Abstract
Aerobic exercise is typically associated with expansion of the mitochondrial protein pool and improvements in muscle oxidative capacity. The impact of aerobic exercise intensity on the synthesis of specific skeletal muscle protein subfractions is not known. We aimed to study the effect of aerobic exercise intensity on rates of myofibrillar (MyoPS) and mitochondrial (MitoPS) protein synthesis over an early (0.5–4.5 h) and late (24–28 h) period during postexercise recovery. Using a within-subject crossover design, eight males (21 ± 1 yr, V̇o2peak 46.7 ± 2.0 ml·kg−1·min−1) performed two work-matched cycle ergometry exercise trials (LOW: 60 min at 30% Wmax; HIGH: 30 min at 60% Wmax) in the fasted state while undergoing a primed constant infusion of l-[ring-13C6]phenylalanine. Muscle biopsies were obtained at rest and 0.5, 4.5, 24, and 28 h postexercise to determine both the “early” and “late” response of MyoPS and MitoPS and the phosphorylation status of selected proteins within both the Akt/mTOR and MAPK pathways. Over 24–28 h postexercise, MitoPS was significantly greater after the HIGH vs. LOW exercise trial (P < 0.05). Rates of MyoPS were increased equivalently over 0.5–4.5 h postexercise recovery (P < 0.05) but remained elevated at 24–28 h postexercise only following the HIGH trial. In conclusion, an acute bout of high- but not low-intensity aerobic exercise in the fasted state resulted in a sustained elevation of both MitoPS and MyoPS at 24–28 h postexercise recovery.
Keywords: Aerobic exercise intensity, myofibrillar and mitochondrial protein synthesis
adaptations to aerobic-based exercise include increases in mitochondrial protein content (both size and number of mitochondria) and subsequent improvements in muscle oxidative capacity and resistance to fatigue (20). Additionally, traditional aerobic exercise (16, 18) as well as high-intensity “sprint” training (19, 33) can also enhance skeletal muscle hypertrophy, an adaptation that would be contingent upon stimulation of myofibrillar protein synthesis (MyoPS) and expansion of the myofibrillar protein pool (31). Specific phenotypic outcomes (i.e., improved oxidative capacity and muscle hypertrophy) in response to divergent exercise stimuli must relate to changes in the synthesis of specific muscle protein subfractions and may be altered by the intensity of exercise (35). For example, work-matched performance of high-intensity resistance exercise results in greater rates of MyoPS than low-intensity resistance exercise (6, 22). An increase in the rate of mixed muscle protein synthesis has been reported after an acute bout of aerobic-based exercise (8, 17, 27); however, such measures preclude insight into the synthetic response of specific muscle protein subfractions, including mitochondrial and myofibrillar proteins. Whether manipulation of aerobic exercise intensity alters the synthesis of specific muscle protein subfractions is unknown.
Phosphorylation resulting in activation/deactivation of proteins in the Akt-mTOR pathway has been shown to be critical in the regulation of contraction-mediated increases in protein synthesis (12). Other contraction-dependent signaling pathways, such as the MAPK pathway, may also be involved in regulating contraction-mediated translational control (34, 36). Phosphorylation of p38 MAPK can affect transcription factors such as peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) (25, 34, 36). As a primary regulator of mitochondrial biogenesis, PGC-1α coordinates transcriptional activity and assists in coordinating the transcription of mitochondrial and nuclear DNA for mitochondrial biogenesis (13, 30). Higher-intensity aerobic exercise has been demonstrated to result in a greater increase in the mRNA abundance of PGC-1α compared with lower-intensity exercise (13), but whether aerobic exercise intensity alters rates of mitochondrial protein synthesis (MitoPS) is unknown.
The purpose of the present study was to examine the effect of acute bouts of work-matched aerobic exercise of different intensities on rates of MyoPS, MitoPS, and the phosphorylation status of signaling molecules of the Akt-mTOR and MAPK pathways during early (4 h) and late (24 h) postexercise recovery. To examine the independent effects of exercise, we chose to study subjects in the fasted state. We hypothesized that high-intensity (HIGH) cycle ergometry would elicit greater increases in MyoPS and MitoPS than work-matched low-intensity (LOW) cycling exercise. Additionally, we hypothesized that signaling molecule phosphorylation would align with the intensity-dependent differences in protein synthesis with greater activation of the Akt/mTOR and MAPK pathways and increased nuclear PGC-1α accumulation following HIGH exercise.
METHODS
Participants
Eight healthy, recreationally active men (means ± SE; 21 ± 1 yr, 82.5 ± 3.8 kg, 181 ± 2 cm, V̇o2peak 46.7 ± 2.0 ml·kg−1·min−1) were recruited to participate in the study. Participants reported participating in unstructured moderate-intensity aerobic exercise 1–2 times per week. All participants were informed of the purpose of the study, experimental procedures, and associated risks prior to participation and exercise testing. All participants gave verbal and written consent to a protocol approved by the Hamilton Health Science Research Ethics Board, conforming to the standards for the use of human subjects in research as outlined in the Declaration of Helsinki and with current Canadian Tri-Council Research Agency guidelines for use of human participants in research (http://www.pre.ethics.gc.ca/eng/policy-politique/initiatives/tcps2-eptc2/Default/).
Experimental Design
The study consisted of prestudy maximal aerobic capacity measures, a brief familiarization session to assess the aerobic exercise intensities for the trials, and finally two infusion trials for resting and postexercise metabolic investigation per exercise intensity (4 infusion trials total). Each participant completed both exercise intensity trials, making this study a within-subject crossover design.
Maximal aerobic capacity measurements.
Two weeks prior to the first infusion trial, participants reported to the laboratory and completed a V̇o2peak test on a cycle ergometer (Lode, Groningen, Netherlands) with continuous oxygen uptake measurements (Ergocard Professional; Medisoft, Sorinnes, Belgium). The test began at 50 W and increased 1 W every 2 s until voluntary fatigue. Fatigue was defined by a respiratory exchange ratio greater than 1.1 and the inability to maintain 60 rpm on the cycle ergometer despite vigorous verbal encouragement. Peak power output in watts (Wmax), maximum heart rate (HRmax), and average cadence were recorded. Participants were asked to maintain a constant cadence (within 5–10 rpm) between 70 and 100 rpm. The positions of the saddle and handlebars were recorded for each participant and were repositioned accordingly for each subsequent exercise bout. The Wmax for each participant was used to determine the workload for the relative high- (HIGH, 60% Wmax) and low-intensity (LOW, 30% Wmax) exercise trials.
Familiarization trial.
A familiarization session (∼15 min) was carried out with each participant with the exercise intensity that was performed on the days of metabolic investigation and also to confirm the relative intensity of the exercise based on heart rate (HR) and oxygen consumption (V̇o2). One week prior to the first infusion trial, participants completed a short bout of exercise at LOW and then HIGH workloads. HR was measured throughout the familiarization, and V̇o2 was also measured in the last 2 min of both HIGH and LOW. The participants were asked to maintain the same constant pedaling cadence that was comfortable for them during the maximum aerobic capacity test, which was also maintained during exercise trials.
Metabolic investigation and infusion protocol.
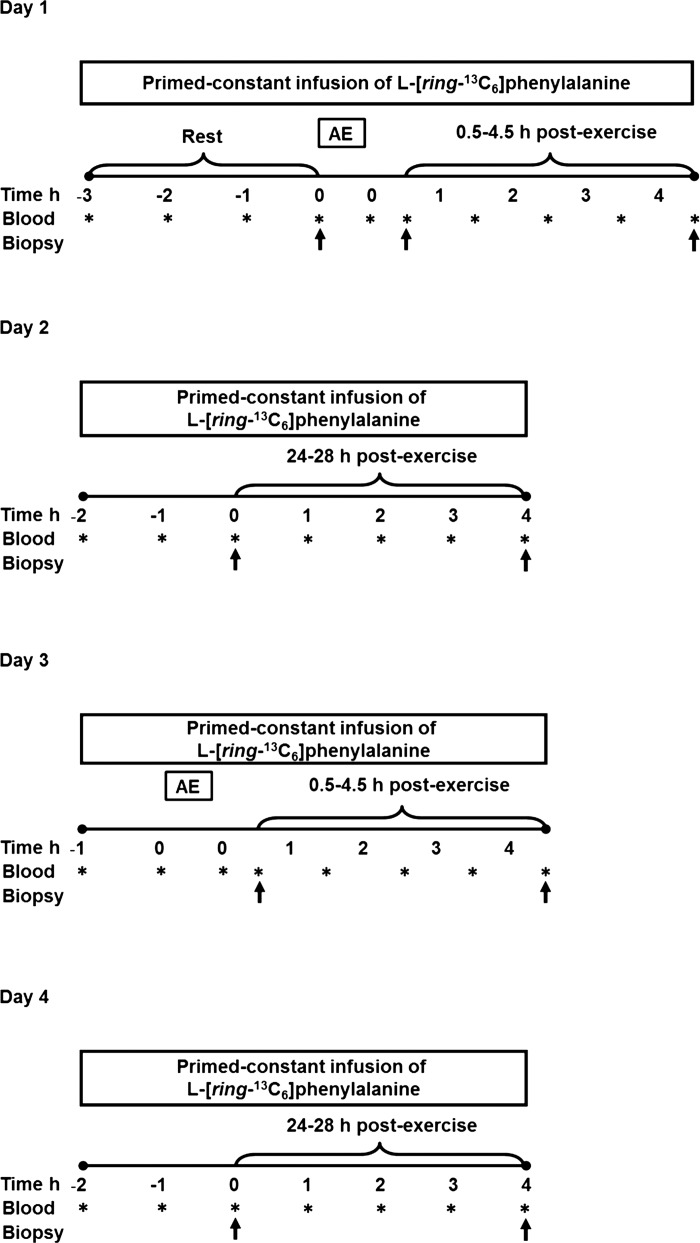
Participants underwent two experimental infusions on sequential days for both HIGH and LOW exercise (4 trials total) to study the response of both MyoPS and MitoPS during early (0.5–4.5 h) and later (24–28 h) postexercise recovery. At least 10 days separated the trials for the two intensities. Participants were asked to keep a diet record for the 48-h period preceding the first infusion protocol corresponding to each of the exercise trials (day 1 and day 3, respectively). A standardized meal representing 30% of each subject's energy requirements (64% CHO, 17% PRO, 19% fat) was provided and consumed by 2000 on the evening before the first infusion trial for each condition. After an overnight fast and after refraining from physical activity for 2 days prior to the trial, participants reported to the laboratory at 0600 for day 1 (Fig. 1). A 20-gauge catheter was inserted into an antecubital vein of one arm, and a baseline blood sample was obtained. The catheter was kept patent with a 0.9% saline drip for repeated blood sampling. A second catheter was then inserted into the other arm for a primed constant infusion of l-[ring-13C6]phenylalanine (prime: 2 μmol/kg; infusion: 0.05 μmol/kg; Cambridge Isotope Laboratories, Cambridge, MA), which passed through a 0.2-μm filter. Participants rested on a bed until 3 h into the infusion, at which point a biopsy (∼100–150 mg) was obtained from the vastus lateralis for fasted resting measurements. Muscle biopsies were obtained under local anesthesia (2% xylocaine) using a 5-mm Bergström needle modified for manual suction. Tissue obtained was blotted, freed of any visible connective tissue and fat, and immediately frozen in liquid N2 and stored at −80°C until analysis. After the resting biopsy, participants began the exercise protocol on the same cycle ergometer that had been used in the V̇o2peak test. Participants were randomized to complete the HIGH or LOW protocol during their first trial. The HIGH protocol consisted of 30 min at 60% Wmax, and the LOW protocol consisted of 60 min at 30% Wmax. By work-matching the protocols, we aimed to remove the influence of total energy expenditure during exercise as a potential confounding variable. Energy expenditure did not differ between the two exercise trials (Table 1). Measurements of HR were taken throughout the exercise bout, and V̇o2 measurements were obtained three times during each ride. The participants returned to a bed to rest until 30 min after exercise, at which point the second biopsy was obtained. After another 4 h of tracer infusion, a third biopsy was obtained, and then the infusion was terminated. The diet was standardized for the infusion and subsequent day by providing participants with a meal immediately after the trial representing 50% of their daily caloric requirements and then providing a meal of identical macronutrient and caloric composition to consume in the evening before 2200. The next day, participants returned to the laboratory at 0700 after an overnight fast to undergo a second infusion (day 2; Fig. 1). The infusion protocol was carried out as on day 1, with biopsies obtained at 1.5 h and 5.5 h into the infusion to obtain 24- to 28-h postexercise measurements. Approximately 2 wk later, the participants returned to the laboratory to complete day 3 and day 4 (Fig. 1), performing the opposite exercise intensity to their first trial. The first biopsy was obtained 2 h into the infusion on both days, with exercise beginning at the appropriate time so that the biopsy was obtained 30 min postexercise.
Fig. 1.
Schema of the experimental infusion study design. Asterisks represent blood draws; single arrows represent muscle biopsies.
Table 1.
Characteristics of low- and high-intensity exercise trials
| LOW | HIGH | |
|---|---|---|
| Workload, W | 99 ± 4 | 198 ± 7 |
| Time, min | 60 | 30 |
| Average %Vo2 peak | 48 ± 1 | 76 ± 3* |
| Average %HRmax | 66 ± 2 | 90 ± 1* |
| Work, kJ | 367 ± 19 | 369 ± 19 |
Values are means ± SE
Significantly different from LOW intensity, P < 0.001.
Blood and Muscle Analysis
All blood samples were collected in heparinized evacuated containers and kept on ice until they were centrifuged to obtain plasma, which was subsequently aliquoted, frozen, and stored at −20°C until further analysis. Plasma [ring-13C6]phenylalanine enrichments were determined as previously described (6). Muscle intracellular (IC) free amino acids were extracted from a 10- to 15-mg piece of wet muscle with ice-cold 0.6 M perchloric acid (PCA) and purified as previously described (5, 6). Purified free amino acids were then converted to their heptafluorobutyrate (HFB) derivatives and analyzed for [ring-13C6]phenylalanine enrichment by a GC-MS (GC:6890, MS:5973; Hewlett-Packard, Palo Alto, CA) as previously described (29).
An ∼100-mg piece of wet muscle was homogenized using a glass homogenizer in ice-cold homogenization buffer (10 μl/mg; 0.067 M sucrose, 0.05 M Tris·HCl, 0.05 M KCl, 0.01 M EDTA) with protease and phosphatase inhibitor cocktail tablets (Complete Mini, PhosSTOP; Roche Applied Science, Mannheim, Germany). The homogenate was transferred to an Eppendorf tube and centrifuged at 700 g for 15 min at 4°C to pellet myofibrillar proteins. The supernatant was transferred to another Eppendorf tube and centrifuged at 12,000 g for 20 min at 4°C to pellet mitochondria. Both the extract and the supernatant were frozen at −80°C until further analysis.
Amino acids were obtained from the mitochondrial pellet as described previously (4–6). Briefly, the pellet was washed twice with ice-cold homogenization buffer, once with ethanol, and then dried under vacuum. Proteins were hydrolyzed by adding 6 M HCl and heating at 110°C for 18 h. From the myofibrillar enriched pellet, nuclear proteins were extracted. The myofibrillar enriched pellet was washed with ice-cold homogenization buffer and centrifuged at 700 g for 10 min at 4°C. Three times the pellet was washed with ice-cold PBS containing protease and phosphatase inhibitors and centrifuged at 15,000 g for 5 min at 4°C. The pellet was fully resuspended in 4 μl of high-salt buffer (HSB; 0.05 M Tris·HCl, 0.4 M NaCl, 0.001 M DTT, 0.001 M EGTA, 0.001 M EDTA, 0.1% SDS; and added protease and phosphatase inhibitors) for every 1 mg of original wet tissue weight. The resuspended pellet was incubated on ice for 20 min and was vortexed twice throughout. The Eppendorf tube was then placed in a sonication bath for 20 min at 4°C followed by vortexing. The resuspended pellet was again incubated on ice for 20 min, vortexing every 10 min, and then was centrifuged at 15,000 g for 10 min at 4°C. The resulting supernatant (nuclear extract) was transferred to an Eppendorf tube and a 100-μl 1:10 dilution was made for use in a BCA assay. Both the extract and the diluted supernatant were frozen at −80°C until further analysis.
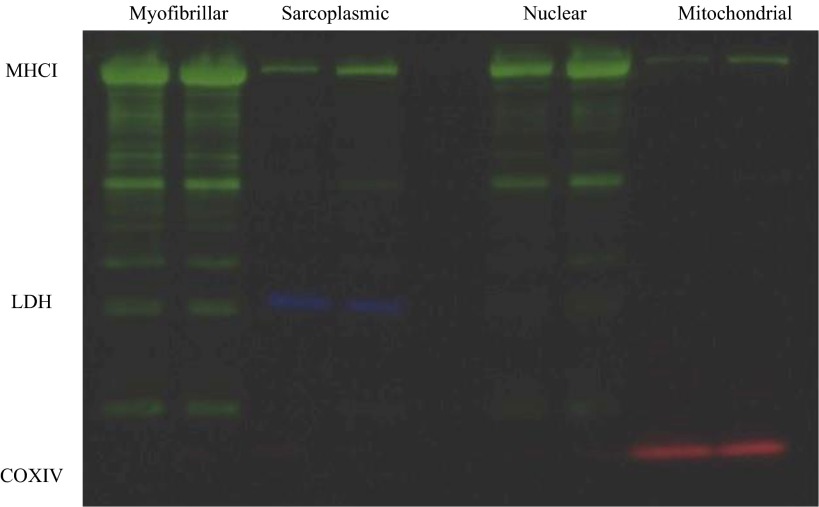
The myofibrillar enriched pellet was washed with H2O and centrifuged at 15,000 g for 5 min at 4°C. Myofibrillar proteins were further extracted and hydrolyzed as described previously (4–6). The free amino acids from the mitochondrial and myofibrillar enriched fractions were purified using cation exchange chromatography (Dowex 50WX8-200 resin; Sigma-Aldrich, St. Louis, MO) and converted to their N-acetyl-n-propyl ester derivatives for GC-combustion isotope ratio MS (GC: 6890; Hewlett Packard; IRMS: Delta Plus XP; Thermo Finnigan, Waltham, MA). The enrichment of the myofibrillar and mitochondrial protein fractions using these methods were confirmed with Western blotting using myosin heavy chain I (MHCI), cyclooxygenase IV (COXIV), and lactate dehydrogenase (LDH) as respective myofibrillar, mitochondrial, and sarcoplasmic markers, respectively. By these methods, only MHCI was detectable in the myofibrillar protein fraction. In some samples (∼15%), trace amounts of MHCI were present in the mitochondrial protein fraction; thus, the isolated mitochondrial fraction was highly enriched with COXIV proteins, and there was no detectable LDH; we have previously confirmed the purity of these protein fractions (35).
Immunoblot Analysis
Both sarcoplasmic and nuclear extracts were used for immunoblot analysis for presence and/or phosphorylation of signaling molecules. The protein concentration of the extracts was determined using the BCA assay (Thermo Fisher Scientific, Rockford, IL). Samples were prepared to the same concentration by dilution with distilled deionized H2O and denatured with Laemmli sample buffer and heated to 95°C. On a 10% SDS-PAGE gel, 20–40 μg of protein (depending on the protein target) was loaded and run at 120 V for 1–1.5 h. Proteins were transferred onto a PVDF membrane using Fast Semi-Dry Transfer (Thermo Fisher Scientific). Membranes were blocked at room temperature (RT) for 1 h using 5% wt/vol milk or BSA in Tris-buffered saline with 0.1% Tween 20 (TBST). Membranes were incubated in primary antibody in TBST at 4°C overnight (Santa Cruz Biotechnology, Santa Cruz, CA) rabbit polyclonal phospho-p70 S6K1 (Thr389; 1:1,000 in TBST, #SC11759-R; Abcam, Cambridge, MA) rabbit polyclonal histone 2B (H2B; 0.1 μg/ml in TBST, #ab1790; Cell Signaling Technology, Danvers, MA), rabbit polyclonal phospho-mTOR (Ser2448; 1:1,000 in TBST, #2971), rabbit monoclonal phospho-p38 MAPK (Thr180/Tyr182; 1:1,000 in TBST; #4511), rabbit polyclonal phospho-ERK1/2 (Thr202/Tyr204; 1:1,1000 in TBST, #9101), and rabbit monoclonal α-tubulin (1:2,000 in TBST, #2125). Membranes were washed with TBST and then incubated with secondary anti-rabbit HRP-linked antibody (1:10,000 in TBST; GE Healthcare Life Sciences, Baie D'Urfe, QC, Canada; NA934) at RT for 1 h. After a washing, membranes were visualized using chemiluminescence (Supersignal West Dura Extended Substrate, Thermo Fisher Scientific) and imaged using Fluorochem SP Imaging system (Protein Simple; Alpha Innotech, Santa Clara, CA). Images were quantified using National Institutes of Health ImageJ software and normalized to the appropriate loading control. α-ubulin and H2B were used as loading controls in sarcoplasmic and nuclear protein samples respectively. Both α-tubulin and H2B were demonstrated to be valid loading controls (i.e., representative of total protein loaded, determined by quantifying Ponceau staining) for sarcoplasmic and nuclear protein extracts, respectively. The degree of enrichment of sarcoplasmic and nuclear protein extracts was determined using Western blotting using MHC1, LDH, and COXIV as markers. Most important to our analysis, the sarcoplasmic fraction was free of detectable COXIV (mitochondrial proteins), and the nuclear extract was free of detectable LDH (sarcoplasmic proteins).
Calculations
The fractional synthetic rates (FSR) of myofibrillar and mitochondrial proteins were calculated using the precursor-product equation
where E2b and E1b are the bound protein enrichments at times 2 and 1, respectively, and Ep is the average enrichment of the precursor, intracellular phenylalanine, during steady state. Since participants were “tracer naïve”, the baseline preinfusion blood sample enrichment represents the naturally abundant 13C enrichments and was used for E1b to determine resting FSR. In this calculation, we used an incorporation time from 30 min after the start of the infusion to the time of the biopsy, which has been previously validated (3).
Statistical Analysis
Aerobic exercise trial data were analyzed using two-tailed paired sample Student's t-test. Data within an exercise trial (%HRmax, %V̇o2peak, and plasma enrichment) were analyzed using a two-way repeated-measures ANOVA. To isolate differences between means for which there was not a resting value in each condition, immunoblot and FSR data were analyzed using a one-way ANOVA, with structured contrasts to determine time- and condition-dependent differences. When appropriate, post hoc analysis was performed with a Student-Newman-Keuls test to isolate significant pairwise differences. Correlations were two-tailed Pearson correlations. All statistical analyses were performed using SPSS Statistics (v. 19; IBM, Armonk, NY, USA). All data are presented as means ± SE. Statistical significance was accepted at P < 0.05.
RESULTS
Aerobic Exercise Trial
All participants completed the exercise at the prescribed intensity. The average %V̇o2peak and %HRmax were significantly higher in the HIGH trial than in the LOW trial (%V̇o2peak 76 ± 3 vs. 48 ± 1, %HRmax 90 ± 1 vs. 66 ± 2, P < 0.001; Table 1). During the HIGH trial, %V̇o2peak was higher in the final 5 min of the bout than at 10 min into the exercise bout (P < 0.05). The same was also observed for %HRmax during the HIGH exercise trial (P < 0.05). No change in %V̇o2peak was observed during the LOW exercise bout. Total work was not different between HIGH and LOW trials (Table 1; P = 0.46).
Plasma and Intracellular Enrichments
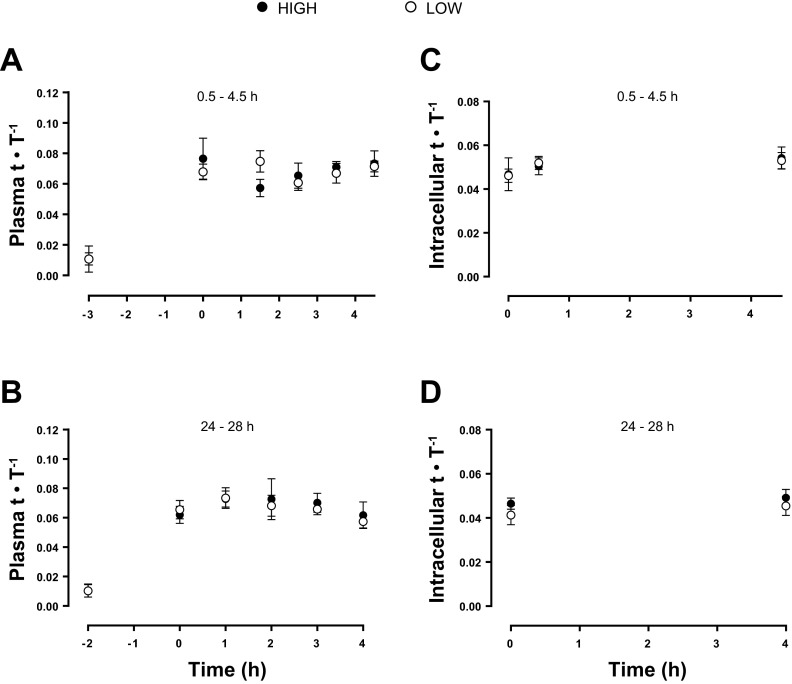
The free plasma tracer enrichment was not different between the 0.5- to 4.5- and 24- to 28-h postexercise incorporation times.
Protein Synthesis
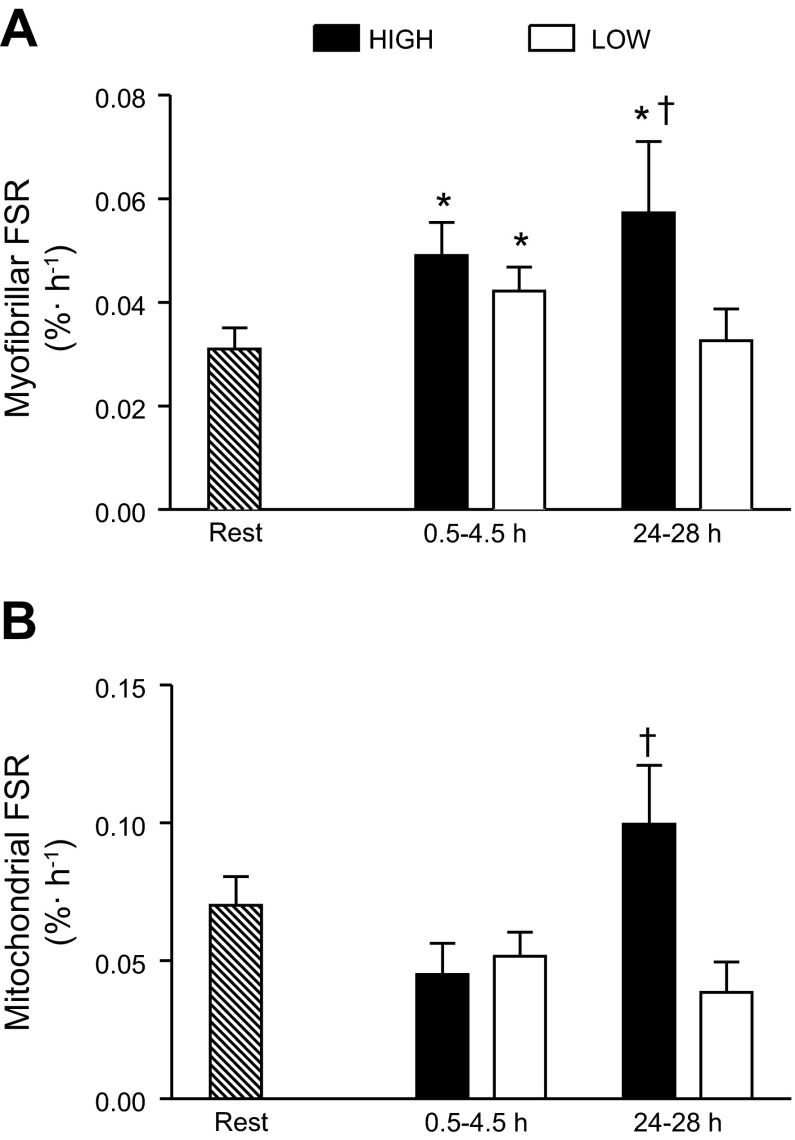
Myofibrillar FSR was increased in early recovery (0.5–4.5 h) compared with rest in both exercise trials (P < 0.05; Fig. 2A). In late recovery (24–28 h), myofibrillar FSR returned to rest in the LOW trial, but remained elevated with HIGH exercise (P = 0.05). Mitochondrial FSR was significantly different between HIGH and LOW conditions in late recovery (P < 0.05; Fig. 2B). Western blot images of MHCI (shown in green), LDH (shown in blue), and COXIV (shown in red) in the myofibrillar, sarcoplasmic, nuclear, and mitochondrial preparations are shown in Fig. 3.
Fig. 2.
Myofibrillar (A) and mitochondrial (B) fractional synthesis rate (FSR; %/h) at rest and during early and late recovery from high-intensity (HIGH) and low-intensity (LOW) exercise. *Significantly different from rest (P < 0.05); †significantly different from LOW at the same time point (P < 0.05).
Fig. 3.
Western blot images of myosin heavy chain I (MHCI; shown in green), lactate dehydrogenase (LDH; shown in blue), and cyclooxygenase IV (COXIV; shown in red) in myofibrillar, sarcoplasmic, nuclear, and mitochondrial preparations.
Cell Signaling
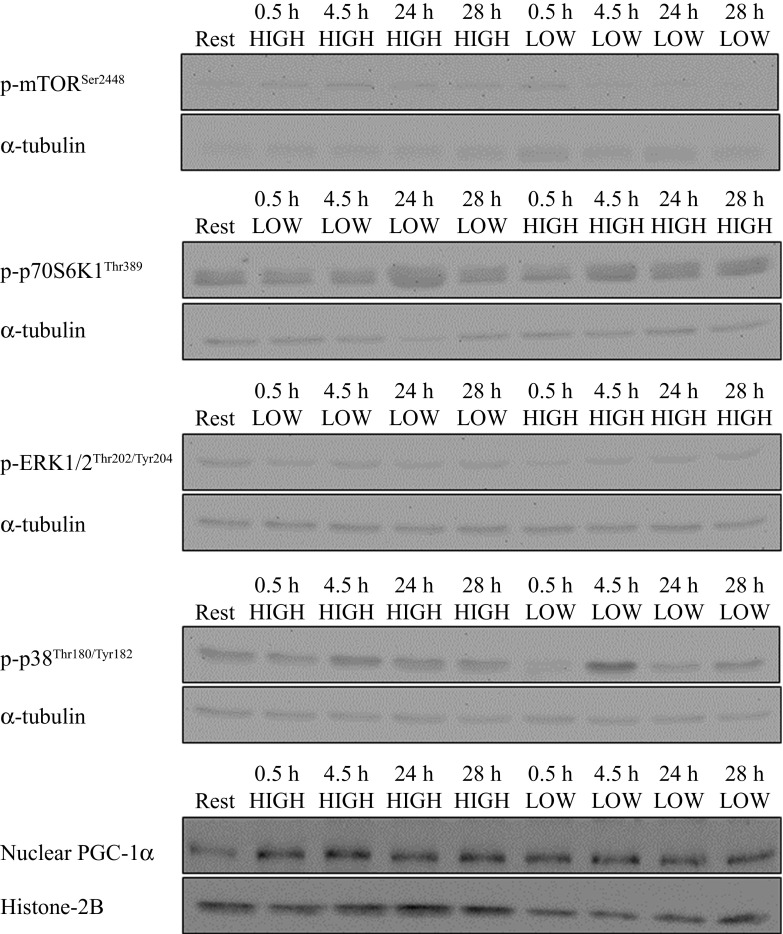
There was a significant difference at 0.5 h postexercise between the HIGH and LOW trials for phospho-mTORSer2448 (P < 0.005; Fig. 4A). This effect was no longer present at 4.5 h postexercise. At 24 and 28 h postexercise, phospho-mTORSer2448 was not different from resting levels in either condition. There was no effect of the exercise on phospho-p70 S6K1Thr389 at the time points measured (Fig. 4B). Myofibrillar FSR in early and late recovery after HIGH exercise was positively correlated with phospho-mTORSer2448 at 0.5 h postexercise (r = 0.953, P < 0.001 and r = 0.866, P < 0.01, respectively). No relationship was observed between phospho-p70 S6K1Thr389 and myofibrillar FSR (data not shown). Aerobic exercise did not result in an increase in the phosphorylation of ERK1/2Thr202/Tyr204 (Fig. 4C). There was a main effect of time for the phosphorylation status of p38Thr180/Tyr182 (P < 0.005; Fig. 4D), where phosphorylation was significantly higher at 4.5 h postexercise compared with rest and 0.5, 24, and 28 h postexercise (P < 0.05). There was no effect of aerobic exercise on nuclear PGC-1α content at the time points examined (Fig. 4E). Representative blot images are shown in Fig. 5.
Fig. 4.
Cell signaling molecule phosphorylation (expressed as phosphorylated protein normalized to α-tubulin content) of mTORSer2448 (A), p70 S6K1Thr389 (B), ERK1/2Thr202/Tyr204 (C), p38Thr180/Tyr182 (D), and nuclear PGC-1α content expressed as PGC-1α normalized to histone 2B (H2B) content. *Significantly different from LOW at time point (P < 0.05);, †significantly different from all other time points (P < 0.05).
Fig. 5.
Representative unaltered Western blot images. Spliced portions of the same gel or sample are demarked by black lines around each image portion.
DISCUSSION
We report here that both high- and low-intensity aerobic exercise stimulate increases in MyoPS during early (0.5–4.5 h) recovery, whereas in late recovery (24–28 h) a sustained elevation in MyoPS was observed only in the HIGH trial (Fig. 2A). We observed an increase in the phosphorylation status of mTORSer2448 only after the HIGH trial, the extent of which was correlated with rates of MyoPS during early and late recovery. This observation is interesting in light of the knowledge that aerobic exercise can serve as a stimulus to promote muscle hypertrophy under certain conditions (16, 18). Thus, it is possible that higher-intensity aerobic exercise may, over time, induce a degree of muscle hypertrophy not seen with low-intensity aerobic exercise. Alternatively, the sustained elevation in MyoPS rates at 24 h postexercise following the HIGH trial may reflect an increase in muscle protein turnover to assist in remodeling and protein renewal. Our findings demonstrate that aerobic exercise intensity influenced the synthesis of specific muscle protein fractions, which has bearing on the interpretation of findings from studies of mixed muscle protein synthesis following endurance exercise (17, 27). It has previously been reported that in the fed state, rates of MyoPS are increased above resting values 24 h after both resistance exercise (4, 6) and high-intensity aerobic exercise (11, 28). Thus, loading appears to “sensitize” the muscle to protein provision late into the postexercise recovery period, which also appears true even with low-intensity endurance exercise (15). In support of this notion, we observed intensity-dependent differences in the phosphorylation of mTORSer2448 at 0.5 h following the HIGH, but not the LOW, exercise trial (Fig. 4A). An increase in the phosphorylation of mTORSer2448 after cycling exercise has been observed previously (7, 9, 10, 26, 27, 35), but the response appears transient with aerobic compared with resistance exercise (7). To our knowledge, ours is the first report of an aerobic exercise intensity-dependent effect on mTORSer2448 phosphorylation. Interestingly, mTORSer2448 phosphorylation at 0.5 h was correlated to rates of MyoPS in early and late recovery from the HIGH exercise trial. However, the phosphorylation of p70 S6K1Thr389, which is often taken as a proxy of mTOR activity and is a known regulator of mRNA translation initiation and elongation (21), was not elevated. This finding is in agreement with previous work of Mascher et al. (2007), who reported that a 1-h bout of cycle ergometer exercise at ∼75% V̇o2 max increased the phosphorylation status of mTORSer2448 at 0.5 h but had no effect on p70 S6K1Thr389 (26). The lack of change in p70 S6K1Thr389 phosphorylation also corroborates results from previous studies of aerobic exercise in which biopsies were taken at similar time points (7, 9, 10). It should, however, be acknowledged that a single phosphorylation site (Ser2448) of mTOR was measured; other phosphorylation sites that regulate mTOR activity were not measured. It is not clear what role, if any, the early divergent response of mTORSer2448 phosphorylation may have played in mediating the divergent protein synthesis responses observed in late recovery. More research is required to determine the mechanisms determining the sustained increases in MyoPS during late (e.g., 24 h) postexercise recovery.
Fig. 6.
Plasma (A and B) and intracellular (C and D) free [ring-13C6]phenylalanine enrichment during early (A and C) and late (B and D) postexercise recovery. Data are shown as tracer (t)-to-tracee (T) ratios.
In the present study, aerobic exercise, irrespective of intensity, did not elicit a significant increase from rest in fasted-state measures of MitoPS during early or late recovery, although there was a trend for such a response (Fig. 2B; P = 0.18). We did however, observe divergent responses in late recovery, whereby MitoPS rates were higher after the HIGH vs. LOW exercise trial. These results are in contrast with our previous findings of a stimulation of MitoPS following aerobic exercise (35); however, our previous study was performed under conditions of sustained hyperinsulinemia and hyperaminoacidemia, which may explain the divergent findings. Others have reported that protein provision before high-intensity repeated sprinting (9) and protein plus carbohydrate ingestion after prolonged higher-intensity aerobic exercise (2) did not affect the synthesis of mitochondrial proteins vs. nonprotein control conditions. However, in neither of these previous studies (2, 9) was a resting rate of MitoPS reported, and so it is not possible to ascertain whether exercise per se, regardless of the nutrition provided, resulted in a stimulation of MitoPS. Sustained hyperinsulinemia and hyperaminoacidemia support increased MitoPS at rest (32), an effect that appears to require amino acids (1). Whether there is a necessity for protein provision to robustly stimulate MitoPS, as there is with MyoPS (29), is currently unknown. Given the relative size of the skeletal muscle mitochondrial protein pool (4–8%) vs. the myofibrillar protein pool (60–70%), it would seem that amino acids would not likely be rate limiting for MitoPS. The physiological relevance of the difference in MitoPS between HIGH and LOW exercise trials in late recovery (i.e., 24 h) is unclear from our acute measurements; however, our results may have implications for phenotypic adaptations following a period of chronic training. Further investigations are required to determine: whether amino acid provision alters the exercise-mediated mitochondrial response, particularly in the late phase of recovery, and whether chronic performance of higher vs. lower intensities of aerobic exercise (even if energy matched) result in divergent increases in mitochondrial content.
An important regulator of mitochondrial biogenesis, PGC-1α, is thought to coordinate both nuclear and mitochondrial gene expression to induce mitochondrial biogenesis (14). In contrast to previous studies (23, 24), and in line with the absence of an early postexercise increase in MitoPS, we found that nuclear PGC-1α content was not increased after exercise (Fig. 5). The exercise models used in previous studies (23, 24) were of higher intensity than that used in the present study, which may explain the discrepant findings. While exercise intensity-dependent PGC-1α nuclear localization has not been reported, the response of PGC-1α mRNA postexercise is intensity dependent, with higher-intensity exercise inducing a 2.5-fold greater increase in mRNA 3 h postexercise than lower-intensity exercise (13). Another factor that may have contributed to the apparent lack of change in PGC-1α nuclear abundance is the timing of biopsy sampling. Nuclear localization of PGC-1α has been observed immediately and 3 h after high-intensity interval exercise (23, 24), which leads to increases in mitochondrial protein content at 24 h postexercise (23).
In conclusion, we present data demonstrating that MyoPS is elevated early during postexercise recovery following both HIGH and LOW bouts of aerobic exercise performed in the fasted state; however, only HIGH exercise extended the duration of the elevated MyoPS response. We did not observe an increase in rates of MitoPS or a change in PGC-1α nuclear localization after exercise performed in the fasted state. The greater rates of MitoPS after HIGH compared with LOW exercise during late postexercise recovery may serve to enhance the mitochondrial protein pool following chronic training.
GRANTS
This work was supported by a grant from the National Sciences and Engineering Council (NSERC) of Canada and the Canadian Foundation for Innovation to S. M. Phillips. D. M. Di Donato was supported by an Ontario Graduate Scholarship; T. A. Churchward-Venne was supported by an NSERC Post-Graduate Scholarship (Doctoral), and D. W. D. West by a Canadian Institutes of Health Research Canada Graduate Scholarship (Doctoral); all wish to acknowledge that source of funding during the conduct of this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.M.D.D., D.W.D.W., T.A.C.-V., L.B., S.K.B., and S.M.P. conception and design of research; D.M.D.D., D.W.D.W., T.A.C.-V., L.B., S.K.B., and S.M.P. performed experiments; D.M.D.D., D.W.D.W., and T.A.C.-V. analyzed data; D.M.D.D., D.W.D.W., T.A.C.-V., L.B., and S.M.P. interpreted results of experiments; D.M.D.D., T.A.C.-V., and S.M.P. prepared figures; D.M.D.D., T.A.C.-V., and S.M.P. drafted manuscript; D.M.D.D., D.W.D.W., T.A.C.-V., L.B., S.K.B., and S.M.P. edited and revised manuscript; D.M.D.D., D.W.D.W., T.A.C.-V., L.B., S.K.B., and S.M.P. approved final version of manuscript.
REFERENCES
- 1.Barazzoni R, Short KR, Asmann Y, Coenen-Schimke JM, Robinson MM, Nair KS. Insulin fails to enhance mTOR phosphorylation, mitochondrial protein synthesis, and ATP production in human skeletal muscle without amino acid replacement. Am J Physiol Endocrinol Metab 303: E1117–E1125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breen L, Philp A, Witard OC, Jackman SR, Selby A, Smith K, Baar K, Tipton KD. The influence of carbohydrate-protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. J Physiol 589: 4011–4025, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burd NA, Groen BB, Beelen M, Senden JM, Gijsen AP, van Loon LJ. The reliability of using the single-biopsy approach to assess basal muscle protein synthesis rates in vivo in humans. Metabolism 61: 931–936, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, Cashaback JG, Potvin JR, Baker SK, Phillips SM. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol 588: 3119–3130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr 141: 568–573, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLos One 5: e12033, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG. Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc 42: 1843–1852, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol Endocrinol Metab 259: E470–E476, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Coffey VG, Moore DR, Burd NA, Rerecich T, Stellingwerff T, Garnham AP, Phillips SM, Hawley JA. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur J Appl Physiol 111: 1473–1483, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J 20: 190–192, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Cuthbertson DJ, Babraj J, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie M. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab 290: E731–E738, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 106: 1374–1384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O'Gorman DJ. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol 588: 1779–1790, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 56: 1615–1622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harber MP, Konopka AR, Douglass MD, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol 297: R1452–R1459, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harber MP, Konopka AR, Jemiolo B, Trappe SW, Trappe TA, Reidy PT. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am J Physiol Regul Integr Comp Physiol 299: R1254–R1262, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol 113: 1495–1504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heydari M, Freund J, Boutcher SH. The effect of high-intensity intermittent exercise on body composition of overweight young males. J Obes 2012: 480467, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Resp Envir Exerc Physiol 56: 831–838, 1984 [DOI] [PubMed] [Google Scholar]
- 21.Kimball SR, Jefferson LS. Control of translation initiation through integration of signals generated by hormones, nutrients, and exercise. J Biol Chem 285: 29027–29032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587: 211–217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300: R1303–R1310, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588: 1011–1022, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long YC, Widegren U, Zierath JR. Exercise-induced mitogen-activated protein kinase signalling in skeletal muscle. Proc Nutr Soc 63: 227–232, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Mascher H, Andersson H, Nilsson PA, Ekblom B, Blomstrand E. Changes in signalling pathways regulating protein synthesis in human muscle in the recovery period after endurance exercise. Acta Physiol (Oxf) 191: 67–75, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Mascher H, Ekblom B, Rooyackers O, Blomstrand E. Enhanced rates of muscle protein synthesis and elevated mTOR signalling following endurance exercise in human subjects. Acta Physiol (Oxf) 202: 175–184, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567: 1021–1033, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol 587: 897–904, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol 66: 799–828, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100: 7996–8001, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trapp EG, Chisholm DJ, Freund J, Boutcher SH. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes 32: 684–691, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Widegren U, Ryder JW, Zierath JR. Mitogen-activated protein kinase signal transduction in skeletal muscle: effects of exercise and muscle contraction. Acta Physiol Scand 172: 227–238, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu M, Stepto NK, Chibalin AV, Fryer LG, Carling D, Krook A, Hawley JA, Zierath JR. Metabolic and mitogenic signal transduction in human skeletal muscle after intense cycling exercise. J Physiol 546: 327–335, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]