Abstract
Migration of medial vascular smooth muscle cells (VSMCs) into the intimal layer contributes to pathological remodeling of the blood vessel in arterial hypertension and atherosclerosis. It is well established that reorganization of cytoskeletal networks is an essential component of cellular motile events. However, there is currently a lack of insight into the cellular characteristics of VSMC migration under three-dimensional environments. Here, we investigated the mechanisms of VSMC migration and remodeling using two different collagen matrix assays as in vitro models: migration of VSMCs within a collagen matrix for VSMC invasion and contraction of a collagen gel by VSMCs for VSMC remodeling and contraction. We found that nonmuscle myosin IIA (NMIIA) and nonmuscle myosin IIB (NMIIB) differentially contribute to the migratory capacity of VSMCs via NMII isoform-dependent cytoskeletal reorganization. Depletion of NMIIA by short hairpin RNA revealed a unique interplay between actomyosin and microtubules during VSMC migration. On the other hand, NMIIB was required for the structural maintenance of migrating VSMC. Interestingly, there was a significant difference between NMIIA and NMIIB knockdown in the VSMC migration but not in the VSMC-mediated collagen gel contraction. Furthermore, depletion of zipper-interacting protein kinase by short hairpin RNA resulted in an impairment of VSMC migration and a substantial decrease of VSMC-mediated collagen gel contraction. These results suggest that NMIIA and NMIIB uniquely control VSMC migration and may contribute to vascular remodeling, which are both regulated by zipper-interacting protein kinase.
Keywords: vascular smooth muscle cell, migration, cytoskeleton, nonmuscle myosin II, zipper-interacting protein kinase, three-dimensional
migration of vascular smooth muscle cells (VSMCs) from their normal location in the media into the intima of blood vessels contributes to the intimal thickening that is considered a risk factor for cardiovascular diseases such as hypertension, restenosis, and atherosclerosis (11, 35, 37, 41). However, the precise mechanisms that regulate pathological vascular remodeling remain unknown. Patients with hypertension displayed an accumulation of VSMCs in the arterial thickened intima (16). It has been reported that VSMCs from hypertensive animals appear to have a higher migratory capacity and growth rate than VSMCs from normotensive animals (17, 19). During the development of vascular diseases, VSMCs change from the physiological contractile phenotype [differentiated smooth muscle cells (SMCs)] to the synthetic phenotype (dedifferentiated SMCs), which is characterized by a loss in contractility and acquisition of the ability to migrate as well as proliferate (11, 13, 41).
It is generally accepted that cell migration is regulated by reorganization of the actin cytoskeleton, which is mediated by the myosin II motor (50). However, the role of myosin II in cytoskeletal reorganization during VSMC migration has not been thoroughly investigated. Previous works have shown that genetic ablation of nonmuscle myosin II (NMII) isoforms (NMIIA and NMIIB) yields different mouse phenotypes. NMIIA gene ablation leads to a failure of the visceral endoderm (7), whereas knockout of NMIIB results in impairments of the heart (45) and brain (36). These results suggest that NMII isoforms play differential roles in regulating cellular motile events. Since VSMC migration in vivo occurs in three dimensions, how the cytoskeletal reorganization is regulated during three-dimensional (3-D) VSMC migration is thus an important step toward a better understanding of the pathophysiological mechanism of VSMC migration.
It is known that NMIIA and NMIIB (encoded by different genes) are expressed in SMCs in addition to smooth muscle myosin II (6, 12, 21). In smooth muscle and vertebrate nonmuscle cells, it is thought that the force generated by the actomyosin II system plays a fundamental role in the various types of cellular motility (18, 29, 38) and that the contractility and stability of actomyosin filaments are regulated by phosphorylation of the myosin light chain (MLC) (42, 46). However, several studies (5, 9, 40, 43) have shown that there are cell type-specific roles of NMII isoforms in cell migration. Despite their biological importance, the roles of NMII isoforms in VSMC migration under a 3-D environment have not yet been studied.
We (24) have previously found that zipper-interacting protein kinase (ZIPK) is a central upstream regulator of MLC phosphorylation in two-dimensional (2-D) cell migration processes such as wound healing and chemomigration. Thus, the present study was designed to investigate the roles of NMII isoforms and ZIPK in VSMC invasion and actomyosin reorganization using two different collagen matrix assays as in vitro models: 3-D migration for VSMC invasion and collagen gel contraction for vessel remodeling and contraction.
MATERIALS AND METHODS
Antibodies.
NMIIA, NMIIB, and α-tubulin antibodies were purchased from Abcam (Cambridge, MA) and Cell Signaling Technology (Beverly, MA). Phospho-Ser19 antibody and phospho-Thr18/Ser19 antibody specifically recognize phosphorylated MLC at Ser19 (26) and Thr18/Ser19 (24), respectively. Nonmuscle myosin heavy chain (nmMHC) and smooth muscle myosin heavy chain (smMHC) antibodies were purchased from Covance (Richmond, CA) and Thermo Fisher Scientific (Rockford, IL). ZIPK antibody was produced as previously described (39).
Immunofluorescence staining.
Immunocytochemistry was performed as previously described (14, 26) with slight modifications. For immunostaining analysis of the 3-D collagen matrix, samples were fixed by solution I (4% formaldehyde, 2 mM MgCl2, and 1 mM EGTA in PBS) for 20 min and then washed twice with PBS. After being extensively washed, samples were permeabilized with 0.1% Triton X-100 in PBS for 15 min. After permeabilization, samples were washed three times with PBS containing 0.6 M KCl (PBS + K) and incubated for 30 min with 1% BSA in PBS + K. Samples were coated with primary antibodies and incubated overnight at 4°C. The unbound primary antibodies were washed three times with PBS + K. Samples were incubated with fluorescence dye-conjugated second antibodies (Molecular Probes) for 4 h at 4°C. All of the samples, after three washes with PBS, were mounted with solution II [10% PBS (vol/vol), 90% glycerol (vol/vol), and 2.5% (wt/vol) triethylenediamine].
Image processing.
Differential interference contrast and fluorescence images were viewed using a Leica DM IRB laser spectral scanning confocal microscope controlled by Leica TCS SP II systems (Leica Microsystems, Heidelberg, Germany) equipped with a 65-mW argon laser and two HeNe lasers (1.2 and 10 mW). All images were taken with the same laser output to directly compare with the fluorescence signal intensities (24). For 3-D reconstruction, a series of optical sections that were obtained by a confocal microscope were collected at 0.5- or 1-μm intervals moving progressively across the cells. Images were reconstructed using LCS 3-D software (Leica Microsystems) and processed using Adobe Photoshop 5.5 software (Adobe Systems). Fluorescence signals and the length of distance were measured using LCS 3-D software (Leica Microsystems) (25).
Production of recombinant adenovirus short hairpin RNAs.
To produce adenovirus short hairpin (sh)RNAs for human NMIIA and NMIIB, the targeting sequences of 5′-GGACTTGTCCCAAGTCTGA-3′ for NMIIA and 5′-GGATCGCTACTATTCAGGA-3′ for NMIIB were used, as previously published (2, 51). The targeting sequence of human ZIPK, 5′-AAGACGGACGTGGTCCTCATC-3′, in which four nucleotides were different from those of mouse/rat ZIPK (24), was used. These targeting sequences were synthesized by Invitrogen (Carlsbad, CA) and subcloned into an RNAi-Ready pSIREN-DNR-DsRed expression vector to make adeno-shRNA, according to the protocol provided by the manufacturer (Clontech). A luciferase shRNA was purchased from Clontech and used as a negative control shRNA. After subcloning, adeno-shRNAs were generated using Knockout Adenoviral RNAi System 2 (Clontech) and amplified with the recombinant adenovirus in human embryonic kidney-293 cells. The amplified adenoviruses were purified by an Adeno-X virus purification kit (Clontech).
Cell culture and infection.
Normal human coronary artery SMCs (HCASMCs) were purchased from PromoCell (Heidelberg, Germany) and maintained in SMC Growth Medium 2 (PromoCell).
For suppression of targeting proteins, cells on coverslips or six-well culture plates were infected with adeno-shRNAs [multiplicity of infection (MOI): 750] using the calcium phosphate precipitate method (31, 48). In double knockdown of NMIIA and NMIIB, cells were infected with adeno-shRNAs (MOI, 1,500 for NMIIA/NMIIB mixture; each, 750 MOI). Since the suppression of target proteins by adeno-shRNAs took 4–5 days, cells were harvested at 4 days after the infection, cultured in collagen matrixes, and then subjected to a migration assay on the following day as described below.
Migration and collagen contraction assays.
Collagen gels were prepared as previously described (14) with slight modifications. Neutralized solutions of collagen type I (1.5 mg/ml, Upstate Biotechnology) containing cells (4 × 106 cells/ml) were placed in a silicon template. It should be noted that the migration assay used the same number of cells, which were treated with NMIIA, NMIIB, ZIPK, and control shRNA, respectively. After 30 min of polymerization at 37°C in a 5% CO2 humidified incubator, matrixes were gently released from the silicon template. Matrixes with cells were cultured in basal medium for 24 h to allow remodeling and contraction, referred to as dermal equivalents. For platelet-derived growth factor (PDGF) stimulation, dermal equivalents were cultured for 18 h in DMEM supplemented with 0.5% fatty acid-free BSA (starvation medium). Collagen solution (10 μl, 1.5 mg/ml) was placed in the center of the 35-mm dishes with 7-mm glass bottoms (MatTek). Serum-starved dermal equivalents were placed on top of 10 μl of collagen solution and covered with 40 μl of collagen solution. After 40 min of polymerization at 37°C, starvation medium with 30 ng/ml PDGF was added for 2–3 days.
In the collagen gel contraction assay, collagen gels were prepared as described above. The mixture of collagen (1.5 mg/ml) and HCASMCs (2.5 × 105 cells/ml) was placed in 96-well plates. After 30 min of polymerization at 37°C in a 5% CO2 humidified incubator, matrixes were gently released from the well. Matrixes with cells were cultured in basal medium with FBS for 20 h to allow remodeling and to undergo free contraction in the presence or absence of inhibitors. Contracted collagen matrixes were fixed with solution I and stained with 0.1% Ponceau S (Sigma). Areas of the gels were measured with ImageJ software (National Institutes of Health).
Western blot analysis.
Cellular proteins were separated by SDS-PAGE in a 7.5–20% polyacrylamide gradient slab gel (28) and transferred to a nitrocellulose membrane. Immunoblot analysis was done as previously described (26). Protein band amounts were determined by scanning densitometry using ImageJ software (25).
Quantification and statistical analysis.
For the quantification of cell migration, microscopic fields were selected around the dermal equivalent (14) and recorded by a confocal fluorescence microscope. Cells were judged to have migrated if the position of the nucleus was outside the edge of the dermal equivalent. The fraction of migrating cells was defined as the percentage of cells out of the dermal equivalent in the response to PDGF. The number of cells (fluorescence signals of nucleus stained with Sytox green) out of the edge of the dermal equivalent was counted by “the Particle analysis” function in ImageJ, and the percentage of cells was calculated (23). For shRNA-treated cells, the population of migrating cells was estimated by two criteria: the number of DsRed-positive cells that migrated out of the dermal equivalent was determined as a reference of control shRNA (estimate 1) and the number of DsRed-positive cells was divided by green-positive cells but not DsRed-positive cells (estimate 2). For the quantification of cell width, the maximum width of the anterior region of motile cells, excluding around the nucleus area, was measured using Leica image processing and analysis software (Leica Microsystems). For the quantification of microtubule distribution, the fluorescence intensity of microtubule was measured from the images using ImageJ software. The percentage of microtubule distribution was determined by dividing the fluorescence intensity of microtubules at the anterior region by that of the entire region. All experiments were carried out multiple times. Data are expressed as means ± SD. Statistical significance was tested with a two-tailed Student's t-test for comparing two groups and one-way ANOVA followed by the Tukey-Kramer test for comparing multiple groups. P values of <0.05 were considered significant.
RESULTS
Cytoskeletal structure of migrating VSMCs in the collagen matrix.
Dynamic remodeling of the actin cytoskeleton and the mechanical force generated by the myosin II motor play an important role in the cell migration processes (50). To investigate cell migration and actin reorganization, we first examined the localization of NMIIA and NMIIB isoforms in migrating VSMCs within a collagen matrix that was stimulated with PDGF. The administration of PDGF significantly enhanced the migration of VSMCs within a collagen matrix (11-fold increase compared with PDGF-untreated cells; see Fig. 2A). Figure 1A shows representative projection images of confocal z-sections of HCASMCs, which showed spindle-shaped morphology in the collagen matrix. This morphology has been observed for a number of VSMCs when they are migrating in 3-D environments but not on a 2-D surface (11). Double immunolabeling and confocal microscopy of motile cells revealed different localization patterns of NMIIA and NMIIB isoforms (Fig. 1A). Line fluorescent intensity scan analysis showed that the fluorescent signals of NMIIA at the anterior region of motile cells were higher than those of NMIIB (Fig. 1A,d and bottom left green line graph). On the other hand, the fluorescent intensity of NMIIB at the cortical region of motile cells was significantly higher than that of NMIIA (Fig. 1A,d and bottom middle purple line graph). Higher-magnification images of migrating VSMCs clearly showed the differential localization of these two isoforms [Fig. 1B; NMIIA (green), NMIIB (red), and F-actin (cyan)]. To further analyze the differential localization of NMIIA and NMIIB, a ratio image of NMIIA to NMIIB was generated by dividing the signal intensity pattern of NMIIA by that of NMIIB. This analysis confirmed that NMIIA and NMIIB are enriched in the anterior region and at the cortical region, respectively (ratio image in Fig. 1B, arrows and arrowheads).
Fig. 2.
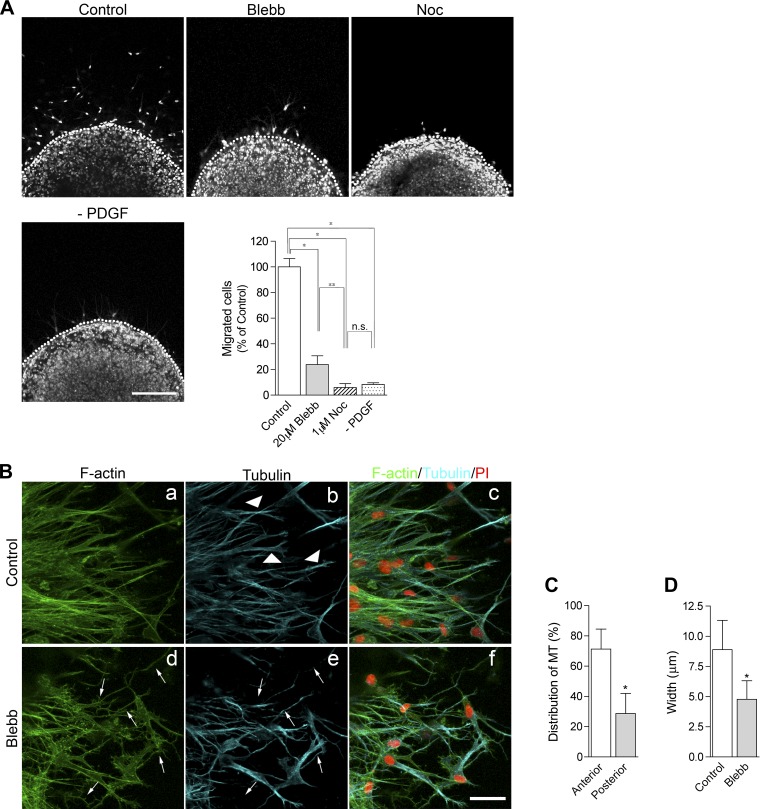
Effects of inhibitions of myosin II or microtubules (MTs) on VSMC migration. A: effects of inhibitors on cell migration. After serum starvation, HCASMCs were treated with or without 30 ng/ml PDFG for 48 h in the presence of 0.1% DMSO (control), 20 μM blebbistatin (Blebb), or 1 μM nocodazole (Noc). At the end of the incubation, cells were fixed and stained with Sytox green for nuclear staining (dotted lines show the boundary between the dermal equivalents and outer matrixes). Bar = 200 μm. The graph shows statistical analysis of the migrating cells. The number of migrated cells out of the dermal equivalent was counted, and the percentage of migrated cells was determined by dividing the number of control migrating cells. Values are means ± SD from three independent experiments. P values were determined by one-way ANOVA with a Tukey-Kramer post hoc test. *P < 0.001; **P < 0.05. n.s., not significant. B: effect of myosin II inhibition on MT structures. Cells were stained with Alexa fluor488-phalloidin for F-actin (green; a and d), α-tubulin (blue; b and e), and propidium iodide (PI; red; merged images in c and f). Bar = 40 μm. C: distribution of MTs. The percentage of MT distribution was determined by dividing the fluorescence intensity of MTs at the anterior region by those of the entire region. Values are means ± SD; n = 30 cells. *P < 0.001. D: average width of the anterior region. The average width of the anterior region of motile cells was determined from 51 cells for each condition. Values are means ± SD. P < 0.001.
Fig. 1.
Platelet-derived growth factor (PDGF)-induced vascular smooth muscle cell (VSMC) migration in a three-dimensional (3-D) collagen matrix. A, top: distribution of nonmuscle myosin IIA (NMIIA) and nonmuscle myosin IIB (NMIIB) in migrating cells. After serum starvation, human coronary artery smooth muscle cells (HCASMCs) were treated with 30 ng/ml PDFG to induce cell migration and then stained with NMII isoform-specific antibodies (a and b) and F-actin (c). The multiple z-series sections were projected onto a plane. d, Merged image; e, differential interference contrast (DIC) image. Bar = 20 μm. Bottom, graphs showing line profiles of the fluorescence intensities of NMIIA and NMIIB from A,d (purple, green, and orange lines, respectively). B: high-magnification images of NMIIA and NMIIB. Arrows and arrowheads indicate the anterior regions and cortical actin bundles of motile cells, respectively. Bars = 10 μm. C: comparison of NMIIA and phosphorylated (p) myosin light chain (MLC; pSer19). Cells were stained with anti-pSer19 MLC antibody (pSer19, red), NMIIA (green), and F-actin (blue). The multiple z-series sections were projected onto a plane. Arrows and arrowheads indicate the anterior regions and cortical actin bundles of motile cells, respectively. Bar = 10 μm.
It is well known that the assembly of myosin filaments and the motor activity of myosin II molecule, except for striated myosin, are regulated by phosphorylation of MLC (42, 46). A confocal imaging of migrating cells revealed that phosphorylated MLC at Ser19 (at the activation site) was colocalized with NMIIA at the anterior regions as well as cortical regions of motile cells rather than the inner region of the spindle-shaped cell body (Fig. 1C, arrows and arrowheads, respectively). It is also possible that phosphorylated MLC at Ser19 of NMIIB and/or smMHC might contribute to the actomyosin contractility at the cell cortex. Since NMIIA but not NMIIB was enriched toward the anterior protrusive branches, the results suggest that NMIIA-driven force contributes to the actomyosin remodeling and contractility at the anterior region of motile cells.
Effects of myosin II and microtubule inhibitors on 3-D migration and cytoskeletal structures.
Next, we determined whether NMII isoforms are necessary for VSMC migration in a 3-D environment. Pharmacological inhibition of NMII with blebbistatin, a myosin II-specific inhibitor, significantly decreased the population of migrating cells (76.2 ± 6.9% decrease of the control; Fig. 2A). Disruption of microtubules by nocodazole treatment also decreased the population of migrating cells (94.1 ± 3.1% decrease of the control; Fig. 2A). The administration of nocodazole caused a more severe effect on cell migration than blebbistatin treatment (P < 0.05 by one-way ANOVA with the Tukey-Kramer post hoc test; Fig. 2A), suggesting that the extension of microtubules is very important for cell migration within the collagen matrix. Immunostaining analysis showed that microtubules were enriched preferentially toward the anterior region of migrating cells (Fig. 2B,b, arrowheads; see also Fig. 5A,b for a magnified image). The fluorescence signals of microtubules at the anterior region of motile cells were ∼70% of the entire signal (Fig. 2C). Interestingly, treatment with blebbistatin led to the change from spindle-shape morphology to skinny morphology (Fig. 2B,d, arrows). Along with this morphological change, microtubules often showed elongated thin structures (Fig. 2B,e, arrows). The width of the anterior region of motile cells was significantly decreased by the administration of blebbistatin (control: 8.9 ± 2.4 μm and blebbistatin: 4.8 ± 1.5 μm, ∼46% decrease of the control, P < 0.001; Fig. 2D). These results suggest that a force produced by the NMII motor protein is involved in the maintenance of the overall structure and the proper formation of microtubules in migrating cells.
Fig. 5.
NMIIA and NMIIB differently contribute to maintain the morphology of migrating cells. A: depletion of NMIIA and NMIIB led to aberrant morphology of migrating cells. HCASMCs were stained with F-actin (a, d, and g) and α-tubulin (b, e, and h) antibodies. DsRed (c, f, and i) was used as an expression marker. Bar = 20 μm. B and C: statistical analyses of cells with abnormal MTs (B) and having elongated morphology (C), respectively. Values are means ± SD from four independent experiments; n > 100 cells. P values were determined by one-way ANOVA with a Tukey-Kramer post hoc test. *P < 0.001; **P < 0.05. D: average width of the anterior region. The average width of the anterior region of motile cells was determined from 51 cells for each condition. Values are means ± SD. P values were determined by one-way ANOVA with a Tukey-Kramer post hoc test. *P < 0.001; **P < 0.01.
NMII isoform-dependent VSMC migration.
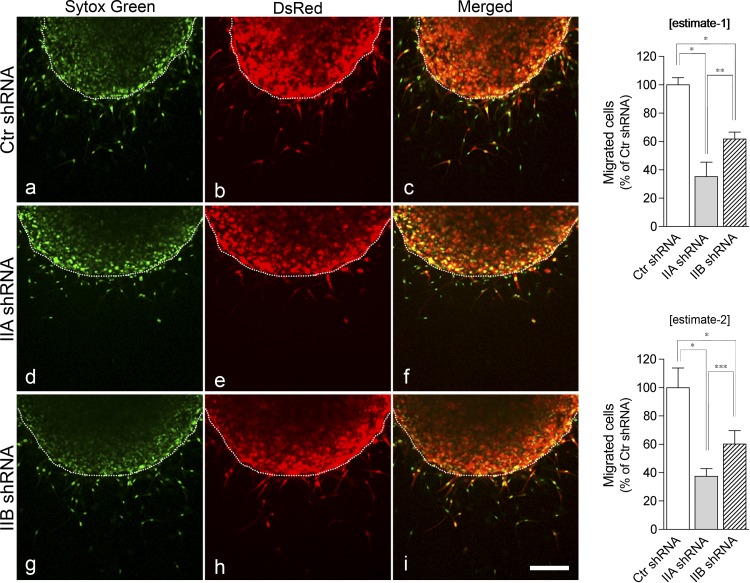
Because different localization patterns were observed for the two NMII isoforms (Fig. 1), we next determined whether the special localizations of NMII isoforms affected VSMC migration and reorganization of the actin cytoskeleton. To examine roles of NMII isoforms in the regulation of VSMC migration within the collagen matrix, we reduced the expression of NMII isoforms using isoform-specific shRNA. To achieve high transfection efficiency, we used an adenovirus vector (adeno-shRNA), which expressed both specific shRNA and red fluorescent protein (DsRed) as an expression marker. Cells infected with adeno-shRNA were identified by DsRed. As shown in Fig. 3A, isoform-specific adeno-shRNA selectively disrupted NMIIA (62 ± 5.9% decrease) and NMIIB (73 ± 2.2% decrease) compared with control adeno-shRNA without influencing the proliferation of knockdown cells (P > 0.05; Fig. 3B, left). The effects were highly specific to the target genes, which is consistent with previous reports (2, 51). Furthermore, depletion of each protein did not affect the expression of smooth muscle myosin II isoforms (Fig. 3A). It should be noted that double knockdown of NMIIA and NMIIB led to a decrease in cell proliferation (∼40% decrease of the control, P < 0.001; Fig. 3B, right). Therefore, it was difficult to accurately analyze the specific effect(s) of double knockdown of NMIIA and NMIIB on cell migration. Previously, it has been reported that NMIIA knockout embryonic stem cells (NMIIA-null cells) and depletion of NMIIA by small interfering RNA in normal human fibroblast cells, respectively, display extremely elongated trailing tails and loss of stress fiber formation, but this was not observed in NMIIB-depleted cells (9). Interestingly, either NMIIA- or NMIIB-depleted VSMCs on a 2-D surface (Fig. 3, C and D) did not notably alter the formation of actin stress fibers compared with control adeno-shRNA-infected cells, suggesting that each NMII isoform can compensate for the lack of the other isoform in the maintenance of actomyosin filaments in the 2-D condition. On the other hand, depletion of NMIIA or NMIIB by specific shRNAs significantly attenuated cell migration (Fig. 4). Depletion of NMIIA decreased the population of migrating cells (estimate 1: 64.8 ± 10.1% and estimate 2: 62.6 ± 5.4% decrease) more than that of NMIIB-depleted cells (estimate 1: 38.3 ± 4.9% and estimate 2: 39.8 ± 9.4% decrease, P < 0.01 for estimate 1 and P < 0.05 for estimate 2 by one-way ANOVA with a Tukey-Kramer post hoc test), whereas shRNA-induced knockdown was more pronounced for NMIIB than for NMIIA. These results suggest that NMII isoforms have distinct roles in the regulation of VSMC migration.
Fig. 3.
Depletion of NMII isoforms does not affect the actomyosin structure on two-dimensional cultured VSMCs. A: depletion of NMII isoforms by recombinant adenovirus short hairpin (sh)RNA (adeno-shRNA). HCASMCs were infected with the following adeno-shRNAs: control (Ctr) shRNA, NMIIA (IIA shRNA), or NMIIB (IIB shRNA). Immunoblot analysis of total cell lysates from VSMCs infected with Ctr, NMIIA, or NMIIB adeno-shRNAs using smooth muscle myosin heavy chain (smMHC), NMIIA, NMIIB, or α-tubulin antibodies is shown. Values are means ± SD from three independent experiments. P values were determined by one-way ANOVA with a Tukey-Kramer post hoc test. *P < 0.001. B: effects of NMII depletion on proliferation. The graphs show the cell viability of NMIIA or NMIIB knockdown cells (left) and double-knockdown cells (right), respectively. Values are means ± SD from three independent experiments. C and D: immunostaining of NMII isoforms. HCASMCs infected with adeno-shRNAs were stained with either NMIIA (C) or NMIIB (D) antibodies. a–c, Ctr shRNA; d–f, NMIIA shRNA; g–i, NMIIB shRNA. DsRed (red fluorescent protein) was used an expression marker. Bars = 40 μm.
Fig. 4.
Depletion of NMII isoforms differently affects cell migration. Left: HCASMCs were infected with Ctr shRNA (a–c), NMIIA shRNA (d–f), or NMIIB shRNA (g–i). Cells were treated with 30 ng/ml PDGF to induce cell migration in a collagen matrix and stained with Sytox green for nuclear staining (dotted lines show the boundary between dermal equivalents and outer matrixes). DsRed was used as an expression marker. Bar = 200 μm. Right: statistical analyses of migrating cells. The two graphs show estimate 1 (top) and estimate 2 (bottom), respectively (see materials and Methods). Values are means ± SD from four independent experiments. P values were determined by one-way ANOVA with a Tukey-Kramer post hoc test. *P < 0.001; **P < 0.01; ***P < 0.05.
The present study demonstrated that NMII inhibition by blebbistatin affected microtubule formation in cells migrating within the collagen matrix. Therefore, we investigated whether NMII depletion influences the intracellular distribution of microtubules. In control shRNA-infected cells (Fig. 5A,a and b), although microtubules were more concentrated at the anterior region than the posterior region, microtubules were found throughout the cell. On the other hand, the fluorescence signals of microtubules in NMIIA-depleted cells were barely detectable at the central/posterior zone (14.3 ± 2.0%, P < 0.001 by one-way ANOVA with a Tukey-Kramer post hoc test; Fig. 5, A,e and B), and microtubules were concentrated at the anterior region, similar to control cells. Interestingly, the shape of NMIIB-depleted cells appeared to be relatively slender (23.0 ± 3.3%, P < 0.05 by one-way ANOVA with a Tukey-Kramer post hoc test; Fig. 5, A,g and h, and C), which was a similar tendency to those treated with blebbistatin (Fig. 2). In support of this notion, depletion of NMIIA resulted in a decrease in the width of the anterior region compared with that of control shRNA (control: 9.5 ± 2.3 μm and NMIIA: 8.2 ± 2.2 μm, ∼14% decrease of the control, P < 0.01; Fig. 5D). The width of the anterior region in NMIIB-depleted cells decreased by ∼30% of control cells (NMIIB: 6.8 ± 2.1 μm, P < 0.001). Furthermore, it should be noted that depletion of NMIIB affected the cellular width more than that of NMIIA (P < 0.01; Fig. 5D).
Effects of ZIPK on VSMC migration and matrix contractions.
We have previously reported that ZIPK is involved in the phosphorylation of NMII in PDGF-induced fibroblast cell migration (24) and responsible for Ca2+-independent myosin phosphorylation and contraction in smooth muscle (39). Thus, we examined if ZIPK plays a role in PDGF-induced VSMC migration in a 3-D matrix. As shown in Fig. 6A, adeno-shRNA targeting human ZIPK (ZIPK shRNA) suppressed MLC phosphorylation (64.8 ± 7.6% decrease) and ZIPK (77.3 ± 8.8% decrease) compared with control adeno-shRNA without affecting the proliferation of ZIPK-depleted cells (P > 0.05; Fig. 6B). These results indicate that ZIPK shRNA is highly specific for the depletion of ZIPK in VSMCs derived from a human artery. Immunostaining analysis showed that depletion of ZIPK resulted in a decrease in signals of MLC phosphorylation and abnormal actin structures (Fig. 6C), indicating that ZIPK appears to be the kinase responsible for the proper formation of actomyosin filaments in dedifferentiated VSMCs. This result was consistent with previously reported results in fibroblasts (24). As shown in Fig. 7, A and B, elimination of ZIPK expression markedly diminished cell migration (estimate 1: 90.7 ± 2.2% decrease and estimate 2: 95.2 ± 1.4% decrease), indicating that ZIPK plays a pivotal role in PDGF-induced VSMC migration. In addition, pharmacological inhibition of MLCKs with 20 μM but not 10 μM ML-7 significantly decreased the population of migrating cells (75.8 ± 7.4% decrease; Fig. 7C). It should be noted that 10 μM ML-7 is sufficient for MLCK inhibition and that high concentrations of ML-7 inhibit ZIPK in addition to MLCK (24).
Fig. 6.
Effects of zipper-interacting protein kinase (ZIPK) depletion on actomyosin structure and focal adhesion. A: effect of ZIPK depletion on the MLC phosphorylation. HCASMCs were infected with either Ctr shRNA or adeno-shRNA for ZIPK (ZIPK shRNA). Immunoblot analysis of total cell lysates from VSMCs infected with adeno-shRNAs using ZIPK, smMHC, nonmuscle myosin heavy chain (nmMHC), tubulin, MLC, and pMLC at Thr18 and Ser19 (pTS) antibodies is shown (24). Values are means ± SD from three independent experiments. *P < 0.001 by Student's t-test. B: effect of ZIPK depletion on cell proliferation. Values are means ± SD from three independent experiments. C: immunostaining of ZIPK-depleted VSMCs. Cells were stained with pTS (a and e), F-actin (b and f). Merged images are shown in c and g. DsRed (d and i) was used as an expression marker. Bar = 50 μm.
Fig. 7.
ZIPK depletion inhibits cell migration. A: HCASMCs were infected with either Ctr shRNA (a–c) or ZIPK shRNA (d–f). Dotted lines show the boundary between dermal equivalents and outer matrixes. Bar = 200 μm. B: quantification of migrating cells. The two graphs showed estimate 1 (right) and estimate 2 (left), respectively (see “Materials and Methods). Values are means ± SD from four independent experiments. *P < 0.001 by Student's t-test. C: effects of ML-7 on cell migration. Values are means ± Sd from three independent experiments. P values were determined by one-way ANOVA with a Tukey-Kramer post hoc test. **P < 0.001.
Since vascular remodeling associated with VSMC migration is one of the key factors for the development of hypertension as well as restenosis and atherosclerosis (4, 11, 34, 37), we investigated the mechanism of VSMC remodeling using a collagen matrix contraction assay, which is used as an in vitro model for vessel remodeling (8, 10, 32, 33, 49). Cells were cultured within a collagen matrix in the presence of various inhibitors. Areas of matrix contraction were measured after 20 h of culture. As shown in Fig. 8A, the area of the collagen matrix in control cells (control) showed a decrease of 50.9 ± 1.6% relative to the area of the collagen matrix without cells (non cells). Both blebbistatin and nocodazole treatment significantly inhibited matrix contraction, suggesting that myosin II and microtubules play important roles in VSMC-induced matrix contraction. Y-27932, a Rho-associated kinase (ROCK) inhibitor, blocked contraction by 55.1 ± 6.8% compared with the control contraction (P < 0.001 by one-way ANOVA with a Tukey-Kramer post hoc test), whereas 20 μM ML-7, but not 10 μM ML-7, inhibited VSMC-induced matrix contraction, suggesting that ROCK, but not MLCK, is involved in dedifferentiated VSMC matrix contraction. This is consistent with a previous report (33) showing that a high concentration of ML-7 (20 μM), but not 10 μM, could inhibit contraction of a collagen matrix by bovine aortic SMCs (33). We (24) have previously reported that ML-7 can inhibit ZIPK at a relatively high concentration in addition to MLCK. Therefore, we next examined whether depletion of ZIPK as well as NMII isoform knockdown influences VSMC-induced matrix contraction. As shown in Fig. 8B, depletion of ZIPK and NMII isoforms by specific shRNA abolished VSMC-mediated collagen gel contraction, in contrast to control shRNA-treated cells, in which the matrix contracted to an extent similar to that of cells without shRNA (no shRNA). Interestingly, no significant differences in gel contraction were observed among knockdowns of NMIIA, NMIIB, and ZIPK (P > 0.05 by one-way ANOVA with a Tukey-Kramer post hoc test), indicating that there were no compensatory effects on gel contraction. These results suggest that NMII isoforms regulated by ZIPK contribute to VSMC-induced matrix contraction.
Fig. 8.
ZIPK depletion affects VSMC-induced matrix contraction. A, top: effects of inhibitors on VSMC-induced matrix contraction. HCASMCs were cultured within a collagen matrix in the presence of 0.1% DMSO (control), 20 μM Blebb, 1 μM Noc, 10 μM Y-27839, or 10–40 μM ML-7. At the end of the incubations, collagen matrixes were fixed and staining with Ponceau S. Bottom, statistical analysis of gel contraction. Values are means ± SD from three independent experiments. P values were determined by one-way ANOVA with a Tukey-Kramer post hoc test. *P < 0.001. B, top: effect of ZIPK depletion on VSMC-induced matrix contraction. HCASMCs were infected with either adeno-shRNAs or vehicle control. Collagen matrixes were fixed and stained with Ponceau S. Bottom, statistical analysis of gel contraction. Values are means ± SD from three independent experiments. P values were determined by one-way ANOVA with a Tukey-Kramer post hoc test. *P < 0.001; **P < 0.01.
DISCUSSION
NMII isoforms differently regulate cytoskeletal structures during VSMC migration in a 3-D environment.
VSMCs invade into the tunica intimal layer of the diseased artery or the injured blood vessel through the extracellular matrix (ECM) (11, 35, 37, 41). Thus, it is crucial to study the mechanism of 3-D cell migration rather than conventional 2-D cell migration to better understand the physiological mechanism of VSMC migration. The present study demonstrated that NMII isoforms differently affect VSMC migration in a 3-D environment.
While depletion of NMIIA or NMIIB by shRNA led to a deficiency of VSMC migration, NMIIA depletion had a more severe effect on VSMC migration than NMIIB depletion. The different effects of NMII depletion could be reflected in the differential distribution of NMII isoforms during VSMC migration. Although the subcellular localization of NMIIA overlapped with that of NMIIB in migrating VSMCs, NMIIA but not NMIIB significantly localized at the anterior region, suggesting that NMIIA can at least partially compensate for the migration defect due to the disruption of NMIIB. Interestingly, it has been previously reported that ablation of NMIIA facilitates extension of the microtubule toward the anterior region of motile fibroblast cells, resulting in an enhancement of random motility (51). In contrast, NMIIA depletion decreases the population of migrating VSMCs. It is thought that this is likely due to abnormal VSMC morphology, which is, at least in part, caused by an impairment of the formation of microtubule network at the central/posterior region.
In support of this view, it has been previously shown that inhibition of microtubule dynamics with taxol, a microtubule-stabilizing drug, inhibits PDGF-induced VSMC chemoinvasion and neointimal VSMC accumulation after balloon dilatation of the rat carotid artery (44). Another possibility is that the force generated by NMIIA at the anterior region of migrating cells might require migration into the collagen matrix, since immunostaining analysis revealed that phosphorylated MLC was also localized at the anterior regions of migrating VSMCs.
Recently, it has been reported that NMIIB participates in the tension maintenance during tonic smooth muscle contraction (54), suggesting that NMIIB may function in maintaining the cytoskeletal structure of actomyosin filaments. Consistent with this view, loss of NMIIB resulted in a cell shape change from a spindle shape into an elongated thin shape. This result suggests that NMIIB plays a prominent role in the structural maintenance of proper cell morphology, which might be necessary to migrate VSMCs in 3-D environments.
Although depletion of NMIIA or NMIIB by shRNA decreased the population of migrating cells, the effects of NMIIA or NMIIB depletion on cell migration were less severe than that of blebbistatin treatment. These results suggest that NMII isoforms could be partially compensating each other in VSMC migration, although we cannot exclude the probability that the amount of remaining endogenous NMII is still capable of supporting VSMC migration. In support of this idea, defects in the brain but not the heart in NMIIB knockout mice can be partially rescued by the expression of NMIIA under control of the endogenous NMIIB promoter (3). It is also likely that both isoforms are required for proper migration.
An important remaining question is how NMII isoforms differently contribute to cell migration in 3-D environments. Several factors are probably involved in the differential roles of these isoforms. One is the differential localization of NMII isoforms. In support of this view, we found that NMII isoforms displayed distinct localization in HCASMCs during cell migration. Another factor is the motor activity of the isoforms, which are different in both cycling rate and duty ratio (22, 27, 52), which are related to force maintenance and contraction. It should also be considered that while NMII isoforms are regulated by MLC phosphorylation, they are differently regulated by heavy chain phosphorylation in the nonhelical tail regions and their binding proteins (30, 50). Understanding the cross-talk between various kinase- and binding protein-mediated regulations of NMII isoforms during VSMC migration will be interesting subjects for future research.
ZIPK is important for VSMC migration and vascular remodeling.
Our results demonstrated that depletion of ZIPK reduces cell motility and contractility. These findings suggest that ZIPK is not only pivotal in regulating PDGF-induced VSMC migration but that it also participates in actomyosin-dependent VSMC remodeling. It is known that MLC phosphorylation determines both the motor activity and filament stability of all myosin II isoforms (smooth muscle myosin II, NMIIA, and NMIIB) in vitro (50). Therefore, it is reasonable that depletion of ZIPK exhibits a more severe effect on VSMC migration than that of individual NMII isoform depletion. Consistent with this view, it has been previously demonstrated by us and other groups that up- and/or downregulation of myosin phosphorylation have an influence on actomyosin filaments (1, 24, 47, 53). Our results also showed that NMII isoforms could not compensate for each other in collagen gel contraction. This suggests that each NMII isoform might have specific functions in vascular remodeling associated with reorganization of the ECM by VSMCs, which is regulated by ZIPK.
Our pharmacological study demonstrated that ROCK, but not MLCK, influences matrix contraction, which is consistent with a previous report (33). Since ROCK regulates MLC phosphorylation through regulation of MLP phosphatase (MLCP) but not direct MLC phosphorylation in both smooth muscle (20) and nonmuscle cells (24, 53), the effect of ROCK inhibition on matrix contraction is in part via MLCP regulation. On the other hand, it has also been previously reported that ROCK phosphorylates ZIPK at activation sites in vitro (15). Therefore, the effect of ROCK inhibition on matrix contraction may be in part due to inhibition of ZIPK. It is plausible the Rho/ROCK/ZIPK signaling pathway regulates VSMC migration and contraction. ZIPK may also modulate MLC phosphorylation through other pathways, including MLCP (39) and integrin-linked kinase. Understanding the signaling pathway of ZIPK-dependent regulation during VSMC migration requires further studies.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R21-HL-094983 (to S. Komatsu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.K. conception and design of research; S.K. performed experiments; S.K. analyzed data; S.K. interpreted results of experiments; S.K. prepared figures; S.K. drafted manuscript; S.K. edited and revised manuscript; S.K. approved final version of manuscript.
REFERENCES
- 1.Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells 3: 177–188, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Andzelm MM, Chen X, Krzewski K, Orange JS, Strominger JL. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J Exp Med 204: 2285–2291, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao J, Ma X, Liu C, Adelstein RS. Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice. J Biol Chem 282: 22102–22111, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bauters C, Isner JM. The biology of restenosis. Prog Cardiovasc Dis 40: 107–116, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Betapudi V, Licate LS, Egelhoff TT. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res 66: 4725–4733, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Bresnick AR. Molecular mechanisms of nonmuscle myosin-II regulation. Curr Opin Cell Biol 11: 26–33, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem 279: 41263–41266, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Defawe OD, Kenagy RD, Choi C, Wan SY, Deroanne C, Nusgens B, Sakalihasan N, Colige A, Clowes AW. MMP-9 regulates both positively and negatively collagen gel contraction: a nonproteolytic function of MMP-9. Cardiovasc Res 66: 402–409, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol 9: 299–309, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Franco C, Ho B, Mulholland D, Hou G, Islam M, Donaldson K, Bendeck MP. Doxycycline alters vascular smooth muscle cell adhesion, migration, and reorganization of fibrillar collagen matrices. Am J Pathol 168: 1697–1709, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res 100: 607–621, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG, Goldin E, Conti MA, Sellers JR, Adelstein RS. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J Biol Chem 279: 2800–2808, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Gorski DH, Walsh K. Mitogen-responsive nuclear factors that mediate growth control signals in vascular myocytes. Cardiovasc Res 30: 585–592, 1995 [PubMed] [Google Scholar]
- 14.Grinnell F, Rocha LB, Iucu C, Rhee S, Jiang H. Nested collagen matrices: a new model to study migration of human fibroblast populations in three dimensions. Exp Cell Res 312: 86–94, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hagerty L, Weitzel DH, Chambers J, Fortner CN, Brush MH, Loiselle D, Hosoya H, Haystead TA. ROCK1 phosphorylates and activates zipper-interacting protein kinase. J Biol Chem 282: 4884–4893, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa K, Fujiwara H, Doyama K, Inada T, Ohtani S, Fujiwara T, Hosoda K, Nakao K, Sasayama S. Endothelin-1-selective receptor in the arterial intima of patients with hypertension. Hypertension 23: 288–293, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Haudenschild CC, Grunwald J, Chobanian AV. Effects of hypertension on migration and proliferation of smooth muscle in culture. Hypertension 7: I101–104, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Horwitz AR, Parsons JT. Cell migration–movin' on. Science 286: 1102–1103, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Hsieh CC, Lau Y. Migration of vascular smooth muscle cells is enhanced in cultures derived from spontaneously hypertensive rat. Pflügers Arch 435: 286–292, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Iizuka K, Yoshii A, Samizo K, Tsukagoshi H, Ishizuka T, Dobashi K, Nakazawa T, Mori M. A major role for the rho-associated coiled coil forming protein kinase in G-protein-mediated Ca2+ sensitization through inhibition of myosin phosphatase in rabbit trachea. Br J Pharmacol 128: 925–933, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamoto S, Adelstein RS. Chicken nonmuscle myosin heavy chains: differential expression of two mRNAs and evidence for two different polypeptides. J Cell Biol 112: 915–924, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KY, Kovacs M, Kawamoto S, Sellers JR, Adelstein RS. Disease-associated mutations and alternative splicing alter the enzymatic and motile activity of nonmuscle myosins II-B and II-C. J Biol Chem 280: 22769–22775, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Komatsu S, Ikebe M. The phosphorylation of myosin II at the Ser1 and Ser2 is critical for normal platelet-derived growth factor induced reorganization of myosin filaments. Mol Biol Cell 18: 5081–5090, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komatsu S, Ikebe M. ZIP kinase is responsible for the phosphorylation of myosin II and necessary for cell motility in mammalian fibroblasts. J Cell Biol 165: 243–254, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komatsu S, Miyazaki K, Tuft RA, Ikebe M. Translocation of telokin by cGMP signaling in smooth muscle cells. Am J Physiol Cell Physiol 283: C752–C761, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Komatsu S, Yano T, Shibata M, Tuft RA, Ikebe M. Effects of the regulatory light chain phosphorylation of myosin II on mitosis and cytokinesis of mammalian cells. J Biol Chem 275: 34512–34520, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Kovacs M, Thirumurugan K, Knight PJ, Sellers JR. Load-dependent mechanism of nonmuscle myosin 2. Proc Natl Acad Sci USA 104: 9994–9999, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 29.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell 84: 359–369, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Lee CS, Choi CK, Shin EY, Schwartz MA, Kim EG. Myosin II directly binds and inhibits Dbl family guanine nucleotide exchange factors: a possible link to Rho family GTPases. J Cell Biol 190: 663–674, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JH, Welsh MJ. Enhancement of calcium phosphate-mediated transfection by inclusion of adenovirus in coprecipitates. Gene Ther 6: 676–682, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Lee RT, Berditchevski F, Cheng GC, Hemler ME. Integrin-mediated collagen matrix reorganization by cultured human vascular smooth muscle cells. Circ Res 76: 209–214, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Li S, Moon JJ, Miao H, Jin G, Chen BP, Yuan S, Hu Y, Usami S, Chien S. Signal transduction in matrix contraction and the migration of vascular smooth muscle cells in three-dimensional matrix. J Vasc Res 40: 378–388, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Libby P, Tanaka H. The molecular bases of restenosis. Prog Cardiovasc Dis 40: 97–106, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Louis SF, Zahradka P. Vascular smooth muscle cell motility: from migration to invasion. Exp Clin Cardiol 15: e75–85, 2010 [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X, Bao J, Adelstein RS. Loss of cell adhesion causes hydrocephalus in nonmuscle myosin II-B-ablated and mutated mice. Mol Biol Cell 18: 2305–2312, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald RA, Hata A, MacLean MR, Morrell NW, Baker AH. MicroRNA and vascular remodelling in acute vascular injury and pulmonary vascular remodelling. Cardiovasc Res 93: 594–604, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell 84: 371–379, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Niiro N, Ikebe M. Zipper-interacting protein kinase induces Ca2+-free smooth muscle contraction via myosin light chain phosphorylation. J Biol Chem 276: 29567–29574, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Sandquist JC, Swenson KI, Demali KA, Burridge K, Means AR. Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J Biol Chem 281: 35873–35883, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest 100: S87–89, 1997 [PubMed] [Google Scholar]
- 42.Sellers JR. Regulation of cytoplasmic and smooth muscle myosin. Curr Opin Cell Biol 3: 98–104, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Shih W, Yamada S. Myosin IIA dependent retrograde flow drives 3D cell migration. Biophys J 98: L29–31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sollott SJ, Cheng L, Pauly RR, Jenkins GM, Monticone RE, Kuzuya M, Froehlich JP, Crow MT, Lakatta EG, Rowinsky EK, et al. Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J Clin Invest 95: 1869–1876, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda K, Kishi H, Ma X, Yu ZX, Adelstein RS. Ablation and mutation of nonmuscle myosin heavy chain II-B results in a defect in cardiac myocyte cytokinesis. Circ Res 93: 330–337, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Tan JL, Ravid S, Spudich JA. Control of nonmuscle myosins by phosphorylation. Annu Rev Biochem 61: 721–759, 1992 [DOI] [PubMed] [Google Scholar]
- 47.Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol 150: 797–806, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toyoda K, Andresen JJ, Zabner J, Faraci FM, Heistad DD. Calcium phosphate precipitates augment adenovirus-mediated gene transfer to blood vessels in vitro and in vivo. Gene Ther 7: 1284–1291, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Travis JA, Hughes MG, Wong JM, Wagner WD, Geary RL. Hyaluronan enhances contraction of collagen by smooth muscle cells and adventitial fibroblasts: role of CD44 and implications for constrictive remodeling. Circ Res 88: 77–83, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778–790, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol 176: 573–580, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang F, Kovacs M, Hu A, Limouze J, Harvey EV, Sellers JR. Kinetic mechanism of non-muscle myosin IIB: functional adaptations for tension generation and maintenance. J Biol Chem 278: 27439–27448, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Xia D, Stull JT, Kamm KE. Myosin phosphatase targeting subunit 1 affects cell migration by regulating myosin phosphorylation and actin assembly. Exp Cell Res 304: 506–517, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Yuen SL, Ogut O, Brozovich FV. Nonmuscle myosin is regulated during smooth muscle contraction. Am J Physiol Heart Circ Physiol 297: H191–H199, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]