Abstract
Adult obese Zucker rats (OZR; >12 wk) develop elevated sympathetic nerve activity (SNA) and mean arterial pressure (MAP) with impaired baroreflexes compared with adult lean Zucker rats (LZR) and juvenile OZR (6–7 wk). In adult OZR, baroreceptor afferent nerves respond normally to changes in MAP, whereas electrical stimulation of baroreceptor afferent fibers produces smaller reductions in SNA and MAP compared with LZR. We hypothesized that impaired baroreflexes in OZR are linked to reduced activation of brain stem sites that mediate baroreflexes. In conscious adult rats, a hydralazine (HDZ)-induced reduction in MAP evoked tachycardia that was initially blunted in OZR, but equivalent to LZR within 5 min. In agreement, HDZ-induced expression of c-Fos in the rostral ventrolateral medulla (RVLM) was comparable between groups. In contrast, phenylephrine (PE)-induced rise in MAP evoked markedly attenuated bradycardia with dramatically reduced c-Fos expression in the nucleus tractus solitarius (NTS) of adult OZR compared with LZR. However, in juvenile rats, PE-induced hypertension evoked comparable bradycardia in OZR and LZR with similar or augmented c-Fos expression in NTS of the OZR. In urethane-anesthetized rats, microinjections of glutamate into NTS evoked equivalent decreases in SNA, heart rate (HR), and MAP in juvenile OZR and LZR, but attenuated decreases in SNA and MAP in adult OZR. In contrast, microinjections of glutamate into the caudal ventrolateral medulla, a target of barosensitive NTS neurons, evoked comparable decreases in SNA, HR, and MAP in adult OZR and LZR. These data suggest that OZR develop impaired glutamatergic activation of the NTS, which likely contributes to attenuated baroreflexes in adult OZR.
Keywords: autonomic, baroreflex, obesity, sympathetic nerve activity, c-Fos, ventrolateral medulla
accumulation of excess body fat is an independent risk factor for increased basal sympathetic nerve activity (SNA) and elevated mean arterial pressure (MAP) in humans and animals (8, 39, 49, 55). In addition, obesity is associated with impaired short-term control of MAP by arterial baroreflexes (4, 5, 19, 47, 59). In obese subjects, evoked changes in MAP yield significantly blunted compensatory baroreflex-mediated changes in SNA and heart rate (HR). In humans and animal models of obesity, impaired baroreflex-mediated regulation of HR is associated with reduced variability in HR, a hallmark for increased risk of poor outcomes in patients with cardiovascular disease (29, 36, 59). These baroreflexes provide powerful moment-to-moment buffering against changes in MAP, and increased variability of MAP is a significant independent risk factor for end-organ damage and detrimental cardiovascular incidents (34, 41). Therefore, understanding the basis for obesity-related impairment of baroreflexes is essential for reducing morbidity and mortality.
The use of obese animal models greatly facilitates the invasive study of mechanisms underlying altered autonomic regulation of cardiovascular function. In one such model, obese Zucker rats (OZR), excess weight gain occurs due to the mutation of the leptin receptor, which promotes hyperphagia compared with lean Zucker rats (LZR) with functional leptin receptors (26, 36). Adult OZR display many of the abnormal physiological attributes observed in obese humans, including hyperlipidemia and insulin resistance with eventual hyperglycemia (10, 14). Although OZR begin to weigh more than LZR shortly after weaning at 4 wk of age (10), autonomic and cardiovascular deficits emerge later in life, suggesting these deficiencies are not due to the mutation of the leptin receptor itself but rather are a consequence of the progressing metabolic syndrome. Juvenile OZR (7–8 wk old) have SNA, MAP, and baroreflex control of HR and SNA that are comparable to age-matched LZR (35, 47). However, by 12 wk of age, adult OZR have elevated renal and splanchnic SNA and MAP with significantly impaired baroreflex control of HR and SNA compared with age-matched LZR and juvenile OZR (9, 33, 35, 47).
The mechanisms underlying impaired baroreflex-mediated control of SNA and HR in adult OZR are not known. We recently reported that baroreceptor afferent nerves, as represented by whole aortic depressor nerve (ADN) activity, appear to respond normally to acute, evoked changes in MAP (22). In addition, the threshold MAP for the onset of afferent activity and gain of ADN activity in relation to MAP is comparable in adult OZR and LZR. In contrast, direct electrical stimulation of baroreceptor afferent fibers evokes blunted decreases in SNA and MAP in adult OZR compared with LZR (22). These data suggest that OZR have impaired baroreflexes due to changes in processing of baroreceptor inputs by the brain.
Three brain stem nuclei are essential for baroreflex-induced changes in SNA to cardiovascular targets (e.g., 16). Increased MAP stimulates baroreceptor afferent nerves to excite second-order neurons in the NTS by activation of glutamatergic receptors (1, 62). Second-order neurons in the NTS provide a glutamatergic activation of GABAergic inhibitory neurons in the caudal ventrolateral medulla (CVLM). These inhibitory neurons in the CVLM, in turn, project to the rostral ventrolateral medulla (RVLM) to inhibit the activity of presympathetic RVLM neurons and decrease SNA, HR, and MAP (16). Conversely, lowering MAP decreases baroreceptor afferent nerve activity to reduce tonic activation of critical neurons in the NTS and CVLM, thereby allowing activation of presympathetic RVLM neurons to increase SNA and raise HR and MAP. Altered central processing of baroreceptor inputs could potentially arise from changes in any of these brain stem regions.
The present study examines whether activation of the brain stem by acute changes in MAP is altered in adult OZR. Specifically, we determined whether hypotension-induced activation of the RVLM, as evidenced by c-Fos expression, is blunted in adult OZR compared with LZR. Furthermore, we sought to determine whether acutely raising MAP results in reduced expression of c-Fos in the NTS of adult OZR compared with LZR. In addition, we sought to determine whether the ability of exogenous glutamate microinjected into the NTS or CVLM to decrease SNA, HR, and MAP is attenuated in adult OZR compared with LZR.
MATERIALS AND METHODS
Animals.
Male OZR (Leprfa), LZR (Lepr fa/Lepr+ and Lepr+/Lepr+), and Sprague-Dawley rats were purchased from Harlan (Indianapolis, IN). Rats were housed in centralized animal care facilities that were kept at a consistent humidity (60 ± 5%), temperature (24 ± 1°C), and light cycle (0600–1800). Rats were given free access to tap water and standard rat chow (Teklad 8640 or Purina 5GL3). Lean and obese rats were housed separately, with rats housed 2–4 in a cage. Experiments were performed on age-matched juvenile (6–7 wk old) Zucker rats, adult (13–17 wk old) Zucker rats, and Sprague-Dawley rats. All experiments were performed in accordance with the National Institutes of Health's Guide for Care and Use of Laboratory Animals and the American Physiological Society's “Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training.” The animal protocols were reviewed and approved by the Institutional Animal Care and Use Committees at University of North Texas Health Science Center and Medical College of Georgia.
Evoked changes in MAP to induce c-Fos expression in conscious rats.
Under isoflurane anesthesia (initially with 5% in 100% oxygen in a secured box and then maintenance with 2.5% through a nose cone), catheters were inserted into a femoral artery and into a femoral vein for recording arterial pressure and infusing drugs, respectively. The catheters were tunneled beneath the skin to exit between the scapulae. To allow free movement of the rat in the conscious state, catheters were run through a tether attached to a swivel (Instech Solomon, Plymouth Meeting, PA). The rats regained consciousness in individual cylindrical Plexiglas cages (MTANK, Instech Solomon) and recovered 1 day prior to experiments. The baseline arterial pressure, MAP, and HR were monitored in quiet conditions for 60 min. Baseline values were recorded for 4 min prior to the onset of a treatment. Rats were subjected to one of two treatments for 90 min; a phenylephrine (PE)-induced increase in MAP or a hydralazine (HDZ)-induced decrease in MAP. Control rats received volume- and rate-matched infusions of saline. Juvenile and adult rats were examined in age-matched pairs of LZR and OZR separated by 30 min to accommodate perfusions and alternating whether a treated or control rat started first. The PE (0.5 mg/ml) was infused through the venous line to increase and maintain MAP ∼40 mmHg from baseline (4–30 μl/min; Razel pump). During the last 30 min of infusion, the PE-filled syringe was exchanged with a saline-filled syringe to flush the PE from the line connecting the syringe to the rat. The HDZ (7–15 mg/kg in 2 ml) was infused through the venous line over 1 min. A single infusion of HDZ produces a significant decrease in MAP that is sustained over the 90-min protocol period (17). After 90 min, rats were anesthetized with urethane (1.5 g/kg iv) and perfused transcardially with PBS (250 ml, pH 7.4) followed by 4% phosphate-buffered formaldehyde (500 ml; Electron Microscopy Sciences). The brain was removed and fixed for 48 h in the same formaldehyde solution for later histological analysis.
Histology for c-Fos and tyrosine hydroxylase.
Brain stems were sectioned in the coronal plane (30 μm) with a Vibratome and stored in a cryoprotectant solution at −20°C (52). Histological protocols were performed using a subset of one in every six sections free floating at room temperature on an orbital shaker in solutions prepared in TBS (pH 7.4) unless otherwise noted. Sections were incubated with 1% hydrogen peroxide (30 min) to block endogenous peroxidases, rinsed in TBS, and then incubated in 10% horse serum (45 min) to block nonspecific staining. For detection of c-Fos, sections were incubated with a goat-anti c-Fos primary antibody (1:2,000; 48 h; 4°C; Santa Cruz Biotechnology, Santa Cruz, CA; sc-52G), followed by a biotinylated donkey anti-goat secondary antibody [1:400; 1 h; Jackson 705–066-147 (Jackson Laboratories, Bar Harbor, ME) or Invitrogen D-20698 (Invitrogen, Carlsbad, CA)], and then an avidin-biotin solution (1 h; PK-6100; Vector Laboratories, Burlingame, CA). Immunoreactivity for c-Fos was revealed by incubation with a nickel-intensified 3–3′diaminobenzadine (DAB) solution for ∼8–10 min. The reaction was terminated by rinsing with TBS.
Presympathetic RVLM neurons comprise C1 adrenergic neurons and noncatecholaminergic neurons (43), so the catecholaminergic phenotype of the c-Fos-positive (Fos+) neurons in the RVLM was also examined by staining for tyrosine hydroxylase (TH). At this rostrocaudal level of the ventrolateral medulla, all neurons that express TH are C1 neurons (50). Tissue was incubated simultaneously with primary antibodies for c-Fos and TH (mouse anti-TH, 1:2,000; MAB5280; Chemicon, Temecula, CA). The protocol for detection of c-Fos in the nucleus was performed to completion, as previously described, followed by detection of TH to avoid false-positive staining for TH in the soma and dendrites. After rinsing sections from the nickel-DAB solution, sections were incubated with a biotinylated donkey anti-mouse secondary antibody (1:400; Jackson Laboratories), followed by an avidin-biotin solution (1 h; Vector, PK-6100). Immunoreactivity for TH was revealed by incubation with a DAB solution for ∼8–10 min. This double-staining protocol produced black c-Fos+ nuclei in brown TH-positive neurons for activated C1 neurons.
After completion of immunohistochemical protocols, the sections were mounted onto gelatin-coated slides and serially dehydrated and delipidated in alcohols and xylenes. Coverslips were affixed with DPX mounting media (Sigma-Aldrich). Sections were examined in brightfield using an Olympus BX40 microscope. c-Fos immunoreactive (Fos+) neurons were mapped and counted in the NTS of rats treated with PE and in the RVLM of rats treated with hydralazine using the Neurolucida system (MicroBrightfield, Williston, VT), as previously described (52). In addition, TH-positive neurons with or without c-Fos immunoreactivity were mapped and counted in the RVLM. To account for activation unrelated to evoked changes in MAP, Fos+ neurons were also counted in the NTS and RVLM of saline-treated rats. Several rostrocaudal levels of the NTS and the RVLM were analyzed separately, and bilateral counts were averaged for each section. Typical examples were photographed (Magnafire SP camera and Magnafire software, Optronics) and imported into Adobe Photoshop. Images were converted to grayscale for sections containing only Fos+ neurons. The output levels on all images were adjusted to the range of levels containing pixels. Contrast, brightness, and sharpness were also individually adjusted to best reflect the original material.
Microinjections into brain stem of anesthetized LZR and OZR.
We sought to determine whether reduced PE-induced c-Fos expression in the NTS of adult OZR was due to an attenuated ability of glutamate to activate the NTS, as indicated by evoked changes in SNA, HR, and MAP. To determine whether altered responses to activation of the NTS could be due to changes at the CVLM, a target of NTS neurons (16), we examined physiological responses to direct glutamatergic activation of the CVLM in adult Zucker rats. We also examined glutamatergic activation of the NTS in juvenile rats, because at this age, baroreflexes are comparable in OZR and LZR (47), and preliminary data showed that PE-induced c-Fos expression was not blunted in juvenile OZR vs. LZR. Anesthetized rats were used in these experiments to facilitate direct measurement of SNA and acute microinjections into the exposed brain stem. We have previously shown that intravenously administered urethane does not prevent the observations of elevated baseline SNA and MAP or reduced baroreflexes in adult OZR vs. LZR (47).
Each rat was initially anesthetized with 5% isoflurane in 100% O2 in a secured box. Then the rat was moved to a heated pad and received 2.5–3.5% isoflurane through a nose cone. Adequate anesthesia was confirmed by absence of leg flexion in response to a firm toe pinch. Catheters were implanted into the femoral artery and vein to record MAP and inject drugs, respectively. The trachea was cannulated toward the lungs, and rats were ventilated at ∼1 ml/100 g body wt with 2.0–2.5% isoflurane in 100% O2 (model 683; Harvard Apparatus, Holliston, MA). Respiratory frequency was adjusted to maintain end-tidal CO2 at 3.8–4.2% (CapStar-100; Charles Ward Electronics). The rat was placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) with the bite bar at −11 mm. The left greater splanchnic sympathetic nerve was isolated, placed on two silver wires (Teflon-coated and bared 250 μm at tips, A-M Systems), and covered with Kwik-Sil (World Precision Instruments, Sarasota, FL). The wound was closed to maintain core temperature and prevent desiccation. A partial occipital craniotomy was performed to expose the dorsal surface of the brain stem caudal to the cerebellum.
After surgical procedures were completed, isoflurane anesthesia was replaced by urethane (1.5 g/kg body wt of LZR in 1.5 g/5 ml at 50 μl/min iv), as previously described (23, 47). Once anesthetized with urethane, rats were allowed to recover for 30–45 min. After confirmation of an adequate level of anesthesia (<10 mmHg change in MAP to firm toe pinch, lack of corneal reflex, and stable MAP and HR), the neuromuscular blocker, pancuronium, was administered (1 mg/kg iv; supplemented hourly at one-third the initial dose). Rectal temperature was maintained at 37°C (TC-1000, Charles Ward Electronics) throughout the experimental protocols.
Microinjections were performed using single-barrel glass pipettes pulled and cut to a 50-μm diameter tip. Glutamate was dissolved in artificial cerebrospinal fluid to deliver 1 nmol in 50 nl into previously established stereotaxic coordinates for the NTS and CVLM (46). Coordinates for the NTS were 0.5 mm lateral to the midline, 0.5 mm rostral to calamus scriptorius (caudal tip of area postrema), and 0.5 mm ventral to the dorsal surface of the brain stem. Coordinates for the CVLM were 1.3 mm rostral to calamus scriptorius, 1.9 mm lateral to the midline, and 2.4–2.8 mm ventral to the dorsal surface of the brain stem. In this case, three depths were explored (2.4 mm, 2.6 mm, and 2.8 mm), and the site producing the largest depressor response was used for analysis. Drugs were microinjected over 4–6 s by pressure (Pressure System IIe, Toohey), and the volume was estimated by observing the movement of the meniscus in the calibrated pipette. The drug solutions contained 5% green latex microspheres (Lumiphore) for histological confirmation of the injection sites as previously shown (32). The drugs were injected on each side of the medulla, and the values of the two responses were averaged for each rat. After the completion of the experimental protocol, the rats were treated with a ganglionic antagonist (mecamylamine; 3 mg/kg iv) to estimate the minimum SNA. Then the rats were deeply anesthetized with the addition of isoflurane and were perfused transcardially with PBS (250 ml, pH 7.4) followed by 4% paraformaldehyde (500 ml). The brains were removed, stored in fixative for 48 h, and then sectioned using a Vibratome (50-μm sections, coronal plane). The sections were mounted onto glass slides, and coverslips were applied with Krystalon. The microinjection sites were visualized via epifluorescence (Olympus BX40) and were verified to be in the region of the NTS or CVLM.
Data collection and statistical analysis.
To measure arterial pressure, the catheter line from the femoral artery was connected to a pressure transducer and amplifier (Neurolog System, Digitimer). The MAP and HR were derived from the arterial pressure pulse using an integrator and a spike trigger, respectively (Neurolog System, Digitimer). The SNA was amplified 25,000 times and filtered at 10–3 kHz with a 60-Hz notch filter (Differential AC amplifier 1700, A-M Systems). For integrated SNA, the raw signal was full-wave rectified and averaged into 1-s bins (Digitimer). The baseline integrated SNA (100%) was defined as the activity 2 min preceding each stimulus. The minimum (0%) SNA was measured after ganglionic blockade with mecamylamine. Changes in integrated SNA were measured as % change from baseline. Differences in baseline raw SNA were measured from the full-wave rectified voltage with the voltage due to noise subtracted (23). All analog signals were converted to digital (Micro 1401, Cambridge Electronic Design) and viewed online using Spike2 software (Cambridge).
All group data are expressed as means ± SE. Significant statistical difference was set at P < 0.05. Comparisons of baseline parameters or changes in SNA, MAP, and HR with microinjections between age-matched OZR and LZR were performed using unpaired t-tests. Comparisons of changes in MAP or HR along the 90-min protocol and the expression of Fos+ neurons at multiple rostrocaudal levels were performed using two-way ANOVA repeated measures followed by Bonferroni post hoc tests. Statistical analyses were performed with SigmaStat software version 3.5.
RESULTS
Baseline values for conscious and anesthetized rats are shown in Tables 1 and 2, respectively. Juvenile and adult OZR had higher body weights compared with age-matched LZR in all experiments. Conscious juvenile OZR had a higher MAP compared with age-matched LZR, but under anesthesia, baseline MAP was not different in juvenile OZR and LZR, as previously reported (47). In the adults, OZR had a significantly higher MAP than age-matched LZR in the conscious state and under anesthesia. There were no differences in HR between age-matched OZR and LZR at either age range or anesthetic condition, as previously reported (47).
Table 1.
Baselines of conscious juvenile and adult OZR and LZR
| Group | n | Weight, g | MAP, mmHg | HR, beats/min |
|---|---|---|---|---|
| Juvenile | ||||
| LZR | 7 | 175 ± 4 | 108 ± 1 | 408 ± 5 |
| OZR | 7 | 233 ± 1* | 121 ± 2* | 422 ± 2 |
| Adult | ||||
| LZR | 25 | 372 ± 7 | 116 ± 1 | 363 ± 4 |
| OZR | 28 | 540 ± 7* | 128 ± 1* | 360 ± 5 |
Values are expressed as means ± SE; n = number of rats.
P < 0.05 compared to LZR in that age group.
MAP, mean arterial pressure; HR, heart rate; OZR, obese Zucker rats; LZR, lean Zucker rats.
Table 2.
Baselines of anesthetized juvenile and adult OZR and LZR
| Group | n | Weight, g | SNA, μV | MAP, mmHg | HR, beats/min |
|---|---|---|---|---|---|
| Juveniles | |||||
| LZR | 10 | 211 ± 6 | 0.9 ± 0.2 | 118 ± 2 | 457 ± 6 |
| OZR | 10 | 309 ± 1* | 1.4 ± 0.4 | 120 ± 3 | 431 ± 8 |
| Adults | |||||
| LZR | 30 | 375 ± 5 | 1.5 ± 0.2 | 116 ± 3 | 427 ± 3 |
| OZR | 34 | 567 ± 7* | 2.3 ± 0.2* | 127 ± 2* | 418 ± 4 |
Values are expressed as means ± SE; n = number of rats.
P < 0.05 compared to LZR in that age group.
SNA, sympathetic nerve activity.
Hydralazine-induced c-Fos expression in the RVLM of conscious Zucker rats.
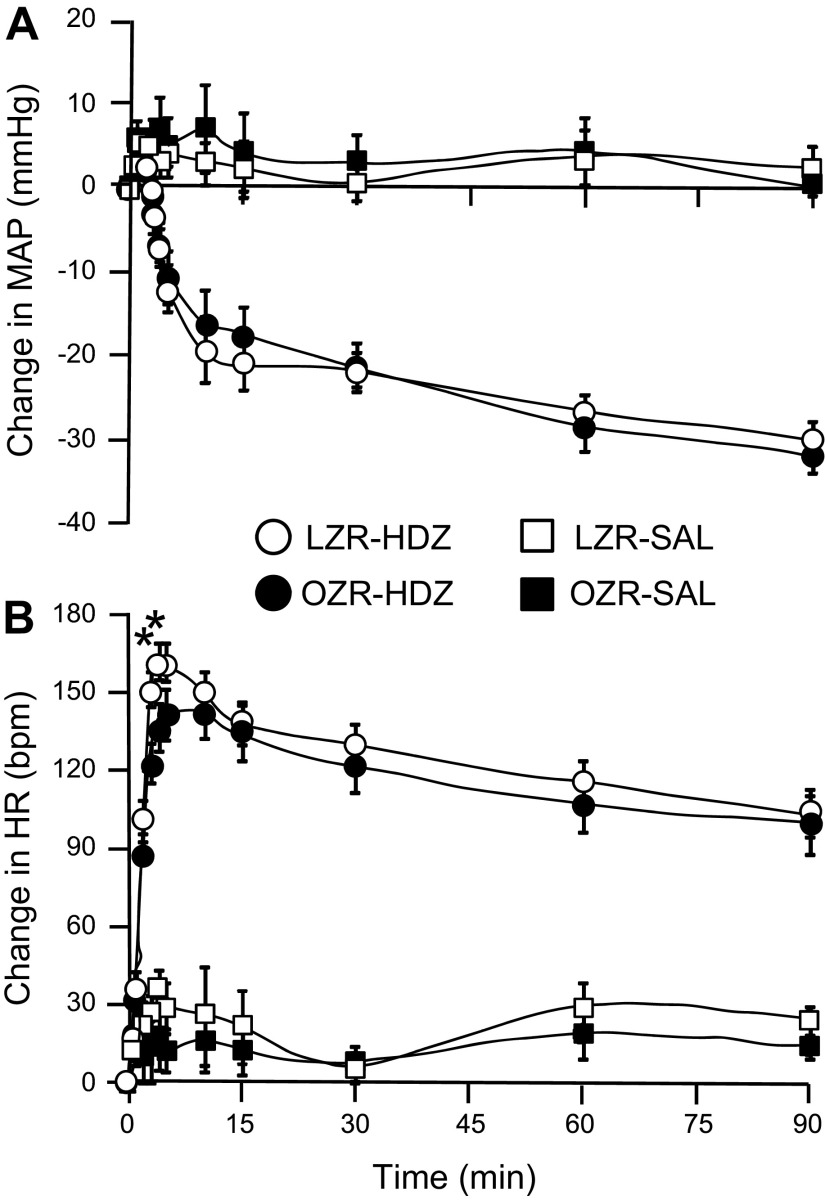
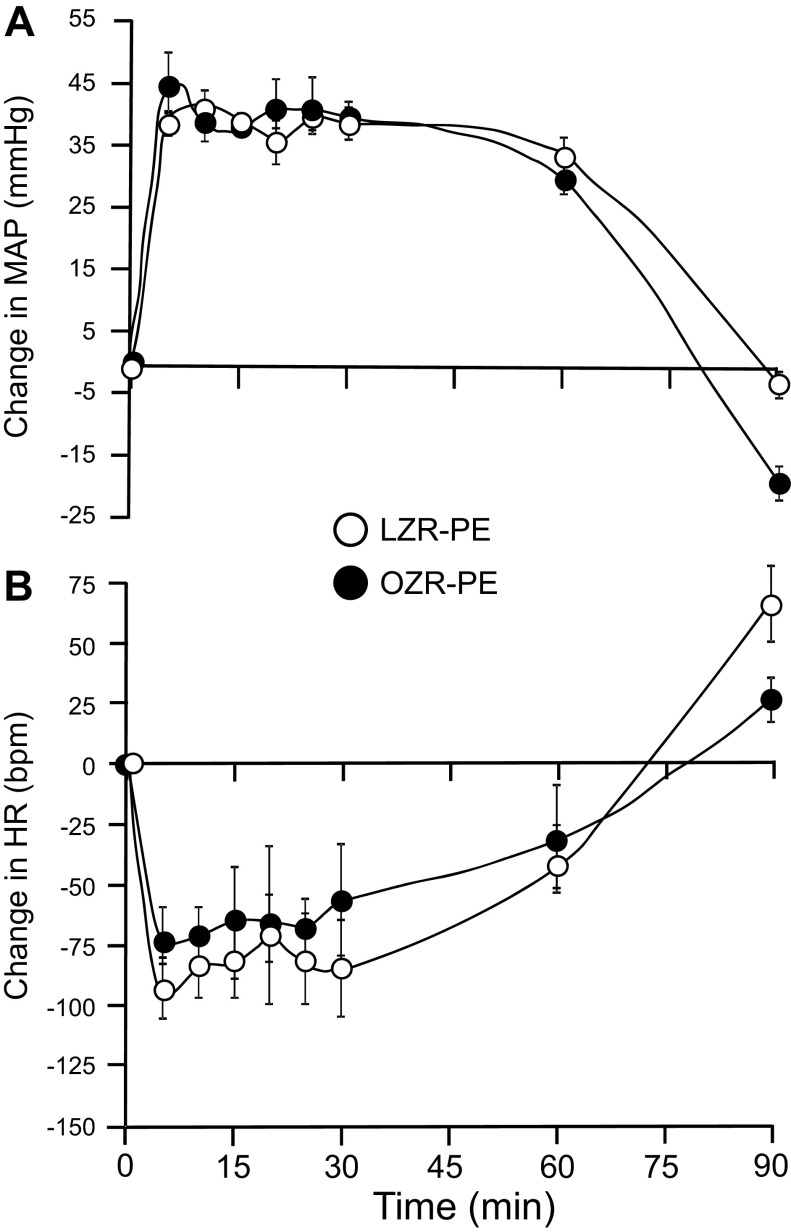
Hydralazine (HDZ) produced a significant decrease in MAP that was comparable in the adult OZR and LZR (Fig. 1A). The initial reduction of ∼20 mmHg was reached within 15 min, and the hypotension was sustained for the 90-min protocol. The HDZ-induced hypotension evoked a considerable baroreflex-mediated tachycardia that was significantly blunted by 16% in the OZR (n = 7) compared with LZR (n = 8) in the initial phase of the response (Fig. 1B). However, the HR values became comparable after 5 min and were not different through the remainder of the 90-min protocol. Infusion of an equivalent volume of saline evoked minimal and comparable changes in MAP and HR in OZR (n =5) and LZR (n = 5; Fig. 1). Because this dose of HDZ did not evoke the expected 40-mmHg reduction in MAP previously reported in conscious Sprague-Dawley rats (17), we examined a set of this strain under the same conditions (n = 8). Interestingly, in Sprague-Dawley rats HDZ (10 mg/kg) reduced MAP 39 ± 4 mmHg within 10 min, but evoked a weaker baroreflex-mediated tachycardia (peak rise was 116 ± 7 beats/min in 3–5 min; compare to Fig. 1B).
Fig. 1.
Changes in mean arterial pressure (A) and heart rate (B) produced by intravenous injection of hydralazine (HDZ) or saline in adult lean Zucker rats (LZR) and obese Zucker rats (OZR). Data are expressed as means ± SE. *Significant difference between LZR and OZR at 3 and 4 min after injection of HDZ, P < 0.05.
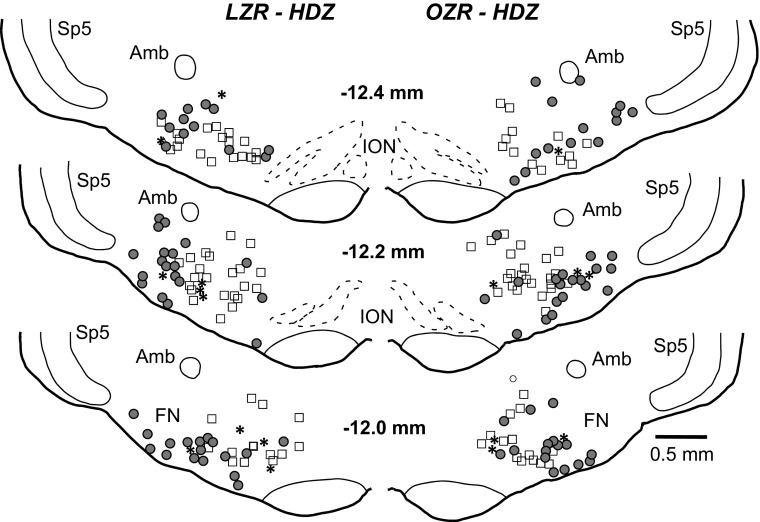
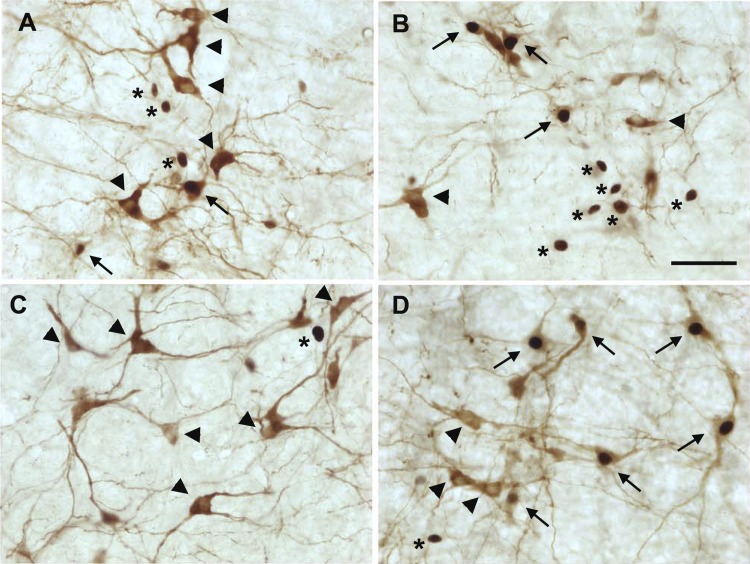
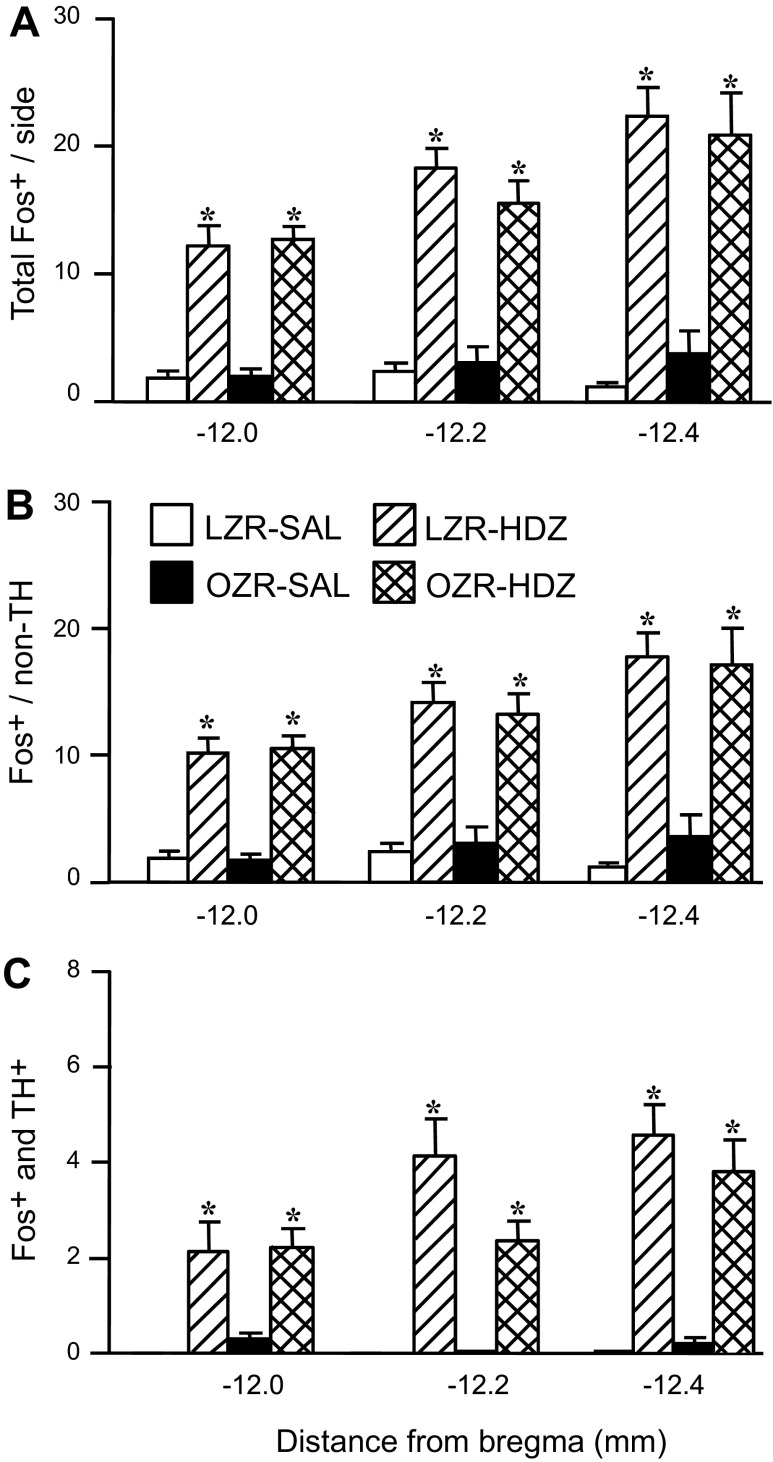
Hydralazine-induced hypotension produced robust c-Fos expression in the RVLM of OZR and LZR (representative maps, Fig. 2; representative photomicrographs, Figs. 3, A and B; group data, Fig. 4). In contrast, rats treated with saline had few Fos+ neurons in the RVLM, and some sections contained no Fos+ neurons (Figs. 3C and 4A). In the HDZ-treated rats, the majority of Fos+ neurons in the RVLM were not catecholaminergic (>80%), and the number of Fos+/non-TH neurons was comparable in OZR and LZR at all levels of the RVLM examined (Fig. 4B). Of the ∼20% of TH+ neurons that were Fos+, there was a trend for a reduced number of Fos+ neurons in the adult OZR compared with LZR, which did not reach statistical significance (Fig. 4C; P = 0.053 at −12.2 mm caudal to bregma). However, the total number of HDZ-induced Fos+ catecholaminergic neurons in the RVLM was low (2–4 on each side of the section; Figs. 2, 3, A and B, and 4C).
Fig. 2.
Representative maps of c-Fos expression in the rostral ventrolateral medulla (RVLM) of an adult lean rat (left) and an adult obese rat (right) after 90 min of HDZ-induced hypotension. Tyrosine hydroxylase (TH) immunoreactivity depicts coincidence of c-Fos expression with the C1 cell group. Open squares (▫) are catecholaminergic [tyrosine hydroxylase-immunoreactive; TH-positive (TH+)] neurons, solid circles (●) are c-Fos-positive (Fos+)/non-TH neurons, and asterisks are Fos+/TH+ neurons. Amb, nucleus ambiguus; FN, facial nucleus; ION, inferior olivary nucleus; sp5, spinal trigeminal nucleus. Location relative to bregma is depicted in millimeters in the middle of each pair of maps.
Fig. 3.
Representative photomicrographs of c-Fos expression (black nuclei) and catecholaminergic neurons (TH+, brown somas and dendrites) in the RVLM of after 90 min of HDZ-induced hypotension or infusion of saline. A: Section from one HDZ-treated LZR. B: Section from one HDZ-treated OZR. C: Section from one saline-treated LZR. D: Section from one HDZ-treated Sprague-Dawley rat. Arrows: Fos+/TH+ neurons; Arrowheads: TH+ neurons without c-Fos; asterisks: Fos+/non-TH neurons. Scale bar is 50 μm.
Fig. 4.
Expression of c-Fos in C1 catecholaminergic and noncatecholaminergic neurons in the RVLM after infusion of HDZ or saline in OZR and LZR. A: Fos+ neurons in the RVLM and three rostrocaudal levels. B: Fos+/non-TH cells/level of RVLM. C: c-Fos in TH+ cells. These are the same rats that are depicted in Fig. 1. Data are expressed as means ± SE. *Significant difference from respective saline-treated group, P <0.05.
Because the percentage of activated C1 neurons was substantially lower than previously reported for Sprague-Dawley rats (80%) (53), we also examined HDZ-induced c-Fos expression in the RVLM in this strain. Although the total number of Fos+ neurons was similar to the Zucker rats (12 ± 1 at −12.0 mm, 17 ± 2 at −12.2 mm, and 24 ± 3 at −12.4 mm, compare to Fig. 4A), the percentage of Fos+ neurons that were catecholaminergic at the most rostral RVLM was ∼60% (example in Fig. 3D). Thus, the activation of the RVLM in terms of c-Fos expression was comparable, but the proportion of C1 vs. noncatecholaminergic RVLM neurons was different between the strains.
Phenylephrine-induced c-Fos expression in the NTS of conscious Zucker rats.
The maximal rise in MAP (40 mmHg) by intravenous injection of PE was reached within 5 min of the onset of the infusion and sustained for at least 60 min in the PE-treated groups (Fig. 5A). The rise in MAP induced a robust baroreflex-mediated bradycardia in both OZR (n = 9) and LZR (n = 7), but the magnitude of the decrease in HR was markedly blunted in OZR for the first 20 min of infusion compared with LZR (47% of LZR response; Fig. 5B). Saline infusion in OZR (n =7) and LZR (n = 5) evoked minimal and comparable changes in MAP and HR (Fig. 5).
Fig. 5.
Changes in mean arterial pressure (A) and heart rate (B) produced by intravenous infusion of PE or saline for 90 min in LZR and OZR. Data are expressed as means ± SE. *Significant difference between LZR-PE and OZR-PE at 5, 10, 15, and 20 min, P < 0.05.
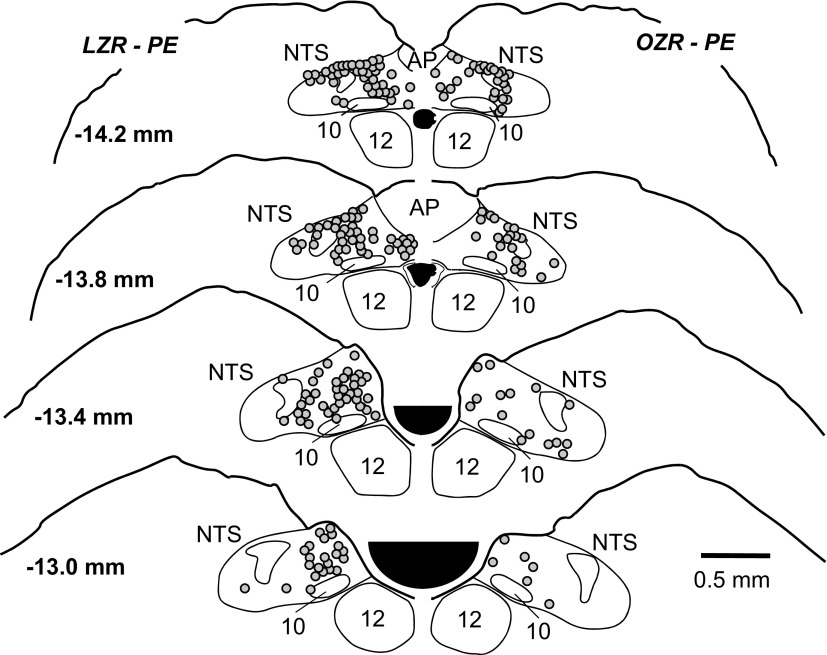
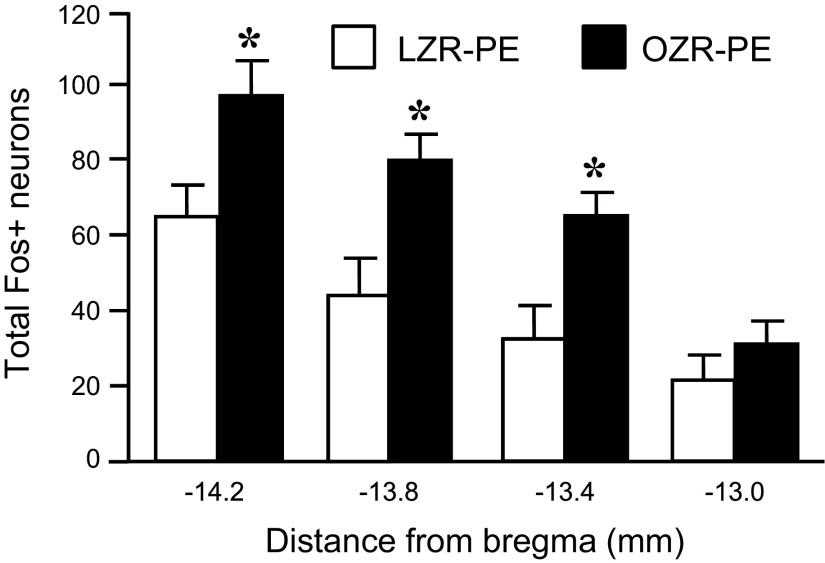
The PE-induced hypertension produced significant expression of c-Fos in the NTS of OZR and LZR (representative maps, Fig. 6; representative photomicrographs, Fig. 7; group data, Fig. 8). In contrast, rate- and volume-matched saline expression yielded little c-Fos expression in the NTS at all levels examined (Fig. 8). In contrast to the comparable HDZ-induced c-Fos expression in the RVLM of OZR and LZR (Fig. 4), the PE-induced c-Fos expression was substantially less in the adult OZR compared with age-matched LZR at all levels of the NTS examined (44% of LZR expression; Figs. 6–8).
Fig. 6.
Representative maps of c-Fos expression in the nucleus tractus solitarius (NTS) of an adult lean rat (left) and an adult obese rat (right) after 90 min of a phenylephrine (PE)-induced increase in mean arterial pressure (MAP). Solid circles (●) represent Fos+ nuclei. Location of section relative to bregma is depicted in millimeters on the left side of the sections. AP, area postrema; 10, dorsal vagal motor nucleus; 12, hypoglossal nucleus.
Fig. 7.
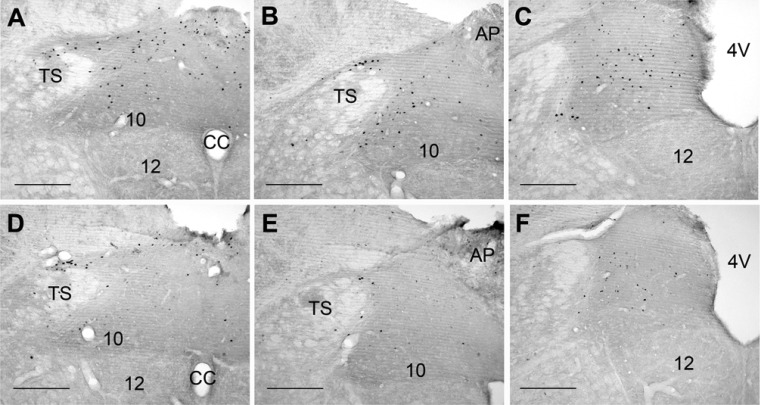
Representative photomicrographs of c-Fos expression in the NTS of an adult lean rat (A–C) and an adult obese rat (D–F) after 90 min of a PE-induced increase in MAP. Photos are from 3 rostrocaudal levels in relation to bregma: −14.2 mm (A and C), −13.8 mm (B and E), and −13.4 mm (C and F). AP, area postrema; CC, central canal; TS, tractus solitarius, 4V, 4th ventricle; 10, dorsal motor nucleus of the vagus; 12, hypoglossal motor nucleus. Scale bars are 250 mm.
Fig. 8.
Expression of c-Fos in the NTS of LZR and OZR after infusion of PE or saline. These are the same rats that are depicted in Fig. 5. Data are expressed as means ± SE. *Significant difference from respective saline-treated rats, P <0.05. #Significant difference from LZR-PE at that bregma level, P <0.05.
Because juvenile OZR appear to have normal baroreflex-mediated responses to evoked increases in MAP (47), we examined whether PE-induced c-Fos expression would be comparable in juvenile OZR and LZR. As seen in the adult rats, PE increased MAP by 40 mmHg within 5 min of the onset of the infusion, and the rise in MAP was sustained for at least 60 min (Fig. 9A). As seen in the adult rats, PE produced a substantial baroreflex-mediated bradycardia in the juvenile OZR and LZR (n = 5 in each group). However, unlike the adult rats, the magnitudes of the PE-induced decreases in HR were comparable in OZR and LZR for the duration of the 90-min protocol (Fig. 9B).
Fig. 9.
Changes in MAP (A) and heart rate (HR; B) produced by intravenous infusion of PE for 90 min in juvenile LZR and OZR. Data are expressed as means ± SE.
In stark contrast to the adult rats and despite the similar changes in MAP and HR, juvenile OZR treated with PE had significantly more Fos+ neurons in the NTS compared with PE-treated juvenile LZR at 3 of the 4 levels examined (Fig. 10). Because the average volume of infusion needed to reach the 40-mmHg increase in MAP was significantly higher in juvenile OZR (1.2 ± 0.13 ml) than in LZR (0.7 ± 0.05 ml), we evaluated c-Fos expression in the NTS in two OZR and LZR that received 0.9% saline solution in the higher volume. In these animals, saline infusion evoked minimal and comparable changes in MAP and HR, and similar c-Fos expression in the NTS (total Fos+ cells for all 4 levels of the NTS combined was ∼30, data not shown).
Fig. 10.
Expression c-Fos in the NTS of juvenile LZR and OZR after intravenous infusion of PE. These are the same rats that are depicted in Fig. 9. Data are expressed as means ± SE. *Significant difference from LZR-PE at that bregma level, P < 0.05.
Microinjections of glutamate into the brain stem of anesthetized Zucker rats.
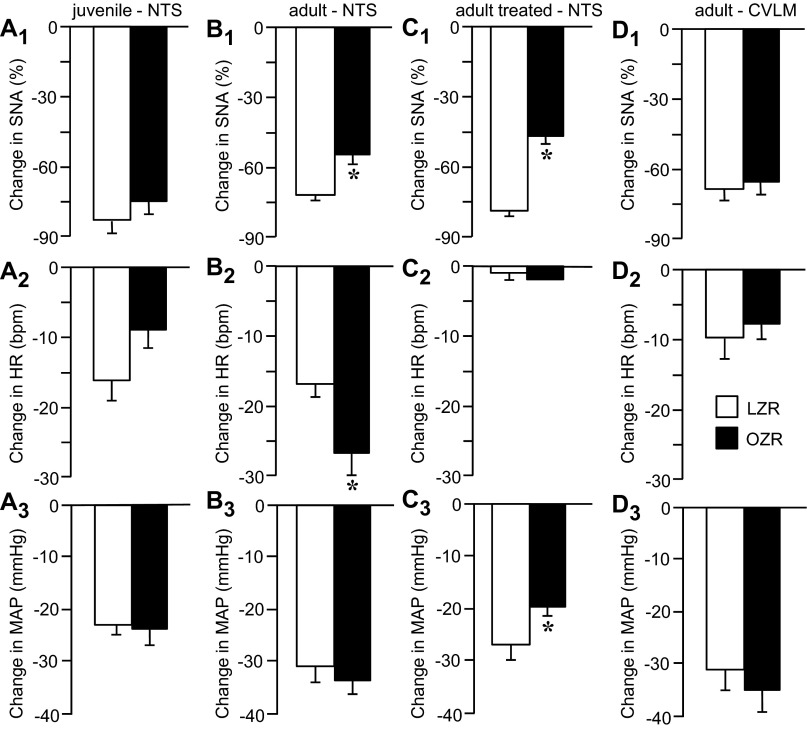
The attenuated PE-induced c-Fos expression in adult OZR suggested that the NTS may be less responsive to excitatory inputs. To examine this possibility, we stimulated the NTS directly by microinjections of glutamate and measured the evoked sympathoinhibition, bradycardia, and hypotension. In juvenile rats, microinjections of glutamate produced comparable physiological responses in OZR and LZR (n = 10 in each group; Fig. 11, A1–A3), in agreement with the equivalent PE-induced bradycardia observed in OZR and LZR of this age (Fig. 9B). In contrast, microinjections of glutamate into the NTS of adult OZR (n = 12) evoked a blunted sympathoinhibition compared with adult LZR (n = 10; Fig. 11B1). This observation is consistent with reduced PE-induced c-Fos expression and markedly attenuated PE-induced bradycardia observed in adult OZR. However, glutamatergic stimulation of the NTS evoked an exaggerated decrease in HR in adult OZR vs. LZR (Fig. 11B2). These two responses together coincided with a comparable decrease in MAP in OZR and LZR (Fig. 11B3).
Fig. 11.
Changes in MAP, HR, and sympathetic nerve activity (SNA) after microinjections of glutamate into the NTS (A–C) or caudal ventrolateral medulla (CVLM; D) in juvenile (A) and adult (B–D) LZR and OZR. In C, rats were treated with atropine and propranolol before microinjections of glutamate into the NTS to prevent changes in HR. Data are expressed as means ± SE. *Significant difference from respective LZR, P < 0.05.
To determine whether the apparent reduced sympathoinhibition in adult OZR had a functional significance for regulation of MAP, we prevented the changes in HR by treating another set of rats with methylatropine (2 mg/kg in 0.1 ml iv) and propranolol (5 mg/kg in 0.1 ml iv) to block parasympathetic and sympathetic inputs to the heart. In this preparation, microinjections of glutamate into the NTS evoked the same attenuated sympathoinhibition observed in the untreated rat (Fig. 11C1 vs. 11B1) and was accompanied by a blunted hypotension in adult OZR (n = 7) compared with LZR (n = 8; Fig. 11C3). These data suggest an impaired ability to inhibit sympathetic vasomotor tone in adult OZR compared with LZR. In these rats, the methylatropine was given before the propranolol, so the rats were also tested with selective antagonism of parasympathetic inputs to the heart. In this condition, microinjections of glutamate evoked comparable reductions in HR in adult OZR and LZR (−8 ± 1 vs. −7 ± 1 beats/min). These data suggest the exaggerated bradycardia evoked by microinjection of glutamate into the NTS was due to enhanced parasympathetically mediated bradycardia in the OZR.
Baroreflex-mediated responses require glutamatergic activation of the CVLM (15), a target of barosensitive glutamatergic NTS neurons (61). To determine whether blunted sympathoinhibition after microinjections of glutamate into the NTS could be due to targets downstream of the NTS, we examined the physiological effects of microinjections of glutamate into the CVLM of adult OZR and LZR. In contrast to the differences observed after stimulation of the NTS (Fig. 11, B and C), activation of the CVLM evoked comparable decreases in SNA, HR, and MAP in adult OZR (n = 14) and LZR (n = 13; Fig. 11, D1–D3). These data suggest that the differences observed after microinjections of glutamate into the NTS between adult OZR and LZR were due to changes at the NTS.
DISCUSSION
Previous studies have shown impaired baroreflex-mediated control of SNA and HR in adult OZR compared with age-matched LZR (3, 9, 47). The present study highlights the disproportionate degree of impairment to evoked increases vs. decreases in MAP in conscious adult OZR and provides insights into potential mechanisms for the compromised responses. Although HDZ-induced tachycardia was significantly attenuated for 5 min in adult OZR, the evoked rise in HR was equivalent to the LZR for the next 85 min of sustained hypotension. This response was reflected in a comparable HDZ-induced c-Fos expression in the RVLM of OZR and LZR. In contrast, the attenuation of PE-evoked bradycardia in adult OZR was much more pronounced and continued for 20 min. In agreement, PE-induced c-Fos expression in the NTS was significantly diminished in adult OZR compared with LZR. Furthermore, the ability of glutamate in the NTS to decrease SNA and MAP was also reduced in adult OZR, suggesting the attenuated c-Fos expression reflected changes in NTS neurons that are relevant to the autonomic control of MAP. In contrast, glutamatergic activation of the CVLM, a target of the NTS in the baroreflex pathway, evoked comparable changes in SNA, HR, and MAP in adult OZR and LZR. Unlike adult rats, PE-induced bradycardia was not impaired in juvenile OZR, as previously reported (47). In agreement, in juvenile OZR and LZR, glutamatergic activation of the NTS evoked equivalent decreases in SNA, HR, and MAP. Unexpectedly, the PE-induced c-Fos expression in NTS of the juvenile OZR was exaggerated compared with LZR. Together, these data suggest that changes are occurring in the NTS of OZR prior to the detection of overt autonomic and cardiovascular deficits and that the NTS is a critical brain stem site for the production of attenuated baroreflexes in adult OZR.
The baroreflex is often depicted as a continuum of changes in HR to induced increases and decreases in MAP, but responses to these opposing changes in MAP involve distinct underlying mechanisms that may be differentially affected by metabolic syndrome. Lowering MAP reduces the ongoing activity of baroreceptor afferent nerves to eventual inactivity (e.g., 22). The observed reflex responses are a reflection of elimination or significant diminution of existing tonic activity in neurons of the baroreflex pathway that are baroreceptor-driven (the NTS and CVLM). Furthermore, hypotension-induced tachycardia is primarily mediated by sympathetic stimulation of the heart, with a smaller contribution from vagal withdrawal (3, 51). The SNA rises due to reduced CVLM-mediated tonic inhibition of presympathetic neurons in the RVLM. The adult OZR appears to have reduced basal tonic CVLM-mediated inhibition of the RVLM, which is also reflected in a reduced drive from the NTS. The diminished tonic influences of the NTS and CVLM may explain the initially blunted HDZ-induced tachycardia observed in adult OZR in the present study, which is in agreement with previous reports of impaired rises in HR that focus on the initial responses observed with brief infusions of nitroprusside (3, 37). Highlighting the relatively small magnitude of the deficit, several reports in obese rats fail to see significant differences in hypotension-induced tachycardia, even when hypertension-induced bradycardia is clearly attenuated (5, 6, 11, 47). Regardless of the differences observed at the onset of hypotension, within minutes, HDZ-induced tachycardia was comparable in OZR and LZR, suggesting other mechanisms activated by hypotension provided a delayed compensation that allowed the OZR to effectively respond to a sustained decrease in MAP.
Infusion of HDZ to decrease MAP produced robust c-Fos expression in the RVLM that was not observed in rats treated with saline, suggesting the hypotension, and not the protocol itself, was the effective stimulus. In agreement with the largely comparable HDZ-induced tachycardia, the HDZ-induced c-Fos expression in the RVLM was not different between adult OZR and LZR. Because the full expression of sympathoexcitatory responses mediated by the RVLM requires activation of both C1 catecholaminergic and noncatecholaminergic neurons (7, 30, 44), both populations were examined. The majority of Fos+ RVLM neurons were not catecholaminergic (80%), and there was no significant difference in the c-Fos expression of either cell group between adult OZR and LZR. Thus, the two populations of presympathetic RVLM neurons each appear to respond comparably to hypotension in adult OZR vs. LZR. With the substantial and sustained hypotension used for activating c-Fos expression in the RVLM, the observed c-Fos expression is likely to be more representative of the upper plateau of the baroreflex rather than the gain, which is more readily observed with the onset of changes in MAP.
The more striking deficit in the adult OZR lies in their reduced ability to combat a hypertensive stimulus. In contrast to hypotension-evoked responses, which rely upon the reduction of ongoing baroreceptor afferent nerve activity to the brain stem, raising MAP stimulates baroreceptor afferent nerves to produce a glutamatergic excitation of the NTS (1, 2, 54, 62). The sympathetic response is highly affected by the ability to acutely activate the NTS and its target in the baroreflex pathway, GABAergic neurons of the CVLM. Although the ability of GABA to inhibit the RVLM can also alter the efficacy of baroreflex-mediated reductions in SNA, we have previously shown that GABAergic inhibition of the RVLM produces comparable decreases in SNA, HR, and MAP in adult OZR and LZR (23). In contrast, the NTS appears to become less responsive to glutamatergic activation in the OZR at the same age when PE-induced bradycardia and c-Fos expression in the NTS become reduced (Figs. 5–10) (47). The attenuated baroreceptor-mediated activation of the NTS in OZR does not appear to be due to impaired baroreceptor afferent function (22) or diminished release of glutamate in the NTS, because direct application of exogenous glutamate into the NTS also produced a smaller reduction in sympathetic vasomotor tone in the adult OZR. Furthermore, the impaired regulation of SNA appears to be localized to the NTS, because activation of target neurons in the CVLM by microinjections of glutamate evoked comparable decreases in SNA, HR, and MAP in adult OZR and LZR. Further study will be needed to determine the cellular mechanisms that underlie the reduced physiological responses to glutamate in the NTS of adult OZR.
Baroreflex-mediated reductions in HR are predominantly produced by activation of vagal parasympathetic efferents to the heart with a smaller contribution from withdrawal of cardiac sympathetic tone (3, 51). Activation of parasympathetic control to the heart is initiated by baroreceptor-driven glutamatergic stimulation of NTS neurons, which, in turn, provide glutamatergic activation of vagal motor neurons in the nucleus ambiguus (e.g., 60). In agreement, selective antagonism of vagal inputs to the heart greatly reduces the bradycardia evoked by raising MAP with PE (3) or by microinjecting glutamate into the NTS (present study). After blockade of sympathetic inputs to the heart with propranolol, PE-induced bradycardia is still smaller in adult OZR compared with LZR, suggesting a reduced vagally mediated inhibition of HR contributes to the blunted baroreflex in OZR. In addition, impaired PE-mediated sympathoinhibition in the adult OZR also appears to contribute, because after selective antagonism of parasympathetic inputs to the heart with atropine, adult OZR still have smaller reductions in HR to evoked rises in MAP compared with LZR (3). Impaired sympathoinhibitory responses extend beyond the heart because PE-induced rises in MAP also produce blunted inhibition of splanchnic SNA in adult OZR (47). Although the diminished PE-evoked sympathoinhibition in adult OZR appears to be mediated by impaired glutamatergic activation of the NTS, whether the same mechanism attenuates vagal responses to increased MAP in adult OZR is not known.
Unexpectedly, although PE-induced bradycardia was significantly reduced in adult OZR vs. LZR, glutamatergic activation of the NTS produced an exaggerated bradycardia in adult OZR. The enhanced glutamate-induced bradycardia appeared to be vagally mediated because blockade of parasympathetic inputs to the heart with methylatropine equalized the responses in OZR and LZR. It is most likely that microinjections of glutamate into the NTS stimulated neurons in addition to those that mediate arterial baroreflexes. Interestingly, not all vagal inputs to the NTS evoke significantly blunted bradycardia in adult OZR. Activation of the Bezold-Jarisch reflex produces a similar pattern to that observed with glutamatergic activation of the NTS with blunted sympathoinhibition, a trend for larger bradycardia, and comparable depressor response in adult OZR vs. LZR (22). The Bezold-Jarisch reflex, which is initiated by stimulation of cardiopulmonary vagal afferents to the NTS, utilizes a brain stem pathway that is parallel to and overlapping with the arterial baroreflex. Within the NTS, distinct neurons process the two types of inputs, whereas neurons in the CVLM and RVLM respond to activation of both reflexes (38, 45, 48, 57, 58). Electrical stimulation of whole vagal afferent nerves in adult OZR and LZR elicit comparable, blunted, or exaggerated changes in HR in OZR, depending on the frequency of the stimulation (22), highlighting the heterogeneity of vagal inputs and their processing in the NTS. In obese rats, the arterial baroreflex is not the only impaired sympathoinhibitory reflex that occurs by vagal activation of the NTS. Rats made obese by a high-fat diet with impaired arterial baroreflexes also show blunted sympathoinhibitory responses to gastric CCK, a reflex that is initiated by gastric vagal afferents to the NTS (21). Thus, it appears that metabolic syndrome differentially affects the processing of functionally diverse vagal inputs to distinct NTS neurons that regulate autonomic function to cardiovascular targets. To our knowledge, it is not known whether arterial baroreceptor-driven control of sympathetic and parasympathetic outflows is directed by common or distinct NTS neurons. Clearly, a more cellular analysis of NTS neurons with identified inputs and projections would elucidate synaptic mechanisms underlying impaired baroreceptor-mediated activation of the NTS in adult OZR.
The underlying causative attributes of metabolic syndrome that lead to the development of impaired NTS function and baroreflexes are also not known. Hypertension and blunted baroreflexes often occur together, but in OZR, the development of impaired baroreflexes and changes in NTS function precede measurable differences in MAP between OZR and LZR. At ∼7 wk of age, the gain of the sympathetic baroreflex is reduced in OZR compared with LZR (47), and PE-induced c-Fos expression in the NTS is different between the two groups (Fig. 10). However, significant differences in MAP cannot be reliably detected in conscious undisturbed OZR and LZR until 10 wk of age (28), with a clear separation of MAP levels by 12 wk of age (35). In agreement, rats made obese by a high-fat diet develop impaired baroreflexes within 4 wk on the diet (63), prior to the onset of elevated MAP that is present after 13 wk (21, 49). Just as animal models of obesity show a separation of baroreflexes and elevated MAP, obese patients can have significantly impaired baroreflexes in the absence of hypertension (18). Conversely, impaired baroreflexes in OZR are coincident with diminished basal tonic inhibition of SNA by the NTS and CVLM and reduced tonic GABAergic inhibition of the RVLM neurons that drive SNA to maintain MAP (23). These changes in the tonic regulation of the baroreflex pathway are likely to contribute to the elevated basal levels of SNA and MAP in adult OZR.
In contrast to the onset of cardiovascular deficits in OZR, metabolic parameters are altered at an earlier age, and many of these attributes have been shown to affect basal SNA and MAP, as well as baroreflex efficacy. At 7 wk of age, fasted OZR have elevated plasma levels of triglycerides and insulin (12, 35, 42). Although reports of differences in fasted levels of blood glucose between OZR and LZR at 7 wk are inconsistent, insulin resistance is unmistakable as evidenced by the hyperinsulinemia and impaired glucose tolerance (12, 35, 42). By adulthood, OZR have fasting hyperglycemia with elevated blood cholesterol and nonesterified fatty acids compared with juvenile OZR and age-matched LZR (12, 37). In addition, compared with LZR, adult OZR have elevated fasting plasma levels of proinflammatory markers, such as interleukin-6 and tumor necrosis factor-α (56). Although OZR do not respond to their elevated leptin levels, the excess white adipose tissue in OZR shows increased expression of inducible nitric oxide synthase and reduced expression of adiponectin (but elevated plasma levels) to further promote insulin resistance (24). In addition, whereas young LZR show rising testosterone that remains stable through adulthood, juvenile OZR show a decline in testosterone with levels that are significantly lower than LZR in adulthood (20). These factors, and others not mentioned here, each have the potential to impact baroreflexes in the adult OZR, making unraveling the underlying deleterious mechanisms and restoration of normal function in the obese state a challenge.
Perspectives and Significance
The occurrence of metabolic syndrome or cardiometabolic disease has reached epidemic proportions in the United States and now across the world. From the cardiovascular perspective, elevated arterial pressure is used as a key indicator of the syndrome. However, cardiometabolic disease is also highly and independently associated with impaired short-term control by baroreflexes, which are vital for stabilization of MAP. When baroreflex sensitivity is diminished, spontaneous variability of MAP is increased and HR variability is reduced (e.g., 25, 27). In humans, these attributes are highly predictive of increased risk for poor cardiovascular outcomes (31, 40). Patients treated for hypertension with residual increased variability of MAP are at higher risk for deleterious cardiovascular events (41). Like obese humans, OZR develop metabolic syndrome complete with elevated MAP and significantly impaired baroreflexes, making the OZR an excellent model to investigate mechanisms and effective treatments for the cardiovascular attributes of cardiometabolic disease. This study illustrates the development of an impaired baroreflex in OZR and pinpoints a region of the brain stem, the NTS, that appears to be a key mediator of the observed deficits in short-term, and perhaps long-term control of MAP. Future studies will be necessary to determine the causes and cellular mechanisms of impaired glutamatergic activation of the NTS in the adult OZR.
GRANTS
This study was funded by a grant from the Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL-086759 to A. M. Schreihofer). P. S. Guimaraes was a recipient of PDSE fellowship (1203-09-6) from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.S.G., D.A.H., and A.M.S. conception and design of research; P.S.G. and D.A.H. performed experiments; P.S.G., D.A.H., and A.M.S. analyzed data; P.S.G., D.A.H., and A.M.S. interpreted results of experiments; P.S.G., D.A.H., and A.M.S. prepared figures; P.S.G. drafted manuscript; P.S.G., D.A.H., M.J.C.-S., and A.M.S. edited and revised manuscript; P.S.G., D.A.H., M.J.C.-S., and A.M.S. approved final version of manuscript.
REFERENCES
- 1.Andresen MC, Doyle MW, Jin YH, Bailey TW. Cellular mechanisms of baroreceptor integration at the nucleus tractus solitarius. Ann NY Acad Sci 940: 132–141, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Aylwin ML, Horowitz JM, Bonham AC. NMDA receptors contribute to primary vsiceral afferent transmission in the nucleus of the solitary tract. J Neurophysiol 77: 2539–2548, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Barringer DL, Buñag RD. Uneven blunting of chronotropic baroreflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol 256: H417–H421, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Beske SD, Alvarez GE, Ballard TP, Davy KP. Reduced cardiovagal baroreflex gain in visceral obesity: implications for the metabolic syndrome. Am J Physiol Heart Circ Physiol 282: H630–H635, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Buñag RD, Eriksson L, Krizsan D. Baroreceptor reflex impairment and mild hypertension in rats with dietary-induced obesity. Hypertension 15: 397–406, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Buñag RD, Meyer M, Vansell N, Kerecsen L. Conscious obese rats have impaired reflex bradycardia and enhanced norepinephrine sensitivity. Am J Physiol Regul Integr Comp Physiol 271: R654–R660, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Burke PG, Neale K, Korim WS, McMullan S, Goodchild AK. Patterning of somatosympathetic reflexes reveals nonuniform organization of presympathetic drive from C1 and non-C1 RVLM neurons. Am J Physiol Regul Integr Comp Physiol 301: R1112–R1122, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Carlson SH, Shelton J, White CR, Wyss JM. Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension 35: 403–408, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Davis G. Baroreflex and somato-reflex control of blood pressure, heart rate and renal sympathetic nerve activity in the obese Zucker rat. Exp Physiol 96: 623–634, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Durham HA, Truett GE. Development of insulin resistance and hyperphagia in Zucker fatty rats. Am J Physiol Regul Integr Comp Physiol 290: R652–R658, 2006 [DOI] [PubMed] [Google Scholar]
- 11.El-Wazir YM, Li SG, Smith R, Silcox DL, Brown DR, Randall DC. Parasympathetic response to acute stress is attenuated in young Zucker rats. Auton Neurosci 143: 33–39, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisbee JC. Hypertension-independent microvascular rarefaction in the obese Zucker rat model of the metabolic syndrome. Microcirculation 12: 383–392, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Gadegbeku CA, Dhandayuthapani A, Sadler ZE, Egan BM. Raising lipids acutely reduces baroreflex sensitivity. Am J Hypertens 15: 479–485, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Goodwill AG, Frisbee SJ, Stapleton PA, James ME, Frisbee JC. Impact of chronic anticholesterol therapy on development of microvascular rarefaction in the metabolic syndrome. Microcirculation 16: 667–684, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon FJ. Aortic baroreceptor reflexes are mediated by NMDA receptors in caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 252: R628–R633, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Gordon FJ, Sved AF. Neurotransmitters in central cardiovascular regulation: Glutamate and GABA. Clin Exper Pharmacol Physiol 29: 522–524, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Graham JC, Hoffman GE, Sved AF. c-Fos expression in brain in response to hypotension and hypertension in conscious rats. J Auton Nerv Syst 55: 92–104, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension 25: 560–563, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Grassi G, Seravalle G, Dell'Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension 36: 538–542, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Honnma H, Endo T, Kiya T, Shimizu A, Nagasawa K, Baba T, Fujimoto T, Henmi H, Kitajima Y, Manase K, Ishioka S, Ito E, Saito T. Remarkable features of ovarian morphology and reproductive hormones in insulin-resistant Zucker fatty (fa/fa) rats. Reprod Biol Endocrinol 8: 73, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.How JM, Pumpa TJ, Sartor DM. Renal sympathoinhibitory and regional vasodilator responses to cholecystokinin are altered in obesity-related hypertension. Exp Physiol 98: 655–654, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol 588: 1515–1525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber DA, Schreihofer AM. Altered regulation of the rostral ventrolateral medulla in hypertensive obese Zucker rats. Am J Physiol Heart Circ Physiol 301: H230–H240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iannitti T, Graham A, Dolan S. Increased central and peripheral inflammation and inflammatory hyperalgesia in Zucker rat model of leptin receptor deficiency and genetic obesity. Exp Physiol 97: 1236–1245, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Iellamo F, Manzi V, Caminiti G, Sposato B, Massaro M, Cerrito A, Rosano G, Volterrani M. Dose-response relationship of baroreflex sensitivity and heart rate variability to individually tailored exercise training in patients with heart failure. Int J Cardiol 166: 334–339, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Substitution a codon 269 (glutamine → proline) of the leptin receptor (OB-R) cDNA is the only mutation found in the Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun 224: 597–604, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Johnson MS, DeMarco VG, Heesch CM, Whaley-Connell AT, Schneider RI, Rehmer NT, Tilmon RD, Ferrario CM, Sowers JR. Sex differences in baroreflex sensitivity, heart rate variability, and end organ damage in the TGR(mRen2)27 rat. Am J Physiol Heart Circ Physiol 301: H1540–H1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan O, Riazi S, Hu X, Song J, Wade JB, Ecelbarger CA. Regulation of renal thiazide-sensitive Na-Cl cotransporter, blood pressure and natriuresis in obese Zucker rats treated with rosiglitizone. Am J Physiol Renal Physiol 289: F442–F450, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Lanfranchi PA, Somers VK. Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol Regul Integr Comp Physiol 283: R815–R826, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Madden CJ, Sved AF. Cardiovascular regulation after destruction of the C1 cell group of the rostral ventrolateral medulla in rats. Am J Physiol Heart Circ Physiol 285: H2734–H2748, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Mancia G, Parati G. The role of blood pressure variability in end-organ damage. J Hypertens Suppl 21: S17–S23, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Mandel DA, Schreihofer AM. Glutamatergic inputs to the CVLM independent of the NTS promote inhibition of sympathetic vasomotor tone in rats. Am J Physiol Heart Circ Physiol 295: H1772–H1779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan DA, Anderson EA, Mark AL. Renal sympathetic nerve activity is increased in obese Zucker rats. Hypertension 25: 834–838, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Oparil S. New challenges in blood pressure goals and assessment. Nat Rev Cardiol 8: 74–75, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Osmond JM, Mintz JD, Dalton B, Stepp DW. Obesity increases blood pressure, cerebral vascular remodeling, and severity of stroke in the Zucker rat. Hypertension 53: 381–386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overton JM, Williams TD, Chambers JB, Rashotte ME. Cardiovascular and metabolic responses to fasting and thermoneutrality are conserved in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 280: R1007–R1015, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Pamidimukkala J, Jandhyala BS. Evaluation of hemodynamics, vascular reactivity and baroreceptor compensation in the insulin resistant Zucker obese rats. Clin Exp Hypertens 18: 1089–1104, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Paton JF. Convergence properties of solitary tract neurons driven synaptically by cardiac vagal afferents in the mouse. J Physiol 508: 237–252, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocchini AP, Mao HZ, Babu K, Marker P, Rocchini AJ. Clonidine prevents insulin resistance and hypertension in obese dogs. Hypertension 33: 548–553, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues TC, Ehlich J, Hunter CM, Kinney GL, Rewers M, Snell-Bergeon JK. Reduced heart rate variability predicts progression of coronary artery calcification in adults with type 1 diabetes and controls without diabetes. Diabetes Technol Ther 12: 963–966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 375: 938–948, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Ruggeri P, Brunori A, Cogo CE, Storace D, Di Nardo F, Burattini R. Enhanced sympathetic reactivity associates with insulin resistance in the young Zucker rat. Am J Physiol Regul Integr Comp Physiol 291: R376–R382, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol 387: 524–536, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Schreihofer AM, Guyenet PG. Sympathetic reflexes after depletion of bulbospinal catecholaminergic neurons with anti-DβH-saporin. Am J Physiol Regul Integr Comp Physiol 279: R729–R742, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Schreihofer AM, Guyenet PG. Baro-activated neurons with pulse-modulated activity in the rat caudal ventrolateral medulla express GAD67 mRNA. J Neurophysiol 83: 1265–1277, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Schreihofer AM, Ito S, Sved AF. Brain stem control of arterial pressure in chronic arterial baroreceptor-denervated rats. Am J Physiol Regul Integr Comp Physiol 289: R1746–R1755, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol 293: H2543–H2549, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Silva-Carvalho L, Paton JF, Rocha I, Goldsmith GE, Spyer KM. Convergence properties of solitary tract neurons responsive to cardiac receptor stimulation in the anesthetized cat. J Neurophysiol 79: 2374–2382, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Stocker SD, Meador R, Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension 49: 640–646, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Stornetta RL, Akey PJ, Guyenet PG. Location and electrophysiological characterization of rostral medullary adrenergic neurons that contain neuropeptide Y mRNA in rat medulla. J Comp Neurol 415: 482–500, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Stornetta RL, Guyenet PG, McCarty RC. Autonomic nervous system control of heart rate during baroreceptor activation in conscious and anesthetized rats. J Auton Nerv Syst 20: 121–127, 1987 [DOI] [PubMed] [Google Scholar]
- 52.Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/VLGUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol 444: 207–220, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Sved AF, Mancini DL, Graham JC, Schreihofer AM, Hoffman GE. PNMT-containing neurons of the C1 cell group express c-fos in response to changes in baroreceptor input. Am J Physiol Regul Integr Comp Physiol 266: R361–R367, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Talman WT. Kynurenic acid microinjected into the nucleus tractus solitarius of rat blocks the arterial baroreflex but not responses to glutamate. Neurosci Lett 102: 247–252, 1989 [DOI] [PubMed] [Google Scholar]
- 55.Tuck ML. Obesity, the sympathetic nervous system, and essential hypertension. Hypertension 19: I67–I77, 1992 [DOI] [PubMed] [Google Scholar]
- 56.Vendrame S, Daugherty A, Kristo AS, Riso P, Klimis-Zacas D. Wild blueberry (Vaccinium angustifolium) consumption improves inflammatory status in the obese Zucker rat model of the metabolic syndrome. J Nutr Biochem 24: 1508–1512, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Verberne AJ, Guyenet PG. Medullary pathway of the Bezold-Jarisch reflex in the rat. Am J Physiol Regul Integr Comp Physiol 263: R1195–R1202, 1992 [DOI] [PubMed] [Google Scholar]
- 58.Verberne AJ, Sartor DM. CCK-induced inhibition of presympathetic vasomotor neurons: dependence on subdiaphragmatic vagal afferents and central NMDA receptors in the rat. Am J Physiol Regul Integr Comp Physiol 287: R809–R816, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Verwaerde P, Sénard JM, Galinier Rougé P M, Massabuau P, Galitzky J, Berlan M, Lafontan M, Montastruc JL. Changes in short-term variability of blood pressure and heart rate during the development of obesity-associated hypertension in high-fat fed dogs. J Hypertens 17: 1135–1143, 1999 [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann NY Acad Sci 940: 237–246, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Weston M, Wang H, Stornetta RL, Sevigny CP, Guyenet PG. Fos expression by glutamatergic neurons of the solitary tract nucleus after phenylephrine-induced hypertension in rats. J Comp Neurol 460: 525–541, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Mifflin SW. Differential roles for NMDA and non-NMDA receptor subtypes in baroreceptor afferent integration in the nucleus of the solitary tract of the rat. J Physiol 511: 733–745, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao D, McCully & Brooks VL BH. Rosiglitazone improves insulin sensitivity and baroreflex gain in rats with diet-induced obesity. J Pharmacol Exp Ther 343: 206–213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]