Abstract
Substance P (SP) promotes cholangiocyte growth during cholestasis by activating its receptor, NK1R. SP is a proteolytic product of tachykinin (Tac1) and is deactivated by membrane metalloendopeptidase (MME). This study aimed to evaluate the functional role of SP in the regulation of cholangiocarcinoma (CCA) growth. NK1R, Tac1, and MME expression and SP secretion were assessed in human CCA cells and nonmalignant cholangiocytes. The proliferative effects of SP (in the absence/presence of the NK1R inhibitor, L-733,060) and of L-733,060 were evaluated. In vivo, the effect of L-733,060 treatment or MME overexpression on tumor growth was evaluated by using a xenograft model of CCA in nu/nu nude mice. The expression of Tac1, MME, NK1R, PCNA, CK-19, and VEGF-A was analyzed in the resulting tumors. Human CCA cell lines had increased expression of Tac1 and NK1R, along with reduced levels of MME compared with nonmalignant cholangiocytes, resulting in a subsequent increase in SP secretion. SP treatment increased CCA cell proliferation in vitro, which was blocked by L-733,060. Treatment with L-733,060 alone inhibited CCA proliferation in vitro and in vivo. Xenograft tumors derived from MME-overexpressed human Mz-ChA-1 CCA cells had a slower growth rate than those derived from control cells. Expression of PCNA, CK-19, and VEGF-A decreased, whereas MME expression increased in the xenograft tumors treated with L-733,060 or MME-overexpressed xenograft tumors compared with controls. The study suggests that SP secreted by CCA promotes CCA growth via autocrine pathway. Blockade of SP secretion and NK1R signaling may be important for the management of CCA.
Keywords: autocrine, biliary cancer, neuroendocrine, sensory innervation, VEGF
cholangiocytes, which line the intrahepatic and extrahepatic bile ducts (28), are the target of chronic cholestatic liver diseases (i.e., cholangiopathies) that are characterized by biliary inflammation and dysregulation of the balance between cell proliferation and apoptosis (8). Cholangiocarcinoma (CCA) results from the malignant transformation of cholangiocytes (33). The pathogenesis of CCA is linked to chronic biliary inflammation, which occurs in cholangiopathies such as primary sclerosing cholangitis (8, 36). CCA is frequently diagnosed at advanced stages, resulting in limited treatment options and a high mortality (33). In addition, CCA responds poorly to both surgical resection and standard chemotherapy (33). With limited treatment strategies and reports of increasing incidence and prevalence of CCA worldwide (40), it is critical to gain a better understanding of the factors regulating cholangiocyte growth and neoplastic transformation.
Recent studies have shown that proliferating cholangiocytes can serve as a neuroendocrine compartment during the progression of liver diseases by secreting and responding to a number of hormones and neuropeptides (such as serotonin, melatonin, and histamine), thereby contributing to the autocrine and paracrine pathways modulating liver inflammation and fibrosis (3, 14, 22). The majority of intrinsic hepatic nerves are associated with the vascular and biliary systems (43, 51). Two afferent nerve pathways have been identified in the liver: the vagal afferent nerve pathway and the spinal afferent nerve pathway (dorsal root ganglion) (51). Sensory nerves also possess efferent functions that are mediated by the release of sensory neuropeptides [i.e., calcitonin gene-related peptide (CGRP) and substance P (SP)] from their peripheral terminals in tissues they innervate, thereby regulating cellular functions independent of sensation (24).
We have previously shown that knockout of α-CGRP and neurokinin-1 receptor (NK1R) reduces biliary hyperplasia in mice with extrahepatic cholestasis (19, 20). SP is a member of the tachykinin peptide family, which is composed of six members to date: SP, neurokinin A, neurokinin B, neuropeptide K, neuropeptide γ, and hemokinin (39). SP is released from sensory nerve endings at the level of both the spinal cord and peripheral tissues (1). SP is also expressed by nonneuronal cells such as human endothelial cells, immune, and inflammatory cells (35). The tachykinin receptors family consists of three types of G protein-coupled receptors: NK1, NK2, and NK3. SP preferentially binds and signals through the NK1 receptor (1). The NK1 receptor is expressed in the central and peripheral nervous system and is also widely distributed in a number of cell types, including vascular endothelial and immune cells, contributing to the connections between the nervous system and peripheral organ system (35, 39). Previous research in our team supports these extrasensory functions as the knockout of α-CGRP and NK1R reduce biliary hyperplasia in mice with extrahepatic cholestasis, giving support that CGRP and SP may have the capability to induce biliary hyperplasia (19, 20).
SP synthesis is regulated by the expression of tachykinin (Tac1, the gene encoding SP) and membrane metalloendopeptidase (MME), the enzymes responsible for the synthesis and degradation of SP, respectively (11, 47). No information exists regarding the autocrine and paracrine role of SP in the regulation of CCA growth.
Given the observation that CCAs display neuroendocrine phenotypes (2), the aims of our study were to demonstrate that 1) CCA and nonmalignant biliary epithelial cells express NK1R; 2) SP stimulates CCA growth by a receptor-mediated mechanism in vitro; 3) there is enhanced expression of Tac1 and reduced expression of MME in CCA, leading to increased synthesis of SP and augmented CCA growth; and 4) reduction of CCA SP synthesis (by molecular stable silencing) and interaction of SP with NK1R (by NK1R antagonists) reduces CCA growth both in vitro and in xenograft tumor models in vivo.
MATERIALS AND METHODS
Materials.
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Synthetic SP (SZ1062) and the SP EIA kit (EZ-061-05, recognizing human, rat, and mouse species) were purchased from Phoenix Pharmaceuticals (Belmont, CA). The rabbit polyclonal antibody (NA4300-0100) and the blocking peptide (neurokinin 1 receptor rat COOH-terminal pentadecapeptide, as negative control, NP1442-0100) for NK1R were purchased from Biomol International (Plymouth Meeting, PA). The NK1R antagonist (L-733,060) (45) was purchased from Sigma-Aldrich. The mouse monoclonal antibody (ab34199) to CD10 was purchased from Abcam (Cambridge, MA). CD10 (also known as the common acute lymphocytic leukemia antigen, CALLA) is a cell surface enzyme with MME activity that inactivates a variety of biologically active peptides including SP (6).
The selected specific primers were purchased from SABiosciences (Qiagen; Valencia, CA) and designed by using sequences with the following NCBI GenBank Accession numbers: NM_001058 (human NK1R); NM_013998 (human Tac1); NM_000902 (human MME); NM_182649 (human PCNA); NM_002276 (human CK-19); NM_003376 (human VEGF-A); and NM_002046 (human glyceraldehyde-3-phosphate dehydrogenase, GAPDH).
Human CCA cell lines and nonmalignant cholangiocytes.
We used six human CCA cell lines (Mz-ChA-1, SG231, HuCC-T1, TFK-1, HuH-28, and CCLP1) of different origins and the normal human intrahepatic biliary epithelial cell line, HIBEpiC (ScienCell Research Laboratories, Carlsbad, CA) (14). Mz-ChA-1 cells (from human gallbladder) (30) were a gift from Dr. G. Fitz (University of Texas Southwestern Medical Center, Dallas, TX). HuH-28 and TFK-1 cells (from human extrahepatic bile ducts) (32, 42) were acquired from Cancer Cell Repository (Tohoku University, Sendai, Japan). These cell lines were maintained at standard conditions (27). HuCC-T1, CCLP1, and SG231 cells (from intrahepatic bile ducts) were a kind gift from Dr. A. J. Demetris (University of Pittsburgh, Pittsburgh, PA) and were cultured as described (53). HIBEpiC cells were cultured as recommended by the supplier (14).
Expression of NK1R in cell lines.
The expression of NK1R was evaluated by immunofluorescence (14), real-time PCR (using 1 μg total RNA) (14), immunoblots (using 10 μg of protein from whole cell lysate) (14) and FACS analysis (22) in the CCA cell lines and HIBEpiC cells.
Immunofluorescence for NK1R in the selected cell lines was performed as described (14). Following staining, cells were mounted onto microscope slides with Prolong Antifade Gold containing 4′,6-diamidino-2-phenylindole (DAPI) as a nuclear counterstain (Molecular Probes, Eugene, OR). Negative controls were also included. Sections were visualized via an Olympus IX-71 inverted confocal microscope (Tokyo, Japan).
RNA was isolated from the selected cell lines by the RNeasy Kit (Qiagen) and reverse transcribed with the Reaction Ready First Strand cDNA synthesis kit (SABiosciences, Frederick, MD). PCR reactions were used as templates for the PCR assays by using a SYBR Green PCR master mix and specific primers designed against the selected gene analyzed in the real-time thermal cycler (Agilent MX3005P thermocycler, Santa Clara, CA). A ΔΔCT (delta delta of the threshold cycle) analysis was performed using HIBEpiC cells as controls (14). Data are expressed as the fold-change of relative mRNA levels ± SE. Immunoblots were normalized by α-tubulin (the housekeeping gene). Band intensity was determined by scanning video densitometry via the phospho-imager, Storm 860 (GE Healthcare, Piscataway, NJ), and the ImageQuant TL software version 2003.02 (GE Healthcare).
FACS analysis (with 20,000 events in the light scatter, SSC/SSC, acquired) for NK1R was performed in the selected cell lines by using a C6 flow cytometer and analyzed by CFlow Software (Accuri Cytometers, Ann Arbor, MI) (22). The expression of NK1R was identified and gated on FL1-A/Count plots. The relative quantity of NK1R (mean selected protein fluorescence) was expressed as mean FL1-A (samples)/mean FL1-A (secondary antibodies only). The standard errors were calculated as (CV FL1-A) × (Mean FL1-A)/SQR(Count-1), where FL1-A is green fluorescence channel, Count is number of cells examined, CV is standard deviation, SQR is square root, and Count-1 is number of events counted as cholangiocytes (gate 1; we counted 5,000 events under gate 1 in each group to keep consistency in all groups).
Expression of Tac1 and MME and secretion of SP in CCA and normal cholangiocyte lines.
We evaluated the expression of 1) the message (by real-time PCR, see above) (14); and 2) the protein for tachykinin (Tac1, the gene encoding SP) (11) and MME in CCA cell lines and HIBEpiC cells by FACS analysis, see above (41).
CCA and HIBEpiC cells (1 × 107 cells/ml) were incubated for 24 h at 37°C and the amount of SP released into the media was assayed by using a commercially available EIA kit according to the manufacturer's instructions.
Effect of SP on the proliferation of nonmalignant and CCA cells.
The proliferation of CCA cells and HIBEpiC cells was evaluated by MTS assays (14). Data were expressed as fold change of proliferation vs. basal. Briefly, cells were plated in a 96-well plate (7,000 cells per well) and incubated overnight at 37°C. Medium was changed to serum free and cells were further incubated for 24 h prior to stimulation. The selected CCA cell lines were stimulated with different doses (10−6 M to 10−12 M for 48 h) of vehicle or SP before measuring cell growth by MTS assays (14). We also measured the effect of SP (10−11 M for 48 h) on the proliferation of Mz-ChA-1 cells (used in the in vivo experiments) in the absence/presence of L-733,060 (25 μM) (37). In parallel, we evaluated the effect of L-733,060 treatment alone (0, 5, 10, 25, 50, and 100 μM, for 48 h) (37) on the proliferation of CCA cells by MTS assays (14). We also evaluated, by FACS analysis, the effect of L-733,060 (stimulation at 25 μM for 24 and 48 h) on the cell cycle of HIBEpic and Mz-ChA-1 cells (25).
We used human cDNA clone (OriGene Technologies, Rockville, MD) with neomycin resistance to generate and select the stable transfected Mz-ChA-1 cells overexpressing MME (5, 14). Real-time PCR (14) was performed to determine the degree of MME overexpression in Mz-ChA-1 cells. We also evaluated the secretion of SP (by EIA kits) in Mz-MME and Mz-neg control stably transfected Mz-ChA-1 cell lines. In the two cell lines, we evaluated cell proliferation by MTS assays (after incubation for 24, 48, 72, and 7 days) (14).
In vivo studies in nu/nu nude mice.
Eight-week-old male Balb/c nu/nu nude mice (∼30 g; Charles River, Wilmington, MA) were kept in a temperature-controlled environment (20–22°C) with 12-h light-dark cycles with free access to drinking water and standard chow. All the animal experiments were conducted under protocols approved by the Scott & White and Texas A&M HSC IACUC.
Mz-ChA-1 cells (3 × 106) were injected subcutaneously into the flanks of nu/nu nude mice (14). Treatments (by intraperitoneal injections) were performed as follows: 1) mice (n = 4) received 0.9% NaCl (100 μl); and 2) mice (n = 4) were treated with L-733,060 (10 mg/kg ip in 100 μl of NaCl) (4) every other day (4). Injections were performed every other day for 52 days after tumor establishment (day 10). After 52 days, mice were anesthetized with pentobarbital sodium (50 mg/kg ip) and tissues were harvested and used for measuring the selected parameter.
In separate experiments, Mz-MME (cells stably overexpressing MME) and Mz-neg were cultured and prepared for injection into the flanks of nu/nu nude mice as described (14). Tumor measurements began after tumor establishment (day 10) (14). After 21 days, both groups of mice were anesthetized with pentobarbital sodium (50 mg/kg body wt ip) and tissues were harvested.
Tumor parameters were measured twice a week with an electronic caliper and volume was determined as follows: tumor volume (mm3) = 0.5 × [length (mm) × width (mm) × height (mm)]. The tumors harvested were used for measuring the expression of Tac1, MME, NK1R, PCNA, CK-19, and VEGF-A (an important trophic factor for CCA growth) (10) in total tumor samples by real-time PCR (14).
Statistical analysis.
All data are expressed as mean ± SE. Differences between groups were analyzed by the Student's unpaired t-test when two groups were analyzed and by ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test. A value of P < 0.05 was considered significant.
RESULTS
Expression of NK1R, Tac1, and MME in biliary cell lines and secretion of SP in biliary cell lines.
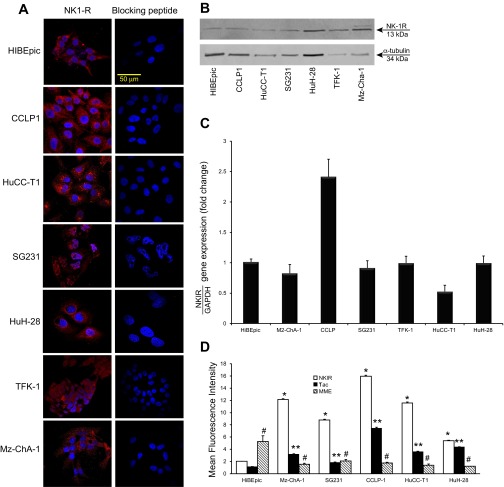
By immunofluorescence, all the CCA cell lines used and HIBEpiC cells showed immunoreactivity for NK1R (red); negative controls with the use of the blocking peptide for NK1R showed no reaction (Fig. 1A). By real-time PCR, immunoblots, and FACS analysis, the expression of NK1R was observed in nonmalignant cholangiocytes as well as in all the CCA cell lines used (Fig. 1, B–D). By FACS analysis, we demonstrated that the expression of NK1R increased in all the CCA cells lines compared with HIBEpiC cells (Fig. 1D).
Fig. 1.
A: by immunofluorescence, HIBEpiC and cholagiocarcinoma (CCA) cell lines show immunoreactivity for NK1R. Negative controls using the blocking peptide for NK1R showed no reaction. Bar = 50 μm. B and C: evaluation of the expression of NK1R in CCA and nonmalignant cell lines by real-time PCR and immunoblots. The expression of NK1R was observed in nonmalignant cholangiocytes as well as in all the CCA cell lines used. Data are mean ± SE of 4 experiments. D: evaluation of the expression of NK1R, Tac1, and membrane metalloendopeptidase (MME) in CCA and nonmalignant cell lines by FACS analysis. The expression of NK1R and Tac1 increased, whereas the expression of MME decreased in CCA cell lines compared with HIBEpiC cells. Data are mean ± SE of 4 experiments. *P < 0.05 vs. HIBEpiC cells; **P < 0.05 vs. corresponding NK1R evaluations; #P < 0.05 vs. corresponding NK1R and Tac evaluations.
By real-time PCR, there was increased expression of Tac1 (the gene encoding SP) (11) and decreased expression of MME mRNA in selected CCA cells used compared with HIBEpic cells (Fig. 2, A and B). By FACS analysis, the protein expression of Tac1 increased, whereas the expression of MME decreased in all CCA cells used compared with HIBEpic (Fig. 1D). Furthermore, all of the CCA cell lines secreted higher levels of SP compared with HIBEpiC cells (Fig. 2C).
Fig. 2.
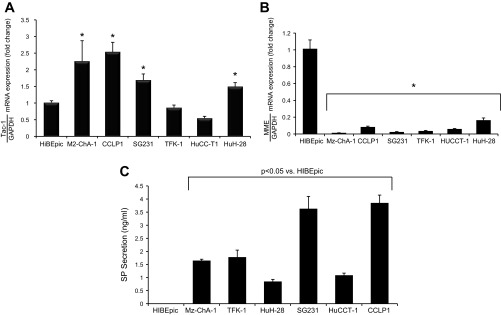
A and B: by real-time PCR, there was an increase in the expression of Tac1 and decreased expression of MME mRNA in most of the cholangiocarcinoma (CCA) used compared with HIBEpiC cells. Data are mean ± SE of 3 experiments. *P < 0.05 vs. HIBEpiC cells. C: CCA cell lines secreted higher levels of substance P (SP) compared with HIBEpiC cells. Data are mean ± SE of 6 experiments.
SP stimulates the proliferation of CCA cells.
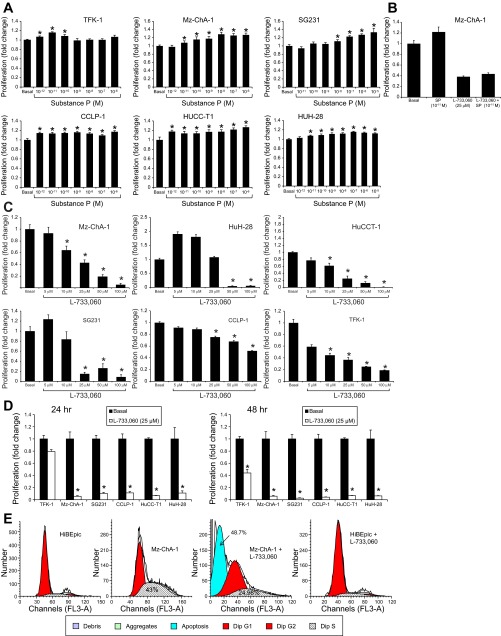
SP (at the doses ranging from 10−6 M to 10−12 M for 48 h) increased the proliferation of CCA cell lines compared with their basal values (Fig. 3A). However, no significant effects for SP were observed in nonmalignant cholangiocytes (data not shown). The stimulatory effect of SP (at 10−11 M at 48 h) on Mz-ChA-1 growth was blocked by the NK1R antagonist, L-733,060 (25 μM) (Fig. 3B); at the same dose L-733,060 inhibited Mz-ChA-1 growth (Fig. 3B). When CCA cell lines were treated with L-733,060 (at 5, 10, 25, 50, and 100 μM) there was a dose-dependent decrease in cell proliferation compared with the relative basal values (Fig. 3C). At the dose of 25 μM, L-733,060 induced a significant decrease in the proliferation of selected CCA cell lines following incubation for 24 and 48 h (Fig. 3D). By FACS analysis, we also show that L-733,060 decreased the proliferative activity of Mz-ChA-1 but not HIBEpiC cells (Fig. 3E). The S phase of Mz-ChA-1 cells (treated with vehicle, basal value) displayed higher proliferation than HIBEpiC cells due to the high mitosis (Fig. 3E). L-733,060 induced arrest of the G0/G1 phase, and decreased the S phase of Mz-ChA-1 cells, indicating disruption of cell cycle progression in Mz-ChA-1 cells through inhibition of SP signaling; L-733,060 induced a significant increase in apoptosis in Mz-ChA-1 but not in HIBEpiC cells (Fig. 3E).
Fig. 3.
A: SP (10−6 M to 10−12 M for 48 h) increases the proliferation of CCA lines compared with their basal values. Data are mean ± SE of 4 experiments. *P < 0.05 vs. basal values. B: the stimulatory effect of SP (at 10−11 M) on CCA growth was blocked by L-733,060 (25 μM); at the same dose L-733,060 inhibited Mz-ChA-1 growth. Data are mean ± SE of 4 experiments. *P < 0.05 vs. nonmalignant controls. C: when CCA cells were treated with L-733,060 (at 5, 10, 25, 50, and 100 μM for 48 h) there was decreased cell proliferation compared with the corresponding basal values. Data are mean ± SE of 4 experiments. *P < 0.05 vs. the corresponding basal values. D: at the dose of 25 μM, L-733,060 decreased the proliferation of selected CCA cell lines following incubation for 24 and 48 h. *P < 0.05 vs. the corresponding basal values. E: by FACS analysis, we also show that L-733,060 decreased the proliferative activity of Mz-ChA-1 but not HIBEpic cells. Data are mean ± SE of 4 experiments.
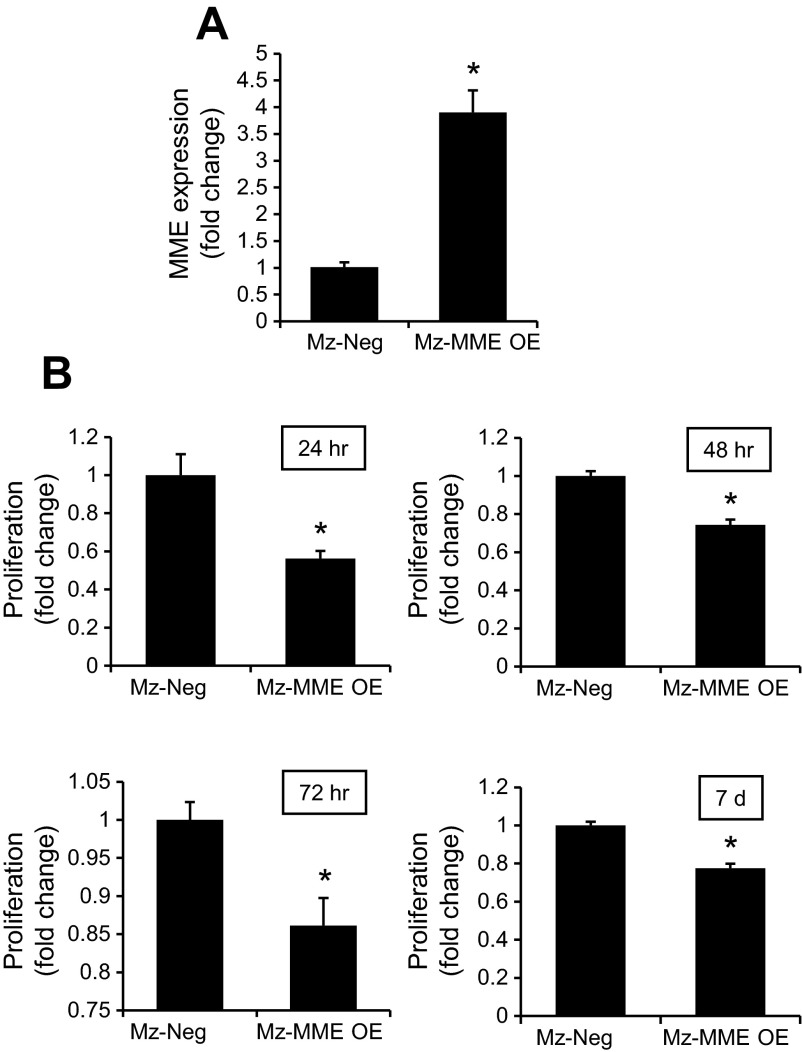
To validate the overexpression of MME in Mz-ChA-1 cells (used in our in vivo studies in nu/nu nude mice) we have shown that Mz-MME cells express higher levels of the message for MME compared with vector transfected cells (Fig. 4A). By MTS assays, there was reduced proliferation of Mz-MME compared with Mz-neg cell lines (Fig. 4B).
Fig. 4.
A: Mz-MME cells express higher levels of the mRNA for MME compared with vector-transfected cells. Data are mean ± SE of 4 experiments. *P < 0.05 vs. Mz-neg. B: by MTS assays, there was reduced proliferation of Mz-MME compared with Mz-neg. Data are mean ± SE of 4 experiments. *P < 0.05 vs. Mz-neg.
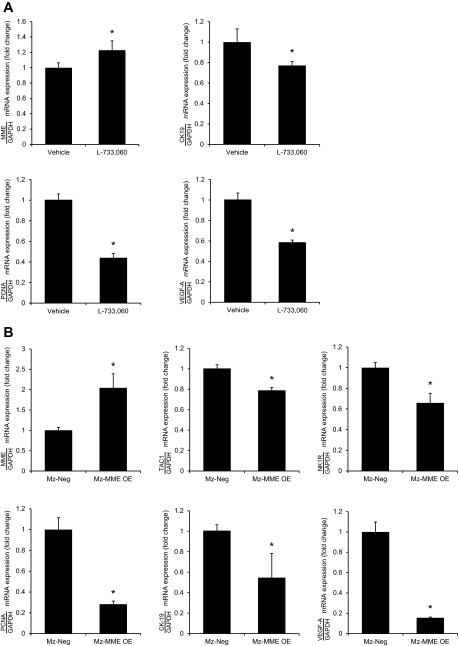
Effects of administration of L-733,060 and overexpression of MME on the proliferation and expression of VEGF-A in Mz-ChA-1 cells implanted in nu/nu nude mice.
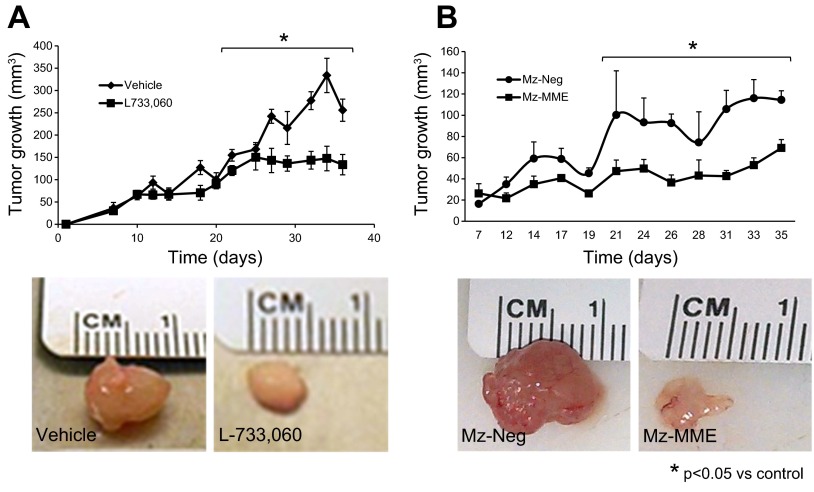
The NK1R inhibitor, L-733,060, had small effects on tumor growth at early time periods but at day 21 significantly decreased xenograft tumor volume throughout the measurement time period compared with the vehicle-treated xenograft tumors (Fig. 5A). When implanted into the flanks of nu/nu nude mice, the tumors derived from Mz-MME cells grew to a lower extent (up to 21 days) than those originating from Mz-neg (Fig. 5B). The expression of MME in Mz-MME cells was significantly different from that of Mz-neg cells both at the time of cell implantation (Fig. 4A) and at the time of tissue collection (Fig. 6B). There was decreased mRNA expression of PCNA, CK-19, and VEGF-A and increased expression of MME mRNA in the tumors from nu/nu nude mice treated with L-733,060 compared with their corresponding controls (Fig. 6A). There was decreased mRNA expression of Tac1, NK1R, PCNA, CK-19, and VEGF-A and increased expression of MME mRNA in the tumors form Mz-MME cells compared with their corresponding controls (Fig. 6B). No significant difference in body and liver weight, and liver to body weight ratio was observed among the four groups of nu/nu nude mice (Table 1).
Fig. 5.
A: L-733,060 had small effects on tumor growth at early time periods, but significantly decreased tumor volume throughout the measurement time period (up to day 21) compared with the vehicle-treated tumors. Data are mean ± SE of values from 4 nu/nu nude mice. *P < 0.05 vs. the corresponding values of nu/nu nude mice treated with vehicle. B: when implanted into the flanks of nu/nu nude mice, the tumors derived from Mz-MME cells grew to a lower extent (up to 21 days) than those originating from Mz-neg. Data are mean ± SE of values from 4 nu/nu nude mice in each treatment group. *P < 0.05 vs. the corresponding values of tumors originating from Mz-neg.
Fig. 6.
A: there was a decrease in the expression of PCNA, CK-19, and VEGF-A and increased expression of MME in the tumors from nu/nu nude mice treated with L-733,060 compared with their corresponding controls. B: there was a decrease in the expression of Tac1, NK1R, PCNA, CK-19, and VEGF-A and increased expression of MME in the tumors from Mz-MME cells compared with their corresponding controls. Data are mean ± SE of 4 experiments. *P < 0.05 vs. the corresponding values of tumors originating from Mz-neg.
Table 1.
Measurement of liver and body weight and liver-to-body weight ratio
| Treatment | Body Weight, g | Liver Weight, g | Liver Weight/Body Weight Ratio, % |
|---|---|---|---|
| Vehicle | 30.9 ± 1.0 | 2.1 ± 0.08 | 6.7 ± 0.1 |
| L733,060 | 28.0 ± 0.8n | 1.6 ± 0.1* | 5.8 ± 0.2* |
| Mz-neg | 33.7 ± 0.9n | 2.7 ± 0.3* | 8.1 ± 0.9* |
| Mz-MME | 34.4 ± 0.2n | 2.3 ± 0.1* | 6.7 ± 0.4* |
Values were obtained from 4 mice for each group.
DISCUSSION
The major findings presented in this study relate to the dysregulation of the SP signaling system in CCA. We demonstrated that the expression of the SP-encoding gene Tac1 and the SP receptor NK1R are upregulated in human CCA tissues and cells. Furthermore, the expression of MME, the enzyme responsible for the deactivation of SP, is downregulated in CCA. Together, these results demonstrated enhanced SP production in human CCA cell lines and tumor tissue. Treatment of human CCA cell lines with recombinant SP significantly increased cell proliferation in vitro, an effect that was prevented by the NK1R antagonist L-733,060. Furthermore, strategies to block endogenous SP effects, namely treatment with L-733,060 alone or stable overexpression of MME, inhibited CCA proliferation in vitro and reduced tumor growth in vivo. These findings suggest that 1) dysregulation of SP signaling may be a key feature associated with the progression of CCA and 2) modulation of this pathway may be a novel approach for the development of effective adjunct therapies to treat this devastating cancer.
Similar to the observations that SP expression is upregulated in CCA, increased SP production and secretion have been discovered in many other types of cancers, including breast, pancreatic, and various gastric cancers (13, 16, 34). However, to our knowledge, the data described in the present study represent new evidence for the role of the SP/NK1R axis in any primary liver tumor. Generally, regardless of the tumor type, high SP and NK1R expression correlated with poor prognosis factors such as tumor development, metastasis, and overall patient survival (13, 16, 34).
Here, we present evidence that SP exerts growth-promoting effects on CCA cells in vitro, and blocking SP activity subsequently inhibits CCA cell proliferation in vitro and tumor growth in vivo. The mechanism by which SP is growth promoting in CCA is unknown; nevertheless, some of the functional effects of the upregulation of SP signaling in other cancers have been elucidated. For example, SP and NK1R are upregulated in HER2-positive breast tumors and are found to transactivate HER2 and EGFR; also, the inhibition of NK1R strongly decreased steady-state expression EGFR and HER2 (16). Similarly, transactivation of EGFR is thought to be partly responsible for the mitogenic actions of SP in glioblastoma cells (9). In addition, SP-mediated activation of the Ca2+/Src/PKCδ/ERK1/2 pathway has been shown to enhance cancer cell proliferation (54). Given that both EGFR activation and ERK1/2 activation are known to increase CCA cell proliferation and tumor growth (15, 44, 52, 55), it is conceivable that SP may be exerting its mitogenic effects via a combination of these pathways. SP had been shown to mediate the antiapoptotic and proliferative responses of human colonocytes via activation of Akt signaling mechanisms (31). Previous studies have shown that VEGF plays an important role in regulating both normal and neoplastic cholangiocyte proliferation (10, 17, 18). SP has been shown to stimulate VEGF expression levels promoting angiogenesis and wound healing (29). This finding may indicate an additional mechanism by which inhibition of SP signaling may decrease VEGF expression and subsequently CCA proliferation. Further studies are warranted to elucidate this potential mechanism mediated by SP.
The upregulation of SP/NK1R have also been observed in chronic liver diseases (19, 50). Indeed, increased SP levels can be detected in the serum of both cholestatic patients and experimental cholestatic rodents (50). Within the liver, SP has been shown to increase cholangiocyte proliferation during cholestasis via the cAMP-dependent phosphorylation of protein kinase A (19). Furthermore, NK1R expression is observed in cholangiocytes and upregulated in a model of extrahepatic biliary obstruction. In addition, NK1R knockout mice have a dampened proliferative reaction to biliary hyperplasia (19). Consistent with these observations, we found that treatment of HIBEpiC cells with recombinant SP also increased proliferation in vitro.
A study has shown that MME, the neutral endopeptidase responsible for cleaving and deactivating SP, is expressed in the bile canaliculi and interlobular ducts (7). The expression of MME is downregulated during liver cirrhosis and hepatocellular carcinoma (38). Furthermore, suppression of MME immunoreactivity has previously been described in extrahepatic bile duct cancers (49). The data presented here demonstrate a suppression of MME expression in CCA, which supports the notion that MME expression may be downregulated in various types of liver cancers. Consistent with this concept, high MME expression in the tumor center or invasion front, but not in the lymph node metastases, was a favorable prognostic indicator in human bladder cancer (46). In contrast, the expression of MME has been shown to decrease in noninvasive low-grade cancers compared with nonneoplastic tissue, but the expression increases again in invasive high-grade tumors (26). This suggests that perhaps MME expression may be a reflection of the grade and invasiveness of the tumor in question. Indeed, MME expression was associated with a more aggressive phenotype in melanoma (48), in colorectal carcinomas (23), and in high-grade sarcoma tissue (12). Interestingly, an explanation as to the disparity in the prognostic value of MME may lie in the predominant cell type within the tumor expressing MME. Specifically, non-small cell lung carcinoma with MME immunoreactivity in the stromal cells had worse prognostic impact decreased survival and disease free survival (21). However, when MME expression was restricted to the epithelial cells, its expression level was a positive prognostic indicator correlating with an increased disease-free survival (21). Taken together, these data indicate that the prognostic value of MME expression is dependent on the cell type expressing MME, the tumor grade, and the tumor type.
In conclusion, the data presented here indicate the existence of a dysregulated SP/NK1R pathway in human CCA compared with nonmalignant human biliary tissues and cells. Furthermore, there is a concomitant increase in NK1R expression in CCA. Conversely, the expression of MME, the enzyme responsible for deactivating SP, is suppressed in human CCA. Specific inhibition of SP function leads to a suppression of tumor growth in a xenograft model of CCA, suggesting that agents that modulate the bioavailability of SP may be the potential therapeutic tools for the treatment of this devastating human cancer.
GRANTS
This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, a VA Research Career Scientist Award and a VA Merit Award to G. Alpini, a VA Merit Award to F. Meng, VA CD2 to H. Francis, VA CDA2 and VA Merit award to S. Glaser, an American Cancer Society Research Scholar award (RSC118760), an NIH K01 award (DK078532) to S. DeMorrow, and the NIH grant DK58411 and DK07698 to G. Alpini and S. Glaser.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). The views presented are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
AUTHOR CONTRIBUTIONS
F.M., S.D., Y.H., S.G., and G.A. interpreted results of experiments; F.M., H.F., K.M., M.M., M.Q., D.R., L.K., S.G., and G.A. edited and revised manuscript; S.D., J.V., G.F., Y.H., H.F., H.S., S. Avila, K.M., M.M., S. Afroze, M.G., M.Q., D.R., L.K., L.H., and S.G. performed experiments; S.D., J.V., G.F., Y.H., H.F., H.S., S. Avila, K.M., M.M., S. Afroze, M.G., M.Q., D.R., L.K., L.H., and S.G. analyzed data; Y.H., H.S., S. Avila, S. Afroze, M.G., S.G., and G.A. prepared figures; S.G. and G.A. conception and design of research; S.G. drafted manuscript; S.G. and G.A. approved final version of manuscript.
REFERENCES
- 1.Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martin JD, Candenas ML. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem 11: 2045–2081, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Alpini G, Invernizzi P, Gaudio E, Venter J, Kopriva S, Bernuzzi F, Onori P, Franchitto A, Coufal M, Frampton G, Alvaro D, Lee SP, Marzioni M, Benedetti A, DeMorrow S. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res 68: 9184–9193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 132: 415–431, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bang R, Biburger M, Neuhuber WL, Tiegs G. Neurokinin-1 receptor antagonists protect mice from CD95- and tumor necrosis factor-alpha-mediated apoptotic liver damage. J Pharmacol Exp Ther 308: 1174–1180, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Barrett KG, Fang H, Gargano MD, Markovich D, Kocarek TA, Runge-Morris M. Regulation of murine hepatic hydroxysteroid sulfotransferase expression in hyposulfatemic mice and in a cell model of 3′-phosphoadenosine-5′-phosphosulfate deficiency. Drug Metab Dispos 41: 1505–1513, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger H, Fechner K, Albrecht E, Niedrich H. Substance P: in vitro inactivation by rat brain fractions and human plasma. Biochem Pharmacol 28: 3173–3180, 1979 [DOI] [PubMed] [Google Scholar]
- 7.Borscheri N, Roessner A, Rocken C. Canalicular immunostaining of neprilysin (CD10) as a diagnostic marker for hepatocellular carcinomas. Am J Surg Pathol 25: 1297–1303, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Bragazzi MC, Cardinale V, Carpino G, Venere R, Semeraro R, Gentile R, Gaudio E, Alvaro D. Cholangiocarcinoma: epidemiology and risk factors. Transl Gastrointest Cancer 1: 21–32, 2012 [Google Scholar]
- 9.Castagliuolo I, Valenick L, Liu J, Pothoulakis C. Epidermal growth factor receptor transactivation mediates substance P induced mitogenic responses in U-373 MG cells. J Biol Chem 275: 26545–26550, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Cherry-Bohannan J, Baker K, Francis H. VEGF and cholangiocarcinoma: feeding the tumor. Transl Gastrointest Cancer 1: 95–102, 2012 [Google Scholar]
- 11.Davidson S, Miller KA, Dowell A, Gildea A, Mackenzie A. A remote and highly conserved enhancer supports amygdala specific expression of the gene encoding the anxiogenic neuropeptide substance-P. Mol Psychiatry 11: 410–421, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Deniz K, Coban G, Okten T. Anti-CD10 (56C6) expression in soft tissue sarcomas. Pathol Res Pract 208: 281–285, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Feng F, Yang J, Tong L, Yuan S, Tian Y, Hong L, Wang W, Zhang H. Substance P immunoreactive nerve fibres are related to gastric cancer differentiation status and could promote proliferation and migration of gastric cancer cells. Cell Biol Int 35: 623–629, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Francis H, DeMorrow S, Venter J, Onori P, White M, Gaudio E, Francis T, Greene JF, Jr, Tran S, Meininger CJ, Alpini G. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut 61: 753–764, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis H, Onori P, Gaudio E, Franchitto A, DeMorrow S, Venter J, Kopriva S, Carpino G, Mancinelli R, White M, Meng F, Vetuschi A, Sferra R, Alpini G. H3 histamine receptor-mediated activation of protein kinase Calpha inhibits the growth of cholangiocarcinoma in vitro and in vivo. Mol Cancer Res 7: 1704–1713, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Recio S, Fuster G, Fernandez-Nogueira P, Pastor-Arroyo EM, Park SY, Mayordomo C, Ametller E, Mancino M, Gonzalez-Farre X, Russnes H, Engel P, Costamagna D, Fernandez PL, Gascon P, Almendro V. Substance P autocrine signaling contributes to persistent HER2 activation that drives malignant progression and drug resistance in breast cancer. Cancer Res 73: 6424–6434, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Franchitto A, Onori P, Ueno Y, Marzioni M, Fava G, Venter J, Reichenbach R, Summers R, Alpini G. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol 291: G307–G317, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, Meininger CJ, Franchitto A, Onori P, Marzioni M, Taffetani S, Fava G, Stoica G, Venter J, Reichenbach R, De Morrow S, Summers R, Alpini G. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 130: 1270–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Glaser S, Gaudio E, Renzi A, Mancinelli R, Ueno Y, Venter J, White M, Kopriva S, Chiasson V, DeMorrow S, Francis H, Meng F, Marzioni M, Franchitto A, Alvaro D, Supowit S, DiPette DJ, Onori P, Alpini G. Knockout of the neurokinin-1 receptor reduces cholangiocyte proliferation in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol 301: G297–G305, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaser S, Ueno Y, DeMorrow S, Chiasson VL, Katki KA, Venter J, Francis HL, Dickerson IM, DiPette DJ, Supowit SC, Alpini G. Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab Invest 87: 914–926, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Gurel D, Kargi A, Karaman I, Onen A, Unlu M. CD10 expression in epithelial and stromal cells of nonsmall cell lung carcinoma (NSCLC): a clinic and pathologic correlation. Pathol Oncol Res 18: 153–160, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Han Y, DeMorrow S, Invernizzi P, Jing Q, Glaser S, Renzi A, Meng F, Venter J, Bernuzzi F, White M, Francis H, Lleo A, Marzioni M, Onori P, Alvaro D, Torzilli G, Gaudio E, Alpini G. Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma: its synthesis is reduced favoring cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol 301: G623–G633, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirano K, Nimura S, Mizoguchi M, Hamada Y, Yamashita Y, Iwasaki H. Early colorectal carcinomas: CD10 expression, mucin phenotype and submucosal invasion. Pathol Int 62: 600–611, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Holzer P. Efferent-like roles of afferent neurons in the gut: blood flow regulation and tissue protection. Auton Neurosci 125: 70–75, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang L, Frampton G, Rao A, Zhang KS, Chen W, Lai JM, Yin XY, Walker K, Culbreath B, Leyva-Illades D, Quinn M, McMillin M, Bradley M, Liang LJ, DeMorrow S. Monoamine oxidase A expression is suppressed in human cholangiocarcinoma via coordinated epigenetic and IL-6-driven events. Lab Invest 92: 1451–1460, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang TJ. CD10 is again expressed at a certain stage during the neoplastic process of bladder transitional cell carcinomas. Cancer Res Treat 44: 262–266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanno N, Glaser S, Chowdhury U, Phinizy JL, Baiocchi L, Francis H, LeSage G, Alpini G. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol 34: 284–291, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Kanno N, LeSage G, Glaser S, Alvaro D, Alpini G. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology 31: 555–561, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Kant V, Gopal A, Kumar D, Bag S, Kurade NP, Kumar A, Tandan SK, Kumar D. Topically applied substance P enhanced healing of open excision wound in rats. Eur J Pharmacol 715: 345–353, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, Prellwitz W, Meyer zum Buschenfelde KH. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol 1: 579–596, 1985 [DOI] [PubMed] [Google Scholar]
- 31.Koon HW, Zhao D, Zhan Y, Moyer MP, Pothoulakis C. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc Natl Acad Sci USA 104: 2013–2018, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusaka Y, Tokiwa T, Sato J. Establishment and characterization of a cell line from a human cholangiocellular carcinoma. Res Exp Med (Berl) 188: 367–375, 1988 [DOI] [PubMed] [Google Scholar]
- 33.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology 128: 1655–1667, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Li X, Ma G, Ma Q, Li W, Liu J, Han L, Duan W, Xu Q, Liu H, Wang Z, Sun Q, Wang F, Wu E. Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Mol Cancer Res 11: 294–302, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linnik MD, Moskowitz MA. Identification of immunoreactive substance P in human and other mammalian endothelial cells. Peptides 10: 957–962, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol 45: 856–867, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munoz M, Rosso M, Aguilar FJ, Gonzalez-Moles MA, Redondo M, Esteban F. NK-1 receptor antagonists induce apoptosis and counteract substance P related mitogenesis in human laryngeal cancer cell line HEp-2. Invest New Drugs 26: 111–118, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T. Changes in expression of bile canalicular CD10 and sinusoidal CD105 (endoglin) in peritumoral hepatic tissue. Tumori 95: 495–500, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Palma C. Tachykinins and their receptors in human malignancies. Curr Drug Targets 7: 1043–1052, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2: 10, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renzi A, Glaser S, DeMorrow S, Mancinelli R, Meng F, Franchitto A, Venter J, White M, Francis H, Han Y, Alvaro D, Gaudio E, Carpino G, Ueno Y, Onori P, Alpini G. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol 301: G634–G643, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saijyo S, Kudo T, Suzuki M, Katayose Y, Shinoda M, Muto T, Fukuhara K, Suzuki T, Matsuno S. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med 177: 61–71, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Sawchenko PE, Friedman MI. Sensory functions of the liver—a review. Am J Physiol Regul Integr Comp Physiol 236: R5–R20, 1979 [DOI] [PubMed] [Google Scholar]
- 44.Schmitz KJ, Lang H, Wohlschlaeger J, Sotiropoulos GC, Reis H, Schmid KW, Baba HA. AKT and ERK1/2 signaling in intrahepatic cholangiocarcinoma. World J Gastroenterol 13: 6470–6477, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seabrook GR, Shepheard SL, Williamson DJ, Tyrer P, Rigby M, Cascieri MA, Harrison T, Hargreaves RJ, Hill RG. L-733,060, a novel tachykinin NK1 receptor antagonist; effects in [Ca2+]i mobilisation, cardiovascular and dural extravasation assays. Eur J Pharmacol 317: 129–135, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Seiler R, von Gunten M, Thalmann GN, Fleischmann A. High CD10 expression predicts favorable outcome in surgically treated lymph node-positive bladder cancer patients. Hum Pathol 43: 269–275, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Skidgel RA, Engelbrecht S, Johnson AR, Erdos EG. Hydrolysis of substance P and neurotensin by converting enzyme and neutral endopeptidase. Peptides 5: 769–776, 1984 [DOI] [PubMed] [Google Scholar]
- 48.Thomas-Pfaab M, Annereau JP, Munsch C, Guilbaud N, Garrido I, Paul C, Brousset P, Lamant L, Meyer N. CD10 expression by melanoma cells is associated with aggressive behavior in vitro and predicts rapid metastatic progression in humans. J Dermatol Sci 69: 105–113, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Tretiakova M, Antic T, Westerhoff M, Mueller J, Himmelfarb EA, Wang HL, Xiao SY. Diagnostic utility of CD10 in benign and malignant extrahepatic bile duct lesions. Am J Surg Pathol 36: 101–108, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Trivedi M, Bergasa NV. Serum concentrations of substance P in cholestasis. Ann Hepatol 9: 177–180, 2010 [PubMed] [Google Scholar]
- 51.Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. Anat Rec A Discov Mol Cell Evol Biol 280: 808–820, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Werneburg NW, Yoon JH, Higuchi H, Gores GJ. Bile acids activate EGF receptor via a TGF-α-dependent mechanism in human cholangiocyte cell lines. Am J Physiol Gastrointest Liver Physiol 285: G31–G36, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Wu T, Leng J, Han C, Demetris AJ. The cyclooxygenase-2 inhibitor celecoxib blocks phosphorylation of Akt and induces apoptosis in human cholangiocarcinoma cells. Mol Cancer Ther 3: 299–307, 2004 [PubMed] [Google Scholar]
- 54.Yamaguchi K, Richardson MD, Bigner DD, Kwatra MM. Signal transduction through substance P receptor in human glioblastoma cells: roles for Src and PKCdelta. Cancer Chemother Pharmacol 56: 585–593, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Yoon JH, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology 122: 985–993, 2002 [DOI] [PubMed] [Google Scholar]