Abstract
The objective of this study was to determine the effect of increased physical activity on subsequent sleeping energy expenditure (SEE) measured in a whole room calorimeter under differing levels of dietary fat. We hypothesized that increased physical activity would increase SEE. Six healthy young men participated in a randomized, single-blind, crossover study. Subjects repeated an 8-day protocol under four conditions separated by at least 7 days. During each condition, subjects consumed an isoenergetic diet consisting of 37% fat, 15% protein, and 48% carbohydrate for the first 4 days, and for the following 4 days SEE and energy balance were measured in a respiration chamber. The first chamber day served as a baseline measurement, and for the remaining 3 days diet and activity were randomly assigned as high-fat/exercise, high-fat/sedentary, low-fat/exercise, or low-fat/sedentary. Energy balance was not different between conditions. When the dietary fat was increased to 50%, SEE increased by 7.4% during exercise (P < 0.05) relative to being sedentary (baseline day), but SEE did not increase with exercise when fat was lowered to 20%. SEE did not change when dietary fat was manipulated under sedentary conditions. Physical activity causes an increase in SEE when dietary fat is high (50%) but not when dietary fat is low (20%). Dietary fat content influences the impact of postexercise-induced increases in SEE. This finding may help explain the conflicting data regarding the effect of exercise on energy expenditure.
Keywords: sleeping energy expenditure, physical activity, dietary fat
exercise increases resting energy expenditure (REE) acutely, and it remains elevated for a variable period of time after the exercise bout has concluded (3, 4, 6). The postexercise-induced increase in REE can be divided into two phases. The first, acute phase, encompasses the oxygen debt whereby the increased energy expenditure (EE) is attributed to the replenishment of energy stores and the increase in EE is relative to the postabsorptive condition. The second, transient phase, represents a varying increase in REE. This later and persistent rise in REE, commonly termed the excess postexercise oxygen consumption (EPOC), is thought to be due to increased sympathetic nervous system activity, substrate cycling, or both (10, 11).
Although several studies have observed an increase in REE following exercise, not all studies have been able to reproduce this effect. For example, the increase in EE after exercise ranged from 4.7% in the study by Bielinski et al. (9) to 14% in the studies by Bahr et al. (5). In contrast, Segal and colleagues suggested that the magnitude of this effect was trivial (24, 25), and, interestingly, Brehm and Gutin (13) were unable to demonstrate this effect at all. Exercise intensity may be an important determinant of the magnitude of the rise in oxygen consumption following exercise. In an elegant study, Bahr and Sejersted (7) demonstrated that exercise intensities above 40% of maximal were necessary to produce a significant increase in the excess EE after exercise. They also showed that the duration of exercise was important (5).
Dietary fat may also be an important factor. In one of the few studies where dietary fat content in the postexercise period was described, Freedman-Akabas et al. (20) found no EPOC in subjects consuming a high-carbohydrate, low-fat diet. Our group and others (14, 15, 17, 21, 26) have shown that fat oxidation is increased during submaximal exercise while a high-fat diet is consumed. Given that there is an increased oxygen cost associated with lipolysis, we hypothesized that oxygen consumption during submaximal exercise would be increased by a high-fat diet and that this would likely lead to increased EPOC.
The aim was thus twofold: first, to demonstrate an increase in EPOC on EE measured during sleep in a metabolic chamber and second, to test whether EPOC induced by the same level of exercise (1.8 × REE) differs with dietary fat content. Therefore, in this controlled study, we examined the effect of exercise on sleeping energy expenditure (SEE) measured in a respiratory chamber under two dietary conditions, divergent on the basis of dietary fat.
METHODS
Parent project.
These data were collected in a study conducted under USDA Project No. 9634323-3031 entitled “Dietary Fat and Obesity,” whose overall objective was to understand the relationship between dietary fat intake and obesity. This substudy was designed to test the hypothesis that exercise enhances the adaptation to a high-fat diet by studying men in the sedentary and active state in a respiration calorimeter. This unique study design provides the opportunity to investigate the interaction between exercise and dietary fat on postexercise energy expenditure (or oxygen consumption). The study was approved and monitored by the Pennington Biomedical Institutional Review Board and all subjects provided informed consent prior to the start of their participation.
Study subjects.
Subjects were recruited via print advertising targeting healthy males aged 18–30 yr with a body mass index between 19 and 35 kg/m2. Subjects were excluded for smoking, weight fluctuation ≥3kg within the previous 6 mo, significant abnormal laboratory findings, or concerns during physical examination.
Design.
This is a post hoc analysis performed in 2012 of a study that was conducted in 1997 prior to clinicaltrials.gov. Six male subjects participated in a 2 × 2 factorially designed study. Each subject completed an 8-day diet and exercise protocol on four occasions separated by a washout period of at least 7 days (Fig. 1). The first 4 days were outpatient and the remaining 4 days were spent in a metabolic chamber. For days 1–5 (4 days outpatient + 1 day in the chamber), subjects consumed a standard weight maintenance diet that provided 37% of energy from fat. For the following 3 days, the subjects were assigned in random order to one of the following diet/activity conditions: high-fat/exercise, high-fat/sedentary, low-fat/exercise, or low-fat/sedentary. Dietary fat content was either raised to 50% or decreased to 20% for the high-fat and low-fat conditions, respectively. In all diets, protein intake was held constant and relative to the total EE throughout the study. Each diet condition was repeated under low (sedentary) and high (exercise) levels of physical activity. Exercise in each condition was standardized on the basis of exercise EE. The exercise condition required EE equivalent to REE × 1.8 or a high level of physical activity. Given that subjects confined in a metabolic chamber have reduced levels of physical activity, exercise was also performed in the sedentary condition, such that exercise EE reached REE × 1.4, or a low level of physical activity, in order to mimic free-living conditions (see Eq. 1). All exercise EE was achieved by treadmill walking at 3 mph and 3% incline (calculated by Eq. 2). The rate of EE (kcal/min) was determined in a submaximal exercise test to determine the treadmill time needed to achieve the desired level of physical activity. All energy expended in exercise was replaced by energy in the diet to preserve 24-h energy balance.
Fig. 1.
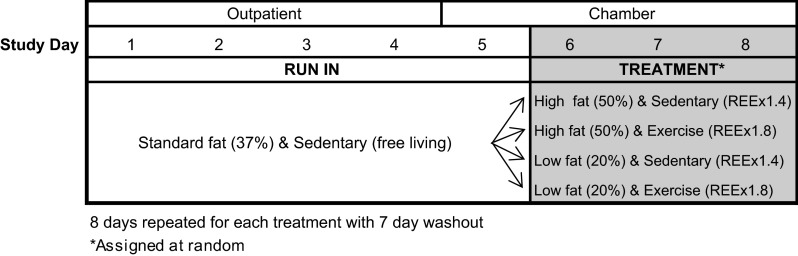
Study design.
Prediction of energy requirements prior to entry into the metabolic chamber.
The expected EE for individuals in the chamber was estimated from REE, measured after an overnight fast in a semirecumbent position using a ventilated hood system (model 2900Z Metabolic Cart; Sensormedics, Yorba Linda, CA) and a 3-day measurement of free-living EE using a triaxial activity monitor (Tritrac; Hemokinetics, Madison, WI). To account for the 15% reduction in physical activity in the metabolic chamber compared with free-living conditions or on the metabolic ward (19), the expected EE in the chamber was calculated as in Eq. 1: expected sedentary EE in metabolic chamber = (REE + free-living EE by Tritrac) × 0.85.
The energy cost of physical activity during the standardized exercise protocol (treadmill walking at 3 mph and 3% incline) was calculated by measuring steady-state V̇o2 with a metabolic cart (Sensormedics Vmax series 29). Briefly, the test began with a warm-up at 3 mph and 0% incline for 2 min. The incline was raised to 3% and EE measured continuously for the next 10 min. The steady-state EE during the 10 min period was used to calculate the treadmill time needed to achieve daily EE of REE × 1.4 for the sedentary condition and REE × 1.8 for the exercise condition as in Eq. 2: treadmill time (min) = [daily EE (REE × PAL, kcal/24 h) − expected sedentary EE in metabolic chamber, kcal/24 h]/(exercise EE @ 3 mph and 3% incline, kcal/min).
Maximal V̇o2 (V̇o2max) was measured during exercise treadmill testing to exhaustion at the same visit using the same equipment. The exercise intensity for the chamber exercise protocol corresponded to ∼35% of the V̇o2max for this group of subjects.
Chamber protocol and maintenance of energy balance.
For each diet and exercise condition, subjects spent 4 consecutive days in the metabolic chamber. For day 1, subjects entered the chamber in the morning (0800) after a 10-h fast and overnight stay in the inpatient unit. On each day in the chamber, three meals (breakfast, lunch, and dinner) were provided at scheduled intervals: 0900, 1230, and 1800. Snacks (provided at 1600 and 2100) were used to buffer the energy expended during the exercise and to maintain energy balance. For exercise, to achieve a total daily EE at the targeted level (REE × 1.4 for sedentary conditions or REE × 1.8 for exercise conditions), subjects walked on the treadmill at 3 mph and 3% incline three times each day (midmorning, midafternoon, and prior to the bedtime snack). The last exercise bout was performed at 2000 and occurred between dinner and snack; ∼6 h prior to sleeping (S)EE measurement. Lights were turned off at 2230. SEE was calculated from the EE recorded between 0200 and 0500 for all minutes with activity <1% (measured by radar) and extrapolated to 24h. At 0700 each morning, the subjects were awakened and exited the chamber for 60 min to be able to bathe and for the chamber to be cleaned and calibrated. Vital signs and body weight were measured at this time; however, physical activity on the metabolic ward was restricted. The EE while outside the chamber for these 60 min was extrapolated using the EE data obtained from 0900 to 1030. At the end of each 24-h period, 24-h EE was compared with energy intake to estimate energy balance. For the purposes of this interim calculation, metabolizable energy intake was assumed to be 90% of the total daily energy intake. Based on the interim energy balance results, three options were available. If the energy balance was less than ±100 kcal, the energy intake and exercise time were not changed. If the energy balance was estimated to be >100 kcal, either energy intake was decreased and/or the treadmill time increased to move the subject toward energy balance (within ±100 kcal). If the energy balance was estimated to be <−100 kcal, either energy intake was increased and/or the treadmill time decreased to move the subject toward energy balance. Only rarely was an adjustment of greater than ±300 kcal necessary.
Body composition.
Body composition was measured by dual-energy X-ray absorptiometry (DEXA) using a QDR 2000 (Hologic, Waltham, MA). Body weight was measured fasting each day prior to entry into the metabolic chamber and upon completion of the 4-day metabolic chamber stay.
Aerobic fitness.
Aerobic fitness was assessed by measuring V̇o2max by Sensormedics metabolic cart during a graded treadmill exercise testing (TrueMax 2400; ParvoMedics, Salt Lake City, UT) to exhaustion.
Design of metabolic diets.
For the run-in period (first 5 days of each 8-day protocol: 4 outpatient days + 1 chamber day), diets were designed to achieve 37% fat, 48% carbohydrate, and 15% protein. The high-fat diet condition provided 50% fat, 35% carbohydrate, and 15% protein; the low-fat diet condition provided 20% fat, 65% carbohydrate, and 15% protein. Protein content remained fixed, and the adjusted fat percentage was compensated for by an increase or decrease in carbohydrate content. For each subject and for each day in the chamber, duplicate meals were prepared and sent to the Food Chemistry Laboratory for chemical analysis.
Calculation of energy and macronutrient balances.
EE and substrate oxidations were calculated from V̇o2, V̇co2, and urinary nitrogen using the equations of Acheson et al. (2, 13). The calculated macronutrient oxidations (g) were converted to kilocalories using the Atwater factors, (4.442/4.183/9.461 kcal/g for protein, carbohydtrate, and fat, respectively). Urine was collected for each 24-h period, and nitrogen was measured on each sample to calculate protein oxidation. The values for macronutrient oxidation were then subtracted from the energy, fat, carbohydrate, and protein intake determined by chemical analysis to calculate daily energy and macronutrient balances.
Analytic methods.
Urinary nitrogen was measured using pyrochemiluminescence on an Antek 735 nitrogen analyzer (Antek Instruments, Houston, TX). Urinary creatinine was measured using the Jaffe rate reaction on a Beckman Synchron CX7 (Beckman Instruments, Brea, CA).
Food composites were collected, weighed, homogenized, and frozen at −20°C until analyzed. Composites were analyzed for moisture, protein, fat, and ash. Moisture was analyzed using a Labwave 9000 microwave oven (CEM, Matthews, NC). Protein was analyzed using a combustion method on a PerkinElmer Series II 2410 nitrogen analyzer (PerkinElmer, Norwalk, CT). Ash was measured using a MAS 7000 microwave muffle furnace (CEM). Carbohydrate was calculated by difference: carbohydrate = 100 − (ash + protein + fat + moisture). Energy content was determined using standard formulas (9 kcal/g fat, 4 kcal/g protein, and 4 kcal/g carbohydrate).
Statistical methods.
Data were analyzed using SAS (version 6.12). The response variables were analyzed using PROC MIXED with day, activity, and diet as the variables. Bonferoni-Dunn was used to correct for multiple comparisons. Baseline values were included as covariates for all of the models. Significance was set a priori at a P value of <0.05. All values are presented as mean ± SE unless otherwise noted.
RESULTS
The study population included healthy young men with similar anthropometric characteristics (Table 1). These healthy, but sedentary, subjects were able to complete successively the protocol with minimal musculoskeletal complaints related to the treadmill exercise. Body weight was maintained throughout study participation; mean weight change was −0.2 ± 1.1 kg. The average time on the treadmill during the exercise conditions (REE × 1.8) was 147 ± 12 min compared with 33 ± 10 min for the sedentary conditions (REE × 1.4). An assessment of indirect calorimetry during the exercise protocol showed that exercise was undertaken at a low exercise intensity, or ∼34.8 ± 2.1% of the V̇o2max.
Table 1.
Characteristics of the study population (n = 6)
| Population Characteristics | Mean ± SD | Range |
|---|---|---|
| Age, yr | 24 ± 4.8 | 19–27 |
| Height, m | 1.75 ± 0.005 | 1.68–1.83 |
| Weight, kg | 71.5 ± 5.9 | 61.2–81.4 |
| BMI, kg/m2 | 23.6 ± 5.8 | 19.8–27.7 |
| Body fat, % | 15.3 ± 5.9 | 8.3–25.7 |
| V̇o2max, ml/kg/min | 50.4 ± 5.9 | 39.9–55.2 |
| Postabsorptive RQ | 0.84 ± 0.06 | 0.76–0.92 |
| Sleeping EE, (kcal/day, day 1) | 1694 ± 116 | 1478–1858 |
| Sleeping npRQ (day 1) | 0.864 ± 0.026 | 0.81–0.92 |
| Activity EE (by Tri-Trac, kcal/day) | 2490 ± 369 | 2053–3128 |
| EE/RMR ratio | 1.48 ± 0.22 | 1.2–1.8 |
Data are presented as mean ± SD; n = 6. EE/RMR ratio is calculated by dividing the activity energy expenditure (EE) by Tri-Trac (kcal/day) by the resting metabolic rate (RMR). This value provides an index of free-living activity corrected for body size (RMR). npRQ, nonprotein respiratory quotient.
Chemical analysis of the food composites showed that dietary fat intake was not different from the target value of 20% during the low-fat diet or 50% during the high-fat diet. As shown in Table 2, daily energy intake was closely matched to daily EE, deviating by less than 250 kcal on any given day, which is less than 10% of 24-h EE. Energy balance was not significantly different between the chamber days in any of the four diet and exercise conditions (P > 0.05; Table 2).
Table 2.
Energy balance
| Study Day 5BL (Chamber day 1) | Study Day 6 (Chamber day 2) | Study Day 7 (Chamber day 3) | Study Day 8 (Chamber day 4) | |
|---|---|---|---|---|
| High fat (50%) | ||||
| Sedentary | 289 ± 85 | 199 ± 116 | 187 ± 43 | 183 ± 46 |
| Exercise | 213 ± 101 | −9 ± 122* | 146 ± 86 | 213 ± 52 |
| Low fat (20%) | ||||
| Sedentary | 181 ± 86 | 64 ± 48 | 246 ± 34 | 185 ± 28 |
| Exercise | 191 ± 35 | 160 ± 57 | 272 ± 52 | 197 ± 47 |
Data are presented as mean ± SE in kcal/day; n = 6. Energy balance was calculated as follows: total daily energy expenditure (Vo2 measured in the metabolic chamber) − [intake (duplicate meal analysis) − fecal energy (bomb calorimetry)].
Significant difference from baseline chamber day (study day 5). BLEnergy balance of baseline (37% dietary fat and sedentary conditions) measurement prior to each condition listed.
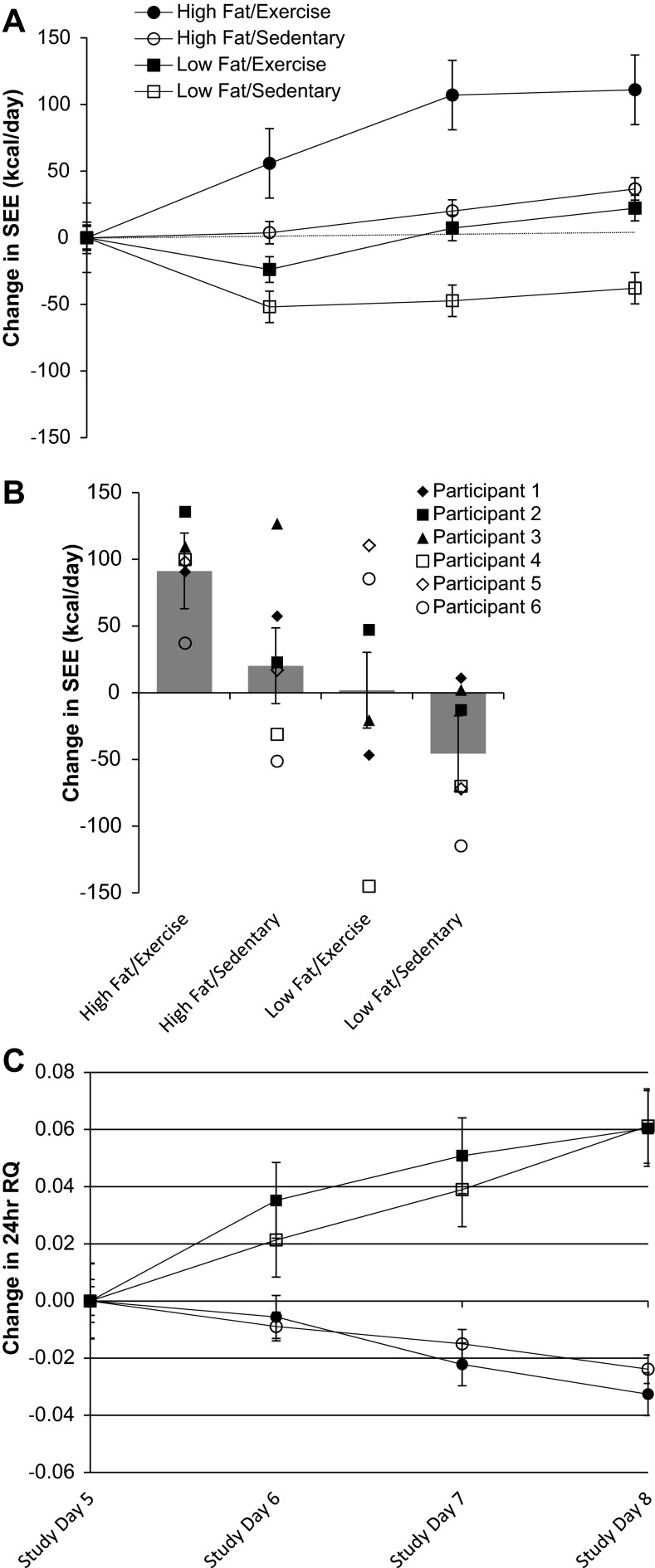
As shown in Fig. 2A, while maintaining 24-h energy balance, the addition of exercise to the high-fat diet significantly increased SEE, whereas exercise in conjunction with a low-fat diet failed to change SEE from the baseline condition. Figure 2B shows that the individual level data and the response to the high-fat/exercise condition and low-fat/sedentary condition had the smallest degree of intraindividual variability. The individual responses to the high -fat/sedentary and low-fat/exercise were more variable (Fig. 2B). Repeated-measures ANOVA showed a significant interaction effect for diet/exercise condition and day (P = 0.02) and a main effect for diet/exercise condition (P < 0.0001) but no significant effect for day (P = 0.26). The post hoc analysis comparing diet/exercise conditions indicated that the mean increase in SEE with the high-fat/exercise condition differed significantly from the mean change in SEE following the other three conditions: high-fat/sedentary condition (P = 0.002), low-fat/exercise condition (P < 0.001), and low-fat/sedentary condition (P < 0.0001). As for within-group effects, the change in SEE from baseline was significant only in the high-fat/exercise condition (P < 0.0001). The changes in SEE in the high-fat/sedentary and low-fat/exercise conditions were not significant (P = 0.5 and P = 0.3, respectively).
Fig. 2.
Mean (A) and individual changes in sleeping energy expenditure (SEE; B) and mean respiratory quotient (RQ; C) in response to exercise and dietary fat over 3 days in a metabolic chamber; n = 6. SEE (kcal/day) measured from 0200 to 0500. Data are presented as means ± SE. *Significant change from baseline.
As expected, a decrease in dietary fat increased respiratory quotient (RQ) under both sedentary and exercise conditions (24-h RQ; Fig. 2C). There was no additional effect of exercise on RQ in either the high-fat or low-fat conditions. The results of the high-fat diet on macronutrient oxidation have been previously reported (27); yet pertinent to this paper is the significant increase in fat oxidation with a high-fat diet, where an increase in fat oxidation was observed from a significant decrease in nonprotein respiratory quotient (npRQ) from baseline (P < 0.05) under exercise vs. sedentary conditions.
DISCUSSION
The purpose of our study was to evaluate the increase in SEE in response to exercise under controlled conditions in a metabolic chamber and to determine whether the effect of exercise-induced SEE was different with diets containing either low or high fat. Using a single-blind, repeated-measures, crossover design, we found that SEE was increased in response to exercise by ∼7.4% (P < 0.05) when energy from dietary fat was increased to 50%. However, exercise did not increase SEE when dietary fat was decreased to 20%. To the contrary, when the energy from fat was reduced to 20%, we observed a slight decrease in SEE from baseline. This interaction between dietary fat and exercise was observed consistently across three consecutive days in each diet/exercise condition. We hypothesized that the oxygen cost of exercise while consuming a high-fat diet would be increased owing to the likely increase in fat oxidation and associated lipolysis. In support of previous studies, we observed that consumption of a high-fat diet for 3 days resulted in higher fat oxidation, as demonstrated by the significant decrease in RQ with the high-fat diet conditions. However, this is obviously not the only mechanism to explain the increased SEE with the high-fat/exercise condition, since we did not observe differences in RQ or substrate oxidation between the high-fat/exercise or high-fat/sedentary conditions. Exercise alone is a stimulus for EPOC; however, we can rule out that exercise intensity contributed to our findings. This study was not designed to determine the mechanisms by which increased physical activity leads to increased postexercise EE, and unfortunately we do not have additional measures needed to explore this further. Several investigators have proposed that EPOC is due to an increase in sympathetic nervous system (SNS) outflow. Meals high in dietary fat are likely to have a lower rather than a higher stimulation of SNS activity (18), as suggested by our results. One alternative explanation is that the high-fat diet, through the delay in gastric emptying induced by the fat, results in a delayed thermic effect of food (TEF). Although we cannot directly rule out this possibility, we believe it is unlikely for three reasons. First, the last food was consumed ∼5 h before the measurement of SEE. Second, the thermic effect of food is smaller with high-fat than with low-fat diets (1, 23). Third, SEE was not increased during the sedentary highfat (50%) condition. If delayed gastric emptying/TEF contributed to the observed increase in SEE, we would have expected an increase in SEE when fat was increased, but the subjects were sedentary. This was not the case.
We acknowledge that the “standard” approach to studying the increase in energy expenditure following exercise (EPOC) is to use a single bout of exercise followed by serial measures of REE with a bedside calorimeter. Our approach was to increase physical activity over the course of the day and measure EE continuously following daily exercise as SEE in a respiratory chamber while maintaining daily energy balance. It could be argued that measurement of SEE ∼6 h after the last bout of exercise underestimates the magnitude of increase in exercise-induced EE. Nonetheless, our protocol has the advantage that the exercise was similar to many types of leisure time activities and the measurement of SEE authenticates the estimate of REE because it is the most robust measurement of EE, as it is obtained during a natural daily occurrence, i.e., sleep. Furthermore, the chamber allows for controlled scheduling of meals, exercise times, and adjustments of both energy intake and EE to achieve a daily energy balance and therefore tease out the direct effect of exercise and diet on exercise-induced changes in SEE.
This study is therefore one of a kind. Performing serial measurements of whole body EE in human subjects with controlled diets is very costly and burdensome; hence, the number of subjects in this study is small. However, despite the small number of subjects studied, the crossover and repeated-measures design adds to the statistical power of the findings. As shown, the physiological responses in each diet/exercise condition were not significantly different across the three study days. It is important to note the high fitness level of the study's subjects (as shown by V̇o2max) compared with the general population. While this fitness status was not a goal for enrollment, it is important to be aware of when considering the results of this study. Due to the low exercise intensity of the study's exercise condition (∼35% V̇o2max), it would be reasonable to assume for an individual with a lower fitness level, exercise intensity would be increased to a moderate intensity (no more than 50% V̇o2max) under the same exercise conditions. However, the goal of this study was to assess the interaction of diet and exercise on EPOC while subjects were in energy balance, and we would assume that for an individual with a lower fitness level, exercising at a higher intensity would have the same findings, as energy balance would be maintained with caloric intake being adjusted accordingly.
This study is important because it demonstrates that the macronutrient composition of the diet can influence EE after exercise during conditions of energy balance. Most previous studies on the effect of exercise on postexercise EE did not control for dietary macronutrient intake preceding or after the exercise period or attempt to replace the calories expended in the exercise bout. For those studies that attempted to measure this effect on subsequent days, most did not report the dietary intake or diet composition. Our results suggest that dietary macronutrient composition is important and should be considered in the design of experiments that attempt to measure EPOC over an extended time period. In support of our observation, a recent report by Børsheim et al. (12) showed that manipulation of a moderate- to high-fat diet (40% of energy) with fatty acids can influence EPOC. Using a cross-sectional design, a 9% increase in EPOC was observed following exercise performed while subjexts consumed a diet enriched with oleic acid compared with a diet enriched with palmitic acid, that showed no measureable increase in EPOC (12). Although this result did not quite reach statistical significance (P = 0.06), the study reinforces in a cross-sectional design the current hypothesis that dietary composition, and in particular dietary fat, can influence EPOC following aerobic exercise.
Indeed, previous work has indicated that EPOC may be evident only beyond a particular threshold of aerobic exercise capacity (i.e., 40% V̇o2max) and following an exercise bout of a particular exercise duration (7); however, a study of young, sedentary, overweight African American subjects showed no change in EPOC following resistance training with equated work of either low or high intensity (29). Our study subjects' exercise intensity was maintained at ∼35% of V̇o2max in each treatment condition, a low intensity. Furthermore, exercise duration was duplicated in the two exercise conditions (147 ± 12 min); therefore, our data do not support the notion that exercise intensity or duration of exercise are determinants of EPOC.
In an attempt to unravel the inconsistent reports of exercise on EPOC, recent studies have examined the determinants of EPOC on the basis of cardiorespiratory fitness and body composition. (16, 28). However, body composition, in particular fat-free mass, has been shown to be positively associated with EPOC following aerobic exercise independently of maximal aerobic capacity (16). Furthermore, after adjustment of EPOC for fat-free mass, the reportedly higher EPOC in men compared with women is no longer evident (22). In this study, energy balance was maintained throughout each diet/exercise condition, and we observed no significant change in body weight or composition during the study. Therefore, our data do not support the notion that interindividual differences in body weight might explain the presence of exercise-induced EPOC. Furthermore, we did not observe an association between fat-free mass and EPOC (data not shown); however, we acknowledge our sample size is small, but nevertheless there was a large variability in fat-free mass (51.8–68.4 kg) among our subjects, such that, if present, a relationship with EPOC and fat-free mass could be detected.
In summary, our results demonstrate an interaction between dietary fat content and physical activity on SEE. When dietary fat is low, increased SEE is not apparent 8–11 h later. When dietary fat is high, an exercise-induced increase in SEE is apparent. Moreover, while previous studies suggest that body weight and composition are determinants of exercise-induced EPOC, our cross-over study design in six healthy young men in energy balance showed that diet composition, and not body weight or composition, is a potential source of variance in previous reports. This study demonstrates that dietary fat content should be considered in future studies of EPOC, particularly when examining the late phase of EPOC (more than 8 h after exercise). Furthermore, previous findings on the presence of EPOC should be interpreted with consideration of dietary intake and energy balance.
GRANTS
This work was supported by the US Department of Agriculture Grant 96034323-3031. L. M. Redman is supported by NIH Pathway to Independence award, R00 HD-060762.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.A.F., L.M.R., and S.R.S. interpreted results of experiments; E.A.F. edited and revised manuscript; L.M.R. and S.R.S. drafted manuscript; L.d.J., J.C.R., J.J.Z., J.V., and G.A.B. conception and design of research.
ACKNOWLEDGMENTS
We acknowledge the participation of the subjects, and Susan Mancuso RN for detailed study coordination.
REFERENCES
- 1.Abbott WG, Howard BV, Ruotolo G, Ravussin E. Energy expenditure in humans: effects of dietary fat and carbohydrate. Am J Physiol Endocrinol Metab 258: E347–E351, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Acheson KJ, Schutz Y, Bessard T, Ravussin E, Jéquier E, Flatt JP. Nutritional influences on lipogenesis and thermogenesis after a carbohydrate meal. Am J Physiol Endocrinol Metab 246: E62–E70, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Bahr R. Excess postexercise oxygen consumption—magnitude, mechanisms and practical implications. Acta Physiol Scand Suppl 605: 1–70, 1992 [PubMed] [Google Scholar]
- 4.Bahr R, Gronnerod O, Sejersted OM. Effect of supramaximal exercise on excess postexercise O2 consumption. Med Sci Sports Exerc 24: 66–71, 1992 [PubMed] [Google Scholar]
- 5.Bahr R, Ingnes I, Vaage O, Sejersted OM, Newsholme EA. Effect of duration of exercise on excess postexercise O2 consumption. J Appl Physiol 62: 485–490, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Bahr R, Sejersted OM. Effect of feeding and fasting on excess postexercise oxygen consumption. J Appl Physiol 71: 2088–2093, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Bahr R, Sejersted OM. Effect of intensity of exercise on excess postexercise O2 consumption. Metabolism 40: 836–841, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Bielinski R, Schutz Y, Jequier E. Energy metabolism during the postexercise recovery in man. Am J Clin Nutr 42: 69–82, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Borsheim E, Bahr R, Hansson P, Gullestad L, Hallén J, Sejersted OM. Effect of beta-adrenoceptor blockade on post-exercise oxygen consumption. Metabolism 43: 565–571, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Borsheim E, Bahr R, Knardahl S. Effect of beta-adrenoceptor stimulation on oxygen consumption and triglyceride/fatty acid cycling after exercise. Acta Physiol Scand 164: 157–166, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Borsheim E, Kien CL, Pearl WM. Differential effects of dietary intake of palmitic acid and oleic acid on oxygen consumption during and after exercise. Metab Clin Exper 55: 1215–1221, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brehm BA, Gutin B. Recovery energy expenditure for steady state exercise in runners and nonexercisers. Med Sci Sports Exerc 18: 205–210, 1986 [PubMed] [Google Scholar]
- 14.Burke LM, Hawley JA, Angus DJ, Cox GR, Clark SA, Cummings NK, Desbrow B, Hargreaves M. Adaptations to short-term high-fat diet persist during exercise despite high carbohydrate availability. Med Sci Sports Exerc 34: 83–91, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Cameron-Smith D, Burke LM, Angus DJ, Tunstall RJ, Cox GR, Bonen A, Hawley JA, Hargreaves M. A short-term, high-fat diet up-regulates lipid metabolism and gene expression in human skeletal muscle. Am J Clin Nutr 77: 313–318, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Campos EZ, Bastos FN, Papoti M, Freitas Junior IF, Gobatto CA, Balikian Junior P. The effects of physical fitness and body composition on oxygen consumption and heart rate recovery after high-intensity exercise. Int J Sports Med 33: 621–626, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Cooper JA, Watras AC, Shriver T, Adams AK, Schoeller DA. Influence of dietary fatty acid composition and exercise on changes in fat oxidation from a high-fat diet. J Appl Physiol 109: 1011–1018, 2010 [DOI] [PubMed] [Google Scholar]
- 18.de Jonge L, Agoues I, Garrel DR. Decreased thermogenic response to food with intragastric vs. oral feeding. Am J Physiol Endocrinol Metab 260: E238–E242, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr 53: 1368–1371, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Freedman-Akabas S, Colt E, Kissileff HR, Pi-Sunyer FX. Lack of sustained increase in VO2 following exercise in fit and unfit subjects. Am J Clin Nutr 41: 545–549, 1985 [DOI] [PubMed] [Google Scholar]
- 21.Hansen KC, Zhang Z, Gomez T, Adams AK, Schoeller DA. Exercise increases the proportion of fat utilization during short-term consumption of a high-fat diet. Am J Clin Nutr 85: 109–116, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Lamont LS, Romito R, Rossi K. Fat-free mass and gender influences the rapid-phase excess postexercise oxygen consumption. Appl Physiol Nutr Metab 35: 23–26, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Schwartz RS, Ravussin E, Massari M, O'Connell M, Robbins DC. The thermic effect of carbohydrate versus fat feeding in man. Metab Clin Exper 34: 285–293, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Segal KR, Gutin B, Albu J, Pi-Sunyer FX. Thermic effects of food and exercise in lean and obese men of similar lean body mass. Am J Physiol Endocrinol Metab 252: E110–E117, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Segal KR, Gutin B, Nyman AM, Pi-Sunyer FX. Thermic effect of food at rest, during exercise, and after exercise in lean and obese men of similar body weight. J Clin Invest 76: 1107–1112, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith SR, de Jonge L, Zachwieja JJ, Roy H, Nguyen T, Rood J, Windhauser M, Volaufova J, Bray GA. Concurrent physical activity increases fat oxidation during the shift to a high-fat diet. Am J Clin Nutr 72: 131–138, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Smith SR, de Jonge L, Zachwieja JJ, Roy H, Nguyen T, Rood J, Windhauser M, Volaufova J, Bray GA. Concurrent physical activity increases fat oxidation during the shift to a high-fat diet. Am J Clin Nutr 72: 131–138, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Tahara Y, Moji K, Honda S, Nakao R, Tsunawake N, Fukuda R, Aoyagi K, Mascie-Taylor N. Fat-free mass and excess post-exercise oxygen consumption in the 40 minutes after short-duration exhaustive exercise in young male Japanese athletes. J Physiol Anthropol 27: 139–143, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Thornton MK, Rossi SJ, McMillan JL. Comparison of two different resistance training intensities on excess post-exercise oxygen consumption in African American women who are overweight. J Strength Conditioning Res 25: 489–496, 2011 [DOI] [PubMed] [Google Scholar]