Abstract
Episodic increases in cerebrovascular perfusion and shear stress may have beneficial impacts on endothelial function that improve brain health. We hypothesized that water immersion to the level of the right atrium in humans would increase cerebral perfusion. We continuously measured, in 9 young (means ± SD, 24.6 ± 2.0 yr) healthy men, systemic hemodynamic variables along with blood flows in the common carotid and middle and posterior cerebral arteries during controlled filling and emptying of a water tank to the level of the right atrium. Mean arterial pressure (80 ± 9 vs. 91 ± 12 mmHg, P < 0.05), cardiac output (4.8 ± 0.7 vs. 5.1 ± 0.6 l/min, P < 0.05) and end-tidal carbon dioxide (PetCO2, 39.5 ± 2.0 vs. 44.4 ± 3.5 mmHg, P < 0.05) increased with water immersion, along with middle (59 ± 6 vs. 64 ± 6 cm/s, P < 0.05) and posterior cerebral artery blood flow velocities (41 ± 9 vs. 44 ± 10 cm/s, P < 0.05). These changes were reversed when the tank was emptied. Water immersion is associated with hemodynamic and PetCO2 changes, which increase cerebral blood velocities in humans. This study provides an evidence base for future studies to examine the potential additive effect of exercise in water on improving cerebrovascular health.
Keywords: cerebral blood flow, water immersion, cardiovascular, hemodynamics
there is a growing body of literature on the beneficial impacts of exercise on brain health, including decreased risk of cerebrovascular events and positive impacts on cognitive function (19, 24, 34). Recent animal studies have related these improvements to enhanced endothelial function (6, 12, 13, 17, 28). Shear stress on the artery wall is the physiological stimulus that improves endothelial function (35). In the peripheral circulation, exercise training enhances both macro- and microvascular endothelial function (18, 39), a phenomenon that is related to episodic increases in shear and nitric oxide bioavailability (9, 41). These findings raise the possibility that increases in flow through cerebral arteries may induce improvements in cerebrovascular health.
Previous studies examining head-out water immersion in humans have assessed impacts on musculoskeletal and joint function and the risk of injury and falls in older or clinically compromised populations (26, 36). While cardiovascular changes occur as a result of water immersion, findings of previous studies are conflicting regarding effects on central hemodynamics, due largely to measurement limitations and methodological differences (e.g., level and duration of immersion, water temperature) (15). In addition, the effect of graded euthermic water immersion on cerebral blood flow velocity (CBFV) has not previously been examined. The purpose of the current study was to assess the integrative cardiovascular responses and changes in CBFV during carefully controlled water immersion in resting humans. We hypothesized that the hydrostatic pressure associated with immersion in water would increase CBFV. If this hypothesis is supported, it would provide a basis for further studies relating to the impact of aquatic exercise as a therapy to enhance cerebrovascular function and brain health.
MATERIALS AND METHODS
Ethical Approval
This study complied with the Declaration of Helsinki and the Human Research Ethics Committee of the University of Western Australia approved the experimental protocol. All subjects provided written, informed consent before participating in the study.
Subject Characteristics
Nine young, healthy recreationally active (≤2 h of physical activity per week) males were recruited (24.6 ± 2.0 yr). The participant's average heights and weights were 1.74 ± 0.09 m and 76 ± 9 kg, respectively, with the average body mass index (BMI) being 25.0 ± 1.7 kg/m2. Subjects had no history of cardiovascular, musculoskeletal or metabolic disease, did not smoke, or take medication. Women were excluded from this study due to the effects of estrogen on hemodynamic and vascular variables.
Experimental Procedures
Subjects arrived at the laboratory after having fasted for a minimum of 8 h and abstained from alcohol, caffeine, and vigorous exercise for at least 24 h. Upon arrival, subjects were seated and instrumented (∼30 min). They were then positioned in a tank (1.4 m diameter, 1.55 m height, 2, 386 liters) in a standing position with their arms resting comfortably on a platform at heart level. Subjects were asked to remain as stationary as possible throughout the experiment and to avoid excessive movement. This experimental approach avoided the potential for confounding effects of movement into, or out of, the tank on hemodynamics and CBFV. After a 10-min baseline period of quiet rest, three submersible water pumps (KPA 600A; Grundfos, South Australia) filled the tank at a constant rate with euthermic water (30°C) to the level of the right atrium (RA), a process that was completed in 7 min. This water temperature was selected on the basis of experiments in our laboratory which indicated that basal skin temperatures average 30.1 ± 1.6°C. The subjects remained immersed at the level of the RA for 10 min while remaining in a stationary standing posture, after which time the pumps were reversed and water rapidly evacuated at a constant rate. Subjects attended the laboratory wearing shorts and a tee-shirt. The average ambient air temperature throughout the testing sessions was 26.0 ± 3.4°C. Before the commencement of baseline recording, after instrumentation, tee-shirts were removed for the duration of the testing session. Data were measured and recorded continuously throughout the entire protocol.
Experimental Measures
Systemic hemodynamics.
A Finometer PRO (Finapres Medical Systems, Amsterdam, The Netherlands) was used to measure changes in mean arterial pressure (MAP), heart rate (HR), cardiac output (CO), and stroke volume (SV) via photoplethysmography. These data were exported to a data acquisition system PowerLab (LabChart 7, ADInstruments, Sydney, Australia) in real time. The finger cuff was placed around the middle finger of an arm supported at right atrium level on a platform. The subject was instructed not to move their arm or finger during recording. All summary data were time averaged for 1–2 min.
Assessment of middle and posterior cerebral artery velocities.
Middle and posterior cerebral artery velocities (MCAV and PCAV, respectively) were measured using a 2-MHz pulsed ST3 transcranial ultrasound system (Spencer Technologies, Seattle, WA). Search techniques adopted to identify the MCA and PCA are described in detail elsewhere. (2) A Marc 600 headframe (Spencer Technologies) was secured to allow for adjustments to the insonation angle until an optimal M-mode image was found. Raw analog MCAV and PCAV cerebral velocity traces were exported from PowerLab to LabChart for post hoc analysis. All data at respective time points were averaged for 1–2 min.
End-tidal carbon dioxide (PetCO2) was collected in seven subjects via a sampling tube connected to a Hans Rudolph mask and was measured by an online gas analyzer (ML206; ADinstruments).
Assessment of common carotid artery diameter and velocity.
After the 10-min baseline period, common carotid artery (CCA) diameter and velocities were simultaneously recorded throughout the protocol using a 10-MHz multifrequency linear array probe attached to a high-resolution ultrasound machine (T3000; Terason, Burlington, MA). Recording began following optimization of the longitudinal B-mode image of the lumen-arterial walls. Concurrently, Doppler velocity assessments were collected using the lowest possible insonation angle (always <60°). Subjects' arms were supported at heart level throughout the test.
Analysis of artery diameter and flow were performed using custom-designed edge-detection and wall-tracking software, which is independent of investigator bias and has previously been comprehensively described (8, 43). From synchronized diameter and velocity data, blood flow (the product of lumen cross-sectional area and Doppler velocity) was calculated at 30 Hz. Reproducibility of diameter measurements using this semiautomated software is significantly better than manual methods, reduces observer error and bias significantly, and possesses an intraobserver CV of 6.7% (43).
Statistics
Statistical analysis was performed using SPSS 19.0 (SPSS, Chicago, IL) software. Repeat-measure ANOVAs were performed with post hoc analysis t-tests used where significant values were found. Statistical significance was assumed at P < 0.05. All data are reported as means ± SD unless stated otherwise.
RESULTS
Middle and Posterior Cerebral Artery Velocities
A repeated-measures ANOVA revealed a significant increase in MCAV from baseline throughout the protocol (P < 0.05, Fig. 1A). Post hoc analysis revealed MCAV increased significantly during filling to the hip, upon immersion to the RA, and after 5 and 10 min at RA level (all P < 0.05). Once the tank was emptied, MCAV returned to baseline levels (P < 0.05). Similarly, PCAV increased (P < 0.05) with differences evident during filling to the hip, upon immersion to the RA, and after 5 and 10 min at RA level (P < 0.05). Consistent with the MCAV response, PCAV returned to baseline levels once the tank was emptied (P < 0.05, Fig. 1B). PetCO2 values increased during immersion (P < 0.05) with differences evident upon immersion to the RA and after 5 and 10 min of continuous immersion at the RA (all P < 0.05, Fig. 1D). However, no change in respiratory rate was evident throughout the immersion protocol (P = 0.97, Table 1).
Fig. 1.
Middle cerebral (A) and posterior cerebral artery (B) velocities, mean arterial pressure (C), and end-tidal carbon dioxide (PetCO2) (D) at rest, during, and after water immersion. Repeated-measures ANOVA revealed that immersion significantly increased middle and posterior cerebral artery velocities, mean arterial pressure and PetCO2 (all P < 0.05). *Significantly different from rest at P < 0.05. Data are means ± SE.
Table 1.
Cardiovascular variables before, during, and after water immersion
| Variable | Baseline | Hip | 0–1 min | 3–5 min | 8–10 min | Rest |
|---|---|---|---|---|---|---|
| Cardiac output, l/min | 4.8 ± 0.7 | 4.7 ± 0.5 | 5.1 ± 0.8 | 5.0 ± 0.6 | 5.1 ± 0.6∗ | 4.9 ± 0.6 |
| Stroke volume, ml | 62 ± 9 | 70 ± 9∗ | 81 ± 8∗ | 83 ± 7∗ | 82 ± 5∗ | 63 ± 7 |
| Heart rate, beats/min | 78 ± 12 | 67 ± 12∗ | 63 ± 11∗ | 60 ± 10∗ | 62 ± 10∗ | 79 ± 12 |
| Carotid artery diameter, mm | 5.74 ± 0.49 | 5.99 ± 0.36∗ | 6.05 ± 0.43∗ | 6.12 ± 0.34∗ | 5.92 ± 0.28 | |
| Carotid artery flow, ml/min | 458 ± 162 | 486 ± 139 | 490 ± 132 | 503 ± 127 | 457 ± 99 | |
| Respiratory rate, breaths/min | 15 ± 3 | 15 ± 5 | 16 ± 5 | 14 ± 5 | 15 ± 4 | 15 ± 4 |
Values are means ± SD. n = 7 for respiratory rate variables.
Significantly different from rest at P < 0.05.
Impact of Water Immersion on Systemic Hemodynamics
Repeated-measures ANOVA revealed a significant increase in MAP from baseline throughout the protocol (P < 0.05, Fig. 1C). Post hoc analysis indicated that MAP was significantly elevated above baseline during filling (at the level of the hip), upon immersion to the RA, and after 5 and 10 min of immersion (all P < 0.05). Once the tank was emptied, MAP decreased significantly compared with immersion data (P < 0.05). In keeping with MAP data, CO increased (P < 0.05), with differences evident after 10 min of immersion (P < 0.05, Table 1). CO decreased as a result of tank emptying and was not significantly different from baseline levels after 5 min.
SV also increased (P < 0.05), with differences during immersion to the hip, upon immersion to the RA, and after 5 and 10 min at RA level (P < 0.05, Table 1). However, SV returned to baseline values 5 min postemptying. HR decreased during immersion (P < 0.05) with significant differences during filling (hip), upon immersion to the RA, and after 5 and 10 min at RA level (all P < 0.05) (Table 1). Once the tank was emptied, HR returned to baseline values.
Common Carotid Artery Diameter and Blood Flow
Common carotid diameter increased significantly (P < 0.05) with differences upon immersion to the RA and after 5 and 10 min (all P < 0.05) (Table 1). However, there was no significant change in common carotid flow throughout the protocol (Table 1).
Correlations With Cerebral Blood Flow Velocities
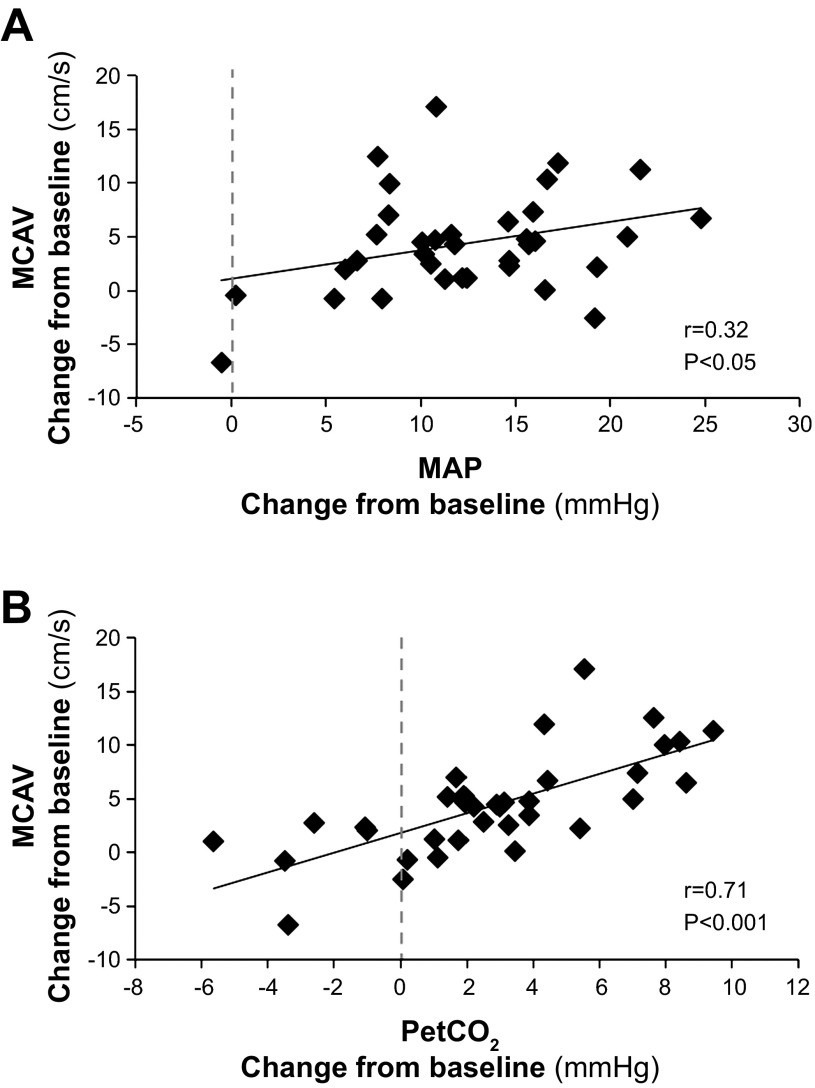
Correlations and multiple regression analysis were performed using change from baseline values at each data collection point. Significant correlations existed between changes in MCAV and changes in MAP (Fig. 2, r = 0.32, P < 0.05), changes in PCAV and MAP (r = 0.50, P < 0.01), changes in PetCO2 and MCAV (r = 0.71, P < 0.001), and changes in PetCO2 and PCAV (r = 0.68, P < 0.001). Multiple regression analysis using PetCO2 and MAP as predictors of MCAV produced R2 = 0.509, F = 16.60, P < 0.001. This analysis revealed only PetCO2 added significantly to the MCAV prediction (P < 0.001), whereas MAP did not (P = 0.95).
Fig. 2.
Pearson correlations calculated from data derived from n = 7 subjects, for changes from baseline at each data collection point. Significant relationships existed between changes in middle cerebral artery velocities (MCAV) in response to water immersion and changes in both mean arterial pressure (MAP) (A) and PetCO2 (B).
DISCUSSION
The aim of the present study was to comprehensively examine the cardiovascular response to graded water immersion in standing subjects using a rapid filling and emptying protocol. The tank was filled with euthermic water (30°C) to avoid any reflex responses elicited by cold or heat and to provide a model allowing for the direct and accurate examination of hydrostatic effects per se. Our principle finding is that water immersion at rest has significant impacts on hemodynamic variables and PetCO2, which are associated with increases in CBFV.
Two important stimuli that can elicit changes in CBFV are blood CO2 levels and MAP. In the current study we observed significant changes in CBFV that were consistent, and correlated with, changes in MAP. Traditionally, on the basis of steady-state measures made in patients with hypertensive and hypotensive disorders, CBFV was considered to be autoregulated such that relatively constant levels were maintained across a wide range of MAP (60–150 mmHg) (23). However, subsequent technical advances revealed that CBFV can respond rapidly to dynamic changes in MAP, a concept termed dynamic cerebral autoregulation (1, 40). In contrast to previous studies, which have either assessed CBFV responses to static or dynamic stimuli, the present study utilized a novel approach that assessed the impact of both rapid and sustained changes in MAP on CBFV. Interestingly, we observed no evidence of autoregulation in response to either rapid or sustained filling in the current study. However, the relationship between MAP and CBFV must be also considered in the context of increases in PetCO2. It is known that water immersion alters the mechanics of respiration. For example, the centralized shift in blood volume to the thorax along with the ascent of the diaphragm has been shown to significantly reduce functional residual volume and increase pulmonary capillary blood volume (3, 7, 15), whereas a further study reported no change in tidal volume during immersion in 25–30°C water (20). In the current study, water immersion was associated with an increase in PetCO2. Breathing rate did not change significantly throughout the immersion protocol, suggesting that changes in tidal volume may have been responsible for the rise in PetCO2. Future studies are required to elucidate the casual pathways. Importantly, the changes in PetCO2 were correlated with those in CBFV, and a multiple regression analysis revealed it was the strongest predictor for changes in MCAV. We can therefore not exclude the possibility that increases in PetCO2 masked autoregulatory responses to changes in MAP. For example, it is known that even small elevations in PetCO2 can reduce the effectiveness of cerebral autoregulation (4). Regardless of the mechanism(s) responsible, our principle finding was that water immersion increased CBFV, and this is the important functional outcome of the study.
While our principle interest was changes in CBFV, our experiment provides an insight into the central hemodynamic effects of water immersion. There have been numerous studies examining the effects of water immersion on cardiovascular variables; however, there are notable discrepancies in their findings. For example, several studies have reported increases in CO following water immersion (5, 7, 10, 15, 27, 33, 42, 44), whereas others have reported decreases (37) or no change (21, 29). HR responses are similarly conflicting, with studies reporting increases (5, 7, 10, 11, 29, 44) and decreases (14–16, 22, 27, 33, 37, 38). Finally, MAP responses have also reported increases (5, 33), decreases (25, 32), or no change (10, 16, 31). These conflicting results are related to differences in methodology, in particular posture, the duration of immersion, and water temperature, as highlighted in a study by Park et al. (33), all of which may elicit reflex changes. In the present study we aimed to clarify the hydrostatic impacts of water immersion per se in upright humans by performing a comprehensive integrative examination of the systemic cardiovascular response to immersion in euthermic water. By employing a filling and emptying protocol we were able to demonstrate reversibility, reinforcing the validity of our measures. We also used independent techniques to measure related variables. For example, cerebral velocities were assessed by transcranial Doppler, whereas common carotid blood flows were assessed using linear array duplex ultrasound. The concordance between these measures reassures us that our data are robust. We also observed increases in CO, SV, and MAP as a result of water immersion, which were reversed when the tank was emptied. It is presumed that a cephaloid redistribution of blood volume as a result of immersion increases preload and SV, in accordance to the Frank-Starling mechanism. The decrease in HR we observed is likely to be a reflex response to the increase in MAP. Our finding of changes in CCA diameter during immersion is consistent with baroreflex elicitation.
A limitation of the present study is our cardio- and cerebrovascular responses to water immersion were collected in young healthy individuals, and our results are therefore not necessarily translatable to other population or clinical groups, i.e., the elderly or heart failure groups.
Implications
Our findings of increased CBFV during water immersion at rest raise the possibility of acute and chronic impacts of water immersion on cerebrovascular function. There is evidence for basal vascular nitric oxide-mediated tone in the cerebrovasculature (13) and exercise improves cerebrovascular endothelial function by upregulating endothelial nitric oxide synthase expression in animals (13, 17). Acute increases in blood flow and shear in peripheral arteries modulate endothelial function (41), and chronic episodic exposure to shear stress induces endothelial adaptation (30, 41). We speculate that water immersion may amplify effects on CBFV, including those associated with exercise training.
Perspectives and Significance
This study indicates that euthermic water immersion in resting humans increases MAP, PetCO2, and, consequently, CBFV. Given that increases in blood flow and shear in the peripheral vasculature are potent stimuli for endothelial adaptation, our observation that CBFV increases during water immersion has potential implications for the use of aquatic exercise as a modality that promotes brain health.
GRANTS
D. J. Green is supported by research funding from the National Health and Medical Research Council of Australia Grant 1045204.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). The results of the present study do not constitute endorsement by the American College of Sports Medicine.
AUTHOR CONTRIBUTIONS
Author contributions: H.H.C., A.L.S., P.N.A., and D.J.G. conception and design of research; H.H.C., A.L.S., C.J.P., and L.H.N. performed experiments; H.H.C. and C.J.P. analyzed data; H.H.C., A.L.S., C.J.P., P.N.A., L.H.N., and D.J.G. interpreted results of experiments; H.H.C., L.H.N., and D.J.G. prepared figures; H.H.C., A.L.S., C.J.P., P.N.A., L.H.N., and D.J.G. drafted manuscript; H.H.C., P.N.A., L.H.N., and D.J.G. edited and revised manuscript; H.H.C., A.L.S., C.J.P., P.N.A., L.H.N., and D.J.G. approved final version of manuscript.
REFERENCES
- 1.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke 20: 45–52, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 57: 769–774, 1982 [DOI] [PubMed] [Google Scholar]
- 3.Agostoni E, Gurtner G, Torri G, Rahn H. Respiratory mechanics during submersion and negative-pressure breathing. J Appl Physiol 21: 251–258, 1966 [DOI] [PubMed] [Google Scholar]
- 4.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Arborelius M, Ballidin UI, Lilja B, Lundgren CE. Hemodynamic changes in man during immersion with the head above water. Aerospace Med 43: 592–598, 1972 [PubMed] [Google Scholar]
- 6.Austin SA, Santhanam AV, Katusic ZS. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ Res 107: 1498–1502, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Begin R, Epstein M, Sackner MA, Levinson R, Dougherty R, Duncan D. Effects of water immersion to the neck on pulmonary circulation and tissue volume in man. J Appl Physiol 40: 293–299, 1976 [DOI] [PubMed] [Google Scholar]
- 8.Black MA, Cable NT, Thijssen DHJ, Green DJ. Importance of measuring the time-course of flow-mediated dilation (FMD) in humans. Hypertension 51: 203–210, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Black MA, Green DJ, Cable NT. Exercise training prevents age-related decline in nitric oxide (NO)-mediated vasodilator function in human microvessels. J Physiol 586: 3511–3524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boussuges A. Immersion in thermoneutral water: effects on arterial compliance. Aviat Space Environ Med 77: 1183–1187, 2006 [PubMed] [Google Scholar]
- 11.Christie JL, Sheldahl LM, Tristani FE, Wann LS, Sagar KB, Levandoski SG, Ptacin MJ, Sobocinski KA, Morris RD. Cardiovascular regulation during head-out water immersion exercise. J Appl Physiol 69: 657–664, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Chu Y, Heistad DD. NO answer to Alzheimer's disease. Circ Res 107: 1400–1402, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Endres M, Gertz K, Lindauer U, Katchanov J, Schultze J, Schröck H, Nickenig G, Kuschinsky W, Dirnagl U, Laufs U. Mechanisms of stroke protection by physical activity. Ann Neurol 54: 582–590, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Epstein M, Pins DS, Arrington R, Denunzio AG, Engstrom R. Comparison of water immersion and saline infusion as a means of inducing volume expansion in man. J Appl Physiol 39: 66–70, 1975 [DOI] [PubMed] [Google Scholar]
- 15.Farhi LE, Linnarsson D. Cardiopulmonary readjustments during graded immersion in water at 35°C. Resp Physiol 30: 35–50, 1977 [DOI] [PubMed] [Google Scholar]
- 16.Gabrielsen A, Johansen LB, Norsk P. Central cardiovascular pressures during graded water immersion in humans. J Appl Physiol 75: 581–585, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schrock H, Ji S, Milosevic M, Harms C, Bohm M, Dirnagl U, Laufs U, Endres M. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res 99: 1132–1140, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Green DJ, Maiorana AJ, O'Driscoll G, Taylor R. Topical Review: Effects of exercise training on vascular endothelial nitric oxide function in humans. J Physiol 561: 1–25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature 9: 58–65, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Hong SK, Cerretelli P, Cruz JC, Rahn H. Mechanics of respiration during submersion in water. J Appl Physiol 27: 535–538, 1969 [DOI] [PubMed] [Google Scholar]
- 21.Hood WBJ, Murray RH, Urchel CW, Bowers JA, Goldman JK. Circulatory effects of water immersion upon human subjects. Aerospace Med 39: 579–584, 1968 [PubMed] [Google Scholar]
- 22.Lange L, Lange S, Echt M, Gauer OH. Heart volume in relation to body posture and immersion in a thermo-neutral bath. A roentgenometric study. Pflügers Arch 352: 219–226, 1974 [DOI] [PubMed] [Google Scholar]
- 23.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 39: 183–238, 1959 [DOI] [PubMed] [Google Scholar]
- 24.Lautenschlager N, Cox K, Kurz A. Physical activity and mild cognitive impairment and Alzheimer's disease. Curr Neur Neurosci Rep 10: 352–358, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Leung WM, Logan AG, Campbell PJ, Debowski TE, Bull SB, Wong PY, Blendis LM, Skorecki KL. Role of atrial natriuretic peptide and urinary cGMP in the natriuretic and diuretic response to central hypervolemia in normal human subjects. Can J Physiol Pharmacol 65: 2076–2080, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Liebs TR, Herzberg W, Rüther W, Haasters J, Russlies M, Hassenpflug J. Multicenter randomized controlled trial comparing early versus late aquatic therapy after total hip or knee arthroplasty. Arch Phys Med Rehabil 93: 192–199, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Löllgen H, Nieding G, Koppenhagen K, Kersting F, Just H. Hemodynamic response to graded water immersion. Klin Wochenschr 59: 623–628, 1981 [DOI] [PubMed] [Google Scholar]
- 28.Mayhan WG, Sun H, Mayhan JF, Patel KP. Influence of exercise on dilatation of the basilar artery during diabetes mellitus. J Appl Physiol 96: 1730–1737, 2004 [DOI] [PubMed] [Google Scholar]
- 29.McArdle WD, Magel JR, Lesmes GR, Pechar GS. Metabolic and cardiovascular adjustment to work in air and water at 18, 25, and 33 degrees C. J Appl Physiol 40: 85–90, 1976 [DOI] [PubMed] [Google Scholar]
- 30.Naylor LH, Carter HH, FitzSimons MG, Cable NT, Thijssen DHJ, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol 300: H664–H669, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Norsk P, Bonde-Petersen F, Warberg J. Arginine vasopressin, circulation, and kidney during graded water immersion in humans. J Appl Physiol 61: 565–574, 1986 [DOI] [PubMed] [Google Scholar]
- 32.Parati G, Grassi G, Coruzzi P, Musiari L, Ravogli A, Novarini A, Mancia G. Influence of cardiopulmonary receptors on the bradycardic responses to carotid baroreceptor stimulation in man. Clin Sci (Lond) 72: 639–645, 1987 [DOI] [PubMed] [Google Scholar]
- 33.Park KS, Choi JK, Park YS. Cardiovascular regulation during water immersion. Appl Human Sci 18: 233–241, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 104: 5638–5643, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Prins J, Cutner D. Aquatic therapy in the rehabilitation of athletic injuries. Clin Sport Med 18: 447–461, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Rennie DW, Prampero Pd, Ceretelli P. Effects of water immersion on cardiac ouput, heart rate, and stroke volume of man at rest and during exercise. Med Sport (Torino) 24: 223–228, 1971 [Google Scholar]
- 38.Risch W, Koubenec HJ, Beckmann U, Lange S, Gauer O. The effect of graded immersion on heart volume, central venous pressure, pulmonary blood distribution, and heart rate in man. Pflügers Arch 374: 115–118, 1978 [DOI] [PubMed] [Google Scholar]
- 39.Thijssen DHJ, Maiorana AJ, O'Driscoll G, Cable NT, Hopman MTE, Green DJ. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol 108: 845–875, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke 26: 1014–1019, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Tinken TM, Thijssen DHJ, Hopkins ND, Dawson EA, Cable NT, Green DJ. Shear stress mediates vascular adaptations to exercise training in humans. Hypertension 55: 312–318, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Weston CF, O'Hare JP, Evans JM, Corrall RJ. Haemodynamic changes in man during immersion in water at different temperatures. Clin Sci 73: 613–616, 1987 [DOI] [PubMed] [Google Scholar]
- 43.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Yun SH, Choi JK, Park YS. Cardiovascular responses to head-out water immersion in Korean women breath-hold divers. Eur J Appl Physiol 91: 708–711, 2004 [DOI] [PubMed] [Google Scholar]