Abstract
Genetic hypercalciuric stone-forming (GHS) rats demonstrate increased intestinal Ca absorption, increased bone resorption, and reduced renal tubular Ca reabsorption leading to hypercalciuria and all form kidney stones. GHS have increased vitamin D receptors (VDR) at these sites of Ca transport. Injection of 1,25(OH)2D3 (1,25D) leads to a greater increase in urine (u)Ca in GHS than in control Sprague-Dawley (SD), possibly due to the additional VDR. In GHS the increased uCa persists on a low-Ca diet (LCD) suggesting enhanced bone resorption. We tested the hypothesis that LCD, coupled to inhibition of bone resorption by alendronate (alen), would eliminate the enhanced 1,25D-induced hypercalciuria in GHS. SD and GHS were fed LCD and half were injected daily with 1,25D. After 8 days all were also given alen until euthanasia at day 16. At 8 days, 1,25D increased uCa in SD and to a greater extent in GHS. At 16 days, alen eliminated the 1,25D-induced increase in uCa in SD. However, in GHS alen decreased, but did not eliminate, the 1,25D-induced hypercalciuria, suggesting maximal alen cannot completely prevent the 1,25D-induced bone resorption in GHS, perhaps due to increased VDR. There was no consistent effect on mRNA expression of renal transcellular or paracellular Ca transporters. Urine CaP and CaOx supersaturation (SS) increased with 1,25D alone in both SD and GHS. Alen eliminated the increase in CaP SS in SD but not in GHS. If these results are confirmed in humans with IH, the use of bisphosphonates, such as alen, may not prevent the decreased bone density observed in these patients.
Keywords: vitamin D, calcium, kidney stones, intestinal absorption, bone resorption
hypercalciuria is the most common metabolic abnormality observed in patients who form calcium-based kidney stones (14, 49, 50). The increased levels of urine (u) calcium (Ca) enhance the probability for nucleation and growth of calcium oxalate (CaOx) and/or calcium hydrogen phosphate (CaHPO4, brushite) crystals into clinically relevant kidney stones (14). Patients with idiopathic hypercalciuria (IH), defined as excessive uCa without a demonstrable metabolic cause, generally have normal serum (s) Ca, normal or elevated s1,25(OH)2D3 (1,25D), normal or elevated serum parathyroid hormone (sPTH), normal or low serum phosphate (sP), and low bone mass (14, 47, 60). IH exhibits a polygenic mode of inheritance (47, 48, 60).
We have established a strain of hypercalciuric rats by selectively inbreeding Sprague-Dawley (SD) rats for increased uCa excretion (2, 3, 5, 11–13, 15–22, 24, 25, 29, 33, 36, 37, 40–42, 44, 52, 62, 66, 67). Our hypercalciuric rat colony has been maintained by continual selection and inbreeding for over 90 generations; each rat now consistently excretes approximately 8 to 10-fold more uCa than SD controls when fed an ample Ca diet (2, 3, 5, 11–13, 15–22, 24, 25, 29, 33, 36, 37, 40–42, 44, 52, 62, 66, 67). These rats all develop kidney stones (2, 17, 18, 21) and have been named genetic hypercalciuric stone-forming (GHS) rats (2, 3, 5, 11–13, 15–20, 22, 24, 25, 29, 33, 36, 37, 40–42, 44, 52, 62, 66, 67).
Like patients with IH, GHS rats exhibit normal sCa (15), increased intestinal Ca absorption (44) and bone resorption (42), decreased renal tubule Ca reabsorption (62), and normal s1,25D levels (40, 41, 44, 66, 67) in addition to decreased bone mineral density (24, 33). Hypercalciuria is also a polygenic trait in GHS rats (36). GHS rats have elevated levels of vitamin D receptor (VDR) protein in Ca-transporting organs including the kidney, intestine, and bone (40, 42, 44).
Administration of 1,25D to normal subjects leads to hypercalciuria with changes in intestine, kidney, and bone Ca transport typical of those observed in IH (1, 46). This increased uCa indicates that the action of 1,25D in increasing intestinal Ca absorption (35) and bone Ca resorption (1, 46) exceeds any 1,25D-mediated change in renal tubular Ca reabsorption (8). While most IH patients have normal s1,25D levels (68), some have elevated s1,25D levels that may account for the phenotype (6, 9, 39, 59). High VDR levels have been found in male IH stone formers in at least one study (26), suggesting elevated VDR levels may play a role in hypercalciuria in some human stone formers.
Administration of 1,25D to GHS and SD rats fed a normal Ca diet (NCD) leads to a greater increment in hypercalciuria in GHS rats than in SD (29). This observation is consistent with a model in which the greater number of VDR in GHS rats is relatively undersaturated with 1,25D under basal conditions, but with addition of more 1,25D, the mass action of the increased VDR leads to enhanced uCa in GHS rats. To identify the source(s) of the increased uCa, a low-Ca diet (LCD) was utilized to remove the contribution of increased intestinal Ca absorption to the hypercalciuria. The hypercalciuria persisted in GHS either with or without additional 1,25D (30), suggesting that the increased uCa was derived from increased bone resorption. In this study we tested the hypothesis that in rats fed LCD, inhibition of bone resorption with a maximal dose of the bisphosphonate alendronate (alen) would result in equalization of uCa between GHS and SD rats.
METHODS
Animals.
The genetic GHS rats were derived from SD rats (Charles River Laboratories, Kingston, NY) by successively inbreeding the most hypercalciuric progeny of each generation (12, 15, 17, 25, 33, 41, 42, 44, 62). Eight-week-old male GHS rats from the 91st generation and 8-wk-old male SD rats (Charles River Laboratories, Kingston, NY) were used in this study.
Experimental conditions.
At the start of the study (day 0), 16 SD and 16 GHS rats, all 25D replete, were placed in metabolic cages and fed 13 g/day low-Ca diet (LCD; 0.02% Ca, providing a maximum of 2.6 mg Ca/day; Harlan-Teklad, Indianapolis, IN) and given deionized, distilled water ad libitum. Also starting on day 0, by random allocation, eight rats in each group were injected intraperitoneally daily with 1,25D (25 ng/100 g body wt; American Regent, Shirley, NY) in saline and eight rats in each group with only saline. This dose of 1,25D elicits a maximal physiologic response (27, 28, 66). Starting on day 8, urine from each rat was collected for four 24-h periods. On days 9 and 11, urine was acidified with HCl and on days 10 and 12 urine was collected in thymol. Collections in thymol were used for pH and Cl and collections in HCl for all other measurements. After the first urine collection, all rats were injected with a maximal dose of alen (5 mg/100 g/day) (4, 21, 45, 51, 58) for 8 days with continued feeding of LCD; injections of 1,25D were continued in the previously injected rats. Beginning at day 16, urine from each rat was again collected over 4 days, as described above. All rats were then euthanized, blood was collected, and the kidneys were quickly removed. Any animal that ate <10 g/day food or drank <15 ml/day water would have been excluded from further analysis; however, all rats met these prespecified criteria during the entire study. All procedures were approved by the University of Rochester Committee for Animal Resources.
Urine and serum chemistries.
Urine Ca, Mg, P, ammonia, and creatinine were measured spectrophotometrically using a Beckman CX5 Pro autoanalyzer (Beckman Instruments, Brea, CA). Urine K, Cl, and Na were measured by ion specific electrodes on the Beckman CX5. Urine pH was measured using a glass electrode and citrate, oxalate, and sulfate were measured by ion chromatography using a Dionex ICS 2000 system (Dionex Corporation, Sunnyvale, CA). Serum Ca and P were determined colorimetrically (BioVision, Milpitas, CA). Serum parathyroid hormone (PTH) was determined by EIA for intact-PTH (ALPCO, Salem, NH). We have used these methods previously (2, 3, 24, 29, 30).
Urine supersaturation.
The CaOx and CaHPO4 (CaP) ion activity product were calculated using the computer program EQUIL 2 (65) as we have done previously (11–13, 16, 19–22, 29, 30). Ratios of 1 denote a sample at equilibrium, >1 denotes supersaturation (SS), and <1 denotes undersaturation. We have found excellent correspondence between calculated and experimentally measured saturation in urine and blood and in bone culture medium (2, 3, 24).
RNA purification.
Kidneys were bisected and placed in 2 ml RNAlater (Ambion, Grand Island, NY) at 4°C overnight and then transferred to −70°C until purification. Each kidney was homogenized in 6 ml TriZol (Invitrogen, Grand Island, NY) using a glass homogenizer, and RNA purification was conducted according to manufacturer's instructions. Aqueous and phenol phases were separated by centrifugation after addition of 1-bromo-3-chloropropane. RNA was precipitated from the aqueous layer with isopropyl alcohol and washed with 75% ethanol. DNA contaminants were removed by on-column digestion with DNase 1 followed by purification using Qiagen RNeasy mini columns (Qiagen, Valencia, CA).
Quantitative real-time polymerase chain reaction.
Kidney RNA was transcribed to cDNA using an iScript kit (Bio-Rad, Hercules, CA). Gene specific targets were amplified and analyzed by real-time polymerase chain reaction with a MyIQ cycler (Bio-Rad) and Sybr Green (IQ Supermix; Bio-Rad). To normalize gene expression, the geometric mean of expression of RNA for β-actin, ribosomal protein L13a, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide, and succinate dehydrogenase complex, subunit a, flavoprotein was calculated for each sample (63). Primer sets used were described previously (29). All expression values were calculated relative to the mean expression in SD + vehicle.
Statistics.
Values were compared by ANOVA using the Bonferroni correction for multiple comparisons, with a conventional computer program (Statistica; StatSoft, Tulsa, OK). Values are expressed as means ± SE. P ≤ 0.05 considered significant.
RESULTS
Urine and serum chemistry.
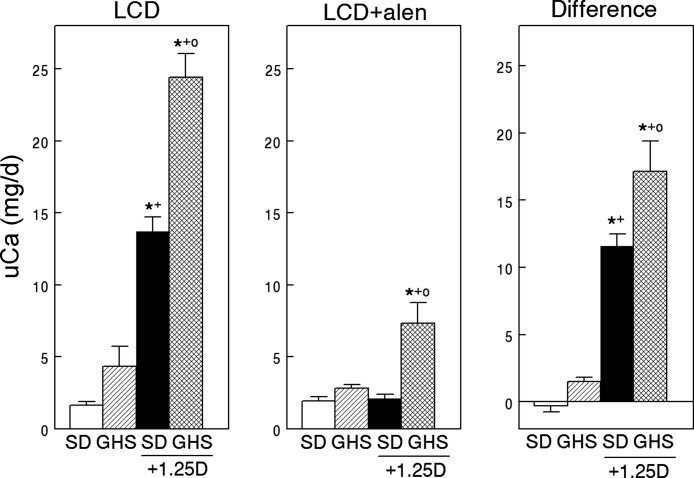
On LCD, the GHS rats excreted 4.3 ± 0.5 mg/day of Ca (Fig. 1, left). As total available dietary Ca with LCD was only 2.6 mg/day, the GHS were in negative total body Ca balance. There were no differences in uNa among the four groups over the two time periods (Table 1), which provides evidence for a constant amount of food intake. Injection of 1,25D increased uCa to 24.4 ± 1.6 mg/day in GHS and from 1.6 ± 0.2 to 13.7 ± 1.0 mg/day in SD indicating that this hormone increased the negative Ca balance in GHS rats and induced negative Ca balance in SD rats.
Fig. 1.
Urine Ca excretion in Sprague-Dawley (SD) and genetic hypercalciuric stone-forming rats fed low-Ca diet (LCD), without or with exogenous 1,25(OH)2D3 (1,25D), before and during alendronate (alen). On day 0, 8 rats in each group were injected intraperitoneally daily with 1,25D (25 ng/100 g body wt) in saline and 8 rats in each group with only saline. Starting on d 8, urine was collected and Ca measured (means ± SE, left). After the first urine collection, all rats were injected with alen (5 mg·100 g−1·day−1) for 8 days with continued feeding of LCD and urine was again collected (middle). The difference (right) was calculated as (uCa before alen) − (uCa after alen). Significance was measured within groups. *P < 0.05, compared with SD; +P < 0.05, compared with GHS; oP < 0.05 compared with SD+1,25D.
Table 1.
Values in LCD and alendronate groups for vehicle and 1,25D groups
| LCD |
LCD + Alen |
|||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle |
1,25D |
Vehicle |
1,25D |
|||||
| SD | GHS | SD | GHS | SD | GHS | SD | GHS | |
| sCa, mg/dl | 9.08 ± 0.30 | 8.51 ± 0.32 | 9.77 ± 0.25† | 9.78 ± 0.33† | ||||
| sP, mg/dl | 6.9 ± 0.5 | 7.6 ± 0.4 | 7.6 ± 0.6 | 7.3 ± 0.8 | ||||
| sPTH, pg/ml | 500 ± 48 | 333 ± 78 | 200 ± 21* | 165 ± 34* | ||||
| s25D, pg/ml | 30.6 ± 1.6 | 41.8 ± 2.9* | 31.5 ± 2.6 | 45.7 ± 2.9*‡ | ||||
| Urine volume, ml | 25.2 ± 5.2 | 24.2 ± 1.8 | 19.7 ± 1.5 | 29.2 ± 2.3 | 39.3 ± 5.3 | 30.0 ± 3.1 | 35.3 ± 4.7 | 32.5 ± 3.9 |
| Urine pH | 5.81 ± 0.06 | 5.71 ± 0.04 | 5.56 ± 0.07* | 5.34 ± 0.06*† | 6.06 ± 0.07 | 5.79 ± 0.038* | 6.09 ± 0.06† | 5.61 ± 0.05*‡ |
| Citrate, mg/24 h | 2.7 ± 0.5 | 28.5 ± 4.5 | 51.6 ± 4.9* | 94.9 ± 10.2*†‡ | 2.1 ± 0.6 | 34.8 ± 2.5* | 17.0 ± 2.0† | 84.0 ± 7.2*†‡ |
| P, g/24 h | 0.070 ± 0.006 | 0.067 ± 0.003 | 0.074 ± 0.004 | 0.076 ± 0.005 | 0.068 ± 0.002 | 0.076 ± 0.002 | 0.073 ± 0.002 | 0.079 ± 0.001* |
| Oxalate, mg/24 h | 0.35 ± 0.05 | 0.53 ± 0.08 | 0.74 ± 0.03* | 0.94 ± 0.09*† | 0.49 ± 0.06 | 0.80 ± 0.03* | 0.91 ± 0.05* | 0.85 ± 0.06* |
| Cl, mmol/24 h | 1.76 ± 0.19 | 1.82 ± 0.16 | 1.41 ± 0.11 | 1.68 ± 0.08 | 1.90 ± 0.26 | 1.84 ± 0.14 | 1.86 ± 0.24 | 1.98 ± 0.15 |
| NH4, mmol/24 h | 1.00 ± 0.08 | 0.74 ± 0.07 | 0.80 ± 0.05 | 1.04 ± 0.07† | 1.02 ± 0.06 | 0.95 ± 0.03 | 1.16 ± 0.05† | 1.38 ± 0.029*†‡ |
| Na, mmol/24 h | 3.0 ± 0.8 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.1 |
| K, mmol/24 h | 1.30 ± 0.11 | 1.13 ± 0.05 | 1.23 ± 0.05 | 1.13 ± 0.05 | 1.09 ± 0.03 | 1.19 ± 0.05 | 1.16 ± 0.09 | 1.27 ± 0.02* |
| SO4, meq/24 h | 0.48 ± 0.07 | 0.47 ± 0.05 | 0.68 ± 0.05† | 0.81 ± 0.02*† | 0.68 ± 0.06 | 0.72 ± 0.02 | 0.70 ± 0.02 | 0.75 ± 0.03 |
| Cr, mg/24 h | 12.10 ± 0.90 | 10.05 ± 0.45 | 11.38 ± 0.56 | 9.95 ± 0.73 | 12.4 ± 0.32 | 11.67 ± 0.22 | 13.08 ± 0.24† | 12.45 ± 0.37 |
Results are means ± SE. Selected serum and urine values in Sprague-Dawley (SD) and genetic hypercalciuric stone-forming (GHS) rats fed low-Ca diet (LCD), without or with exogenous1,25(OH)2D3 (1,25D), without or with alendronate (alen); s, serum. Significance was measured within groups.
P < 0.05, compared with SD;
P < 0.05, compared with GHS;
P < 0.05, compared with SD +1,25D.
After injection of alen, uCa continued to be significantly elevated with concurrent 1,25D injection in GHS but not in SD (Fig. 1, middle) indicating that while the bisphosphonate completely eliminated the 1,25D-induced hypercalciuria in the SD rats, the GHS rats continued to be hypercalciuric.
Alen inhibited more bone resorption in GHS, as measured by a greater difference in uCa before and after alen in GHS compared with SD rats given 1,25D (Fig. 1, right). However, in the GHS rats, the increase in uCa with 1,25D was of sufficient magnitude that even with inhibition of more bone resorption in GHS than SD rats, alen was unable to completely inhibit the increase in uCa (Fig. 1, middle).
Although there was no significant difference in sCa or sPTH between the GHS and SD rats given alen without exogenous 1,25D, both values trended lower in GHS rats (Table 1). Injection of 1,25D led to a numerical increase in sCa in both GHS and SD; however, the change in sCa was only significant in GHS. There were no differences in sP among the four groups. Injection of 1,25D led to a numerical decrease in sPTH in both GHS and SD, but the change in sPTH was only significant in SD. Both the SD and GHS rats were 25D replete; however, s25D was higher in the GHS rats compared with SD. 1,25D did not alter s25D levels in either group.
Urine SS.
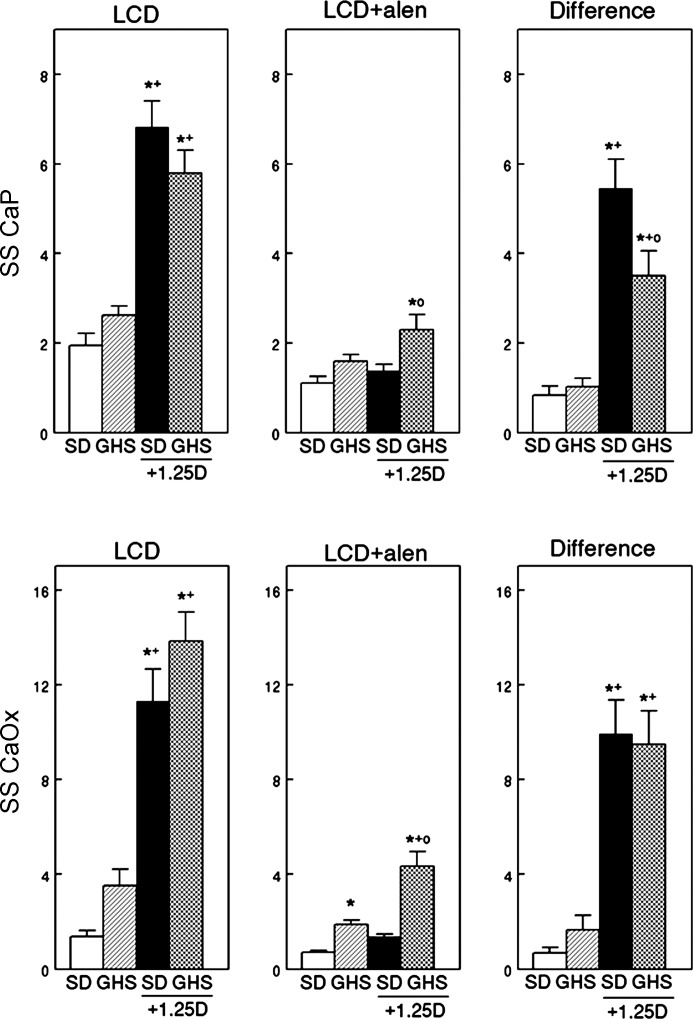
When fed LCD there was no significant difference in CaP or CaOx SS between GHS and SD rats (Fig. 2, left). In the absence of alen, 1,25D increased both CaP and CaOx SS in both SD and GHS, which were not different from each other. With alen, the GHS rats had a significantly higher CaP SS with 1,25D and CaOx SS with or without 1,25D compared with SD (Fig. 2, middle). Alen prevented the increase in CaP and CaOx SS with 1,25D in SD rats. Alen led to a marked difference in both CaP and CaOx SS only in GHS and SD rats given 1,25D (Fig. 2, middle and right).
Fig. 2.
Supersaturation (SS) of calcium phosphate (CaP) and calcium oxalate (CaOx) in SD and GHS rats fed LCD, without or with exogenous 1,25D, before and during alendronate (alen). On day 0, 8 rats in each group were injected intraperitoneally daily with 1,25D (25 ng/100 g body wt) in saline and 8 rats in each group with only saline. Starting on day 8, urine was collected and supersaturation was calculated (means ± SE, left: CaP, top; CaOx, bottom). After the first urine collection, all rats were injected with alen (5 mg·100 g−1·day−1) for 8 days with continued feeding of LCD and urine was again collected (middle). The difference (right) was calculated as (SS before alen) − (SS after alen). Significance was measured within groups. *P < 0.05, compared with SD; +P < 0.05, compared with GHS; oP < 0.05, compared with SD + 1,25D.
Expression of markers of renal calcium transport.
The source of the continued hypercalciuria in the GHS rats on LCD injected with alen and receiving 1,25D must be from bone, the only significant repository of Ca in the body. This continued hypercalciuria indicates that there is residual Ca resorption from bone due to direct effects of 1,25D on bone and/or secondary to incomplete renal reabsorption of filtered Ca. To further define the potential mechanisms of altered renal Ca reabsorption, we determined the effects of alen and 1,25D on expression of genes related to renal Ca transport.
Active transport.
There was no difference in the RNA expression of transient receptor potential vanilloid (TRPV) 5, TRPV6, plasma membrane Ca ATPase (PMCA), or Na/Ca exchanger (NCX1) between GHS and SD rats given alen without additional 1,25D (Table 2). Expression of calbindin D28k was elevated in GHS + alen rats but there was no difference in expression of klotho. With alen, 1,25D increased expression of TRPV6 only in SD rats. NCX expression was decreased in SD rats given both alen and 1,25D. Levels of TRPV5, calbindinD28k, and PMCA were not significantly altered in either SD or GHS rats with alen and 1,25D.
Table 2.
Relative RNA expression of components of renal calcium transport
| SD + Alen | GHS + Alen | SD + Alen + 1,25D | GHS + Alen + 1,25D | |
|---|---|---|---|---|
| TRPV5 | 1.00 ± 0.42 | 0.61 ± 0.07 | 0.50 ± 0.04 | 0.52 ± 0.04 |
| TRPV6 | 1.00 ± 0.12 | 1.48 ± 0.21 | 2.20 ± 0.43* | 1.87 ± 0.2 |
| PMCA | 1.00 ± 0.07 | 1.29 ± 0.12 | 1.01 ± 0.06 | 0.97 ± 0.07 |
| NCX1 | 1.00 ± 0.12 | 0.68 ± 0.16 | 0.30 ± 0.06* | 0.49 ± 0.07* |
| Calbindin D28k | 1.00 ± 0.11 | 2.27 ± 0.4* | 1.08 ± 0.08† | 1.72 ± 0.22 |
| klotho | 1.00 ± 0.08 | 1.14 ± 0.10 | 0.82 ± 0.04† | 0.89 ± 0.05 |
| Claudin 16 | 1.00 ± 0.09 | 1.50 ± 0.36 | 0.68 ± 0.06† | 0.93 ± 0.09 |
| Claudin 19 | 1.00 ± 0.10 | 1.66 ± 0.40 | 1.78 ± 0.20 | 1.62 ± 0.10 |
| Claudin 14 | 1.00 ± 0.20 | 2.26 ± 0.50 | 1.04 ± 0.20 | 1.41 ± 0.20 |
| CaR | 1.0 ± 0.09 | 1.73 ± 0.42 | 0.86 ± 0.05 | 1.05 ± 0.05 |
| ROMK | 1.0 ± 0.13 | 1.28 ± 0.22 | 0.9 ± 0.07 | 1.42 ± 0.13 |
| NKCC2 | 1.00 ± 0.10 | 1.43 ± 0.28 | 0.70 ± 0.03† | 1.15 ± 0.14 |
Results are means ± SE. Relative RNA expression of selected components of renal calcium transport in SD and GHS rats fed LCD, without or with exogenous 1,25D.
TRPV, transient receptor potential vanilloid; PMCA, plasma membrane Ca ATPase; NCX1, Na/Ca exchanger; CaR, calcium-sensing receptor; ROMK, outward modulating K channel; NKCC, Na/K/2Cl transporter.
P < 0.05, compared with SD;
P < 0.05, compared with GHS.
Paracellular Ca transport.
Renal Ca reabsorption occurs in the thick ascending limb of Henle (TALH), via paracellular transport through tight junctions, which contain claudin 16 and claudin 19 (38), and permeability of cations through tight junctions is regulated by claudin 14 (32). Without 1,25D expression of claudins 16, 19, and 14 were not different between GHS and SD (Table 2). 1,25D did not significantly affect expression of claudins 16, 19, or 14. Expression of the calcium-sensing receptor (CaR), the outward modulating K channel (ROMK), and the Na/K/2Cl transporter (NKCC2) did not differ between SD and GHS with or without 1,25D.
DISCUSSION
The phenotype of the GHS rats is very similar to human IH (14), including increased intestinal Ca absorption (44), increased bone resorption (42), and decreased renal tubule Ca reabsorption (62). The levels of serum 1,25D are generally normal in the GHS rats (40, 41, 44, 66, 67); however, we have found that the GHS rats have an increased number of VDR in each of these Ca-transporting organs (31, 40, 42, 44). We hypothesized that in the GHS rats a portion of the increased VDR would not be occupied by endogenous 1,25D and that the addition of exogenous 1,25D would cause a greater biological response in GHS compared with SD rats.
To explore the importance of the dysregulation of Ca transport in each organ in the development of the hypercalciuria, we first placed the GHS rats on a normal Ca diet and determined that the increased number of VDR were biologically active by demonstrating a greater increase in uCa in response to exogenous 1,25D in the GHS rats compared with the control SD rats (29). We then drastically reduced dietary Ca to minimize intestinal Ca absorption and again demonstrated a greater increment of uCa in the GHS rats compared with SD indicating that there was enhanced 1,25D-induced bone resorption and/or a decrease in renal tubular Ca reabsorption (30). In this study we not only reduced intestinal Ca absorption with LCD, but we inhibited bone resorption with a maximal dose of the bisphosphonate alen. In the presence of alen, the hypercalciuria in GHS rats fed LCD was completely inhibited without exogenous 1,25D, and was significantly reduced with administration of 1,25D.
The GHS rats fed LCD and injected with both alen and 1,25D continued to be hypercalciuric, with uCa exceeding available dietary Ca, indicating that they were in negative Ca balance. This elevated uCa must originate principally from enhanced bone resorption, the only source of uCa when dietary Ca is virtually eliminated (21). We utilized a maximal dose of alen that was sufficient to totally inhibit bone resorption in the SD rats, with and without 1,25D, and in the GHS rats without 1,25D. However, in the GHS rats given 1,25D, although there was a greater decrease in uCa with alen than in the SD rats (Fig. 1, right) indicating a greater inhibition of bone resorption, there was continued enhanced bone resorption. This enhanced bone resorption may be the result of 1,25D stimulating the increased number of VDR in bone (42) leading to bone resorption, which could not be completely inhibited by this maximal dose of alen.
In addition to Ca circulating in the blood and that stored in the skeleton, an additional source of Ca might be from soft tissue, particularly vascular, calcification. Price et al. has reported that bisphosphonates inhibit vascular calcification induced by toxic doses of cholecalciferol (53) or by a low protein diet in uremic rats (55). However, Price et al. also found (54) that the dose response for inhibition of vascular calcification by etidronate was identical to the dose response for inhibition of bone resorption, suggesting that the origin of the Ca in soft tissue calcification was from bone. This is consistent with the observation that osteoprotegerin null mice develop both osteoporosis and vascular calcification (10). Our data support the hypothesis that the source of the increased uCa in GHS + 1,25D must be from bone; whether there is intermediate deposition of Ca in soft tissue remains to be determined. We cannot rule out the possibility of direct effects of bisphosphonates on vascular calcification.
Nitrogen-containing bisphosphonates such as alen decrease bone resorption by inhibiting osteoclastic farnesyl diphosphate synthase (FPPS), an enzyme critical in the mevalonate pathway which provides the substrate for the geranylgeranylation of Rac, Rho, and cdc42, small molecules necessary for osteoclastic activity (28, 57). The IC50 for alen inhibition of osteoclast activity in vitro (pit formation on ivory) is 2 nM (45, 58). When comparing several bisphosphonates in vivo (28), the maximal reduction in osteoclasts positive for the mevalonate pathway was achieved with an identical dose of alen used in the current study. An alen dose of 1.9 μmol·100 g−1·day−1 caused a significant decrease in the markers of bone resorption uPyr and uD-Pyr in vivo (4). Muhlbauer et al.(51) found essentially total inhibition of arotinoid-induced bone resorption by an alen dose of 3 μmol·100 g−1·day−1 in vivo. In this study we injected the rats with a dose of alen (15.4 μmol·100 g−1·day−1) which was equal to (28) or far exceeded (4, 51) these doses indicating that the use of more alen would not have led to a greater inhibition of bone resorption.
The marked increase in uCa excretion with 1,25D leads to the increase in CaP SS in both SD and GHS rats before treatment with alen (Fig. 2, top left). That the CaP SS is not higher in the GHS than the SD rats receiving 1,25D is explained by the trend to a higher urine volume and lower urine pH in the GHS group. When treated with alen, uCa falls significantly in the rats treated with 1,25D, leading to lower CaP SS compared with pretreatment with alen.
Although there was no significant difference in uOx between GHS and SD rats, numerically uOx was higher in GHS, consistent with the observation that patients with IH may exhibit mild hyperoxaluria (7). Compared with SD, the GHS rat has increased intestinal Ca absorption (44) leading to more free oxalate in the intestine and greater availability for Ox absorption and excretion. There was no difference in CaOx SS between GHS and SD rats without exogenous 1,25D; administration of the hormone led to a significant increase in CaOX SS in both groups.
Before alen, the molar ratio of Ca to Ox in SD without exogenous 1,25D was 15 to 1, while the Ca to Ox ratio in GHS without exogenous 1,25D was similar, at 18 to 1. With exogenous 1,25D, the ratios for SD and GHS were 40 to 1 and 54 to 1, respectively. This higher ratio drives the increased CaOx SS illustrated in Fig, 2 (bottom left). However, there is such an excess of Ca to Ox that there is insufficient Ox available to drive SS despite the increased uCa (61). With alen, the molar ratio without 1,25D for SD was 10 to 1 and for GHS was 8 to 1. With both alen and 1,25D, the ratio for SD was 5 to 1 while the ratio for GHS increased to 20 to 1. This higher GHS ratio would help account for the elevated CaOx SS seen in GHS plus alen plus 1,25D (Fig. 2, bottom middle).
The enhanced uCa could originate from dysregulation of renal tubular Ca reabsorption by PTH, leading to a lower sCa which stimulated bone resorption. However, in this case sPTH should be increased in the GHS rats given 1,25D. In this study sPTH was not stimulated, consistent with our prior observations (29, 30). To explore whether 1,25D contributes to alterations in renal tubular Ca reabsorption and hypercalciuria in GHS rats, we determined the effects of alen and 1,25D on the expression of genes related to renal Ca transport. In a prior study in the GHS rats fed a NCD we found increases in RNA levels for TRPV5, TRPV6, and calbindin in response to 1,25D (31, 34). However, in this study there were no consistent increases in the RNA levels for these markers of active renal Ca transport. While small changes were found in other components of renal Ca transport, there was no consistent pattern in the comparison of GHS to SD with or without 1,25D. Since changes in RNA abundance do not necessarily reflect the magnitude of Ca reabsorption, further studies examining protein levels and transport activity will be necessary to determine which, if any, of these transporters may be altered in GHS rats. In particular, it would be interesting to directly compare rates of renal tubular Ca reabsorption from GHS and SD rats treated with 1,25D and/or alen. We have previously found that parathyroidectomized GHS rats fed a NCD or LCD had a significant decrease in renal tubular Ca reabsorption compared with SD rats (62).
Bone is a large repository of base (43, 56). Increased bone resorption induced by 1,25D will lead to the release of base into the systemic circulation while inhibition of bone resorption with alen will decrease release of base into the circulation. As the increase in uCa with 1,25D+alen results in net bone resorption, there will clearly be release of base into the systemic circulation which has the potential to increase renal tubular Ca reabsorption and thus decrease uCa excretion. However, it is unclear if the increase in bone resorption is of sufficient magnitude to alter systemic acid-base balance resulting in alterations in uCa. To accurately measure systemic acid-base parameters, we have found it necessary to place indwelling arterial catheters (23, 64), which was not done here. Thus in the current study we cannot determine if alterations in bone resorption induced by 1,25D and alen resulted in changes in systemic acid-base parameters that then affected renal tubular Ca reabsorption.
Since the GHS rats have normal levels of s1,25D, we have postulated that their elevated tissue levels of VDR in the Ca-transporting organs would be relatively undersaturated with 1,25D compared with SD rats. We previously tested the hypothesis that, while fed NCD, administration of 1,25D, which should result in greater VDR saturation, would lead to enhanced uCa excretion in GHS compared with SD rats. We found that administration of 1,25D to the GHS rats fed NCD increased the hypercalciuria to a greater extent than in SD (29), perhaps due to their increased VDR. This additional uCa must come from either enhanced intestinal Ca absorption and/or increased bone Ca resorption. We then utilized LCD to remove the contribution of intestinal Ca absorption to uCa (30). We found that administration of 1,25D to rats fed LCD increased hypercalciuria to a greater extent in GHS than in SD controls; these results provided clear support for enhanced bone resorption as the source of this additional uCa in GHS rats. In the current study we used a maximal dose of the bisphosphonate alen to inhibit bone resorption in rats fed LCD. In SD rats, alen eliminated the 1,25D-induced hypercalciuria; however, in GHS rats the increase in uCa was decreased but not eliminated. These results demonstrate that a maximal dose of alen is unable to completely prevent the 1,25D-induced bone resorption in GHS, perhaps due to the increased VDR in the bone cells of these rats (42). As elevated levels of VDR levels have been found in male IH stone-formers in at least one study (26), if these results are confirmed in similar hypercalciuric humans, they suggest that the clinical use of bisphosphonates, such as alen, may not completely prevent the hypercalciuria and decreased bone density observed in these patients.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-075462.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.K.F., N.S.K., and D.A.B. conception and design of research; K.K.F., J.R.A., C.D.C., and N.S.K. performed experiments; K.K.F., J.R.A., C.D.C., I.G., N.S.K., and D.A.B. analyzed data; K.K.F., J.R.A., N.S.K., and D.A.B. interpreted results of experiments; K.K.F., C.D.C., N.S.K., and D.A.B. prepared figures; K.K.F., N.S.K., and D.A.B. drafted manuscript; K.K.F., J.R.A., I.G., N.S.K., and D.A.B. edited and revised manuscript; K.K.F., J.R.A., C.D.C., I.G., N.S.K., and D.A.B. approved final version of manuscript.
ACKNOWLEDGMENTS
A portion of this work was presented at the annual meeting of the American Society for Nephrology in 2012.
REFERENCES
- 1.Adams ND, Gray RW, Lemann JJ. Effects of calcitriol administration on calcium metabolism in healthy men. Kidney Int 21: 90–97, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Asplin JR, Bushinsky DA, Singharetnam W, Riordon D, Parks JH, Coe FL. Relationship between supersaturation and crystal inhibition in hypercalciuric rats. Kidney Int 51: 640–645, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Asplin JR, Donahue SE, Lindeman C, Michalenka A, Strutz KL, Bushinsky DA. Thiosulfate reduces calcium phosphate nephrolithiasis. J Am Soc Nephrol 20: 1246–1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azuma Y, Chokki M, Ohta T, Kiyoki M. Effects of alendronate on plasma calcium levels, urinary calcium excretion and bone resorption markers in normal rats: comparison with elcatonin, synthetic eel calcitonin. Endocrinology 137: 2586–2592, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Bai S, Wang H, Shen J, Zhou R, Bushinsky DA, Favus MJ. Elevated vitamin D receptor levels in genetic hypercalciuric stone-forming rats are associated with downregulation of Snail. J Bone Miner Res 25: 830–840, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bataille P, Bouillion R, Fournier A, Renaud H, Gueris J, Idrissi A. Increased plasma concentrations of total and free 1,25(OH)2D3 in calcium stone formers with idiopathic hypercalciuria. Contrib Nephrol 58: 137–142, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Bergsland KJ, Zisman AL, Asplin JR, Worcester EM, Coe FL. Evidence for net renal tubule oxalate secretion in patients with calcium kidney stones. Am J Physiol Renal Physiol 300: F311–F318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bindels RJ, Hartog A, Timmermans J, Van Os CH. Active Ca2+ transport in primary cultures of rabbit kidney CCD: stimulation by 1,25-dihydroxyvitamin D3 and PTH. Am J Physiol Renal Fluid Electrolyte Physiol 261: F799–F807, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Broadus AE, Horst RL, Lang R, Littledike ET, Rasmussen H. The importance of circulating 1,25(OH)2D in the pathogenesis of hypercalciuria and renal stone formation in primary hyperparathyroidism. N Engl J Med 302: 421–426, 1980 [DOI] [PubMed] [Google Scholar]
- 10.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12: 1260–1268, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bushinsky DA, Asplin JR. Thiazides reduce brushite, but not calcium oxalate, supersaturation and stone formation in genetic hypercalciuric stone-forming rats. J Am Soc Nephrol 16: 417–424, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Bushinsky DA, Asplin JR, Grynpas MD, Evan AP, Parker WR, Alexander KM, Coe FL. Calcium oxalate stone formation in genetic hypercalciuric stone-forming rats. Kidney Int 61: 975–987, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Bushinsky DA, Bashir MA, Riordon DR, Nakagawa Y, Coe FL, Grynpas MD. Increased dietary oxalate does not increase urinary calcium oxalate saturation in hypercalciuric rats. Kidney Int 55: 602–612, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Bushinsky DA, Coe FL, Moe Nephrolithiasis OW. In: The Kidney, edited by Brenner BM. Philadelphia, PA: W. B. Saunders, 2012, p. 1455–1507 [Google Scholar]
- 15.Bushinsky DA, Favus MJ. Mechanism of hypercalciuria in genetic hypercalciuric rats: inherited defect in intestinal calcium transport. J Clin Invest 82: 1585–1591, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bushinsky DA, Grynpas MD, Asplin JR. Effect of acidosis on urine supersaturation and stone formation in genetic hypercalciuric stone forming rats. Kidney Int 59: 1415–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Bushinsky DA, Grynpas MD, Nilsson EL, Nakagawa Y, Coe FL. Stone formation in genetic hypercalciuric rats. Kidney Int 48: 1705–1713, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Bushinsky DA, Kim M, Sessler NE, Nakagawa Y, Coe FL. Increased urinary saturation and kidney calcium content in genetic hypercalciuric rats. Kidney Int 45: 58–65, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Bushinsky DA, LaPlante K, Asplin JR. Effect of cinacalcet on urine calcium excretion and supersaturation in genetic hypercalciuric stone-forming rats. Kidney Int 69: 1586–1592, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Bushinsky DA, Michalenka AC, Strutz KL, Donahue S, Asplin JR. Effect of bolus and divided feeding on urine ions and supersaturation in genetic hypercalciuric stone-forming rats. Kidney Int 73: 423–429, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Bushinsky DA, Neumann KJ, Asplin J, Krieger NS. Alendronate decreases urine calcium and supersaturation in genetic hypercalciuric rats. Kidney Int 55: 234–243, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Bushinsky DA, Parker WR, Asplin JR. Calcium phosphate supersaturation regulates stone formation in genetic hypercalciuric stone-forming rats. Kidney Int 57: 550–560, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Bushinsky DA, Riera GS, Favus MJ, Coe FL. Evidence that blood ionized calcium can regulate serum 1,25(OH) 2 D 3 independently of PTH and phosphorus in the rat. J Clin Invest 76: 1599–1604, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bushinsky DA, Willett T, Asplin JR, Culbertson C, Che SP, Grynpas M. Chlorthalidone improves vertebral bone quality in genetic hypercalciuric stone-forming rats. J Bone Miner Res 26: 1904–1912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evan AP, Bledsoe SB, Smith SB, Bushinsky DA. Calcium oxalate crystal localization and osteopontin immunostaining in genetic hypercalciuric stone-forming rats. Kidney Int 65: 154–161, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Favus MJ, Karnauskas AJ, Parks JH, Coe FL. Peripheral blood monocyte vitamin D receptor levels are elevated in patients with idiopathic hypercalciuria. J Clin Endocrinol Metab 89: 4937–4943, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Fisher J, Rosenberg E, Santora A, Reszka A. In vitro and in vivo responses to high and low doses of nitrogen-containing bisphosphonates suggest engagement of different mechanisms for inhibition of osteoclastic bone resorption. Calcif Tissue Int 92: 531–538, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Fisher JE, Rodan GA, Reszka AA. In vivo effects of bisphosphonates on the osteoclast mevalonate pathway. Endocrinology 141: 4793–4796, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Frick KK, Asplin JR, Favus M, Culbertson C, Krieger NS, Bushinsky DA. Increased biological response to 1,25(OH)2D3 in genetic hypercalciuric stone-forming rats. Am J Physiol Renal Physiol 304: F718–F726, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frick KK, Asplin JR, Krieger NS, Culbertson C, Asplin DM, Bushinsky DA. 1,25(OH)2D3-enhanced hypercalciuria in genetic hypercalciuric stone-forming rats fed a low calcium diet. Am J Physiol Renal Physiol 305: F1132–F1138, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frick KK, Bushinsky DA. Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol 14: 1082–1095, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J. Claudin-14 regulates renal Ca2+ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 31: 1999–2012, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grynpas M, Waldman S, Holmyard D, Bushinsky DA. Genetic hypercalciuric stone-forming rats have a primary decrease in bone mineral density and strength. J Bone Miner Res 24: 1420–1426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoenderop JG, Bindels RJ. Epithelial Ca2+ and Mg2+ channels in health and disease. J Am Soc Nephrol 16: 15–26, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev 85: 373–422, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Hoopes RR, Jr, Middleton FA, Sen S, Hueber PA, Reid R, Bushinsky DA, Scheinman SJ. Isolation and confirmation of a calcium excretion quantitative trait locus on chromosome 1 in genetic hypercalciuric stone-forming congenic rats. J Am Soc Nephrol 17: 1292–1304, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Hoopes RR, Reid R, Sen S, Szpirer C, Dixon P, Pannet A, Thakker RV, Bushinsky DA, Scheinman SJ. Quantitative trait loci for hypercalciuria in a rat model of kidney stone disease. J Am Soc Nephrol 14: 1844–1850, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci USA 106: 15350–15355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Insogna KL, Broadus AE, Dryer BE, Ellison AF, Gertner JM. Elevated production rate of 1,25-dihydroxyvitamin D in patients with absorptive hypercalciuria. J Clin Endocrinol Metab 61: 490–495, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Karnauskas AJ, van Leeuwen JP, van den Bemd GJ, Kathpalia PP, DeLuca HF, Bushinsky DA, Favus MJ. Mechanism and function of high vitamin D receptor levels in genetic hypercalciuric stone-forming rats. J Bone Miner Res 20: 447–454, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Kim M, Sessler NE, Tembe V, Favus MJ, Bushinsky DA. Response of genetic hypercalciuric rats to a low calcium diet. Kidney Int 43: 189–196, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Krieger NS, Stathopoulos VM, Bushinsky DA. Increased sensitivity to 1,25(OH)2D3 in bone from genetic hypercalciuric rats. Am J Physiol Cell Physiol 271: C130–C135, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Lemann J, Jr, Litzow JR, Lennon EJ. The effects of chronic acid loads in normal man: Further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest 45: 1608–1614, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li XQ, Tembe V, Horwitz GM, Bushinsky DA, Favus MJ. Increased intestinal vitamin D receptor in genetic hypercalciuric rats: a cause of intestinal calcium hyperabsorption. J Clin Invest 91: 661–667, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone 18: 75–85, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Maierhofer WJ, Gray RW, Cheung HS, Lemann J., Jr Bone resorption stimulated by elevated serum 1,25-(OH)2-vitamin D3 concentrations in healthy men. Kidney Int 24: 555–560, 1983 [DOI] [PubMed] [Google Scholar]
- 47.Moe OW, Bonny O. Genetic hypercalciuria. J Am Soc Nephrol 16: 729–745, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Monico CG, Milliner DS. Genetic determinants of urolithiasis. Nat Rev Nephrol 8: 151–162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monk RD, Bushinsky DA. Kidney stones. In: Williams Textbook of Endocrinology, edited by Kronenberg HM, Melmed S, Polonsky KS, Larsen PR. Philadelphia, PA: Saunders, 2011, p. 1350–1367 [Google Scholar]
- 50.Monk RD, Bushinsky DA. Nephrolithiasis and nephrocalcinosis. In: Comprehensive Clinical Nephrology, edited by Frehally J, Floege J, Johnson RJ. St. Louis, MO: Elsevier, 2010, p. 687–701 [Google Scholar]
- 51.Muhlbauer R, Bauss F, Schenk R, Janner M, Bosies E, Strein K, Fleisch H. BM 21.0955, a potent new bisphosphonate to inhibit bone resorption. J Bone Miner Res 6: 1003–1011, 1991 [DOI] [PubMed] [Google Scholar]
- 52.Perry GML, Nehrke KW, Bushinsky DA, Reid R, Lewandowski KL, Hueber P, Scheinman SJ. Sex modifies genetic effects on residual variance in urinary calcium excretion in rat (Rattus norvegicus). Genetics 191: 1003–1013, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price PA, Buckley JR, Williamson MK. The amino bisphosphonate ibandronate prevents vitamin d toxicity and inhibits vitamin D-induced calcification of arteries, cartilage, lungs and kidneys in rats. J Nutr 131: 2910–2915, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Price PA, Omid N, Than TN, Williamson MK. The amino bisphosphonate ibandronate prevents calciphylaxis in the rat at doses that inhibit bone resorption. Calcif Tissue Int 71: 356–363, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Price PA, Roublick AM, Williamson MK. Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int 70: 1577–1583, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Relman AS. The acidosis of renal disease. Am J Med 44: 706–713, 1968 [DOI] [PubMed] [Google Scholar]
- 57.Reszka AA, Rodan GA. Nitrogen-containing bisphosphonate mechanism of action. Mini Rev Med Chem 4: 711–719, 2004 [PubMed] [Google Scholar]
- 58.Sahni M, Guenther HL, Fleisch H, Collin P, Martin TJ. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J Clin Invest 91: 2004–2011, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen FH, Baylink DJ, Nielsen RL. Increased serum 1,25-dihydroxy cholecalciferol (1,25 diOHD3) in patients with idiopathic hypercalciuria (IH). Clin Res 23: 423A, 1975 [Google Scholar]
- 60.Stechman MJ, Loh NY, Thakker RV. Genetics of hypercalciuric nephrolithiasis: renal stone disease. Ann NY Acad Sci 1116: 461–484, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Tiselius HG. An improved method for the routine biochemical evaluation of patients with recurrent calcium oxalate stone disease. Clin Chim Acta 122: 409–418, 1982 [DOI] [PubMed] [Google Scholar]
- 62.Tsuruoka S, Bushinsky DA, Schwartz GJ. Defective renal calcium reabsorption in genetic hypercalciuric rats. Kidney Int 51: 1540–1547, 1997 [DOI] [PubMed] [Google Scholar]
- 63.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weisinger JR, Favus MJ, Langman CB, Bushinsky DA. Regulation of 1,25-dihydroxyvitamin D 3 by calcium in the parathyroidectomized parathyroid hormone replete rat. J Bone Miner Res 4: 929–935, 1989 [DOI] [PubMed] [Google Scholar]
- 65.Werness PG, Brown CM, Smith LH, Finlayson B. Equil2: a BASIC computer program for the calculation of urinary saturation. J Urol 134: 1242–1244, 1985 [DOI] [PubMed] [Google Scholar]
- 66.Yao J, Karnauskas AJ, Bushinsky DA, Favus MJ. Regulation of renal calcium-sensing receptor gene expression in response to 1,25(OH)2D3 in genetic hypercalciuric stone forming rats. J Am Soc Nephrol 16: 1300–1308, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Yao J, Kathpalia P, Bushinsky DA, Favus MJ. Hyperresponsiveness of vitamin D receptor gene expression to 1,25-dihydroxyvitamin D3: a new characteristic of genetic hypercalciuric stone-forming rats. J Clin Invest 101: 2223–2232, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zerwekh JE, Reed BY, Heller HJ, Gonzalez GB, Haussler MR, Pak CY. Normal vitamin D receptor concentration and responsiveness to 1,25-dihydroxyvitamin D3 in skin fibroblasts from patients with absorptive hypercalciuria. Miner Electrolyte Metab 24: 307–313, 1998 [DOI] [PubMed] [Google Scholar]