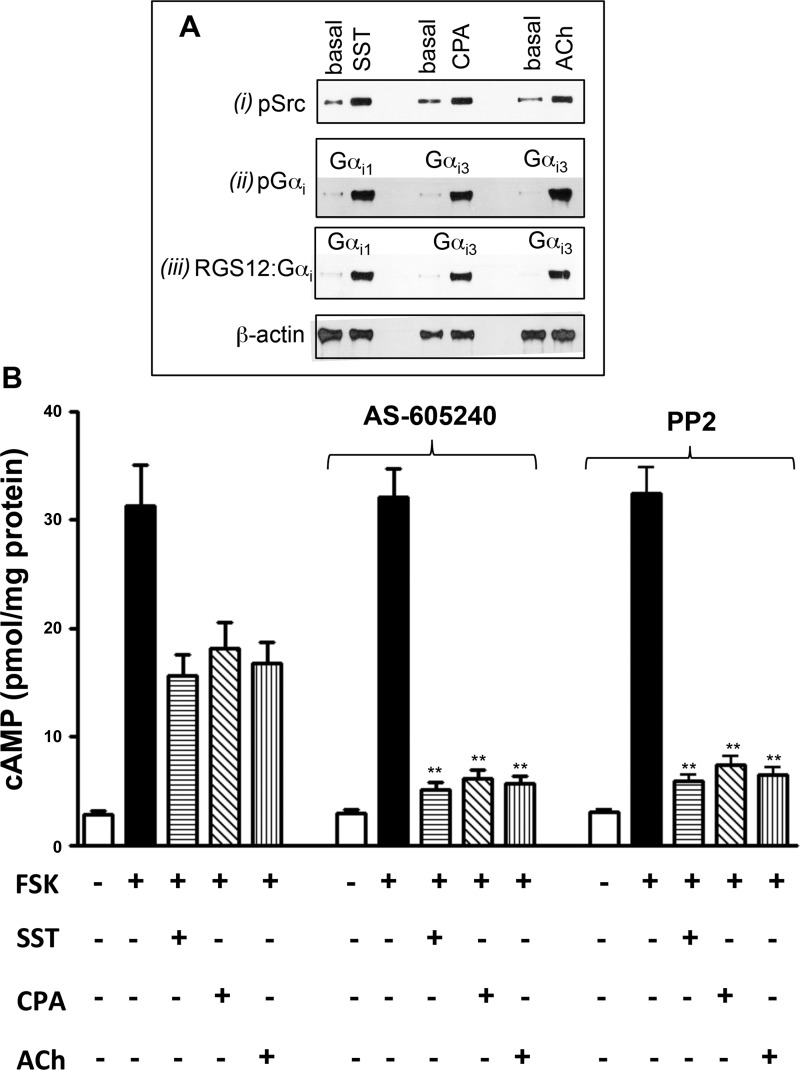
The close homology between Gαi1, Gαi2, and Gαi3 and the presence of tyrosine phosphorylation sites in similar locations in all three subunits suggested that a similar Gβγ-Gαi feedback mechanism was more widely applicable. Accordingly, we compared the effects of DPDPE with the effects of somatostatin, CPA, and ACh to examine whether the feedback regulatory mechanism applies to all Gi subunits. In the gastrointestinal system, somatostatin, δ-opioid, and CPA activate receptors coupled to Gi1, Gi2, and Gi3, respectively (21–23). ACh in the presence of the m3 receptor antagonist 4-DAMP activates m2 receptors coupled to Gi3 (24). Somatostatin induced cSrc activation, tyrosine phosphorylation of Gαi1, and Gαi1-RGS12 association, and CPA and ACh induced cSrc activation, tyrosine phosphorylation of Gαi3, and Gαi3-RGS12 association (Fig. 5A). Thus, Gβγ dimers derived from all three Gi proteins are capable of activating PI 3-kinase-γ and cSrc to induce tyrosine phosphorylation of the corresponding Gαi, recruitment of RGS12, and inhibition of Gαi activity.
Fig. 5.
A: agonist-induced cSrc activation, tyrosine phosphorylation of Gαi1 and Gαi3, and association of Gαi1 or Gαi3 with RGS12. Dispersed smooth muscle cells were treated for 1 min with somatostatin (SST, 1 μM), cyclopentyl adenosine (CPA, 1 μM), or ACh (1 μM) + 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP, 0.1 μM). Activation of cSrc (pSrc) was measured using specific phosphorylated (Tyr416) Src antibody (i). Lysates from control and agonist-treated cells were incubated with antibody to Gαi1 (SST-treated) or Gαi3 (CPA- or ACh-treated), and immunoprecipitates were probed with phosphotyrosine antibody (ii). Lysates from control and agonist-treated cells were incubated with antibody to Gαi1 (SST-treated) or Gαi3 (CPA- or ACh-treated), and immunoprecipitates were probed with RGS12 antibody (iii). Immunoblots are representative of 4 separate experiments. B: inhibition of cAMP induced by Gαi1 and Gαi3 attenuated by Gβγ-dependent activation of PI 3-kinase-γ and cSrc. Dispersed smooth muscle cells were pretreated for 10 min with control buffer containing 10 μM forskolin (FSK) or buffer containing 10 μM forskolin and inhibitors of PI 3-kinase-γ (AS-605240, 25 nM) or cSrc (PP2, 1 μM) followed by addition of somatostatin (1 μM), CPA (1 μM), or ACh (1 μM) + the m3 receptor antagonist 4-DAMP (0.1 μM). cAMP levels were measured by radioimmunassay. Values are means ± SE of 5 experiments. **P < 0.01 compared with control.
Treatment of freshly dispersed smooth muscle cells with somatostatin, CPA, or ACh inhibited forskolin-stimulated cAMP formation to the same extent as DPDPE (Fig. 5B). The inhibition of cAMP was greatly enhanced in cells pretreated with the PI 3-kinase-γ-specific inhibitor AS-605240 (20 nM) (control inhibition of 46 ± 3% to 54 ± 5% with different agonists vs. 86 ± 5% to 93 ± 3% inhibition in the presence of AS-605240, P < 0.01, n = 4) or PP2 (control inhibition of 48 ± 4% to 57 ± 4% with different agonists vs. 83 ± 5% to 90 ± 3% inhibition in presence of PP2, P < 0.01, n = 4; Fig. 5B).
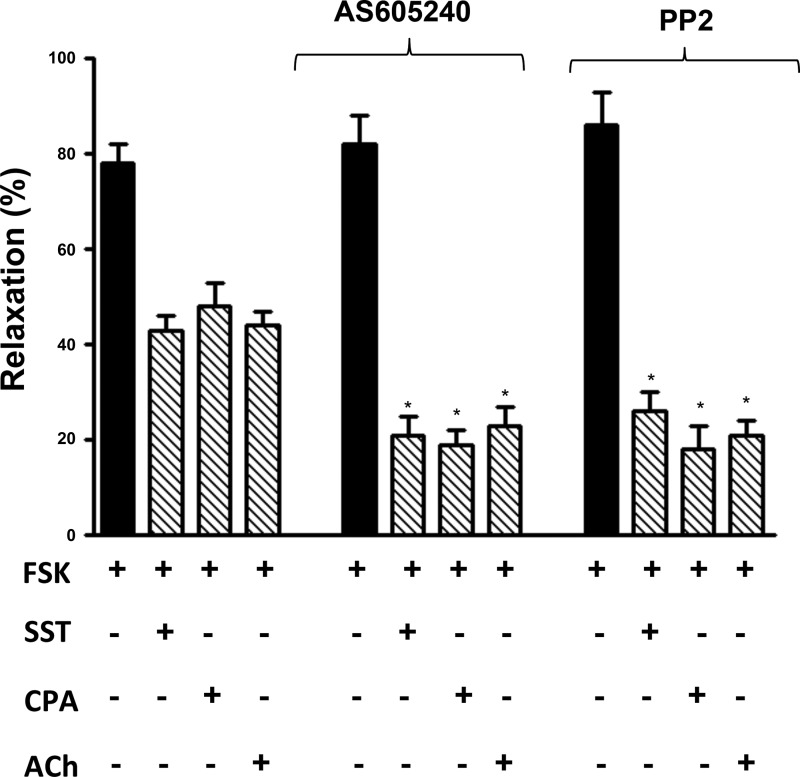
Treatment of freshly dispersed smooth muscle cells with somatostatin, CPA, or ACh inhibited forskolin-stimulated relaxation to the same extent as DPDPE (Fig. 6). The inhibition of relaxation was greatly enhanced in cells pretreated with the PI 3-kinase-γ-specific inhibitor AS-605240 (20 nM) [control inhibition of 39 ± 4% to 44 ± 3% with different agonists vs. 71 ± 4% to 77 ± 5% inhibition in the presence of AS-605240 (P < 0.01, n = 4) or 70 ± 6% to 80 ± 5% inhibition in the presence of PP2 (P < 0.01, n = 4); Fig. 6].
Fig. 6.
Gαi1- and Gαi3-induced inhibition of muscle relaxation attenuated by PI 3-kinase-γ and cSrc. Dispersed gastric smooth muscle cells from rabbit were pretreated for 10 min with control buffer containing 10 μM forskolin in the presence or absence of inhibitors of PI 3-kinase-γ (AS-605240, 25 nM) or cSrc (PP2, 1 μM) followed by addition of somatostatin (1 μM), CPA (1 μM), or ACh (1 μM) + the m3 receptor antagonist 4-DAMP (0.1 μM) for 1 min and KCl for 30 s. Somatostatin and CPA were added in the presence of U73122 (1 μM) to block hydrolysis of phosphoinositides by Gβγ-dependent PLCβ3. Contraction in response to KCl was measured as decrease in cell length by scanning micrometry. Relaxation was measured as decrease in contraction in response to KCl in the presence of forskolin and expressed as percent inhibition of contraction. Contraction in response to KCl was 33 ± 4% decrease in cell length from control cell length of 98 ± 4 μm. Values are means ± SE of 5 experiments. *P < 0.05 compared with control.
DISCUSSION
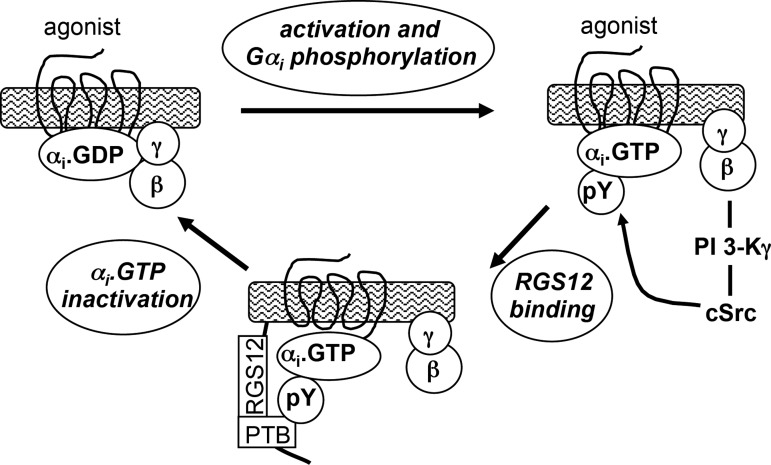
The role of Gβγ heterodimers in G protein-receptor coupling and guanine nucleotide exchange is now well established, as is their role in termination of the cycle and reassociation of the subunits into inactive Gαβγ heterotrimers. The present study in smooth muscle identifies a distinct role for Gβγ in inactivation of Gαi signaling. The extent and duration of Gαi activity were shown to depend on signals emanating from Gβγ to downstream effectors. Gβγ sequentially activated PI 3-kinase-γ and cSrc, leading to tyrosine phosphorylation of Gαi, recruitment of RGS12, and rapid inactivation of Gαi (Fig. 7). Detailed evidence obtained for Gαi2-coupled δ-opioid receptors can be summarized as follows. 1) DPDPE activated cSrc, stimulated tyrosine phosphorylation of Gαi2, and induced Gαi2-RGS12 association; all three events were blocked by PI 3-kinase and cSrc inhibitors and by expression of a Gβγ-scavenging peptide (COOH-terminal sequence of GRK2). 2) Inhibition of forskolin-stimulated cAMP formation by DPDPE was augmented by PI 3-kinase-γ and cSrc inhibitors and by expression of Gβγ-scavenging peptide. 3) Inhibition of forskolin-stimulated muscle relaxation by DPDPE was augmented by PI 3-kinase-γ and cSrc inhibitors. 4) Expression of tyrosine-deficient Gαi2 mutant or knockdown of RGS12 blocked Gαi2 phosphorylation and Gαi2-RGS12 association and caused greater inhibition of cAMP formation. 5) Inhibition of cAMP formation and muscle relaxation by DPDPE was also augmented in mouse and human gastric muscle cells. 6) Expression of PI 3-kinase-γ was demonstrated in rabbit, mouse, and human gastric muscle cells.
Fig. 7.
Model depicting pathway for Gβγ-dependent regulation of Gαi function mediated via sequential activation of PI 3-kinase (PI 3 K)-γ and cSrc, leading to tyrosine phosphorylation of Gαi, recruitment of RGS12, and inactivation of Gαi2. PTB, phosphotyrosine binding.
Corroborating evidence was obtained for Gαi1-coupled somatostatin sstr3 receptors and Gαi3-coupled adenosine A1 and muscarinic m2 receptors (21, 22). This was not unexpected in view of the close homology between Gαi1, Gαi2, and Gαi3 and the presence of tyrosine phosphorylation sites in locations similar to those mutated in Gαi2. The corresponding agonists, somatostatin, CPA, and ACh (the latter in the presence of the selective m3 receptor antagonist 4-DAMP), induced Gαi1-RGS12 or Gαi3-RGS12 association and inhibited forskolin-induced cAMP formation and muscle relaxation; inhibition of cAMP formation and muscle relaxation was increased by the PI 3-kinase-γ-selective inhibitor AS-605240 and by PP2. The effectiveness of AS-605240 underscored the fact that PI 3-kinase-γ, which consists of p110γ catalytic and p101 noncatalytic regulatory subunits, is the singular class 1B member of the PI 3-kinase family activated by Gβγ (2). PI 3-kinase-γ is predominantly expressed in cells of the hematopoietic system, but it is also found in other tissues (1, 3), including gastrointestinal, airway, and vascular smooth muscle (8, 21, 31, 33). PI 3-kinase has been identified as an important regulator of vascular myogenic tone and blood pressure (3, 14).
Previous studies in smooth muscle showed that Gβγ subunits derived from Gi1, Gi2, or Gi3 activate PLCβ3 and PI 3-kinase (8, 20–23). PLCβ3 initiates a signaling cascade that leads to transient activation of a Ca2+-dependent MLC kinase and stimulation of MLC20 phosphorylation and smooth muscle contraction (21–23). PI 3-kinase initiates a distinct signaling cascade that leads to Ca2+-independent, ILK-mediated inhibition of MLC phosphatase and sustained stimulation of MLC20 phosphorylation and smooth muscle contraction (5, 8). When initiated by muscarinic m2 receptors, however, the same cascades are abrogated by a parallel cascade mediated by PI 3-kinase involving sequential activation of Rac1/cdc42, PAK1, and p38 MAP kinase (39). As shown previously (24), PAK1 inactivates Ca2+-dependent MLC kinase, whereas p38 MAP inactivates ILK (8); as a result, m2 receptors are uncoupled from MLC20 phosphorylation and smooth muscle contraction. The present study confirms the existence of a parallel pathway involving sequential activation of PI 3-kinase-γ and cSrc that leads to inactivation of Gαi.
From a physiological point of view, muscle relaxation in the gastrointestinal tract can be regulated by various endogenous agonists, such as vasoactive intestinal peptide and pituitary adenylate cyclase-activating peptide and peptide histidine isoleucine (19). These agonist-specific receptors coupled to Gs, activation of adenylyl cyclase, and cAMP formation (19). Concomitant activation of receptors coupled to Gαi by endogenous agonists such as adenosine, opioid peptides, and somatostatin inhibit cAMP formation. Rapid termination of Gαi signaling by the feedback mechanism contributes to enhance the function of cAMP by minimizing the inhibition of cAMP formation and muscle relaxation. Occurrence of this feedback inhibitory pathway with receptors coupled to Gαi1, Gαi2, or Gαi3 and in different species underscores the importance of this mechanism in the regulation of muscle function by neurotransmitters in the gastrointestinal tract.
The significance of the mechanism identified in this study depends on the convergence and interaction of four signaling proteins: Gβγ derived from Gi, PI 3-kinase-γ, cSrc, and RGS12. The multidomain RGS12 contains a NH2-terminal PDZ domain, dual Ras-binding domains, a COOH-terminal GoLoco motif, and a unique PTB domain; together, these domains endow RGS12 with the ability to interact with a variety of proteins (30). The RGS domain and the GoLoco motif display high affinity for Gαi-GTP and Gαi-GDP, respectively, while the PTB domain interacts with many, but not all, target proteins, with a canonical Asn-Pro-X-(p)Tyr binding motif (13, 27). RGS12 association with Gαi1, Gαi2, and Gαi3 was evident in our study, even though none of the putative tyrosine phosphorylation sites in Gi displayed a canonical PTB motif.
Unlike Gi, cSrc, and RGS12, which are widely expressed, PI 3-kinase-γ, as noted above, appears to exhibit a more limited tissue distribution, which would limit a wider applicability of the mechanism described in this study. Furthermore, consideration should be given to the issue of differential sensitivity of PI 3-kinase-γ to isoforms of Gβγ. Gβ1–4 exhibit close homology, sharing 78–88% amino acid identity, and are widely expressed or coexpressed in various tissues, whereas Gγ1–13 subunits are more diverse in sequence and tissue expression (6, 12, 26). Some Gγ subunits are restricted to the retina (Gγ7,9), others are preferentially expressed in the brain (Gγ2,3,4,7) and olfactory and taste receptor cells (Gγ8,13), while others (Gγ5,10,11,12) are more widely expressed in various tissues (28). In in vitro systems, all four Gβ subunits bound to 9 of 12 Gγ subunits and activate PI 3-kinase-γ with varying potencies (11). Kerchner and co-workers (11) used pure recombinant G protein subunits to examine the ability of various Gβγ combinations to activate PI 3-kinase-γ. When bound to Gγ2, all Gβ subunits, except Gβ5, were equally potent activators of PI 3-kinase-γ. The effectiveness of Gγ isoforms was traced to the isoprenyl group associated with this subunit. Gγ subunits modified by a geranylgeranyl group at their COOH terminus were highly effective, whereas Gγ1,8,11, which are modified by a farnesyl group, were not. Substitution of one prenyl group for the other reversed the ability of Gβγ to activate PI 3-kinase-γ. These findings suggest that the composition of the Gβγ complex and its affinity for PI 3-kinase-γ may be of critical importance for the operation of the feedback mechanism involving Gβγ-mediated regulation of Gαi activity.
In summary, we have identified a novel feedback mechanism for the termination of Gαi signaling in smooth muscle cells. We have provided compelling evidence that Gβγ-dependent activation of the PI 3-kinase-γ-cSrc pathway and tyrosine phosphorylation of Gαi leads to recruitment of GTPase-activating protein RGS12 and inactivation of Gαi. This regulating mechanism attenuates the inhibition of cAMP formation and facilitates muscle relaxation.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-28300 and DK-15564 to K. S. Murthy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.H., A.D.N., S.M., and D.P.K. performed the experiments; J.H., A.D.N., S.M., and K.S.M. analyzed the data; J.H. and A.D.N. interpreted the results of the experiments; J.H. and A.D.N. drafted the manuscript; J.H., A.D.N., S.M., D.P.K., and K.S.M. approved the final version of the manuscript; A.D.N., S.M., D.P.K., and K.S.M. are responsible for conception and design of the research; D.P.K. prepared the figures; K.S.M. edited and revised the manuscript.
REFERENCES
- 1.Bernstein HG, Keilhoff G, Reiser M, Freese S, Wetzker R. Tissue distribution and subcellular localization of a G-protein activated phosphoinositide 3-kinase. An immunohistochemical study. Cell Mol Biol (Noisy-le-grand) 44: 973–983, 1998 [PubMed] [Google Scholar]
- 2.Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, Schultz G, Nürnberg B. Roles of Gβγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase-γ. J Cell Biol 60: 89–99, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carnevale D, Lembo G. PI3Kγ in hypertension: a novel therapeutic target controlling vascular myogenic tone and target organ damage. Cardiovasc Res 95: 403–408, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 390: 88–91, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Deng JT, Van Lierop JE, Sutherland C, Walsh MP. Ca2+-independent smooth muscle contraction: a novel function of integrin-linked kinase. J Biol Chem 276: 16365–16373, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Gautam N, Downes GB, Yan K, Kisselev O. The G-protein βγ complex. Cell Signal 10: 447–455, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Hamm HE. The many faces of G protein signaling. J Biol Chem 273: 669–672, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Mahavadi S, Sriwai W, Hu W, Murthy KS. Gi-coupled receptors mediate phosphorylation of CPI-17 and MLC20 via preferential activation of the PI3K/ILK pathway. Biochem J 396: 193–200, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Abel PW, Toews ML, Deng C, Casale TB, Xie Y, Tu Y. Phosphoinositide 3-kinase-γ regulates airway smooth muscle contraction by modulating calcium oscillations. J Pharmacol Exp Ther 334: 703–709, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston CA, Siderovski DP. Receptor-mediated activation of heterotrimeric G-proteins: current structural insights. Mol Pharmacol 72: 219–230, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Kerchner KR, Clay RL, McCleery G, Watson N, McIntire WE, Myung CS, Garrison JC. Differential sensitivity of phosphatidylinositol kinase p110γ to isoforms of G protein βγ dimers. J Biol Chem 279: 44554–44562, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbé JC, Miller GJ, Hébert TE. The expanding roles of Gβγ subunits in G protein-coupled receptor signaling and drug action. Pharmacol Rev 65: 545–577, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Kimple RJ, De Vries L, Tronchère H, Behe CI, Morris RA, Gist Farquhar M, Siderovski DP. RGS12 and RGS14 GoLoco motifs are Gαi interaction sites with guanine nucleotide dissociation inhibitor activity. J Biol Chem 276: 29275–29281, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates Gβγ-mediated signaling. J Biol Chem 269: 6193–6197, 1994 [PubMed] [Google Scholar]
- 15.Lambright DG, Noel JP, Hamm HE, Sigler PB. Structural determinants for activation of the α-subunit of a heterotrimeric G protein. Nature 369: 621–628, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Mahavadi S, Sriwai W, Huang J, Grider JR, Murthy KS. Inhibitory signaling by CB1 receptors in smooth muscle mediated by GRK5/β-arrestin activation of ERK1/2 and Src. Am J Physiol Gastrointest Liver Physiol 306: G535–G545, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morello F, Perino A, Hirsch E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc Res 82: 261–271, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Murthy KS. Inhibitory phosphorylation of soluble guanylyl cyclase by muscarinic m2 receptors via Gβγ-dependent activation of c-Src kinase. J Pharmacol Exp Ther 325: 183–189, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Murthy KS. Inhibitory phosphorylation of soluble guanylyl cyclase by muscarinic m2 receptors via Gβγ-dependent activation of c-Src kinase. J Pharmacol Exp Ther 325: 183–189, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Murthy KS, Coy DH, Makhlouf GM. Somatostatin receptor-mediated signaling in smooth muscle. Activation of phospholipase C-β3 by Gβγ and inhibition of adenylyl cyclase by Gαi1 and Gαo. J Biol Chem 271: 23458–23463, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Murthy KS, Makhlouf GM. Adenosine A1 receptor-mediated activation of phospholipase C-β3 in intestinal muscle: dual requirement for α and βγ subunits of Gi3. Mol Pharmacol 47: 1172–1179, 1995 [PubMed] [Google Scholar]
- 23.Murthy KS, Makhlouf GM. Opioid-μ, -δ, and -κ receptor-induced activation of phospholipase C-β3 and inhibition of adenylyl cyclase is mediated by Gi2 and Go in smooth muscle. Mol Pharmacol 50: 870–877, 1996 [PubMed] [Google Scholar]
- 24.Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit 1 and protein kinase C/CPI-17 pathway. Biochem J 374: 145–155, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nino G, Hu A, Grunstein JS, McDonough J, Kreiger PA, Josephson MB, Choi JK, Grunstein MM. G protein βγ-subunit signaling mediated airway hyperresponsiveness and inflammation in allergic asthma. PLos One 7: e32078, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poon LS, Chan AS, Wong YH. Gβ3 forms distinct dimers with specific Gγ subunits and preferentially activates the β3 isoform of phospholipase C. Cell Signal 21: 737–744, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Richman RW, Strock J, Hains MD, Cabanilla NJ, Lau KK, Siderovski DP, Diversé-Pierluissi M. RGS12 interacts with the SNARE-binding region of the Cav2.2 calcium channel. J Biol Chem 280: 1521–1528, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Schwindinger WF, Robishaw JD. Heterotrimeric G-protein βγ dimers in growth and differentiation. Oncogene 20: 1653–1660, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Siderovski DP, Hessel A, Chung S, Mak TW, Tyers M. A new family of regulators of G-protein-coupled receptors. Curr Biol 6: 211–222, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heteromeric G-protein α subunits. Int J Biol Sci 1: 51–66, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singer CA, Vang S, Gerthoffer WT. Coupling of M2 muscarinic receptors to Src activation in cultured canine colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 282: G61–G68, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Smrcka AV. G protein βγ subunits: central mediators of G protein coupled receptor signaling. Cell Mol Life Sci 65: 2191–2214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teng B, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 275: G342–G351, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Van Biesen T, Hawes BE, Luttrell DK, Krueger KM, Touhara K, Porfiri E, Sakaue M, Luttrell LM, Lefkowitz RJ. Receptor-tyrosine-kinase- and Gβγ-mediated MAP kinase activation by a common signalling pathway. Nature 376: 781–784, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Wang YX, Dhulipala PD, Li L, Benovic JL, Kotlikoff MI. Coupling of M2 muscarinic receptors to membrane ion channels via phosphoinositide 3-kinase-γ and atypical protein kinase C. J Biol Chem 274: 13859–13864, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Watson N, Linder ME, Druey KM, Kehrl JH, Blumer KJ. RGS family members: GTPase-activating proteins for heterotrimeric G-protein α-subunits. Nature 383: 172–175, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Yamboliev IA, Wiesmann KM, Singer CA, Hedges JC, Gerthoffer WT. Phosphatidylinositol 3-kinases regulate ERK and p38 MAP kinases in canine colonic smooth muscle. Am J Physiol Cell Physiol 279: C352–C360, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Zheng M, Zhang SJ, Zhu WZ, Ziman B, Kobilka BK, Xiao RP. β2-Adrenergic receptor-induced p38 MAPK activation is mediated by protein kinase A rather than by Gi or Gβγ in adult mouse cardiomyocytes. J Biol Chem 275: 40635–40640, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Zhou H, Das S, Murthy KS. Erk1/2- and p38 MAP kinase-dependent phosphorylation and activation of cPLA2 by m2 and m2 receptors. Am J Physiol Gastrointest Liver Physiol 284: G472–G480, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Zhou H, Murthy KS. Identification of the G protein-activating sequence of the single-transmembrane natriuretic peptide receptor C (NPR-C). Am J Physiol Cell Physiol 284: C1255–C1261, 2003 [DOI] [PubMed] [Google Scholar]