Abstract
Necrotizing enterocolitis (NEC) is associated with a high morbidity and mortality in very low birth weight infants. Several hypotheses regarding the pathogenesis of NEC have been proposed but to date no effective treatment is available. Previous studies suggest that probiotic supplementation is protective. We recently reported that probiotic (Bifidobacterium infantis) conditioned medium (PCM) has an anti-inflammatory effect in cultured fetal human intestinal cells (H4) and fetal intestine explants. In this study, we tested in vivo whether PCM protects neonatal mice from developing intestinal inflammation induced by exposure to Cronobacter sakazakii (C. sakazakii), an opportunistic pathogen associated with NEC. We found that infected neonatal mice had a significantly lower body weight than control groups. Infection led to ileal tissue damage including villous rupture, disruption of epithelial cell alignment, intestinal inflammation, apoptotic cell loss, and decreased mucus production. Pretreatment with PCM prevented infection caused decrease in body weight, attenuated enterocyte apoptotic cell death, mitigated reduced mucin production, and maintained ileal structure. Infected ileum expressed reduced levels of IκBα, which could be restored upon pretreatment with PCM. We also observed a nuclear translocation of NF-κB p65 in H4 cells exposed to C. sakazakii, which was prevented in PCM-pretreated cells. Finally, treatment of neonatal mice with PCM prior to infection sustained the capacity of ileal epithelial proliferation. This study suggests that an active component(s) released into the culture medium by B. infantis may prevent ileal damage by a pathogen linked to NEC.
Keywords: neonatal intestinal inflammation, necrotizing enterocolitis, probiotic-conditioned media, Bifidobacterium infantis, Cronobacter sakazakii
the incidence of premature deliveries, and as a result the incidence of necrotizing enterocolitis (NEC) which occurs in almost 10% of premature infants under 1,500 g, has been unchanged over the last several decades in the United States (5, 30). NEC is the most common gastrointestinal disease in neonatal intensive care units (NICU), with each case costing approximately $300,000. The health care expense of NEC accounts for nearly 20% of annual NICU costs (13). Although there are several theories to explain the pathogenesis of NEC, to date there still has been no effective preventive treatment available for the neonatologist.
A prospective, randomized, controlled clinical trial from three different NICUs strongly suggests that a regimen combining the probiotics Bifidobacteria infantis and Lactobacillus acidophilus provides a protective effect in preventing/treating NEC in very low birth weight infants (1, 2, 23, 24). Meta-analyses of multiple studies with a relatively small number of subjects using different probiotics also suggest a beneficial role of probiotics in NEC prevention (8). However, routine administration of live organisms to immunocompromised premature infants is not allowed in the United States because of regulations from the Food and Drug Administration (FDA). We have therefore analyzed conditioned media of these probiotic organisms (PCM) to determine whether they release factors into the culture medium that confer protection. Our recent study has shown that when Bifidobacteria infantis was grown separately, its PCM had a more potent anti-inflammatory effect on a human fetal intestine cell line and in human fetal intestine explants (12) than Lactobacillus alone or both organisms in combination.
In this study, we extended these in vitro observations into an in vivo animal model to determine whether Bifidobacterium infantis PCM also protects against intestinal inflammation in newborn mice when exposed to Cronobacter sakazakii (C. sakazakii), an opportunistic pathogen linked to an outbreak of NEC in newborn infants in the United States (16, 35, 37). We found that administration of B. infantis PCM before C. sakazakii infection reduced inflammation, ameliorated cell death, restored the proliferative capacity of ileal epithelial cells, increased mucin production, and allowed maintenance of body weight. We also found that PCM pretreatment was able to prevent C. sakazakii-induced reduction in levels of IκBα in ileal tissues and attenuated the translocation of NF-κB p65 from the cytoplasm into the nucleus in immature human fetal intestine cells. These data suggest that factors released from the probiotic B. infantis protect against intestinal inflammation and tissue damage in C. sakazakii-infected neonatal mice in vivo and may help to prevent necrotizing enterocolitis in infant in future clinical trials.
MATERIALS AND METHODS
Mice and bacteria.
C57BL/6 mice were obtained from the Jackson Laboratory. C. sakazakii (strain no. 51329) and B. infantis (strain no. 15697) were obtained from American Type Culture Collection (ATCC, Manassas, VA). B. infantis PCM was prepared as described in a previous study (12). Briefly, the inoculum of B. infantis was cultured at 37°C under anaerobic conditions until they reached a midexponential phase of growth. A <30 kDa fraction of PCM was prepared by centrifugation of probiotic cultures at 3,000 RPM for 15 min followed by 0.22-μm filtration and Amicon Ultra-30 centrifugal filter (Merck Millipore, Billerica, MA).
Mouse model of neonatal intestinal inflammation.
All protocols were approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital (A3596-01). Newborn pups were divided into four groups: I (PBS), II (PCM), III (C. sakazakii), and IV (PCM plus C. sakazakii). The C. sakazakii-induced mouse model for intestinal inflammation was developed as described previously with minor modifications (10). In brief, each pup at P1 (postnatal day 1) received PCM or PBS via gavage once per day for 8 days. At P5, each pup in groups III and IV was given a single oral dose of C. sakazakii (1 × 107). At P8, all pups in each group were euthanized and the entire small intestine was collected and stored at −80°C until further analysis.
Human intestinal epithelial cell cultures and immunofluorescence staining.
A human fetal nontransformed primary small intestinal epithelial cell line (H4 cells) was established from a 20-wk-old normal fetus in our laboratory (34). H4 cells were maintained in DMEM supplemented with 10% FBS, 1% nonessential amino acids, 200 mM glutamine, 50 IU/ml penicillin, 50 μg/ml streptomycin, 1% HEPES buffer, and 10 mg/l recombinant human insulin (Roche, Indianapolis, IN) and cultured in a 37°C humidified incubator with 5% CO2 and 95% air. Cells were seeded on glass coverslips at a concentration of 1 × 105 per ml the day before being used for experiments. After three washes in PBS, H4 cells were cultured in antibiotic-free culture medium containing PCM (15% of total cell culture medium in a cell culture incubator for 1 h) and then infected with or without 1 × 107 C. sakazakii for 1 h. Cells were washed in PBS three times, fixed in 4% paraformaldehyde/PBS for 15 min, and then incubated in PBS containing 0.5% Triton X-100 for 5 min. After three washes in PBS, cells were blocked with 5% BSA/PBS for 1 h and incubated with an antibody specific for NF-κB p65 (1:50, Cell Signaling Technology, Danvers, MA). After washes, cells were incubated with a secondary antibody conjugated with Cy-3 (Jackson ImmunoResearch Laboratories, West Grove, PA). Coverslips were mounted with medium containing DAPI (Invitrogen). Immunofluorescent microscopy was performed via a ×60 oil Nikon PL APO CS objective mounted on a Nikon Eclipse 80i microscope. The scanned images from each fluorescence channel were merged by using PhotoShop. Nuclear translocation of NF-κB-p65 was quantified. Cells with or without colocalization of NF-κB-p65 immunoreactivity with DAPI staining were counted from randomly selected imaging fields. A percentage of cells with nuclear translocation of NF-κB-p65 was calculated and graphed. Two-sided χ2 with Yates correction was used for data analysis.
Histopathological examinations and immunohistochemical staining.
Ileum tissues were flushed with cold PBS, the whole gut was fixed in cold 4% paraformaldehyde/PBS overnight, and the paraffin-embedded tissue was stained with hematoxylin and eosin. The stained sections were examined and analyzed blinded to the conditions of treatment. Intestinal histopathology (scale of 0–4 with increments of 1) from normal morphology (grade 0) to complete obliteration of the epithelium and intestinal perforation (grade 4) was graded as previously described (27). The grading was based on the extent of epithelial sloughing, submucosal edema, neutrophil infiltration, and villus architectural destruction in the most affected area. Grade 0: normal architecture, absence of pathology; grade 1: mild, epithelial sloughing; grade 2: moderate, epithelial sloughing, and partial destruction of villus tips; grade 3: submucosal edema, and severe disruption of villus architecture; grade 4: complete obliteration of the epithelium and intestinal perforation.
For detection of proliferative activity in ileal epithelial cells, sections were blocked in 1% normal goat serum and incubated overnight at 4°C with a goat antibody specific for Ki67 (1:50, Santa Cruz, CA) followed by incubation with a horseradish peroxidase-conjugated anti-goat IgG (1:100, Santa Cruz, CA). Sections were stained with DAB (3,3′-diaminobenzidine) to detect dividing cells (Ki67-positive cells) and counterstained with Gill's hematoxylin. Microscopy was performed using a ×40/1.25–0.75 oil Nikon PL APO CS objective mounted on a Nikon Eclipse 80i microscope. Digital images were collected from at least three different fields per section and used for counting Ki67-positive and negative cells of the crypts in each ileal section blinded to treatment conditions. Data were presented as a percent of total crypt cells with positive Ki67 staining.
PAS staining was performed by the NORC-H Morphology Core at Massachusetts General Hospital. Digital images were collected from at least two sections in 10 mice for each group. Data were presented as positive PAS stain per villus.
Real-time quantitative PCR.
RNA was extracted from the ileum by using TRIzol (Invitrogen, Grand Island, NY) or Qiagen RNeasy purification kits (Qiagen, Valencia, CA) according to the supplier's instructions. After treatment with DNase, 1 μg of RNA was converted to cDNA (Applied Biosystems). Real-time PCR was performed with SYBR Green PCR Master-Mix for 40 cycles on an Opticon II DNA engine (MJ Research). Primer sequences for IL-1β: Forward 5′ ACCTGTCCTGTGTAATGAAAGACG; Reverse 5′ TGGGTATTGCTTGGGATCCA; TNF-α: Forward 5′ GTTTGTGGACAATACCAGCAGT; Reverse 5′ GTTCTTCACTACCCTCTGTGC; GAPDH: Forward 5′ GGCAAATTCAACGGCACAGT; Reverse 5′ AGATGGTGATGGGCTTCCC. LightCycler Relative Quantification Software was used to normalize data to the standard glucose-phosphate dehydrogenase mRNA level. Reactions were set up by using QuantiTect SYBR Green RT-PCR kit reagents (Qiagen) following the kit instructions. For all samples, real-time quantitative PCR (qPCR) was performed in duplicate.
Apoptosis assay.
Paraffin-embedded tissues were processed for TUNEL labeling to detect apoptotic cell death by using an ApopTag peroxidase in situ apoptosis detection kit according to the supplier's instructions (Millipore, Billerica, MA). Digital images were obtained by using a Nikon PL APO 10× objective mounted on a Nikon Eclipse 80i microscope. Ten villi and crypts of each section were observed under a light microscope. A ratio of the number of TUNEL-positive cells to the total number of cells in 10 villi and crypts was used as the apoptotic index.
Fluorescent labeled inhibitor of caspases (FLICA) staining was performed on the paraffin-embedded ileum tissues to detect caspase 3&7 activity in living cells. A reaction was set up using FAM FLICA caspase 3&7 assay kit from ImmunoChemistry Technologies (Bloomington, MN) following the kit instructions. Coverslips were mounted with medium containing DAPI (Invitrogen). Immunofluorescent microscopy was performed via a ×20 Nikon PL APO CS objective mounted on a Nikon Eclipse 80i microscope. The scanned images from each fluorescence channel were merged by use of PhotoShop. Activated caspase 3&7 (green) was quantified. A percentage of activated caspase 3&7 (green) cells in the total villi cells was calculated and graphed.
SDS-PAGE and Western blot.
Ileum was washed in PBS and lysed in a NP40 cell lysis buffer (Invitrogen, Camarillo, CA) containing phosphatase (Calbiochem, La Jolla, CA) and protease inhibitor cocktail tablets (Roche, Indianapolis, IN). Lysates were cleared by centrifugation at 12,000 rpm and 4°C for 15 min. The concentration of proteins in postnuclear supernatants was determined by use of Bio-Rad protein assay reagents. Proteins (30 μg) were subjected to SDS-PAGE on a 4–20% gradient Tris-Glycine Gel (Invitrogen) under reducing conditions and then transferred onto a nitrocellulose membrane (Invitrogen). Blots were blocked in TBST (Tris-buffered saline, Tween 20) with 5% nonfat milk for 1 h at room temperature and incubated overnight at 4°C with primary antibodies in TBST containing 5% BSA. Membranes were washed three times in TBST for 5 min and then incubated 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology). Bound antibodies were detected with ECL reagents (GE Healthcare, Milwaukee, WI). The dilutions were 1:1,000 for primary antibodies and 1:5,000 for the secondary antibodies. Primary antibodies to IκBα and β-actin were obtained from Cell Signaling Technology.
Statistical analysis.
Results were shown as a means ± SE. A one-way ANOVA or Student's t-test was used for comparing groups, with P < 0.05 regarded as statistically significance.
RESULTS
PCM protects neonatal mice from C. sakazakii-induced intestinal inflammation.
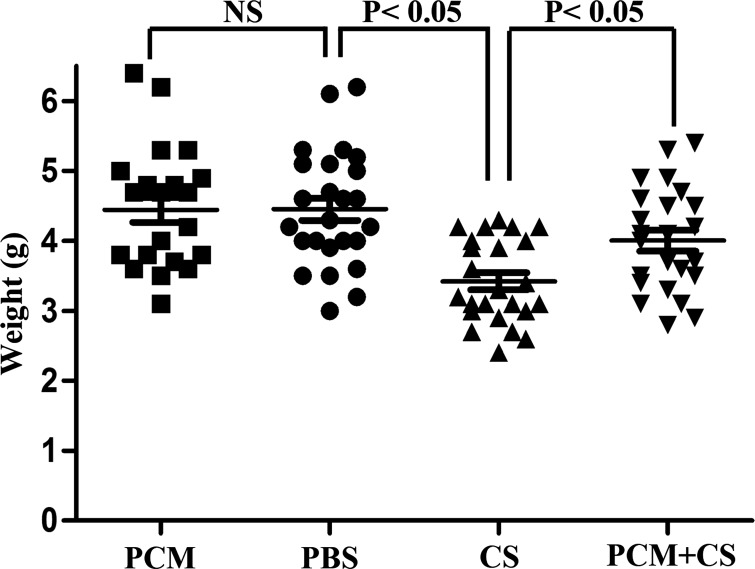
Using a neonatal intestinal inflammation model similar to those established for NEC mouse models (1, 10), we infected pups on postnatal day 5 with an oral inoculation of 1 × 107 C. sakazakii. Three days later, mice were weighed and then euthanized. Jejunum and ileum were collected for histological analysis. Mice infected with C. sakazakii had a significantly lower body weight relative to mice treated with PCM prior to infection with C. sakazakii and PBS-treated mice (Fig. 1).
Fig. 1.
Bifidobacterium infantis conditioned medium prevents a Cronobacter sakazakii-induced decrease in body weight in newborn pups. Pups were divided into 4 groups: I (PBS alone), II [probiotic conditioned medium (PCM) alone], III [C. sakazakii (CS) alone], and IV (PCM plus C. sakazakii). The body weight of each pup was measured at postnatal day 8 (P8) and compared. One-way ANOVA: n = 23–26 per group; post hoc Newman-Keuls multiple-comparison test: P < 0.05; NS, no significance.
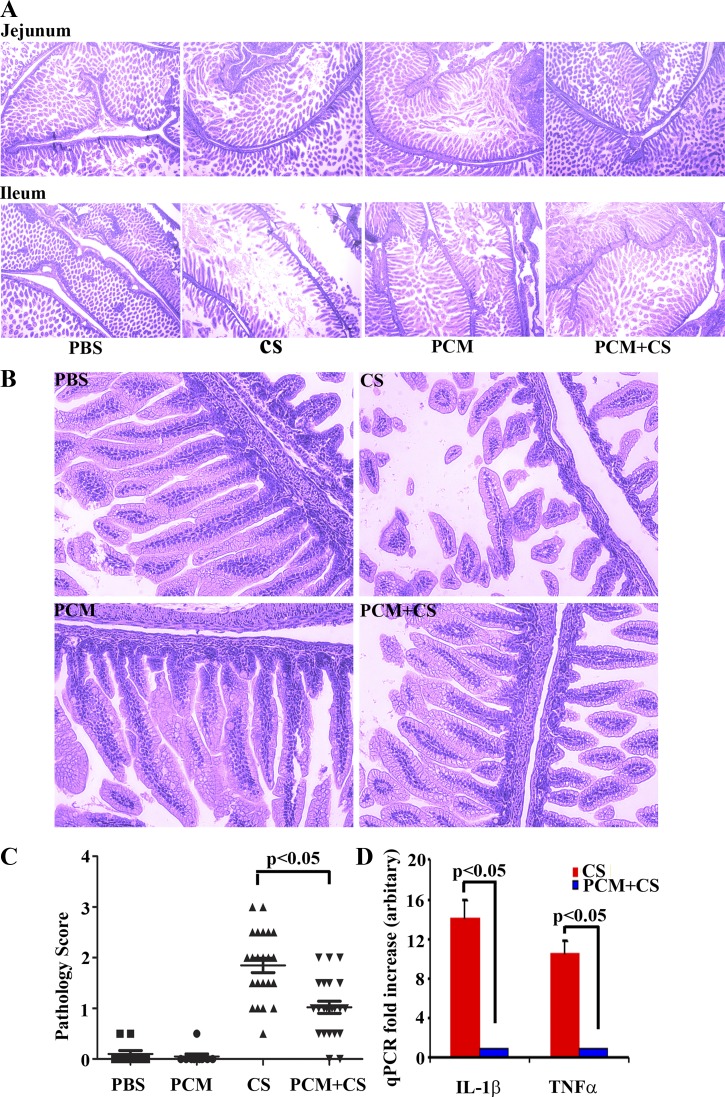
Next, we determined whether C. sakazakii could infect the entire intestine. We fixed the entire intestine and performed hematoxylin and eosin staining. We observed that the entire gut was infected, with the jejunum very mildly and the ileum and colon extensively damaged. The histology analysis, however, was done only on the jejunum and ileum since colon tissue was inadequate for examination (Fig. 2A). Because the ileum had more extensive damage, we compared ileal sections at higher power (×200 magnification) to determine a pathology score (Fig. 2B). Histological examination of ileal sections showed that C. sakazakii infection resulted in substantial pathological changes including villus rupture (epithelial sloughing), disruption of orderly alignment of epithelial cells (villus architecture destruction), and villus cell loss (Fig. 2, B and C). Although villus rupture was noted in mice receiving PCM prior to C. sakazakii infection, they retained an orderly alignment of epithelial cells and cell loss was prevented (Fig. 2B). Using qPCR, we found that levels of IL-1β mRNA and TNF-α mRNA were increased 10- to 12-fold in ileal tissues from C. sakazakii-infected mice compared with those treated with PCM prior to C. sakazakii infection (Fig. 2D). We were not able to detect a signal for IL-1β and TNF-α in ileal tissues of mice treated with PBS or with PCM alone. We also employed qPCR to analyze expression levels of several other inflammatory cytokines, including IL-6, iNOS, IL-12 p40, IL-12 p35, IFN-γ, and MIP3α. However, their expression levels, although increased, were not noted to be statistically significant under the different conditions examined (data not shown).
Fig. 2.
PCM treatment maintains the structural integrity of C. sakazaii-infected ileum. A and B: whole gut embedded in paraffin and processed by hematoxylin and eosin staining. The treatment condition is shown at the bottom of each column (×40 magnification; ×200 magnification) and at top left of each image. Villi displaying epithelial discontinuity, irregular alignment, and/or loss of epithelial cells was observed in the CS-infected group. In contrast, treatment with PCM and CS shows modest villus rupture only, which manifest regular alignment and no apparent loss of epithelial cells. Top, jejunal sections; bottom, ileal sections; ×40 magnification (A), ×200 magnification, ileal section (B). The pathology score (C) was determined according to the extent of epithelial sloughing, submucosal edema, neutrophil infiltration, and villus architecture destruction in the worst affected areas. Mean ± SE pathology score was graphed for 23–26 mice in each group and 2 sections were analyzed in each mouse (1-way ANOVA; post hoc Newman-Keuls multiple-comparison test: P < 0.05). D: real-time quantitative RT-PCR (qPCR) was performed in duplicate to measure the mRNA expression levels of the inflammatory cytokines IL-1β and TNF-α with GAPDH as an internal control. Mean ± SE fold increase was graphed (n = 10 mice in each group, Student's t-test: P < 0.05).
PCM pretreatment mitigated induction of mucin in C. sakazakii-infected mice.
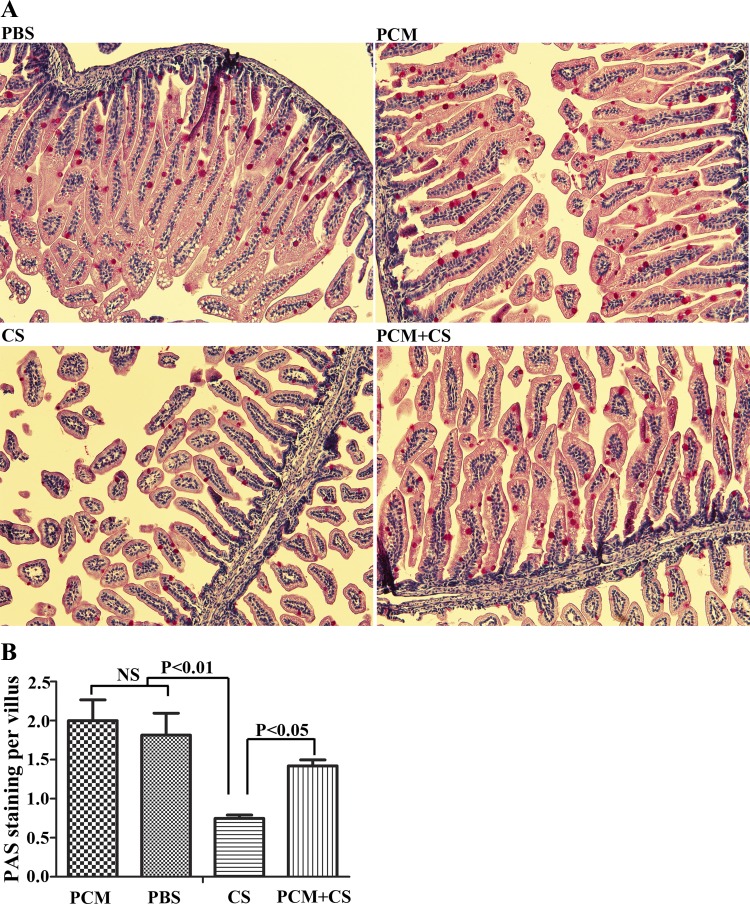
To characterize goblet cell mucus production, PAS staining was performed. Most of the PAS stained cells are located in the ileum and colon. We observed a significant reduction in PAS staining in C. sakazakii-infected ileum as determined by the number of PAS-positive cells per villus. However, PCM pretreatment increased PAS-positive cells per villus in C. sakazakii-infected ileum sections (Fig. 3).
Fig. 3.
PCM pretreatment mitigates against a decrease in mucin production in C. sakazakii infection. Paraffin-embedded ileum tissues under different treatment conditions were processed with periodic acid-Schiff (PAS) stain. The treatment condition is shown at top left of each image (×200 magnification). The percentage of PAS-positive cells per villus with each condition was calculated and graphed (n = 10 mice in each group, 2 sections per animal, means ± SD, 1-way ANOVA).
PCM pretreatment attenuates apoptosis of epithelium cells in C. sakazakii-infected ileum.
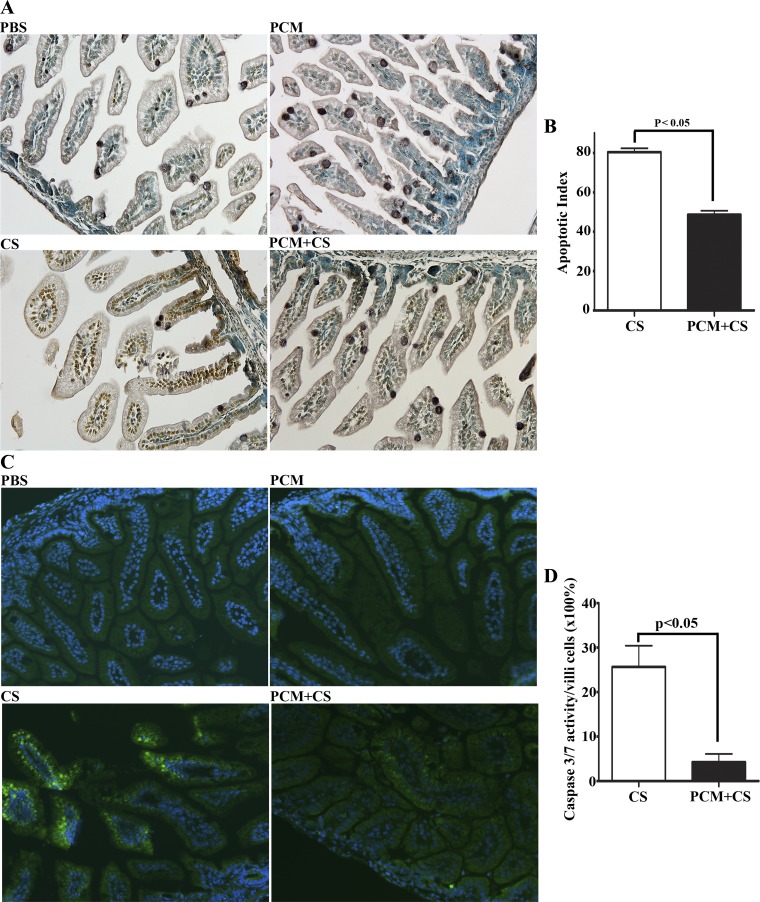
Histological analysis of ileal sections showed that C. sakazakii infection resulted in villus cell loss. We wondered whether apoptosis accounted for the cell loss observed in ileal sections from neonatal mice infected with C. sakazakii. TUNEL labeling of ileal sections followed by immunoperoxidase-based detection revealed a profound apoptosis of epithelial cells in C. sakazakii-infected mice (Fig. 4). Under the same conditions, apoptotic cells were not detectable in the ileum sections from mice treated with PBS or with PCM alone (Fig. 4A). In contrast, ileal sections from mice pretreated with PCM before infection showed very mild signs of apoptosis (Fig. 4A). Quantitation of apoptotic cells in ileal sections from these mice showed that the percentage of apoptotic cells (TUNEL spots) was significantly lower than that in ileal sections from infected mice (Fig. 4B). FLICA staining was also performed on the ileum tissues to detect caspase 3&7 activity. We did not observe activated caspase 3&7 in PBS and PCM control ileum sections. However, C. sakazakii infection induced the caspase 3&7 activation and PCM pretreatment significantly reduced caspase 3&7 activation (Fig. 4, C and D).
Fig. 4.
PCM attenuates apoptotic enterocyte cell death in C. sakazaii-infected ileum. Apoptotic cells in the paraffin-embedded ileum sections from each group were used in an immunoperoxidase-based TUNEL assay (A and B) and an activated caspase enzyme detection fluorescent labeled inhibitor of caspases (FLICA) caspase assay (C and D). A: images collected via a Nikon PL APO ×20 objective (n = 10 mice in each group). No apoptotic cells could be detected in ileal sections of mice treated with PBS or with PCM alone. C. sakasakii infection triggered severe apoptosis of epithelial cells in all examined ileal sections that was attenuated by PCM treatment of mice prior to infection. B: 10 villi and crypts of each section from PCM plus C. sakasakii and C. sakasakii-alone groups were observed under a light microscope, and the ratio of the number of TUNEL-positive cells to the total number of cells in the 10 villi and crypts was assigned as the apoptotic index (n = 10 mice in each group, means ± SE, Student's t-test: P < 0.05). C: active caspase-3 and -7 enzymes in paraffin-embedded ileal sections were labeled by a green fluorescent inhibitor probe FAM-DEVD-FMK (green). Nuclei were indicated with DAPI staining (blue), ×200 magnification. Treatment conditions are shown at bottom left of each image. D: 10 villi of each section from PCM plus C. sakasakii and C. sakasakii-alone groups were observed under a light microscope, and the ratio of the number of activated caspase 3&7 (green) cells to the total number of cells in the 10 villi (×100%) was calculated and graphed (n = 5 mice in each group, means ± SE, Student's t-test: P < 0.05).
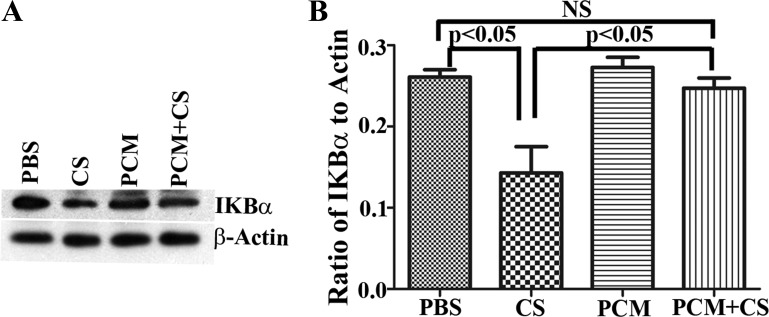
PCM prevents C. sakazakii-induced degradation of IκBα in ileal tissues.
We observed elevated levels of the inflammatory cytokine IL-1β and overt cell death in ileal tissues of C. sakazakii-infected mice. Since the NF-κB pathway plays an important role in innate intestinal inflammation by regulating inflammatory cell infiltration, host cell apoptosis, and production of inflammatory cytokines and chemokines (14), we looked for evidence of NF-κB activation. Western blot analysis of ileum lysates showed that levels of IκBα in mice infected with C. sakazakii were substantially reduced compared with those treated with PBS or PCM alone, a potential basis for increased NF-κB activation (Fig. 5, A and B). Levels of IκBα protein in the ileum of C. sakazakii-infected mice receiving PCM pretreatment were comparable to those in the ileum of PBS- and/or PCM-treated mice (Fig. 5, A and B). PCM attenuates C. sakazakii-triggered nuclear translocation of NF-κB p65 in human immature intestinal H4 cells.
Fig. 5.
PCM prevents a C. sakazakii-triggered decrease of IκBα in the ileum. A: Western blot analysis of ileal lysate showed that there was a substantial decrease in IκBα levels in C. sakazakii-infected ileum whereas levels of IκBα were maintained in mice pretreated with PCM. Data are shown here for 1 animal in each group. B: blots were scanned and signal intensities of IκBα and β-actin were measured with NIH Image J software. Data were represented as a ratio of IκBα to β-actin signal intensity (n = 8 mice in each group, mean ± SD, 1-way ANOVA: P < 0.05).
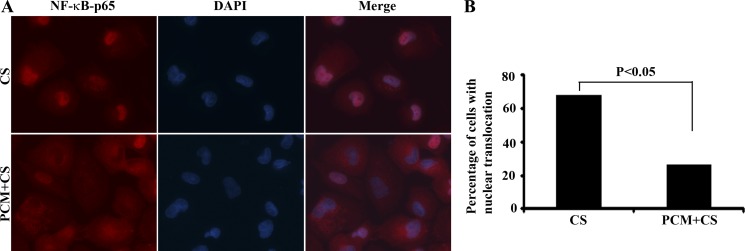
To further demonstrate that PCM pretreatment can abrogate C. sakazakii infection triggered activation of the NF-κB pathway, we looked for evidence of the translocation of NF-κB p65 into the nucleus. Because of significant ileal damage and cell loss in C. sakazakii-infected mice, we used a human immature intestine cell line (H4 cells) that was isolated from a 20-wk-old normal fetus (34). C. sakazakii infection stimulated the translocation of NF-κB p65 from the cytoplasm to the nucleus in 65% of cells (Fig. 6, A and B). However, nuclear translocation of NF-κB p65 was detected in only 25% of cells treated with PCM prior to C. sakazakii infection (Fig. 6, A and B).
Fig. 6.
PCM attenuates C. sakasakii-triggered nuclear translocation of NF-κB-P65 in fetal human intestinal H4 cells. A: treated H4 cells were processed for immunolabeling with antibody against NF-κB p65 (total) (red). Nuclei were indicated with DAPI staining (blue). B: quantitation of cells for nuclear translocation of NF-κB-p65. The percentage of cells with nuclear translocation of NF-κB-p65 was calculated and graphed (n = 110 cells in the C. sakazakii-treated group, n = 119 cells in the PCM plus C. sakazakii-treated group, 2-sided χ2 with Yates correction, χ2 = 38.19, P < 0.05).
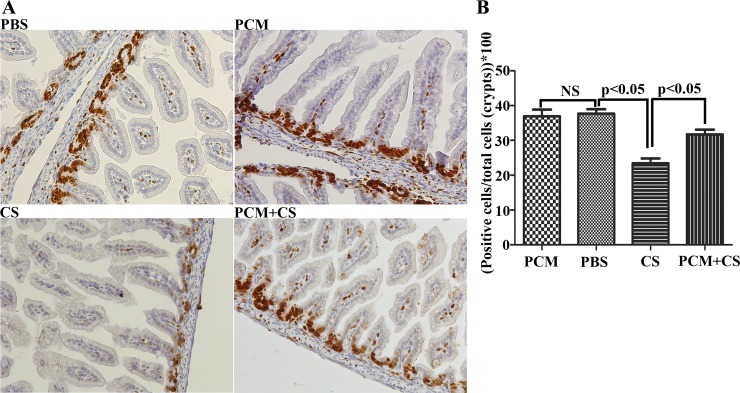
PCM restores the proliferative capacity of epithelial cells in C. sakazakii-infected ileum.
Having shown severe damage of the ileal epithelium in C. sakazakii-infected mice, we then examined whether C. sakazakii infection also affected the capacity of ileal cells to repair the damaged intestinal epithelia. We performed Ki67 staining to detect mitotic cells in ileal crypts. We found that there were fewer Ki67-positive dividing cells in ileal crypts from mice infected with C. sakazakii compared with mice treated with PBS and or with PCM alone (Fig. 7, A and B). In contrast, PCM pretreatment normalized the proliferation of the intestinal cells in the crypts of the C. sakazakii-infected mice (Fig. 7, A and B).
Fig. 7.
Proliferation of enterocytes in C. sakazaii-infected ileum is arrested and partially restored by pretreatment with PCM. A: dividing cells in ileal sections were detected by labeling with anti-Ki67 antibody followed by incubation with a horseradish peroxidase-conjugated secondary antibody and subsequent 3,3′-diaminobenzidine staining with Gill's hematoxylin counterstaining. Shown are digital images of ileal sections from one representative animal in each of 4 groups. B: Ki67-positive (brown and blue) and negative (blue only) cells in the crypts by digital imaging from 10 ileal sections in each group were counted. Means ± SD percent of Ki67-positive cells in ileal crypts were graphed (n = 10 mice in each group by using a 1-way ANOVA and post hoc Newman-Keuls test: P < 0.05; NS, no difference).
DISCUSSION
Bifidobacterium represents a prominent colonizing commensal bacterium found in the gut during the first weeks of life of the full-term human infant. However, colonization by this organism can be delayed and is isolated less often in preterm compared with full-term infants (20, 36). This disruption leads to a delay in adequate initial colonization, which favors colonization by potential pathogenic organisms such as C. sakazakii and is considered a risk factor in the etiology of NEC (15, 16, 18, 21). Butel et al. (3) showed for the first time that Bifidobacteria can have a protective effect in NEC in a gnotobiotic quail model. Other studies of NEC prevention in very low birth weight infants in the NICU setting support the protective effects of probiotics such as Bifidobacteria infantis. In fact, these studies observed that a combination of Lactobacillus acidophilus and Bifidobacteria infantis provides significant protection against NEC (23, 24). However, these studies were all performed outside of the United States because the FDA restricts the use of live organisms in immunocompromised premature infants. We recently showed that a 5- to 10-kDa molecule that is heat and acid stable and resistant to DNAse, RNAse, and protease was detected in the culture medium of these organisms; it significantly attenuated a LPS- and IL-1β-induced inflammatory response in a primary human fetal enterocyte cell line and in immature fetal small intestinal xenografts (12). In these studies we reported that the PCM of Bifidobacteria infantis grown separately from Lactobacillus acidophilus was a more effective anti-inflammatory agent. We have now extended these in vitro observations to an in vivo model of neonatal intestinal inflammation, demonstrating that B. infantis PCM protects neonatal mice from inflammation upon exposure to live C. sakazakii, an organism implicated in NEC (10, 35, 37). We showed that PCM attenuated C. sakazakii-induced inflammation and apoptosis of ileal epithelial cells and stimulated the proliferation of enterocytes in ileal crypts to repair the damaged ileal epithelium.
C. sakazakii, also referred to as Enterobacter sakazakii, was first reported as a neonatal pathogen by Urmenyi and Franklin (38). C. sakazakii is a gram-negative, rod-shaped, non-spore-forming bacterium (17) that is frequently a contaminant in powdered infant formulas, table foods, and kitchen counters (4, 9, 19). C. sakazakii has also been reported to cause meningitis and sepsis and is associated with NEC in preterm infants (11, 37, 39). These features make C. sakazakii a useful organism for developing an animal model to study mechanisms of neonatal inflammation. The dosage of C. sakazakii used to induce intestinal inflammation varied from 103 to 108 CFU (10, 22, 31). In this study, we gave newborn pups at postnatal day 5 a single does of 107 CFU C. sakazakii, which has been shown to cause a NEC-like condition in a previous study (22). Higher doses result in almost 100% mortality. Seventy-two hours after oral administration of C. sakazakii, intestinal tissues were collected for histopathological examination. We found that C. sakazakii-infected jejunum showed mild inflammation whereas ileum exhibited moderate epithelial sloughing, partial or severe disruption of villus architecture, and a neutrophil or macrophage infiltration. An increase in the expression of IL-1β and TNF-α mRNA was also noted. These observations are similar to the intestinal inflammatory characteristics of NEC in human infants (30).
Using this neonatal inflammatory model, we investigated whether and by what mechanism(s) PCM provides a protective effect against inflammation in vivo. We noted that the beneficial effect of PCM occurred in two ways. One was to promote the viability of ileum epithelium cells exposed to C. sakazakii, e.g., an antiapoptotic effect. The other was to nullify the inhibitory effect of C. sakazakii on the proliferation of epithelial cells needed to repair the inflammatory damaged epithelium. Our findings are consistent with other studies showing that certain strains of probiotics dramatically increase enterocyte migration, proliferation, and crypt height compared with vehicle-treated controls (25, 33). However, these studies used intact probiotic bacteria. Here, we suggest that an effective factor(s) of B. infantis (strain no. 15697) secreted into the medium can also provide protection against the development of neonatal inflammation in an in vivo murine model in a manner similar to that provided by live probiotics. Other investigators have reported intestinal protective effects of bacterial secreted factors (26).
We also provided a possible cellular mechanism underlying the protective effect of PCM against inflammation in this mouse model. We showed that PCM could maintain the levels of IκBα in ileal tissues of newborn mice infected with C. sakazakii, thereby preventing nuclear translocation and activation of NF-κB. The NF-κB signaling pathway plays an important role in innate immunity and gut inflammation (32). Both commensal and pathogenic bacteria express microbial-associated molecular patterns, which are recognized by pattern-recognition receptors [e.g., Toll-like receptors (TLRs)] present on the cell surface of intestinal epithelial cells. Once they engage TLRs, MyD88 recruits a cohort of signaling molecules and drives nuclear translocation of NF-κB after Iκβ phosphorylation and disassociation. The observation of a reduced IKBα level in fetal epithelial cells in response to inflammatory stimuli suggests that disruption of this cytoplasmic molecule that binds NF-κB and prevents nuclear translocation may be involved (6). Unlike pathogens that employ TLR pathways to trigger inflammation, commensal bacteria can inhibit the activation of NF-κB (7, 29). In our neonatal inflammation model, we found that B. infantis PCM could inhibit the NF-κB pathway by stabilizing the NF-κB/IκBα complex, which maintains NF-κB in the cytoplasm and prevents the nuclear translocation of NF-κB. The first layer of host defense in the gastrointestinal tract is the mucus layer.
Goblet cells, which are specialized epithelial cells, secrete protective molecules such as mucins and trefoil factor into the intestinal lumen, which coat epithelial villi. These data suggest that goblet cell function is altered during infection and PCM pretreatment may partly restore its function. It will be necessary in future studies to isolate and characterize the bioactive component of B. infantis PCM and determine how it stabilizes IκBα at a cellular level. When successful, the isolated factor could be added to expressed breast milk from mothers delivering premature infants and routinely fed to these infants, if found to be safe and efficacious in an initial pilot clinical trial.
In summary, we used a C. sakazakii-induced mouse model of neonatal intestinal inflammation to verify our previous in vitro observations that B. infantis-conditioned medium is protective against neonatal enterocytes and xenograft intestinal inflammation. This study along with our previous in vitro observations in human fetal intestine (28) suggests that the secreted component(s) of B. infantis-conditioned medium may be effective in preventing and or treating necrotizing enterocolitis in human infants without exposing the premature to live organisms.
GRANTS
This work was supported by grants from the National Institutes of Health R01-HD012437, R01-HD059126, P01 DK033506 and P30 DK040561 (W. A. Walker); R01 DK082427 (H. N. Shi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.W., H.N.S., and W.A.W. conception and design of research; M.W. performed experiments; M.W., H.N.S., and W.A.W. analyzed data; M.W. and H.N.S. interpreted results of experiments; M.W. and H.N.S. prepared figures; M.W. drafted manuscript; K.G., W.Z., H.N.S., and W.A.W. edited and revised manuscript; H.N.S. and W.A.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Bobby Cherayil, Mucosal Immunology and Biology Research Center, Department of Pediatrics, Mass General Hospital for Children, for kindly providing the IKBα antibody, and Patricia Della Pelle, NORC-H Morphology Core, Massachusetts General Hospital, for helping with Ki67 and PAS staining.
REFERENCES
- 1.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 147: 192–196, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Braga TD, da Silva GA, de Lira PI, de Carvalho Lima M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 93: 81–86, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Butel MJ, Roland N, Hibert A, Popot F, Favre A, Tessedre AC, Bensaada M, Rimbault A, Szylit O. Clostridial pathogenicity in experimental necrotising enterocolitis in gnotobiotic quails and protective role of bifidobacteria. J Med Microbiol 47: 391–399, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Chon JW, Song KY, Kim SY, Hyeon JY, Seo KH. Isolation and characterization of Cronobacter from desiccated foods in Korea. J Food Sci 77: M354–M358, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Christensen RD, Gordon PV, Besner GE. Can we cut the incidence of necrotizing enterocolitis in half—today? Fetal Pediatr Pathol 29: 185–198, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Claud EC, Lu L, Anton PM, Savidge T, Walker WA, Cherayil BJ. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc Natl Acad Sci USA 101: 7404–7408, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collier-Hyams LS, Zeng H, Sun J, Tomlinson AD, Bao ZQ, Chen H, Madara JL, Orth K, Neish AS. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-κB pathway. J Immunol 169: 2846–2850, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 125: 921–930, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Drudy D, Mullane NR, Quinn T, Wall PG, Fanning S. Enterobacter sakazakii: an emerging pathogen in powdered infant formula. Clin Infect Dis 42: 996–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Emami CN, Mittal R, Wang L, Ford HR, Prasadarao NV. Recruitment of dendritic cells is responsible for intestinal epithelial damage in the pathogenesis of necrotizing enterocolitis by Cronobacter sakazakii. J Immunol 186: 7067–7079, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Friedemann M. Epidemiology of invasive neonatal Cronobacter (Enterobacter sakazakii) infections. Eur J Clin Microbiol Infect Dis 28: 1297–1304, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Ganguli K, Meng D, Rautava S, Lu L, Walker WA, Nanthakumar N. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am J Physiol Gastrointest Liver Physiol 304: G132–G141, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gephart SM, McGrath JM, Effken JA, Halpern MD. Necrotizing enterocolitis risk: state of the science. Adv Neonatal Care 12: 77–87; quiz 88–79, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol 8: 837–848, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Gothefors L, Blenkharn I. Clostridium butyricum and necrotising enterocolitis. Lancet 1: 52–53, 1978 [DOI] [PubMed] [Google Scholar]
- 16.Howard FM, Flynn DM, Bradley JM, Noone P, Szawatkowski M. Outbreak of necrotising enterocolitis caused by Clostridium butyricum. Lancet 2: 1099–1102, 1977 [DOI] [PubMed] [Google Scholar]
- 17.Iversen C, Waddington M, On SL, Forsythe S. Identification and phylogeny of Enterobacter sakazakii relative to Enterobacter and Citrobacter species. J Clin Microbiol 42: 5368–5370, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelsey MC, Vince AJ. Clostridia in neonatal faeces. Lancet 2: 100, 1979 [DOI] [PubMed] [Google Scholar]
- 19.Kilonzo-Nthenge A, Rotich E, Godwin S, Nahashon S, Chen F. Prevalence and antimicrobial resistance of Cronobacter sakazakii isolated from domestic kitchens in middle Tennessee, United States. J Food Prot 75: 1512–1517, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Kleessen B, Bunke H, Tovar K, Noack J, Sawatzki G. Influence of two infant formulas and human milk on the development of the faecal flora in newborn infants. Acta Paediatr 84: 1347–1356, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Lawrence G, Bates J, Gaul A. Pathogenesis of neonatal necrotising enterocolitis. Lancet 1: 137–139, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Lee HA, Hong S, Park H, Kim H, Kim O. Cronobacter sakazakii infection induced fatal clinical sequels including meningitis in neonatal ICR mice. Lab Anim Res 27: 59–62, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, Tsao LY, Chen CH, Su BH. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 122: 693–700, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, Oh W. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 115: 1–4, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Lin PW, Nasr TR, Berardinelli AJ, Kumar A, Neish AS. The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr Res 64: 511–516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host system. Cell 122: 107–118, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res 92: 71–77, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu K, Uauy R, Llano A, Claud EC. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PloS One 6: E17776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, Rao AS, Madara JL. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science 289: 1560–1563, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 364: 255–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagotto FJ, Nazarowec-White M, Bidawid S, Farber JM. Enterobacter sakazakii: infectivity and enterotoxin production in vitro and in vivo. J Food Prot 66: 370–375, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Pasparakis M. Role of NF-kappaB in epithelial biology. Immunol Rev 246: 346–358, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Preidis GA, Saulnier DM, Blutt SE, Mistretta TA, Riehle KP, Major AM, Venable SF, Finegold MJ, Petrosino JF, Conner ME, Versalovic J. Probiotics stimulate enterocyte migration and microbial diversity in the neonatal mouse intestine. FASEB J 26: 1960–1969, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanderson IR, Ezzell RM, Kedinger M, Erlanger M, Xu ZX, Pringault E, Leon-Robine S, Louvard D, Walker WA. Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci USA 93: 7717–7722, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmons BP, Gelfand MS, Haas M, Metts L, Ferguson J. Enterobacter sakazakii infections in neonates associated with intrinsic contamination of a powdered infant formula. Infect Control Hosp Epidemiol 10: 398–401, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Stark PL, Lee A. The bacterial colonization of the large bowel of pre-term low birth weight neonates. J Hyg (Lond) 89: 59–67, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Townsend S, Hurrell E, Forsythe S. Virulence studies of Enterobacter sakazakii isolates associated with a neonatal intensive care unit outbreak. BMC Microbiol 8: 64, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urmenyi AM, Franklin AW. Neonatal death from pigmented coliform infection. Lancet 1: 313–315, 1961 [DOI] [PubMed] [Google Scholar]
- 39.van Acker J, de Smet F, Muyldermans G, Bougatef A, Naessens A, Lauwers S. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J Clin Microbiol 39: 293–297, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]