Abstract
Serotonergic (5-hydroxytryptamine, 5-HT) neurons of the area postrema (AP) represent one neuronal phenotype implicated in the regulation of salt appetite. Tryptophan hydroxylase (Tryp-OH, synthetic enzyme-producing 5-HT) immunoreactive neurons in the AP of rats become c-Fos-activated following conditions in which plasma sodium levels are elevated; these include intraperitoneal injections of hypertonic saline and sodium repletion. Non-Tryp-OH neurons also became c-Fos-activated. Sodium depletion, which induced an increase in plasma osmolality but caused no significant change in the plasma sodium concentration, had no effect on the c-Fos activity in the AP. Epithelial sodium channels are expressed in the Tryp-OH-immunoreactive AP neurons, possibly functioning in the detection of changes in plasma sodium levels. Since little is known about the neural circuitry of these neurons, we tested whether the AP contributes to a central pathway that innervates the reward center of the brain. Stereotaxic injections of pseudorabies virus were made in the nucleus accumbens (NAc), and after 4 days, this viral tracer produced retrograde transneuronal labeling in the Tryp-OH and non-Tryp-OH AP neurons. Both sets of neurons innervate the NAc via a multisynaptic pathway. Besides sensory information regarding plasma sodium levels, the AP→NAc pathway may also transmit other types of chemosensory information, such as those related to metabolic functions, food intake, and immune system to the subcortical structures of the reward system. Because these subcortical regions ultimately project to the medial prefrontal cortex, different types of chemical signals from visceral systems may influence affective functions.
Keywords: area postrema, circumventricular organs, epithelial sodium channels, nucleus accumbens, ventral tegmental area
the area postrema (ap) is a sensory circumventricular organ (CVO) located in the dorsal midline of the medulla oblongata, which protrudes into the 4th ventricle. Its terminal arterial supply is made up of fenestrated capillaries with pores measuring ∼60–80 nm, which allow the passage of solutes and small peptides from the plasma into the AP, so the local neurons and glial cells are bathed in a similar chemical environment as the plasma. CVO neurons detect small changes in the plasma concentration of many of these chemicals and convert this information into neural activity that is sent via feed-forward excitatory pathways to homeostatic regulatory sites in the hypothalamus. For example, the forebrain CVO neurons, originating in the subfornical organ (SFO) and organum vasculosum of the lamina terminalis (OVLT), project to the vasopressin and oxytocin neurons of the supraoptic and paraventricular hypothalamic nuclei and elicit the release of these hormones that serve to maintain body fluid homeostasis (7, 24). Both of these CVOs are also connected multisynaptically to the sympathetic outflow system (46).
AP has different connectional properties than the SFO or the OVLT. It innervates the nucleus tractus solitarius (NTS), ventrolateral medulla, and parabrachial region (4, 37, 41), and also projects to the pancreatic parasympathetic outflow system (19, 23), but there has been no evidence to indicate that it connects with any of the sympathetic outflow systems. From the functional perspective, the AP affects a range of seemingly unrelated visceral functions, such as the vomiting reflex (2), cardiovascular reflexes (24), and food intake (10, 11, 18), but thus far, no rationale has been presented to explain why such diverse functions are modulated by this CVO.
Because the AP contains an extensive collection of ion channels and receptors implicated in cardiovascular, metabolic, food intake, salt and fluid balance, and immune functions (17), this CVO may function as a pan-chemosensor for the internal milieu. The AP is probably not unique in this regard because the SFO also has a similar assortment of diverse channels and peptide receptors (16). The OVLT has not yet been analyzed in this manner.
A number of questions pertaining to the function of the AP can now be proposed. These are as follows: Is the AP made up of collections of different types of chemical-sensing neurons, each with its own unique set of ion channels and receptors? Do AP neurons sense multiple chemical signals? Do AP neurons sense synergetic chemical signals related to a particular function, such as insulin and amylin? Do AP neurons detect different classes of chemical signals? Can hormones related to immune status and energy balance be detected by the same AP neuron? The answers to these questions are unknown and represent rich areas for future research.
The gene chip studies have added a great amount of information to our understanding of the CVOs, but they have implicitly assumed that the CVOs are homogenous structures (16, 17), and clearly, this is not the case. Each CVO has its own defined cytoarchitectonic regions and contains a heterogeneous group of neurons (24). In the case of the AP, for example, seven different neuronal phenotypes have been identified, including those that express ANG II, cholecystokinin, enkephalin, GABA, glutamate, glycine, and serotonin (21, 22, 30–33, 45). Of particular interest to us has been the serotonin (5-hydroxytryptamine, 5-HT)-containing neurons because of their potential involvement in the regulation of salt appetite (28).
Here, we show that the Tryp-OH-immunoreactive AP neurons became c-Fos-activated in rats undergoing sodium repletion following 8 days of a near-zero-sodium diet and after intraperitoneal injections of hypertonic saline. Second, using the viral transneuronal tracing method, we demonstrate that the Tryp-OH AP neurons are linked to the nucleus accumbens (NAc), which is a cell group that is part of the reward system. Because the connections from the reward system ultimately project to select cortical areas, such as the medial prefrontal cortex, the present findings provide a novel insight on how visceral states may affect complex higher brain functions, such as mood and related cognitive states.
MATERIALS AND METHODS
Animals.
The animal experiments described here were approved by the Washington University School of Medicine Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines. Adult Sprague-Dawley rats (wt = 250–300 g, male, Charles River Laboratories, Wilmington, MA). The rats were housed for 3–7 days in a room maintained at 23°C with an automated lighting system: 12:12-h light-dark schedule (lights on at 5:30 AM; lights off at 5:30 PM). The rats were provided with free access to tap water and standard rat chow (Pico Lab rodent #20, containing 0.33% sodium; Lab-Diet, Richmond, IN).
Sodium deprivation procedure.
During the sodium deprivation period, the rats were housed in individual cages. The wood chip bedding material was changed daily to prevent the rats from eating their waste materials, which contain a residual amount of sodium. The rats had ad libitum access to distilled water and 0.01% sodium chow (no. 85292; Harlan-Teklad, Madison, WI) for 8 days. A 0.01% sodium diet provides rats with an extremely low amount of sodium, which is well below the minimum amount of sodium (0.05%) needed to maintain growth (15). Throughout the remainder of this article, we refer to this diet as “a near zero-sodium diet,” and in Fig. 3, we use the shortened term “No Na+” to indicate that the rats were fed a 0.01% sodium diet.
Fig. 3.
A: Line drawings arranged from rostral to caudal of the dorsomedial medulla from a normal rat. Note there were virtually no c-Fos-activated neurons in the area postrema (AP) or nucleus tractus solitarius (NTS). A: photoimage of the 5-HT neurons in the AP. Gr, gracile nucleus. B: 8 days of near-zero sodium diet resulted in c-Fos activation of the 11-β-hydroxysteroid dehydrogenase type 2 (HSD2) neurons in the NTS. B′: photoimage of 5-HT neurons in the AP from a rat that was sodium-deprived for 8 days. No c-Fos-activated neurons were found in the AP. C: after 8 days of near zero-sodium diet, followed by sodium repletion elicited c-Fos activation in the AP in 5-HT neurons and other AP neurons, especially in the ventrolateral AP. The neuronal phenotype(s) of the latter group is/are unknown. C′: photoimage showing 5-HT AP neurons were c-Fos activated following sodium repletion with 0.9% saline (arrows). D: sodium repletion with 3.0% saline produced a similar c-Fos activation pattern as observed in the 0.9% saline repletion experiments. Both putative 5-HT and non-5-HT neurons in the AP become c-Fos-activated. Sodium repletion elicited c-Fos activation in the medial NTS; this is likely to be the result of saline infusion into the stomach and the subsequent activation of its vagal stretch receptors. In addition, neurons in the dorsal vagal nucleus (DMX) were also c-Fos-activated, probably the result of reflex feedback from the stomach. D′: photoimage of c-Fos-activated 5-HT neurons in the AP (arrows).
On day 8, the rats were presented with two graduated cylinders: one contained distilled water, while the other contained 0.9% or 3.0% sodium; they were allowed to consume these fluids for 2 h. Then, they were anesthetized with 3.5% chloral hydrate (1 ml/100 g body wt ip injection; Sigma, St. Louis, MO) and perfused through the heart with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH = 7.4). The brains were removed and stored in fixative for 3–7 days. The brains were sectioned in the transverse plane at 50 μm on a freezing microtome and immediately processed for immunohistochemistry.
Hypertonic NaCl injections.
A separate group of rats (n = 3) were maintained ad libitum for 8 days on standard rat chow (0.33% Na+ as described above) and tap water. Then, the rats were injected intraperitoneally with 2 ml of 12.0% NaCl made in sterile water. After 2 h, the rats were anesthetized and perfused with saline, followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH = 7.4). The brains were stored in fixative for 1–3 days. Then, the brain stems were cut in the transverse plane, and the sections were processed by a double immunohistochemical method for visualization of c-Fos and Tryp-OH immunoreactivity (for details, see Double indirect immunofluorescent procedure).
Antibodies.
A rabbit antiserum was used to label c-Fos-activated neurons (1:8,000; Ab5; no. PC38: Calbiochem, EMD Chemicals, San Diego, CA), which was raised against a synthetic peptide (SGFNADYEASSSRC) corresponding to amino acids 4–17 of human c-Fos (Calbiochem data sheet). A chicken c-Fos antibody (1:1,250, ab14285; Abcam, Cambridge, MA) was also used, and it was made against a synthetic peptide corresponding to amino acids 168–380 of human c-Fos. In an earlier study, the labeling efficiency of these two antibodies were compared; the chicken antibody colabeled ∼90% of the c-Fos-labeled neurons detected with the rabbit antibody (29).
A monoclonal antibody against Tryp-OH (1:2K; “PH8 antibody”, MAB5278, Chemicon, Temecula, CA) labels a subset of AP neurons (28). As noted before, Tryp-OH is the enzyme that synthesizes serotonin (5-HT). This antibody recognizes both isoforms of Tryp-OH and is regarded as a highly specific reagent for defining 5-HT neurons (36). Originally, this antibody was made against phenylalanine hydroxylase (PH) that had been purified from monkey liver (20), and subsequently, it was shown to bind to PH, tyrosine hydroxylase, and tryptophan hydroxylase (3). In formalin-fixed tissues, however, this antibody labels neurons in the raphe nuclei (13) and in the AP (28, 41), but does not react with any of the tyrosine hydroxylase neurons in the brain stem (13).
The avidity of the antibody for the putative 5-HT cell groups varies. In our experience, raphe neurons were robustly immunostained with a 1:4,000 dilution of this monoclonal antibody, while a 1:2,000 dilution was needed to produce optimal immunostaining of the AP neurons. The reason for the weak immunostaining in the AP is unknown, but it may be due to weak expression of Tryp-OH in these neurons. Others have also noted weak 5-HT expression in these neurons. For example, to enhance 5-HT immunostaining in the AP, rats were pretreated with the monoamine oxidase inhibitor, pargyline (21). Because these AP neurons have different staining characteristics compared with the raphe 5-HT neurons, we use the term “putative 5-HT neurons” throughout the remainder of this paper when referring to these Tryp-OH-immunoreactive neurons.
Antibodies to the rat epithelial sodium channel, α subunit (α-ENaC) protein were purchased from StressMarq Bioscience (Victoria, Canada). The α-ENaC (Scnn1a) antibody (1;1,000; SPC-403D) was produced against a synthetic peptide derived from the NH2 terminus of the α-ENaC (amino acids 46–68, NP_113736; no. 3560–2). Immunostaining of α-ENaC-positive cell groups in the brain stem (namely, hypoglossal and dorsal motor nuclei) was blocked when this antibody was preadsorbed with the α-ENaC subunit peptide with the following sequence: LGKGDKREEQGLGPEPSAPRQPTC-COOH (500 μg/ml; Thermo Fisher Scientific, Rockford, IL). No immunostaining was present in brain stem tissues, when this primary antibody was omitted from this immunohistochemical staining procedure.
Double indirect immunofluorescence procedure.
All solutions used for immunohistochemical reactions were made in a 5% donkey serum in a 0.1 M sodium phosphate buffer (pH = 7.4) containing 0.01% sodium azide. The detergent Triton-X 100 was omitted to prevent the solubilization of ENaCs.
Free-floating sections were incubated in a solution containing mouse antibody to Tryp-OH (1:2,000), reacted overnight, washed in potassium phosphate buffer (KPBS; 0.1 M, pH = 7.4), transferred to a solution of biotinylated donkey anti-mouse (1:500; Jackson Laboratory, Bar Harbor, ME) for 2.5 h, washed in KPBS, transferred to a solution containing the ABC complex (Vectastain kit, Vector Laboratories, Burlingame, CA) for 2 h, washed in KPBS, and transferred to Cy2-streptavidin (1:250; Jackson Laboratory) for 3 h. The sections were placed in a solution of the rabbit anti c-Fos antibody (1:8,000) overnight. The following morning, the sections were washed in KBPS and transferred to a solution of biotinylated donkey anti-rabbit (1:250; Jackson Laboratory) for 2.5 h, washed in KPBS, transferred to the ABC complex (Vectastain kit, Vector Laboratories) for 2 h, washed in KPBS, and transferred to Cy3-streptavidin (1:250; Jackson Laboratory) for 3 h. The sections were washed in buffer and then mounted on gelatin-coated glass slides. After drying, sections were coverslipped using a fade-retardant glycerol mounting solution containing sodium azide and n-propyl gallate.
Triple indirect immunofluorescence procedure.
A three-step immunofluorescence procedure was used to determine whether the 5-HT neurons of the AP, which express α-ENaC and also become c-Fos-activated following hypertonic injections and after sodium repletion following a near-zero-sodium diet.
Free-floating sections were incubated in the mouse anti-TrypOH (1:2,000) overnight, washed in KPBS, transferred to a solution of biotinylated donkey anti-mouse (1:500; Jackson Laboratory) for 2.5 h, washed in KPBS, transferred to the ABC complex (Vectastain kit, Vector Laboratories, Burlingame, CA) for 2 h, washed in KPBS, and moved into a solution of Cy2-streptavidin (1:250; Jackson Laboratory) for 3 h. The following day, the sections were washed in KBPS buffer and transferred to a solution of biotinylated donkey anti-rabbit (1:250; Jackson Laboratory) for 2.5 h, washed in KPBS, transferred to the ABC complex (Vectastain kit, Vector Laboratories, Burlingame, CA) for 2 h, washed in KPBS, and transferred to Cy3-streptavidin (1:250; Jackson Laboratories) for 3 h. In the third step, sections were transferred to a chicken anti c-Fos antibody solution (1:1,250), and the next day, washed in KPBS, transferred to donkey anti-chicken (1:250) for 2.5 h, washed, reacted in the ABC complex for 2 h, washed, and reacted with Alexa Fluor 647-streptavidin (1:250) for 3 h, washed, mounted on glass slides, allowed to air dry, and then, coverslipped using a fade-retardant glycerol mounting solution.
Digital images.
Confocal immunofluorescence images were obtained with an Olympus Fluoview FV500b laser-scanning microscope using either 20× (NA 1.17) or 40× (NA 1.35) oil objective lens in steps of 0.621 or 0.311 μM, respectively, through the tissue section. Image resolution was 1,024 × 1,024 pixels. One pixel in the X-Y plane was the minimum unit of resolution, which covered an area roughly 0.6 μM × 0.6 μM. The z-frames were collapsed into a two-dimensional image. Photomontages were constructed, and adjustments in brightness and contrast were made using the Adobe Photoshop CS3 program (San Jose, CA). Manipulations of the confocal stacks, z-frame projections, and pseudocoloration were performed using MetaMorph software (Molecular Devices, Sunnyvale, CA). Cytoarchitectonic boundaries were added to the photo images with the aid of the Adobe Illustrator program (San Jose, CA).
Brightfield images were taken with a Nikon microscope using a charge-coupled device camera and Nikon ACT-1 software (v2.62). Image cropping, resizing, and adjustments in brightness, contrast, sharpness, and color balance were performed using Adobe Photoshop program. The photo images were superimposed onto computer drawings taken from the rat brain atlas constructed by Paxinos and Watson (34) with the aid of the Adobe Illustrator program.
Plasma sodium and osmolality measurements.
Nineteen rats were used for measurement of plasma sodium levels after various manipulations: sodium deprivation (8 days), 2 h of sodium repletion with 0.9% M NaCl after 8 days of sodium deprivation, and 2 h of sodium repletion with 3.0% M NaCl after 8 days of sodium deprivation. In addition, plasma sodium measurements from the hypertonic saline experiments were made in an earlier study (29), and all of these data are presented in Fig. 5.
Fig. 5.
Bar graphs showing the distribution of c-Fos-activated neurons in the AP under various sodium conditions. Note that the putative 5-HT neurons represent ∼20% of the total population of c-Fos-activated AP neurons. The data for the sodium-deprived and repletion conditions were compared with the values obtained in the control animals. In addition, data from the 12% NaCl injections are presented and compared with control values.
To obtain the plasma samples, the rats were anesthetized with 8% chloral hydrate intraperitoneally, and 3 to 4 ml of blood were drawn from the left ventricle of the heart in a 10-ml syringe containing heparin (50 U in 0.5 ml; Abbott Laboratories, Chicago, IL). Blood plasma was immediately separated by centrifugation (5,000 rpm, 5 min). Plasma samples were aliquoted into two separate tubes. The sample for the plasma sodium concentration was immediately frozen in −80°C until the samples were taken in the Core Laboratory for Clinical Studies in the Department of Medicine (Washington University School of Medicine, St. Louis, MO) for analysis. The second set of plasma samples used to measure plasma osmolality was maintained at room temperature and measured within 3 h after they had been withdrawn from the rats. Plasma osmolality was determined using a Wescor Vapro 5600 vapor pressure osmometer (Logan, UT).
Viral transneuronal tracing experiments.
The viral transneuronal labeling experiments followed the same protocol as described in a previous publication from our laboratory (39). Sprague-Dawley male and female rats (n = 60) were anesthetized with pentobarbital sodium (50 mg/kg ip) and placed in a stereotaxic apparatus. The skull was leveled, and a craniotomy was performed. A glass micropipette (≈25-μm tip diameter) containing a mixture of the Bartha strain of pseudorabies virus (PRV; K. Platt, Iowa State University, Ames, IA) and cholera toxin β-subunit (CTb; 0.1% salt free; no. 103B, List Biological, Campbell, CA) was advanced into the NAc with a micromanipulator. A 40-nl injection of a mixture of the Bartha PRV (5 μl; titer = 1 × 108 plaque-forming units/ml) and 0.1% CTb (2 μl) made in a 0.02 M potassium phosphate buffer was delivered over 15 min using a hand-held air pressure injection system. An oblique approach of 20° from the dorsoventral plane was used. The coordinates were bregma = +1.70 mm; lateral = 4.50 mm, deep = 6.20 mm. The rats were allowed to survive 4 days, and then, were anesthetized and perfused through the heart with saline, followed by buffered 4% paraformaldehyde solution. The brains were removed and fixed for 1 wk.
To evaluate the injection site, forebrain sections at the level of the NAc were reacted with a polyclonal goat anti-CTb (1:25,000, no. 703; List Biological), as described previously (39). After the sections were reacted by the standard ABC procedure with diaminobenzidine (39), they were mounted on gelatin-coated slides and allowed to air dry. Then, the sections were counterstained with 0.1% thionin-buffered solution (pH = 4.6) and coverslipped with DPX mountant (Gallard-Schlesinger Chemical, Carle Place, NY).
A one-in-five series of sections through the AP were processed by a double-immunofluorescence method. Sections were incubated in a solution containing two antibodies: mouse monoclonal antibody to Tryp-OH (1:4,000, Chemicon) and rabbit anti-pseudorabies virus (1:250; Abcam, Cambridge, MA) overnight, washed in KPBS, and reacted first with biotinylated donkey anti-mouse (1:500; Jackson Laboratory) for 3 h, washed in KPBS, followed by Cy3-streptavidin (1:500; Jackson Laboratory) for 3 h, then washed again with KPBS, and transferred to Cy2-donkey anti-rabbit for 3 h, washed, and mounted on gelatinized slides.
RESULTS
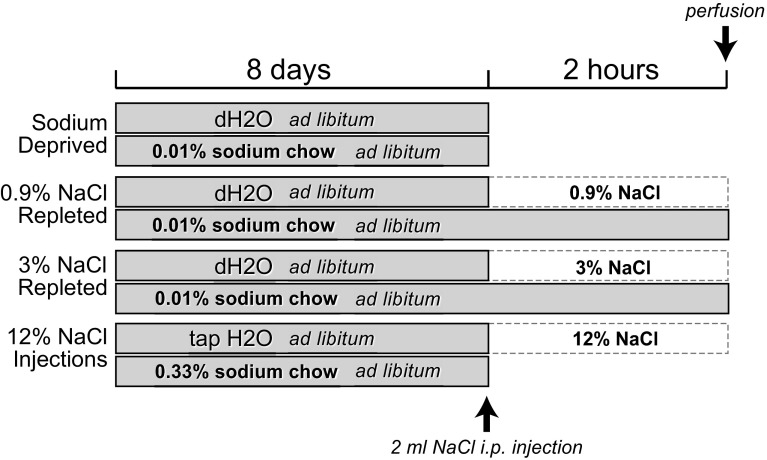
Figure 1 shows the design of the sodium depletion, sodium repletion, and hypertonic saline experiments. Three separate groups of rats were examined: sodium-deprived (n = 10), sodium-deprived followed by sodium repletion with 0.9% saline (n = 5), and sodium-deprived followed by sodium repletion with 3% saline (n = 5). Three rats were used in the hypertonic saline experiments.
Fig. 1.
Flow chart showing the design of the sodium deprivation, sodium repletion, and hypertonic saline experiments.
In a separate group of rats (n = 19), these three experiments were repeated to obtain plasma samples that were used to measure sodium levels and osmolality. As shown in Fig. 2A, sodium levels were maintained between 137 to149 meq/l, which approximates the normal range of 141–147 meq/l that has been reported for Sprague-Dawley male rats. Similar plasma sodium values were obtained in an earlier study in which plasma sodium levels were 146 ± 0.58 meq/l, and plasma osmolality was 297.58 ± 0.72 mosmol/kgH2O 2 h after intraperitoneal injections of 2 M NaCl (29). In the present study, plasma osmolality ranged from 290 to 307 mosmol/kgH2O, which closely matches the normal values (294–313 mosmol/kgH2O), as reported by Hilltop Laboratories (http://hilltoplabs.com/public/blood.html).
Fig. 2.
A: plasma sodium concentration in control rats, after 8 days of near zero-sodium diet, after 2 h of sodium repletion with either 0.9% or 3.0% NaCl following an 8-day period of zero-sodium diet, and 2 h after an intraperitoneal injection of 12% NaCl. B: bar graphs showing the plasma osmolality under the same conditions.
Figure 3 presents a series of line drawings of the dorsomedial medulla showing the c-Fos activation patterns under four conditions: normal, sodium deprivation, sodium repletion with 0.9% saline, and sodium repletion with 3.0% saline. As shown in Fig. 3A, the AP, NTS, and dorsal vagal nucleus of normal rats were virtually devoid of c-Fos-activated neurons, while in Fig. 3B, which shows a series of sections through the dorsal medulla from a rat that was sodium-deprived for 8 days, only the 11-β-hydroxysteroid dehydrogenase type 2 (HSD2) neurons of the NTS were c-Fos-activated, which confirms earlier data (12). Under both conditions, no c-Fos-labeled neurons were found in the AP. In Fig. 3, C and D, the c-Fos pattern of activation in the dorsal medulla following sodium repletion is illustrated. Under both conditions, namely, sodium repletion with either 0.9% or 3.0% saline, c-Fos-activated 5-HT and non-5-HT neurons were present in the AP.
Figure 4 illustrates a hypertonic saline experiment. It shows that both 5-HT and non-5-HT neurons are c-Fos-activated. Furthermore, the distribution of the c-Fos labeling in the AP in the sodium repletion and hypertonic saline experiments is similar, with the bulk of the activated neurons localized in the caudal part of the AP. At the more rostral AP levels, the c-Fos labeling is distributed in the lateral part of the AP.
Fig. 4.
A: line drawings showing the distribution of c-Fos-activated neurons in the dorsomedial medulla 2 h after an intraperitoneal injection of 12% saline. AP, area postrema; Gr, gracile nucleus; CC, central canal; DMX, dorsal vagus nucleus; t, solitary tract; NTS, nucleus tractus solitarius. B: photoimage showing 5-HT AP neurons were c-Fos-activated following an intraperitoneal injection of 12% saline. Some of the 5-HT (green) neurons were c-Fos-activated (red), as indicated by arrows.
Figure 5 shows the bar graphs representing the distribution of c-Fos-activated neurons in the AP. Only ∼20% of the c-Fos-activated neurons express putative 5-HT immunoreactivity, with the remaining 80% being an unknown neuronal phenotype. Similar findings were obtained in the hypertonic saline experiments; namely, ∼23% of the c-Fos-activated neurons expressed putative 5-HT immunoreactivity (Fig. 4).
Because ENaC-expressing CVO neurons may be involved in sodium sensing (29), we examined whether the putative 5-HT AP neurons contain ENaCs, since these channels could be part of the substrate for detection of sodium ions. Figure 6 shows examples of the c-Fos-activated putative 5-HT neurons that coexpress ENaCs. While an exhaustive analysis was not performed, these findings support the hypothesis that ENaCs potentially could participate in the sodium-sensing functions of the putative 5-HT AP neurons.
Fig. 6.
A: line drawing from a triple-color immunohistochemical preparation showing the distribution of neurons that expressed ENaC α-subunit (red), 5-HT (green), c-Fos (blue), and all three markers (yellow stars). This section was from a rat (case no. 6427) that was fed a near zero-sodium diet for 8 days, and then, sodium repleted for 2 h with 0.9% saline. B: photoimages showing AP neurons that expressed ENaC alpha (red), 5-HT (green), c-Fos (blue), and a merged version demonstrating all three markers were present in a single neuron.
Figure 7 presents the data from the viral transneuronal labeling experiments. Stereotaxic injections of PRV were made in the core of the NAc, and after 4 days, transneuronal labeling was identified in the AP. In an earlier report, using the same technique, we observed retrograde transneuronal labeling in the NTS (39); similar results were found in the present study. Here, the focus was solely on the AP, and thus, the SFO and OVLT were not analyzed. In the AP, we found that both putative 5-HT and non-5-HT neurons were labeled after PRV injections, suggesting that both types of neurons are connected to the NAc.
Fig. 7.
A: photoimage of a transverse section through the rat forebrain in case no. 4545 showing an injection site in the nucleus accumbens (NAc). A cocktail of cholera toxin β-subunit (CTb) and pseudorabies virus (PRV) was injected into the core of the NAc. The CTb injection site was immediately lateral to the anterior commissure (ac). B. Four examples of injection sites in the core region of the NAc. A and B: CPu, caudate-putamen. C: Line drawings through the area postrema illustrating the distribution of 5-HT neurons (green dots), PRV-infected neurons (red dots), and colabeled neurons (yellow stars). D: photoimages showing putative 5-HT neurons, PRV-infected neurons, and colabeled neurons (merged).
DISCUSSION
Major findings.
This study demonstrates that 1) putative 5-HT-immunoreactive AP neurons become c-Fos-activated by increases in plasma sodium levels, which are seen after intraperitoneal injections of hypertonic saline and sodium repletion occurring 8 days after a near zero-sodium diet, 2) c-Fos activation of the AP, including the putative 5-HT neurons, was correlated with increases in plasma sodium concentration, but not plasma osmolality; 3) sodium depletion, which causes an increase in plasma osmolality, but no change in sodium plasma levels, did not affect the c-Fos activity in the AP; 4) c-Fos-activated 5-HT neurons coexpress ENaCs; and 5) putative 5-HT and non-5-HT AP neurons are linked multisynaptically to the NAc. When these data are viewed collectively, they suggest that putative 5-HT AP neurons contribute to an ascending pathway that is linked to the NAc, which is a component in the reward network of the brain.
Figure 8 presents a drawing of the hypothesized cell groups that form the AP→NAc pathway. Besides the pathway presented in Fig. 8, there may be alternative pathways that account for the viral tracing findings.
Fig. 8.
A proposed central pathway showing the area postrema (AP) pathway projects by a chain of neurons to the nucleus accumbens (NAc). The AP projection is shown in red, indicating that it may provide inhibitory information to the dorsolateral pons, where it makes close contacts with the FoxP2 neurons that lie in this region (42). The two dorsolateral pontine sites include the external lateral subnucleus of the parabrachial nucleus (PB) and prelocus coeruleus nucleus (pre-LC) (28), project to the ventral tegmental area (VTA) (28). The FoxP2 neurons express mRNA for glutamate vesicular transporter, Vglut2 (Slc17a6), indicating that these neurons use glutamate as a transmitter (26), and have been shown earlier to be one of the glutamatergic afferents projecting to the ventral tegmental area (VTA) (14). This excitatory projection is shown in green. The VTA projects to the nucleus accumbens (NAc). Whether the VTA projection arises from the dopamine neurons has not yet been established. It is shown here as a double-color projection (red and green), suggesting it may produce either positive (green) or negative (red) effect on the affective system. Line drawings modified from the Paxinos rat brain atlas (34). PAG, periaqueductal gray matter; CPu, caudate-putamen; PBel-inner, external lateral subnucleus of the PB. [Adapted from Figs. 12, 38, 54, and 72 of The Rat Brain in Stereotaxic Coordinates, 2nd ed., 1986 (ISBN 9780125476218), Copyright Elsevier, Paxinos and Watson.]
As a first step in considering potential alternate pathways, it is instructive first to review the afferent inputs to the NAc. The major subcortical input to the NAc arises from the ventral tegmental area (VTA), with lesser inputs originating from the paraventricular thalamic nucleus and related midline thalamic nuclei, basolateral amygdala, ventral pallidum, and lateral hypothalamic area (35). Because the parabrachial nucleus (PB) is an integral part of this pathway, the next logical consideration is to determine which of the forebrain cell groups that project to the NAc also receive an input from the PB (40).
One other significant point is there are two subsets of salt-sensitive FoxP2 neurons in the dorsolateral pons, one originating from a restricted part of the PB and the other from prelocus coeruleus (pre-LC) that receive direct inputs from the AP (41). Both groups of FoxP2 neurons project heavily to the VTA (40). Because the VTA plays a key role in appetite-related behavior (8), including regulation of salt intake (28), the FoxP2 neuronal projections to the VTA may serve as one of the critical neuronal relays in the AP→NAc pathway.
Other potential pathways exist, including one made up of the following sites: AP→pre-LC and PB→paraventricular and midline thalamic nuclei→NAc projection. In addition, the FoxP2 neurons of the dorsolateral pons innervate of lateral hypothalamic area (40), and thus, this region may be another alternative relay site. Furthermore, the ventral pallidum receives a weak input from the FoxP2 neurons of the pre-LC (40), so this forebrain region could be yet another relay site. Since it is unknown whether the FoxP2 neurons of the dorsolateral pons project to the basolateral amygdala, there is no information on whether this site could be another relay site. When all of these possibilities are considered in light of the relative density of innervation that the FoxP2 dorsolateral pontine neurons provide to these various forebrain cell groups, which are illustrated in a recent paper (40), the pathway presented in Fig. 8 appears to be the best model that explains present data. Future experiments will be needed to confirm which subcortical cell groups contribute to this pathway.
Because the putative 5-HT AP neurons were activated under conditions of increased plasma sodium levels, we speculate that these neurons are part of a central pathway that transmits information related to salt-intake satiety to higher brain centers. The situation is likely to be far more complex because the AP projection system may provide collateral inhibition of a second pathway that originates from the HSD2 neurons of the NTS. The latter pathway is likely to be a feed-forward excitatory projection signaling higher brain levels that there is a need to find and consume salt (41). Furthermore, it is worth noting that while our focus has been on the central processing of sensory signals related to plasma sodium levels, this may not be the sole function of the putative 5-HT AP neurons, as noted below.
The present study has limitations, and one of them is the need to establish whether 5-HT per se is the neurotransmitter used by the Tryp-OH AP projection neurons that innervate the dorsolateral pons. The “PH8” monoclonal antibody to Tryp-OH is generally regarded as the standard antibody for identification of 5-HT neurons (36). The putative 5-HT AP neurons were weakly immunostained with this reagent, possibly because of low expression of Tryp-OH in these cells. Others have had similar experience using another monoclonal antibody to 5-HT (21).
Pharmacological studies have documented that a 5-HT mechanism is operative within the PB that affects salt intake (25). Bilateral activation of 5-HT1A receptors in the PB results in an increase in sodium appetite (5), while a similar treatment with 5-HT2A and 5-HT2C receptor blockade drugs inhibits salt intake (6). The salt-sensitive FoxP2 neurons originating from the PB and pre-LC, two sites that receive direct inputs from the AP (41), project heavily to the VTA (40). Earlier work has demonstrated that the VTA plays an important role in appetite-related behavior (8), including regulation of salt intake (28). As a result of these connectional properties, the FoxP2 dorsolateral pontine projections to the VTA may serve as one of the critical neuronal relays in the AP→NAc pathway.
Originally, c-Fos studies demonstrated the two 5-HT-containing sites in the brain stem were involved in modulating salt intake; they resided in the dorsal raphe nucleus and the AP (9). Both sites project to the PB (28, 44), and the 5-HT immunoreactive neurons found in both cell groups contribute to this projection (9, 28). As noted above, the putative 5-HT-immunoreactive AP neurons project to a specialized group of dorsolateral pontine neurons that constitutively express the transcription factor Forkhead box protein P2 (FoxP2); these are localized in the inner part of the external lateral subnucleus of the PB (PB-el inner) and pre-LC (28).
This is the first demonstration that a circumventricular organ, namely, the AP, which is activated by blood-borne chemical messages, is part of a central pathway that is linked to the subcortical structures of the reward system. This pathway may modulate affective behavior because one of the of subcortical cell groups implicated in the reward system—the VTA—sends a major output to the medial prefrontal cortex (38). As demonstrated earlier, the PB provides a very dense input into the VTA (28, 40).
Since the neurons of the AP sense a range of chemical signals, detection of changes in the plasma levels of NaCl may not necessarily be the only chemosensitive modality transmitted in the AP→NAc projection pathway. Other types of chemosensitive information, including signals from the gastrointestinal tract and the immune system, may be transmitted in this pathway to the subcortical structures of the reward system. This system is likely to be complex, and thus, it will be important to know whether these visceral chemical signals act solely on the AP, and/or also influence NTS neurons via inputs from the vagus nerve.
In a previous report from our laboratory (39), the viral transneuronal labeling method was used to demonstrate a NTS→NAc pathway. Part of this multisynaptic projection originates from the 11-β-hydroxysteroid dehydrogenase type 2 (HSD2) neurons of the NTS; these aldosterone-sensitive NTS neurons (12) project via a multisynaptic pathway to the NAc (39). These neurons also were c-Fos-activated during states of sodium depletion (12). The HSD2 pathway targeted mainly to the core of the NAc with the shell region of the NAc being a secondary target. The variations between innervation of the “shell” vs. “core” are difficult to assess because the two NAc regions are interconnected (42). Earlier in a report by Shekhtman et al. (39), we showed in Fig. 5, but did not comment on the fact, that the AP also contained extensive cell body labeling following PRV injections in the NAc. Here, we demonstrated that both putative 5-HT and non-5-HT AP neurons contribute to the AP→NAc projection.
These findings raise a number of new questions: Do the 5-HT AP neurons project to the HSD2 neurons of the NTS? Do the HSD2 and AP neurons participate in reciprocal functions at the level of the PB (41)? Our current hypothesis is that the HSD2 neurons signal the need to consume sodium, while the AP neurons, including the 5-HT AP neurons, may provide inhibitory information that causes a cessation of salt consumption (41). Experiments are needed to test these alternatives. Previous work reported that the AP neurons make “close contacts” with the aldosterone-sensitive NTS neurons that express HSD2 (37), and this observation needs confirmation with the electron microscope to establish whether these contacts represent synaptic terminals. Nevertheless, on the basis of the current anatomical evidence, it is reasonable to hypothesize that specific subsets of AP neurons may provide an “on-off” switch that regulates salt consumption.
ENaCs are highly expressed in the sensory CVOs, including the AP neurons, and we have hypothesized that these channels may participate in plasma sodium sensing that is critical for maintaining sodium metabolism, as well as affect a range of critical brain functions (27, 29). ENaCs are not likely to act alone, and future studies need to test whether sodium-related peptides, such as aldosterone and ANG II influence the overall excitability of these ENaC-expressing neurons (29). ENaCs are a significant factor that determine the resting potential of neurons and other cells, and hence, the number of ENaCs and other ion channels present in the CVO neurons will affect the magnitude of the sodium current (29). Slight increases in the plasma sodium concentration may increase the net excitability of neurons, moving them closer to their action potential threshold. Alternatively, minor decreases in sodium concentration may result in a stable inactivated state. In the future, it will be important to explore the possibility that ENaCs function as sodium detectors in the CVOs.
Here, we show that when rats are deprived of sodium for 8 days, they maintain their plasma sodium concentrations within normal or nearly normal levels. The mechanism for this steady equilibrium is generally thought to be dependent upon a constant infusion from sodium reservoirs located in bone (1), but surely other factors are likely to be affecting this balance.
In the 8-day sodium depletion experiments, plasma sodium was maintained at 137 mmol/l, which is slightly lower than the control rats (140 mmol/l). Earlier, we found that after the 4 wk of sodium depletion, plasma sodium levels were 142 mmol/l vs. control rats that had 140 mmol/l. Normal values for male Sprague-Dawley rats were reported to be between 141 to 147 mmol/l, as determined by Hilltop Laboratories; see the following link (http://hilltoplabs.com/public/blood.html). As shown in Fig. 2, plasma osmolality increased during the 8-day period of sodium deprivation to 297 mosmol/kgH2O, which is considerably higher than the control values, which were 290 mosmol/kgH2O. After 4 wk of sodium deprivation, plasma osmolality reached 296 mosmol/kgH2O vs. the control value, which was 288 mosmol/kgH2O. The normal range for Sprague-Dawley male rats is 290–307 mosmol/kgH2O, as reported by Hilltop Laboratories. Under the state of sodium deprivation, c-Fos-activated neurons were not found in the AP. While the AP neurons appear to be insensitive to plasma osmolality changes, both the SFO and OVLT show unique patterns of c-Fos activation following 1 mo of sodium deprivation (29); these sites were not studied in the 8-day sodium deprivation experiments. For the 8-day zero-sodium experiments, sodium repletion with either 0.9% or 3.0% saline resulted in a 3.3 or 6.4%, respectively, increase in plasma sodium concentration. The former was within the normal range, while the latter was slightly elevated (149 mmol/l) vs. normal values of 141–147 mmol/l.
Perspectives and Significance
The putative 5-HT-expressing AP neurons become c-Fos-activated following intraperitoneal injections of hypertonic saline and after sodium repletion following 8 days of near zero-sodium intake. Both procedures caused an elevation in plasma sodium levels, as well as increases in plasma osmolality. Sodium deprivation alone caused an elevation in plasma osmolality without statistically significant changes in plasma sodium levels and did not induce c-Fos activation of the AP. These findings raise the likelihood that the 5-HT AP neurons function as sodium detectors. Sodium perturbations, such as performed here, are far more complex than merely producing changes in [Na+]. Since these changes are likely accompanied by changes in a host of hormones, which act on AP neurons along with brain volume effects (43), in vitro electrophysiological studies will be needed to examine whether the putative 5-HT neurons detect changes in [Na+].
The viral tracing data presented here demonstrate that the putative 5-HT and non-5HT AP neurons project via a chain of neurons to the NAc. The function of the AP→NAc pathway remains unknown, but we have hypothesized that it is part of a central pathway involved in detecting the reward aspects of salt consumption—perhaps signaling that satiety has been achieved. Moreover, it is highly probable that this chemosensory pathway carries interoceptive information that reaches the medial prefrontal cortex, since this cortical area is a major recipient of afferents from the VTA, which is a component in the subcortical reward circuit. While most of the focus of this study relates to proposed sodium sensing sites in the brain, the AP→NAc pathway may carry a full spectrum of other chemical messages related to other internal bodily functions that also ultimately reach the medial prefrontal cortex, and thus, potentially influence affective functions.
GRANTS
This study was supported by National Institute of Heart, Lung, and Blood of the NIH, Grant #: HL-25449 (ADL).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.L.M. and A.D.L. conception and design of research; R.L.M. performed experiments; R.L.M. and A.D.L. analyzed data; R.L.M. and A.D.L. interpreted results of experiments; R.L.M. and A.D.L. prepared figures; R.L.M. and A.D.L. drafted manuscript; R.L.M. and A.D.L. edited and revised manuscript; R.L.M. and A.D.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Xay Van Nguyen for technical assistance, and Marcy Hartstein for the computer graphics. In addition, we thank Dr. Timothy Holy for the generous use of his osmometer.
REFERENCES
- 1.Bergstrom WH, Wallace WM. Bone as a sodium and potassium reservoir. J Clin Invest 33: 867–873, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borison HL, Brizzee KR. Morphology of emetic chemoreceptor trigger zone in cat medulla oblongata. Proc Soc Exp Biol Med 77: 38–42, 1951 [DOI] [PubMed] [Google Scholar]
- 3.Cotton RG, McAdam W, Jennings I, Morgan FJ. A monoclonal antibody to aromatic amino acid hydroxylases. Identification of the epitope. Biochem J 255: 193–196, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham ET, Jr, Miselis RR, Sawchenko PE. The relationship of efferent projections from the area postrema to vagal motor and brain stem catecholamine-containing cell groups: an axonal transport and immunohistochemical study in the rat. Neuroscience 58: 635–648, 1994 [DOI] [PubMed] [Google Scholar]
- 5.De Gobbi JI, Barbosa SP, De Luca LA, Jr, Thunhorst RL, Johnson AK, Menani JV. Activation of serotonergic 5-HT1A receptors in the lateral parabrachial nucleus increases NaCl intake. Brain Res 1066: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 6.De Gobbi JI, Martinez G, Barbosa SP, Beltz TG, De Luca LA, Jr, Thunhorst RL, Johnson AK, Vanderlei Menani J. 5-HT2 and 5-HT3 receptors in the lateral parabrachial nucleus mediate opposite effects on sodium intake. Neuroscience 146: 1453–1461, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Ferguson AV. Neurophysiological analysis of mechanisms for subfornical organ and area postrema involvement in autonomic control. Prog Brain Res 91: 413–421, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci 30: 289–316, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Franchini LF, Johnson AK, de Olmos J, Vivas L. Sodium appetite and Fos activation in serotonergic neurons. Am J Physiol Regul Integr Comp Physiol 282: R235–R243, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Fry M, Ferguson AV. Ghrelin modulates electrical activity of area postrema neurons. Am J Physiol Regul Integr Comp Physiol 296: R485–R492, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Fukuda T, Hirai Y, Maezawa H, Kitagawa Y, Funahashi M. Electrophysiologically identified presynaptic mechanisms underlying amylinergic modulation of area postrema neuronal excitability in rat brain slices. Brain Res 1494: 9–16, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Geerling JC, Engeland WC, Kawata M, Loewy AD. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. J Neurosci 26: 411–417, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol 518: 1460–1499, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci 27: 5730–5743, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grunert RR, Meyer JH, Phillips PH. The sodium and potassium requirements of the rat for growth. J Nutr 42: 609–618, 1950 [DOI] [PubMed] [Google Scholar]
- 16.Hindmarch C, Fry M, Yao ST, Smith PM, Murphy D, Ferguson AV. Microarray analysis of the transcriptome of the subfornical organ in the rat: regulation by fluid and food deprivation. Am J Physiol Regul Integr Comp Physiol 295: R1914–R1920, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Hindmarch CC, Fry M, Smith PM, Yao ST, Hazell GG, Lolait SJ, Paton JF, Ferguson AV, Murphy D. The transcriptome of the medullary area postrema: the thirsty rat, the hungry rat and the hypertensive rat. Exp Physiol 96: 495–504, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Hoyda TD, Smith PM, Ferguson AV. Gastrointestinal hormone actions in the central regulation of energy metabolism: potential sensory roles for the circumventricular organs. Int J Obes (Lond) 33 Suppl 1: S16–S21, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Jansen AS, Hoffman JL, Loewy AD. CNS sites involved in sympathetic and parasympathetic control of the pancreas: a viral tracing study. Brain Res 766: 29–38, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Jennings IG, Russell RG, Armarego WL, Cotton RG. Functional analysis of the effect of monoclonal antibodies on monkey liver phenylalanine hydroxylase. Biochem J 235: 133–138, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanca AJ, van der Kooy D. A serotonin-containing pathway from the area postrema to the parabrachial nucleus in the rat. Neuroscience 14: 1117–1126, 1985 [DOI] [PubMed] [Google Scholar]
- 22.Lind RW, Swanson LW, Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. An immunohistochemical study. Neuroendocrinology 40: 2–24, 1985 [DOI] [PubMed] [Google Scholar]
- 23.Loewy AD, Haxhiu MA. CNS cell groups projecting to pancreatic parasympathetic preganglionic neurons. Brain Res 620: 323–330, 1993 [DOI] [PubMed] [Google Scholar]
- 24.McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol 172: 1–122, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Menani JV, Johnson AK. Lateral parabrachial serotonergic mechanisms: angiotensin-induced pressor and drinking responses. Am J Physiol Regul Integr Comp Physiol 269: R1044–R1049, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Miller RL, Knuepfer MM, Wang MH, Denny GO, Gray PA, Loewy AD. Fos-activation of FoxP2 and Lmx1b neurons in the parabrachial nucleus evoked by hypotension and hypertension in conscious rats. Neuroscience 218: 110–125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller RL, Loewy AD. ENaC-γ-expressing astrocytes in the circumventricular organs, white matter, and ventral medullary surface: sites for Na+ regulation by glial cells. J Chem Neuroanat: 53: 72–83, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller RL, Stein MK, Loewy AD. Serotonergic inputs to FoxP2 neurons of the pre-locus coeruleus and parabrachial nuclei that project to the ventral tegmental area. Neuroscience 193: 229–240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller RL, Wang MH, Gray PA, Salkoff LB, Loewy AD. ENaC-expressing neurons in the sensory circumventricular organs become c-Fos activated following systemic sodium changes. Am J Physiol Regul Integr Comp Physiol 305: R1141–R1152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton BW, Maley B, Traurig H. The distribution of substance P, enkephalin, and serotonin immunoreactivities in the area postrema of the rat and cat. J Comp Neurol 234: 87–104, 1985 [DOI] [PubMed] [Google Scholar]
- 31.Newton BW, Maley BE. Cholecystokinin-octapeptide like immunoreactivity in the area postrema of the rat and cat. Regul Pept 13: 31–40, 1985 [DOI] [PubMed] [Google Scholar]
- 32.Newton BW, Maley BE. Distribution of neurotensin-like immunoreactivity in rat and cat area postrema. Peptides 6: 301–306, 1985 [DOI] [PubMed] [Google Scholar]
- 33.Oldfield BJ, Ganten D, McKinley MJ. An ultrastructural analysis of the distribution of angiotensin II in the rat brain. J Neuroendocrinol 1: 121–128, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience 16: 275–296, 1985 [DOI] [PubMed] [Google Scholar]
- 36.Sakowski SA, Geddes TJ, Thomas DM, Levi E, Hatfield JS, Kuhn DM. Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Res 1085: 11–18, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Sequeira SM, Geerling JC, Loewy AD. Local inputs to aldosterone-sensitive neurons of the nucleus tractus solitarius. Neuroscience 141: 1995–2005, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35: 27–47, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shekhtman E, Geerling JC, Loewy AD. Aldosterone-sensitive neurons of the nucleus of the solitary tract: multisynaptic pathway to the nucleus accumbens. J Comp Neurol 501: 274–289, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Shin JW, Geerling JC, Stein MK, Miller RL, Loewy AD. FoxP2 brainstem neurons project to sodium appetite regulatory sites. J Chem Neuroanat 42: 1–23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stein MK, Loewy AD. Area postrema projects to FoxP2 neurons of the pre-locus coeruleus and parabrachial nuclei: brainstem sites implicated in sodium appetite regulation. Brain Res 1359: 116–127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Dongen YC, Deniau JM, Pennartz CM, Galis-de Graaf Y, Voorn P, Thierry AM, Groenewegen HJ. Anatomical evidence for direct connections between the shell and core subregions of the rat nucleus accumbens. Neuroscience 136: 1049–1071, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Verbalis JG. Control of brain volume during hypoosmolality and hyperosmolality. Adv Exp Med Biol 576: 113–29; discussion 361–3, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Vertes RP, Kocsis B. Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. J Comp Neurol 340: 11–26, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Walberg F, Ottersen OP. Neuroactive amino acids in the area postrema. An immunocytochemical investigation in rat with some observations in cat and monkey (Macaca fascicularis). Anat Embryol (Berl) 185: 529–545, 1992 [DOI] [PubMed] [Google Scholar]
- 46.Westerhaus MJ, Loewy AD. Sympathetic-related neurons in the preoptic region of the rat identified by viral transneuronal labeling. J Comp Neurol 414: 361–378, 1999 [PubMed] [Google Scholar]