Abstract
The renal glomerulus forms a selective filtration barrier that allows the passage of water, ions, and small solutes into the urinary space while restricting the passage of cells and macromolecules. The three layers of the glomerular filtration barrier include the vascular endothelium, glomerular basement membrane (GBM), and podocyte epithelium. Podocytes are capable of internalizing albumin and are hypothesized to clear proteins that traverse the GBM. The present study followed the fate of FITC-labeled albumin to establish the mechanisms of albumin endocytosis and processing by podocytes. Confocal imaging and total internal reflection fluorescence microscopy of immortalized human podocytes showed FITC-albumin endocytosis occurred preferentially across the basal membrane. Inhibition of clathrin-mediated endocytosis and caveolae-mediated endocytosis demonstrated that the majority of FITC-albumin entered podocytes through caveolae. Once internalized, FITC-albumin colocalized with EEA1 and LAMP1, endocytic markers, and with the neonatal Fc receptor, a marker for transcytosis. After preloading podocytes with FITC-albumin, the majority of loaded FITC-albumin was lost over the subsequent 60 min of incubation. A portion of the loss of albumin occurred via lysosomal degradation as pretreatment with leupeptin, a lysosomal protease inhibitor, partially inhibited the loss of FITC-albumin. Consistent with transcytosis of albumin, preloaded podocytes also progressively released FITC-albumin into the extracellular media. These studies confirm the ability of podocytes to endocytose albumin and provide mechanistic insight into cellular mechanisms and fates of albumin handling in podocytes.
Keywords: albumin, caveoli, endocytosis, podocytes, trafficking
the specialized capillary bed of the renal glomerulus plays an essential role in the formation of urine. Under physiological conditions, the glomerulus readily allows the movement of water, ions, and small solutes from the plasma into the urinary space of the nephron. At the same time, the glomerulus restricts the exodus of cells and larger macromolecules. The glomerular filtration barrier (GFB) is established by three adjacent physiological layers: vascular endothelial cells, glomerular basement membrane (GBM) proteins, and podocyte epithelial cells (21). The first filtration layer, the glomerular endothelial cells are fenestrated, allowing the movement of most of the plasma constituents while still restricting the cellular components of blood to the vascular space. The second filtration layer, the GBM is a meshwork of extracellular matrix proteins, including laminin, collagen IV, nidogen, and heparan sulfate proteoglycan (23). The third filtration layer, podocytes are a specialized epithelial cell type that completely encase the capillary tufts within the glomeruli. This coverage includes extensions of cellular processes out from the cell body. Progressive branching ultimately generates fine foot processes that interdigitate with the fine foot processes of neighboring podocytes. By doing so, podocytes markedly increase the area available for the paracellular flux of the glomerular filtrate. To selectively restrict the paracellular flux of specific solutes, the extracellular domains of specific integral membrane proteins extend between these foot processes to form the slit diaphragm (SD). Under physiological conditions, the SD forms a selective barrier that is highly permeable to water and small solutes but largely reflects macromolecules.
Traditionally, only small amounts of albumin were thought to pass through the GFB and into the glomerular ultrafiltrate (28, 39). Challenging this view, more recent studies have measured the passage of nephrotic levels of albumin through the GFB (32, 34). The flux of albumin across the podocyte layer could arise by either the paracellular movement of albumin through the SD or the transcellular migration of albumin through the podocytes. In support of albumin being transcytosed through podocytes, podocytes in culture endocytose albumin (13, 14) and, in human and animals models under albuminuric conditions, albumin is observed within the cytoplasm of native podocytes (13, 41, 42). In addition, a study by Kinugasa et al. (19) using Evans blue-labeled albumin demonstrated increased endocytosis of albumin by podocytes in vivo in a rat model of minimal change disease and albuminuria was decreased after treatment of proteinuric animals with an antibody that blocks transcytosis. Several cell types are capable of endocytosing albumin but do so by distinct mechanisms. For example, renal proximal cells and alveolar type II cells endocytose albumin via clathrin-mediated endocytosis (7, 25, 47) while cultured astrocytes, endothelial cells, and hepatocytes utilize caveolin-mediated endocytosis to bring albumin into the cell interior (4, 6, 9). Once within the cells, the fate of albumin can vary. In most cell types, albumin is trafficked to lysosomes where it is degraded (16). In cells that express the neonatal Fc receptor (FcRn), FcRn is capable of intercepting albumin from the degradation pathway and trafficking albumin to the plasma membrane for transcytosis (20).
The present study utilized cultured human urine-derived podocyte-like epithelial cells (HUPEC) to examine albumin trafficking pathways in podocytes. HUPEC cells express podocyte markers (33) and respond to albumin in a similar fashion as podocytes derived from human biopsy specimens (26). Findings from these studies provide direct insight into the polarity of albumin uptake, the endocytic pathway responsible for albumin uptake, and the capability of podocytes to perform albumin transcytosis. The findings advance the understanding of podocyte handling of albumin under physiological conditions and may contribute to the discovery of pathogenic mechanisms that underlie chronic kidney diseases that are associated with albuminuria.
MATERIALS AND METHODS
Immunofluorescence imaging of rat renal podocytes.
All procedures involving animals were performed using protocols approved by the Institutional Animal Care and Use Committee at the University of Colorado, Denver. Male Sprague-Dawley rats (200–250 g) were obtained from Harlan Laboratories (Madison, WI). Animals were used to immunoblot or immunolocalize specific proteins within renal tissues, as previously described (12). For the immunolocalization studies, the kidneys were cleared of blood by cannulating the descending aorta and retrograde perfusion of phosphate-buffered saline (PBS) and then fixed by perfusion with 4% paraformaldehyde (Electron Microscopy Sciences; Hatfield, PA) in PBS (pH 7.4). The kidneys were then removed, immersed in 4% paraformaldehyde for 60 min, cut into 3–4 mm cubes, infused with 5% (2 h), 10% (2 h) and 25% (overnight) sucrose, frozen in liquid nitrogen, and cryosectioned (5–8 μm). Kidney sections were blocked [10% normal goat serum and 1% bovine serum albumin (BSA) in PBS] and incubated overnight at 4°C with primary antibody synaptopodin (undiluted, clone G1D4, 10R-S125A, Fitzgerald Laboratories), clathrin (1:100; 23/clathrin heavy chain, Becton-Dickinson; Franklin Lakes, NJ) caveolin-1 (1:100, Abcam, ab2910, Cambridge, MA), and podocin (1:100; PO372, Sigma, St. Louis, MO). After washing, the sections were incubated (30 min, room temperature) with appropriate mix of Alexa 488-conjugated goat anti-rabbit IgG (1:500; Invitrogen, Carlsbad, CA), Alexa 633-conjugated goat anti-mouse IgG (1:500; Invitrogen), and Alexa 546-conjugated phalloidin (1:100; Invitrogen). Sections were then washed with PBS and mounted in Mowiol 4–88 (Calbiochem, Gibbstown, NJ) containing 2.5% 1,4-diazabicyclo[2.2.2]octane (Sigma). Fluorescence images were obtained using Zeiss Axiovert 200M laser-scanning confocal/multiphoton-excitation fluorescence microscope with a Meta spectral detection system (Zeiss NLO 510 with META, Zeiss, Thornwood, NY). The imaging settings were initially defined empirically to maximize the signal-to-noise ratio and to avoid saturation. In comparative imaging, the settings were kept constant between samples. Series of confocal fluorescence images were simultaneously obtained with a Zeiss C-Apochromat 40x/1.2NA water-immersion lens objective. The illumination for imaging is provided by a 30-mW argon laser, HeNe 5 mW (633 nm) and 1 mW (543 nm). Image processing was performed using Zeiss ZEN 2008 software. Figures were mounted using Adobe Photoshop CS4 (Adobe System).
Cell culture.
Human urine derived podocyte-like epithelial cells (HUPEC) carrying genes encoding a temperature-sensitive variant of the simian virus (SV40) large tumor antigen and human telomerase were cultured and maintained, as previously described (33). Briefly, podocytes were grown on type I collagen-coated flasks (Fisher Scientific, Rochester, NY) at 33°C (growth permissive conditions) in growth media [RPMI 1640 medium (Sigma, St. Louis, MO), 10% fetal bovine serum (Hyclone, Logan, UT), 15 mM HEPES, 5 mM l-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin; 5% CO2 atmosphere]. After reaching confluency, cells were seeded onto 35 mm type I collagen-coated dishes and maintained at 37°C (growth restrictive conditions) for 7–12 days to allow for differentiation. All cells were used between passages 17 and 25.
FITC-albumin uptake/degradation/transcytosis.
FITC-labeled albumin was used to monitor and measure the uptake, degradation, and transcytosis of albumin by cultured podocytes. The fate of FITC-albumin was followed both biochemically and by immunofluorescence. Two hours prior to the experimental procedures, the media was changed to Ringer solution (122.5 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 0.8 mM MgCl2, 0.8 mM Na2HPO4, 0.2 mM NaH2PO4, 5.5 mM glucose, and 10 mM HEPES; pH 7.4).
For albumin uptake experiments, podocytes were incubated with 1.5 mg/ml human FITC-albumin (MP Biomedicals, Santa Ana, CA) in Ringer solution at either 4°C or 37°C for 0, 60, and 120 min. For the 4°C condition, cells were kept at 4°C while loading and then for the duration of the experiment. After the specified times, cells were washed 6 times with ice-cold PBS. For spectrofluorometric measurements, podocytes were lysed in 20 mM MOPS with 0.1% Triton X-100. FITC-fluorescence was measured using an excitation wavelength of 490 nm and an emission wavelength of 540 nm. The amount of protein in the lysates was measured using the bicinchoninic acid (BCA) assay (Pierce, Rockford, IL) and the amount of cell associated FITC-albumin at each time point was normalized to the protein content. For Western blot analyses, podocytes were lysed in 50 μl of 5x PAGE [50 mM Tris-base, 5% sodium dodecyl sulfate, 25% sucrose, 5 mM EDTA; pH 8.0; complete protease inhibitors (Roche; Indianapolis, IN)].
For degradation/transcytosis experiments, podocytes were incubated with 1.5 mg/ml human FITC-albumin solution for 90 min at 4°C or 37°C. Further, each group was run either with or without 20 μM leupeptin, an inhibitor of lysosomal protein degradation. Leupeptin was added 15 min prior to loading and was present throughout the experiment. After FITC-albumin loading, podocytes were washed 6 times with ice-cold PBS, and Ringer solution was added for 0, 30 min and 60 min. After the specified times, the Ringer solution from each dish was collected, completely lyophilized, and reconstituted in 50 μl of 2.5x PAGE. The abundance of FITC-albumin released into the media and remaining in the podocytes was measured by Western blotting.
Western blotting.
For podocytes lysed in a 5x PAGE buffer, protein concentrations were measured by BCA assay (Pierce; Rockford, IL), and samples were reduced (10% β-mercaptoethanol). Cell lysates were run on 9% polyacrylamide gels and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). Subsequent blocking, antibody, and wash solutions were diluted in blot buffer (150 mM NaCl, 10 mM Na2HPO4, 5 mM EDTA, 1% Triton X-100; pH 7.4). Membranes were initially blocked (5% nonfat dry milk; 60 min) and then incubated with primary antibody. Primary antibodies include FITC (1:1,000; clone ZF2471–1900, Invitrogen; Carlsbad, CA), synaptopodin (1:1,000; H-140, Santa Cruz Biotech; Santa Cruz, CA), GAPDH (1:1,000; FL-335, Santa Cruz Biotech), actin (1:5,000; JLA20, Chemicon; Temecula, CA), FcRn (1:100; H-247, Santa Cruz Biotech), EEA1 (1:1,000; 14/EEA1, Becton-Dickinson; Franklin Lakes, NJ), LAMP1 (1:1,000; clone LY1C6, Enzo, Farmingdale, NY), caveolin-1 (1:1,000; ab2910, Abcam, Cambridge, MA), and clathrin (1:1,000; 23/clathrin heavy chain, Becton-Dickinson). Blots were then washed, incubated with horseradish peroxidase-conjugated secondary antibodies (1:10,000 dilution; Jackson ImmunoResearch, West Grove, PA), and washed. The antibody complexes were detected using enhanced chemiluminescence (Pierce; Rockford, IL) and captured using a photodocumentation system (UVP; Upland, CA).
Immunofluorescence.
For confocal microscopic imaging of live cells, podocytes were seeded onto glass-bottom collagen-coated MatTek dishes (MatTek, Ashland, MA). Images were acquired on Zeiss LSM 510 confocal microscope (Zeiss NLO 510 with META; Zeiss Plan-Apochromat 63/1.4NA oil; Thornwood, NY) that was equipped with a temperature-controlled incubation chamber (Solent Scientific) as described above.
For fixed-cell images, podocytes were incubated in 4% paraformaldehyde in phosphate-buffered saline (PBS) with 0.5% Triton X-100 (20 min; room temperature), washed, blocked with 10% normal serum, and labeled with primary antibodies. Primary antibodies include synaptopodin (undiluted; clone G1D4, 10R-S125A, Fitzgerald Laboratories), FcRn (1:100; H-247, Santa Cruz Biotech), EEA1 (1:100; 14/EEA1, Becton-Dickinson), LAMP1 (1:100; LY1C6, Enzo, Farmingdale, NY), clathrin (1:100; 23/clathrin heavy chain, Becton-Dickinson), and caveolin-1 (1:100; ab2910, Abcam, Cambridge, MA). Cells were subsequently washed and labeled with the appropriate conjugated secondary antibodies (Alexa Fluor 488, Alexa Fluor 546, Alexa Fluor 633; Invitrogen). F-actin was concurrently stained with Alexa-Phalloidin 633 or Alexa-Phalloidin 546 (Invitrogen).
The degree of colocalization of FITC-albumin with different markers within the cells was quantified by calculating their intensity correlation quotient (ICQ) values (10, 22). Intensity_Correlation_Analysis macros (www.macbiophotonics.ca) for this analysis were applied to ImageJ (National Institute of Mental Health, Bethesda, MD). Values for the product of the differences from the mean (PDM) quantify the degree of pixel-by-pixel synchrony of the fluorophore intensities in the two channels [PDM = (Ri - Rave) × (Gi - Gave); Ri = individual pixel intensity-red, Rave = average pixel intensity-red, Gi = individual pixel intensity-green, Gave = average pixel intensity-green]. The intensity correlation quotient (ICQ) ratio is equal to the ratio of the number of positive PDM values to the total number of pixel values [ICQ = (N+ve/Ntotal); N+ve = number of pixels with a positive PDM value, Ntotal = total number of pixels with a non-zero value in each channel]. The 11 focal planes of 10 individual podocytes for each marker after 0 and 1 h of FITC-albumin uptake were analyzed (ImageJ; NIH).
Total internal reflectance fluorescence microscopy.
Total internal reflectance (TIRF) microscopy was used for live-cell imaging of FITC-albumin internalization along the basal membrane of podocytes. Cells were initially incubated with FITC-albumin for 5 min at 37°C. Unbound extracellular FITC-albumin was then removed by washing with ice-cold PBS. Labeled cells were then placed in RPMI media without phenol red and imaged on a Zeiss TIRF microscope equipped with a temperature and CO2 incubation chamber. Cells were kept at 37°C and 5% CO2 throughout the experiment. Images were acquired with a 100 × 1.45 NA objective under the control of AxioVision V4.5 software (Carl Zeiss, Thornwood, NJ). Laser excitation of FITC-albumin was derived from a multiline argon ion laser. Excitation and emission wavelengths were selected using filter set for FITC (488 nm). Images were acquired every second for a total of 5 min.
Inhibition of clathrin- and caveolin-mediated endocytosis.
The relative contribution of clathrin-mediated and caveolin-mediated endocytosis was evaluated using biochemical inhibitors. Clathrin-mediated endocytosis was inhibited by treating cells with 30 μM Pitstop2 (Abcam, Cambridge, MA; Ref. 44). Caveolin-mediated endocytosis was inhibited by treating cells with 20 μg/ml nystatin (Calbiochem, Gibbstown, NJ; Ref. 29). For both inhibitors, podocytes were pretreated for 15 min with inhibitor, and then 1.5 mg/ml of human FITC-albumin in Ringer was added as described above. The presence of inhibitors was maintained throughout the experiment.
Statistical analysis.
All data are presented as means ± SE. Statistical analysis was performed using t-tests for pairs of data and one-way analysis of variance for data groups of three or more using Prism-4 GraphPad software. Tukey's post hoc test was applied to the ANOVA data. Values were considered statistically significant when P < 0.05.
RESULTS
HUPEC cells express podocyte-specific marker proteins.
Podocytes in kidney glomeruli express a subset of podocyte-specific proteins that can be used to identify cells that have differentiated into podocytes. HUPECs have previously been shown to express podocyte-specific genes (33). To confirm that the HUPEC cells used in this study express podocyte-specific proteins, these cells were evaluated by Western blotting and immunofluorescence imaging for synaptopodin expression. Western blot analysis shows the presence of synaptopodin in rat renal cortex (Fig. 1A) while immunofluorescence staining of rat renal cortex shows that synaptopodin antibody interacts with proteins within the glomerular tufts (Fig. 1B). Other renal cell types within the same section, such as proximal tubule cells, do not show any specific expression of synaptopodin. Western blot analysis of HUPEC lysates shows the presence of a specific synaptopodin band with a similar migration pattern as rat renal synaptopodin (Fig. 1A). Further, HUPEC cells, hereafter referred to as podocytes, were readily immunolabeled by synaptopodin antibodies. At low magnification using epifluorescence, synaptopodin was observed broadly distributed throughout the cultured podocytes (Fig. 1C). At higher magnification with confocal imaging, costaining podocytes with synaptopodin and F-actin staining found that, similar to previous podocyte studies (24), much of the synaptopodin on the outer periphery of the cells colocalized with actin filaments.
Fig. 1.
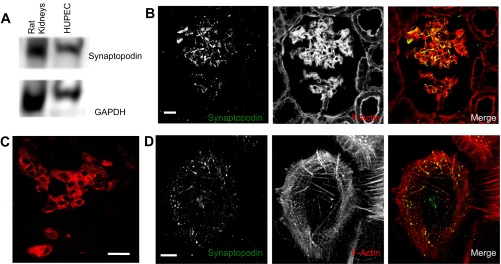
Cultured human urine-derived podocyte-like epithelial cells (HUPEC) cells express synaptopodin. A: Western blotting of rat renal cortex proteins shows the migration position of synaptopodin. Western blotting of cultured HUPEC cells shows these cells express synaptopodin of a similar size. GAPDH is shown as a loading control. B: combined 3 confocal planes of the immunofluorescence image of rat renal cortex sections shows synaptopodin expression is restricted to cells within the glomerular tufts. F-actin staining is used to highlight the cellular and tubular structures within the renal cortex. In the Merge image, synaptopodin (green) colocalizes (yellow) with F-actin (red) along the glomerular tufts. Bar = 10 μm. C: epifluorescent imaging of cultured HUPEC cells shows synaptopodin is broadly expressed and distributed. Bar = 20 μm. D: confocal immunofluorescence imaging of cultured HUPEC cells shows synaptopodin is broadly expressed and distributed. Merged images of synaptopodin (green) and F-actin (red) find much of the peripherally distributed synaptopodin colocalizes with F-actin structures. Bar = 10 μm.
Time course of FITC-albumin uptake by cultured podocytes.
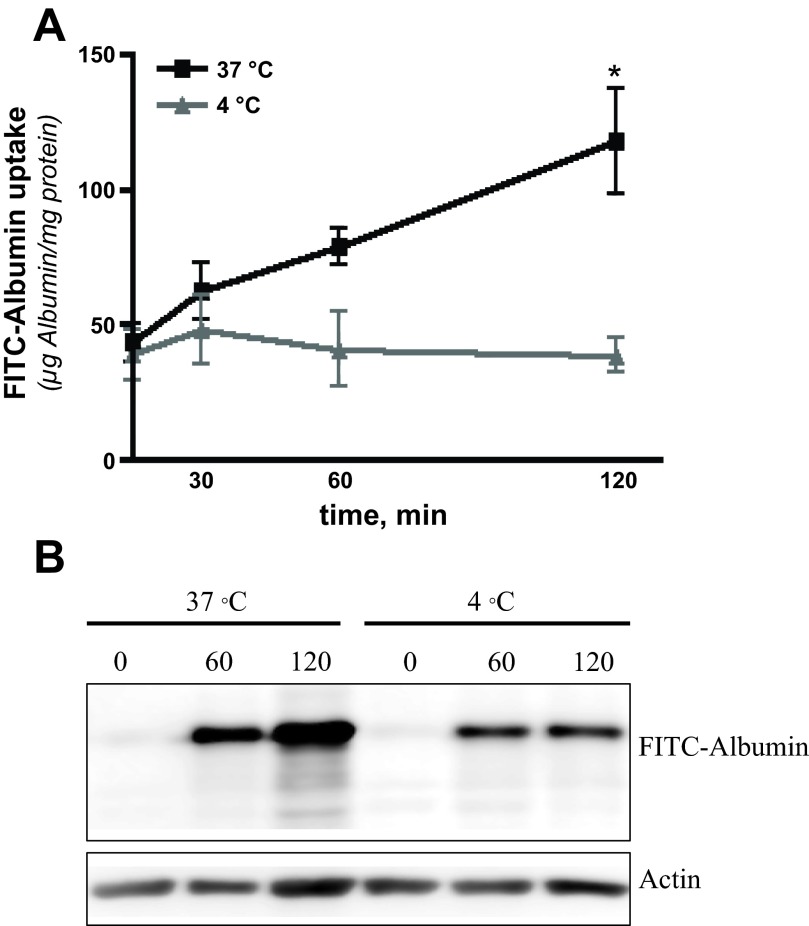
The presence and time course of FITC-albumin uptake by podocytes were evaluated by comparing the amount of cell-associated FITC-albumin when cells were incubated at 37°C (binding and internalization) vs. at 4°C (binding only). Podocytes were incubated with FITC-albumin for 0, 30, 60, or 120 min. Spectrofluorometric analysis of cell lysates (Fig. 2A; n = 4) shows that FITC-albumin was taken up by podocytes in a temperature-sensitive manner. At 4°C, FITC-albumin signal at 0 min (FITC-albumin added and washed off in <1 min) was above background levels (i.e., cell surface binding) but was unchanged after 120 min of additional incubation (i.e., minimal internalization). Podocytes incubated for 0 min at 37°C had a similar amount of FITC-albumin bound as the cells at 4°C. After 60 min of incubation at 37°C, however, the FITC-albumin signal was trending upward and was significantly increased after 120 min of incubation. Similar studies using Western blot analysis had a similar pattern of FITC-albumin uptake (Fig. 2B; n = 5). Podocytes without exposure to FITC-albumin (0 min) showed no signal at either 4°C or 37°C. The FITC-albumin signal in cells incubated at 4°C was similar at the 60 min and 120 min time points while the signal in cells incubated at 37°C showed a progressive increase in these levels over both time points.
Fig. 2.
Cultured podocytes endocytose FITC-albumin. A: spectrophotometric analysis of the binding/uptake of FITC-albumin by podocytes at 4°C showed a significant level of cell-associated FITC-albumin after <1 min of incubation (39.1 ± 9.4 μg FITC-albumin/mg protein) but no increase over the following 120 min. In podocytes at 37°C, the cell-associated FITC-albumin was trending upward after 60 min of incubation (78.8 ± 6.4 μg FITC-albumin/mg protein) and significantly increased after 120 min of incubation (117.9 ± 19.3 μg FITC-albumin/mg protein). n = 5. *P < 0.05 compared with t = 0. B: similarly, Western blot analysis of FITC-albumin binding/uptake by podocytes at 37°C shows a marked increase in FITC-albumin over 2 h of incubation. Podocytes at 4°C again showed initial binding but no change in albumin uptake between the 60 and 120 min time points. The faint laddering under the albumin band at 120 min at 37°C is likely due to albumin degradation within the cell.
Polarity of albumin uptake: basal membrane endocytosis.
Fluorescence imaging of podocytes indicated there was a marked polarity in the uptake of FITC-albumin. Despite a predicted hindrance in accessing the basal membranes, FITC-albumin was preferentially endocytosed along the basal membrane. As shown in fixed-cell confocal images after incubating podocytes with FITC-albumin for 10 min (Fig. 3A), FITC-albumin was abundant and readily observed in optical sections within 2 μm of the basal membrane. In contrast, in optical sections over 8 μm above the basal membrane, comparatively little FITC-albumin was observed. Live-cell TIRF microscopy was able to observe the dynamics of FITC-albumin-laden endosomes as they emerged and trafficked within 100 nm of the basal membranes. Within minutes of adding FITC-albumin to the media, concentrated puncta consistent with FITC-albumin-laden endosomes (Fig. 3B; arrows) emerged from the basal membrane.
Fig. 3.
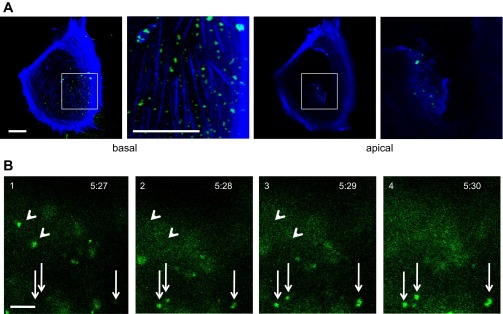
Cultured podocytes preferentially endocytose FITC-albumin at the basal membrane. A: after 10 min of incubation with FITC-albumin, live-cell confocal imaging of the basal domain (<2 μm above the coverslip) of podocytes showed an abundance of FITC-albumin-laden endosomes (green). Concurrent imaging of the apical domain (>5 μm above the coverslip) showed far fewer FITC-albumin-laden endosomes. The second and fourth panels (bar = 10 μm) are magnified images of the white boxes in the first and third panels (bar = 5 μm). B: live-cell total internal reflectance (TIRF) imaging captures the emergence of three FITC-albumin-laden endosomes (arrows) within 100 nm of the basal membrane. Other FITC-albumin-laden endosomes disappeared from the image plane during the same time span (arrowheads). Podocytes were incubated with 1.5 mg/ml FITC-albumin for 5 min and then washed. Time shown as minutes:seconds following addition of albumin. Scale bar = 2 μm.
FITC-albumin undergoes caveolin-mediated uptake in podocytes.
The pathway for albumin endocytosis is cell type dependent and includes either clathrin- or caveolin-mediated endocytosis (4, 6, 11, 38, 46). Confirming previous observations, confocal immunofluorescence imaging of glomeruli in rat kidneys showed both clathrin and caveolin-1 are expressed in glomerular tufts (Fig. 4, A and B). Western blotting of podocyte lysates similarly shows both clathrin and caveolin-1 are expressed in these cultured podocytes (Fig. 5A). Immunofluorescence imaging of the cultured podocytes found a small percentage of FITC-albumin endosomes colocalized with caveolin-1 (Fig. 5B). Little or no colocalization of FITC-albumin was observed with clathrin (Fig. 5C). This low percentage of colocalization could be due either to FITC-albumin not being associated with that pathway or to the transient nature of clathrin or caveolin-1 association with albumin-containing endosomes following endosome formation. Consequently, a biochemical approach was applied to investigate the relative contributions of clathrin- and caveolin-mediated endocytosis in the internalization of FITC-albumin by podocytes.
Fig. 4.
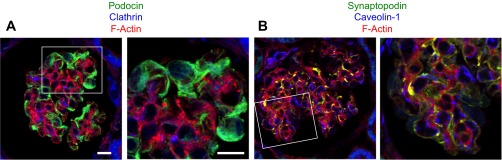
Podocytes in vivo express clathrin and caveolin-1. A: immunostaining of clathrin (blue) in rat renal glomeruli shows specific staining in podocytes. Podocin staining (green) highlights podocytes, and phalloidin staining of F-actin (red) highlights the structure of the glomerular tufts. Bar = 10 μm. The area in the white box is magnified and shown in the second panel. Bar = 5 μm. B: immunostaining of caveolin-1 (blue) in rat renal glomeruli shows specific staining in podocytes. Synaptopodin staining (green) highlights podocytes and phalloidin staining of F-actin (red) highlights the structure of the glomerular tufts. The area in the white box is magnified and shown in the second panel. Same magnification.
Fig. 5.
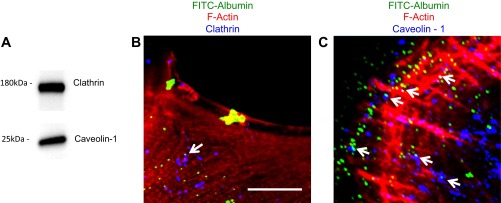
Clathrin and caveolin-1 are expressed in cultured podocytes. A: Western blot analysis shows both clathrin and caveolin-1 are expressed in cultured podocytes. B: in podocytes fixed after 10 min of incubation in FITC-albumin (green), immunostaining of clathrin (blue) shows little or no colocalization of the two proteins at the cell periphery. Phalloidin staining of F-actin (red) highlights the cell perimeter. Bar = 5 μm. C: in podocytes fixed after 10 min of incubation in FITC-albumin (green), immunostaining of caveolin-1 (blue) shows foci of colocalization of the two proteins at the cell periphery (arrows). Phalloidin staining of F-actin (red) highlights the cell perimeter. Same magnification.
To evaluate clathrin-mediated and caveolae-mediated endocytosis of FITC-albumin, podocytes were treated with Pitstop2 or nystatin, respectively. In both cases, cells were pretreated with inhibitors for 15 min, incubated with FITC-albumin for 60 min, and evaluated for uptake using confocal fluorescence microscopy (Fig. 6A). Podocytes incubated at 4°C were used to establish the background fluorescence level. Podocytes incubated at 37°C without inhibitors showed a significant level of FITC-albumin uptake. Podocytes treated with Pitstop2 continued to endocytose FITC-albumin and display the clear presence of FITC-albumin-laden endosomes. Importantly, podocytes treated with nystatin showed a marked decrease in the amount of FITC-albumin endocytosis and generally had FITC-albumin labeling similar to control podocytes at 4°C. To quantify the effects of Pitstop2 and nystatin, FITC-albumin uptake levels by podocytes were evaluated by Western blot analysis (Fig. 6, B and C). Densitometric analysis showed that incubation at 37°C resulted in a significant increase in the accumulation of FITC-albumin when compared with podocytes incubated at 4°C. Inhibition of clathrin-mediated endocytosis with Pitstop2 had variable results with the mean value not being significantly different from mean values of either the 4°C or 37°C control podocytes. Inhibition of caveolae-mediated endocytosis with nystatin, however, had a clear effect with the mean value being significantly different from mean values of 37°C control podocytes. These findings indicate that while clathrin-mediated endocytosis may contribute, caveolae-mediated endocytosis plays the primary role in the internalization of albumin by podocytes.
Fig. 6.
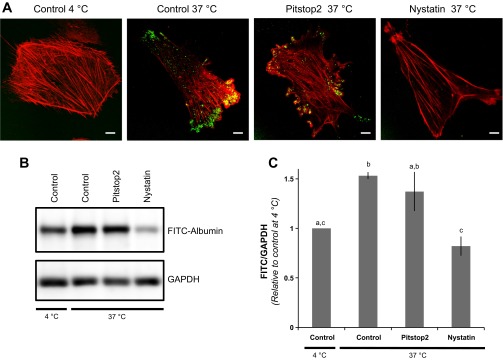
FITC-albumin endocytosis by cultured podocytes is caveolae-dependent. A: when incubated with FITC-albumin (green) for 60 min at 4°C, little or no FITC-albumin is observed within the interior of control podocytes. Incubation for 60 min at 37°C results in a marked level of FITC-albumin associated with the control podocytes. FITC-albumin also associated with podocytes when incubated at 37°C in the presence of Pitstop2, an inhibitor of clathrin-mediated endocytosis. In contrast, podocytes coincubated with nystatin, which inhibits caveolin-dependent endocytosis, had little or no observed endocytosis of FITC-albumin. Bar = 10 μm. F-actin staining is shown in red. B: Western blot analysis shows the comparative FITC-albumin levels in podocytes incubated with FITC-albumin for 60 min. The coincubation of Pitstop2 had no observed effect while nystatin treatment markedly inhibited FITC-albumin uptake. GAPDH served as a loading control. C: densitometric analysis of the Western blots shows control podocytes at 37°C had 53 ± 4% more FITC-albumin than control cells at 4°C. The FITC-albumin uptake in cells coincubated with Pitstop2 (137 ± 19% of control cells at 4°C) was not significantly different from either the 4°C or the 37°C control cells. The coincubation with nystatin resulted in a significant decrease in FITC-albumin uptake (82 ± 9% of control cells at 4°C) compared with control cells at 37°C. n = 6. Bars without a letter in common are significantly different from each other (P < 0.05).
FITC-albumin traffics to early endosomes and lysosomes.
Having established that FITC-albumin preferentially enters podocytes at the basal membrane via caveolae-mediated endocytosis, the intracellular fate of FITC-albumin was investigated. Specifically, it was determined whether FITC-albumin trafficked through the early endosome to the lysosomal compartment and whether FITC-albumin associated with the neonatal Fc receptor (FcRn). In numerous cell types, albumin is generally trafficked to the lysosome and degraded. In specific cell types, FcRn intercepts and redirects albumin for transcytosis and release (2, 5, 8, 18). Western blotting shows human podocytes express EEA1 (an early endosome marker), LAMP1 (a lysosomal marker), and FcRn (Fig. 7A). To determine the intracellular trafficking patterns of albumin in podocytes, podocytes were loaded with FITC-albumin for 10 min, fixed, and imaged. Confocal imaging showed that a portion of the FITC-albumin-containing endosomes colocalized with EEA1 (i.e., early endosomes; Fig. 7B), LAMP1 (i.e., lysosomes; Fig. 7C), and FcRn (i.e., transcytotic endosome; Fig. 7D).
Fig. 7.
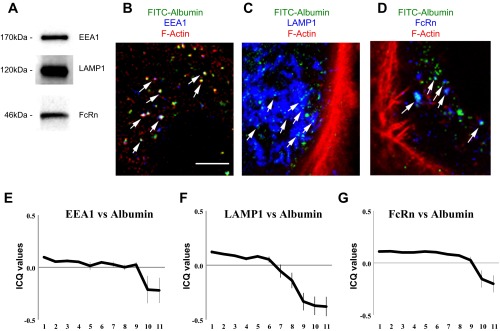
Distribution of FITC-albumin in distinct endosomal compartments. A: Western blotting shows HUPEC podocytes express EEA1, LAMP1, and FcRn. After 10 min uptake, FITC-albumin (green) distributes with markers for early endosomes (EEA1; blue) (B), lysosomes (LAMP1; blue) (C), and the neonatal Fc receptor (FcRn; blue) (D) in the cell interior. F-actin (red) was used as a counterstain for the cells. Bar = 5 μm. Intensity correlation quotient (ICQ) analysis of FITC-albumin vs. the three marker proteins [EEA1 (E), LAMP1 (F), FcRn (G)] was evaluated after 10 min of loading at 1-μm steps along the vertical axis of each podocyte. The modest ICQ values for FITC-albumin vs. each of the three endosomal markers is consistent with only a fraction of the total FITC-albumin being associated with each distinct compartment at any one time. Consistent with the preferential entry of FITC-albumin along the basal membrane, the highest degree of intensity correlation for each marker protein was in the basal sections and diminished in the apical reaches of the podocytes.
ICQ analysis of FITC-albumin vs. the three marker proteins was evaluated after 10 min of loading at 1-μm steps along the vertical axis of each podocyte (Fig. 7, E–G). The modest ICQ values for FITC-albumin vs. each of the three endosomal markers is consistent with only a fraction of the total FITC-albumin being associated with each distinct compartment at any one time. Consistent with the preferential entry of FITC-albumin along the basal membrane, the highest degree of intensity correlation (i.e., colocalization) for each endosomal marker protein was in the basal sections and diminished in the apical reaches of the podocytes. For both EEA1 and FcRn, the fluorescence intensity correlations with FITC-albumin were consistent through the first 9 μm above the basal membrane before falling off. For LAMP1, the fluorescence intensity correlations with FITC-albumin fell off in regions 6 μm above the basal membrane. This may reflect a delay in FITC-albumin progressing through the earlier endosomal compartments and trafficking into the lysosomal compartments within the higher reaches of the podocytes.
Degradation and transcytosis of FITC-albumin by podocytes.
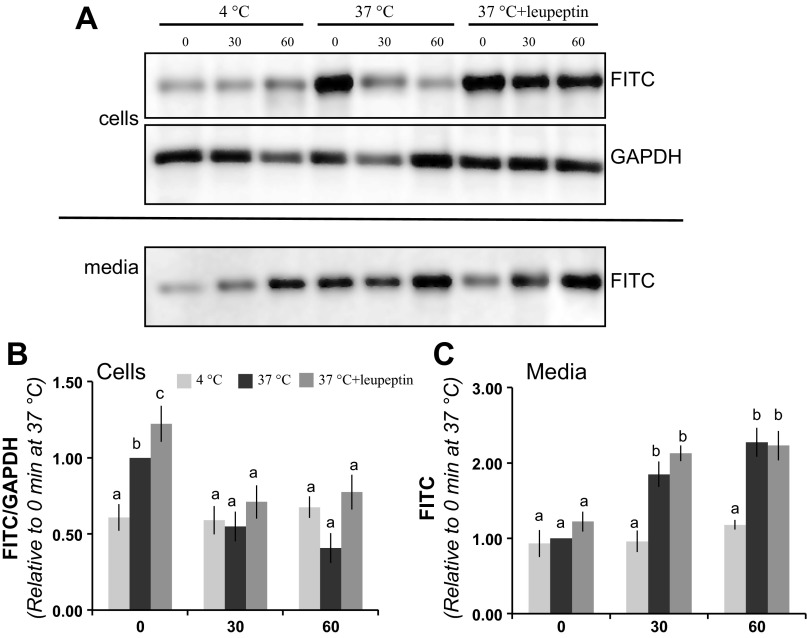
Having observed FITC-albumin colocalizing with putative degradation and transcytotic markers, the fate of the internalized FITC-albumin was investigated. Podocytes were initially incubated and loaded with FITC-albumin at 37°C or 4°C for 90 min. Extracellular FITC-albumin was then washed away and the cells incubated in unlabeled media. Cell lysates and media were collected at 0 min, 30 min, and 60 min and analyzed by Western blotting. Consistent with surface binding and no internalization, podocytes maintained at 4°C showed a modest amount of associated FITC-albumin following the loading period (Fig. 8, A and B). This level remained unchanged over the experimental period. Podocytes loaded at 37°C had significantly more FITC-albumin over the initial incubation period (Fig. 8, A and B). After both 30 and 60 min of postloading incubation, these podocytes showed a significant decrease in the amount of FITC-albumin associated with the podocytes.
Fig. 8.
Lysosomal degradation and transcytosis of FITC-albumin by cultured podocytes. To evaluate the capacities of podocytes to degrade and transcytose FITC-albumin, cells were preincubated (i.e., loaded) with FITC-albumin for 60 min and then the cells and media were harvested at specific time points. A: Western blot analysis of FITC-albumin from podocytes kept at 4°C showed modest loading and minimal change over the subsequent 60 min of incubation. Podocytes at 37°C had robust loading and progressive loss of FITC-albumin after 30 min and 60 min of incubation. Podocytes treated with leupeptin also had robust cell loading but had a reduced rate of loss in the 60 min after loading. Analysis of FITC-albumin in the media shows that cells incubated at 37°C had a progressive release of FITC-albumin in either the absence or presence of leupeptin. B: densitometric analysis showed leupeptin treatment increased the amount of FITC-albumin loading and partially preserved FITC-albumin levels after 60 min of subsequent incubation. Bars without a letter in common are significantly different from each other (P < 0.05, n = 9 experiments). C: analysis of FITC-albumin levels in the postloading media shows a significant amount of release into the media when podocytes are incubated at 37°C. Leupeptin treatment did not significantly impact the level of this release. Bars without a letter in common are significantly different from each other (P < 0.05, n = 9 experiments).
The decrease in FITC-albumin observed following washout could occur either from cellular degradation of FITC-albumin or from transcytosis (i.e., release) of FITC-albumin. To evaluate the contribution of lysosomal degradation, a paired set of podocytes was incubated in the presence of 20 μM leupeptin. Significantly more albumin was found in the cellular fraction of podocytes loaded at 37°C in the presence of leupeptin compared with those loaded at 37°C without leupeptin (Fig. 8, A and B), suggesting that a portion of albumin endocytosed by podocytes is degraded in the lysosome. Sixty minutes after loading, there was a trend toward increased albumin accumulation (which was not statistically significant) in podocytes treated with leupeptin compared with podocytes incubated at 37°C without leupeptin. These data are consistent with either partial inhibition of degradation by leupeptin or removal of albumin from the cell via an additional pathway such as transcytosis. In support of the presence of transcytosis, there was a significant accumulation of full-length FITC-albumin in the media after 30 and 60 min of incubation (Fig. 8, A and C). This indicates that after having taken up FITC-albumin, a portion of the FITC-albumin traffics to the plasma membrane and is released from the podocytes. The levels of FITC-albumin released by podocytes into the medium were not impacted by leupeptin treatment.
DISCUSSION
The long-standing model of the GFB had only a very small percentage of plasma proteins such as albumin traversing the GFB and entering the urinary space. Some tenets of the traditional model have recently been challenged with in vivo multiphoton imaging studies showing nephrotic levels of albumin traversing the GFB in normal animals (32, 34). Albumin has also been found within the podocyte cell body in human and rodent proteinuric kidney disease (19, 41, 43). In vivo two-photon imaging of rat renal glomeruli determined the glomerular sieving coefficient for albumin (GSCA) varies from ∼0.016 to ∼0.034 (32, 34) which is much higher than that obtained by traditional micropuncture techniques, which gave a GSC of 0.00062 in rats (40). Depending on the value used to calculate the GSC (0.00062 to 0.034), the amount of albumin filtered through the glomerulus could range from 3 g to 200 g per day.
While several theories have been proposed to explain why the GFB does not clog with serum proteins such as albumin and IgG (36), podocyte uptake and disposal of serum proteins provides a biologically plausible mechanism for maintaining GFB homeostasis. Evidence for the importance of the podocyte in preventing GFB clogging comes from the following: In animals with heavy proteinuria, albumin-filled vesicles are found within the podocyte cell body (19, 43); IgG appears to collect in the GFB in mice that lack FcRn (1) and podocytes endocytose albumin in vitro (14, 26).
The mechanism by which albumin traverses the podocyte epithelial layer remains to be defined. Although the slit diaphragms between interdigitated podocytes were generally considered to resist the vast majority of the paracellular passage of albumin, scanning electron microscopy indicates that the SD may have pores large enough to allow the diffusion of proteins with the diameter of albumin (15, 31). Alternatively, albumin may cross the epithelium via transcellular transport. Several cell types, including proximal tubule cells (48), endothelial cells (38), and astrocytes (4), are capable of endocytosing albumin by either clathrin-mediated or caveolae-mediated endocytosis. While most cells can traffic albumin to the lysosomal compartment for degradation, proximal tubule cells divert much of the albumin away from lysosomes, traffic albumin to the basal membrane, and release albumin into the serosa. Podocytes are also capable of endocytosing albumin (14, 26). The present study utilized immortalized podocytes that were isolated from the urine of a normal subject to explore and define the polarity, endocytic pathways, and fate of the albumin in human podocytes.
Podocytic uptake of albumin occurred along the basal membrane.
The polarity of albumin uptake has significant implications for our understanding the purpose of the uptake. Preferential uptake at the apical membrane, which faces the urinary space, would suggest that the function of albumin uptake by podocytes mirrors that by proximal tubules and serves to recover albumin that leaks across the GFB and enters the urinary space. Preferential uptake at the basal membrane, which abuts the glomerular basement membrane (GBM), would support the hypothesis that podocytes internalize albumin and other large proteins from the GBM-podocyte interface to reduce the accumulation of proteins along the slit diaphragms and maintain the filtration characteristics of the GFB (1). In the present albumin uptake studies, podocytes were adhered onto collagen-coated coverslips. During the FITC-albumin incubation period, the FITC-albumin was considered to have unfettered access to the apical membrane while diffusional access to the basal membrane was predicted to be hindered. Despite this potential limitation, both fixed-cell confocal microscopy and live-cell TIRF microscopy studies demonstrated preferential uptake of FITC-albumin at the basal membrane (Fig. 3). If representative of native podocytes, this finding is consistent with the hypothesis that podocytes take up albumin, the most abundant protein in serum, in an effort to maintain a clear filtration barrier. If one function of podocyte albumin uptake is to prevent protein clogging of the slit diaphragm, albumin endocytosis is predicted to be particularly enhanced at the extremity of the foot processes (i.e., at the foot process sole and the adjacent lateral aspects) as these regions lie in the space between the GBM and the slit diaphragm. One limitation of the present studies is that cultured podocytes do not manifest well-developed foot processes that would allow this localization to be studied in vitro.
Podocytic uptake of albumin occurred by caveolae-mediated endocytosis.
While a number of distinct cell types endocytose albumin, the pathway for cell entry varies between cell types. More specifically, renal proximal tubules and alveolar epithelial cells use clathrin-mediated endocytosis (7, 46, 48) while astrocytes, cultured hepatocytes and endothelial cells utilize caveolae-mediated endocytosis (4, 6, 38). The present study found marker proteins for both clathrin-coated pits and caveolae were expressed in podocytes (Fig. 5). While both clathrin and caveolin are expressed in podocytes, the present study found that inhibition of clathrin-mediated endocytosis had little or no effect on the level of albumin endocytosis whereas inhibition of caveolae-mediated endocytosis profoundly blunted albumin endocytosis in these cells (Fig. 6).
In other cell types, caveolae-dependent endocytosis is dynamically regulated. Endothelial cell response to oxidative stress includes phosphorylation of caveolin-1 and increased albumin uptake (38). Caveolin-1 is also thought to play a role in transducing stretch and shear stress information to signaling pathways. In vascular endothelial cells, caveolin-1 is required for flow-mediated dilation of the vessel walls (45). In podocytes, caveolin-1 is found at the slit diaphragm where it colocalizes with the slit diaphragm proteins nephrin and CD2AP (37), suggesting that caveolin-1 may be involved in either transducing or responding to alterations in glomerular hemodynamics. In addition, the effects of angiotensin II on podocytes are mediated at least in part by caveolin. Ren et al. (30) have found that angiotensin II upregulates phospho-caveolin 1 both in vivo and in vitro and that silencing caveolin-1 decreases angiotensin II-induced apoptosis in cultured podocytes. It will be of interest to determine if the extracellular albumin load influences glomerular dynamics and rates of albumin endocytosis.
Podocytes both degrade and transcytose albumin.
Studies in other cell type have found that both clathrin-mediated and caveolae-mediated endocytosis can deliver albumin to the lysosomal compartment (6, 27, 48). Colocalization and ICQ analyses of FITC-albumin and LAMP1 in podocytes showed that a portion of the albumin trafficked into the lysosomal compartments (Fig. 7), and inhibition of leupeptin-sensitive proteolysis found ∼20% of the internalized albumin underwent lysosomal degradation (Fig. 8).
In proximal tubule cells, FcRn can rescue albumin from the lysosomal degradation pathway and redirect the albumin for transcytotic release at the basal membrane (3, 35). FcRn also plays a pivotal role in the transcytosis of albumin in endothelial cells (3). In a related model of FcRn-dependent protein clearance, FcRn(−/−) mice have a marked accumulation of IgG within the GBM (1). Like albumin, IgG is a very abundant serum protein. As with other podocyte models (1, 17), HUPEC podocytes also express FcRn, and FcRn colocalizes with a subpopulation of albumin-laden endosomes (Fig. 7). While the present studies did not determine the dependence on FcRn, pulse-chase studies demonstrated that following endocytic loading of FITC-albumin into the cells, podocytes released a significant portion of the FITC-albumin back into the extracellular media (Fig. 8). Our data support the findings of Kinugasa et al. (19), who demonstrated that purine aminonucleoside-induced nephrotic syndrome in in vivo rats that albumin uptake by podocytes is increased. Further, administration of an antibody against FcRn decreased proteinuria in the PAN-treated animals providing additional evidence for a role for FcRn in albumin trafficking in podocytes (19). Taken together, the polarized uptake of albumin from the basal membrane along with the capacity of the podocytes to release full length FITC-albumin back into the extracellular space supports the hypothesis that podocytes actively engage in clearing large serum proteins that make it to the GBM-podocyte interface. These studies contribute new insights into the physiology of podocytes and the molecular and cellular mechanisms that allow for the exquisite control of glomerular filtration.
GRANTS
These studies were supported by NIH grants to R. B. Doctor (DK-080769) and J. Blaine (DK-080989). J. B. Kopp was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program (NIH). Imaging experiments were performed in the University of Colorado Anschutz Medical Campus Advance Light Microscopy Core supported in part by NIH/NCRR Colorado CTSI Grant UL1 RR-025780.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.D., R.B.D., and J.B. conception and design of research; E.D., K.O., R.B.D., and J.B. performed experiments; E.D. and K.O. analyzed data; E.D., K.O., J.B.K., R.B.D., and J.B. interpreted results of experiments; E.D. prepared figures; E.D., R.B.D., and J.B. drafted manuscript; E.D., K.O., J.B.K., R.B.D., and J.B. edited and revised manuscript; E.D., J.B.K., R.B.D., and J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
Contents are the authors' sole responsibility and do not necessarily represent official National Institutes of Health (NIH) views.
REFERENCES
- 1.Akilesh S, Huber TB, Wu H, Wang G, Hartleben Br Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci USA 105: 967–972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen JT, Sandlie I. The versatile MHC class I-related FcRn protects IgG and albumin from degradation: implications for development of new diagnostics and therapeutics. Drug Metab Pharmacokinet 24: 318–332, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Anderson CL, Chaudhury C, Kim J, Bronson CL, Wani MA, Mohanty S. Perspective. FcRn transports albumin: relevance to immunology and medicine. Trends Immunol 27: 343–348, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bento-Abreu A, Velasco A, Polo-Hernández E, Lillo C, Kozyraki R, Tabernero A, Medina JM. Albumin endocytosis via megalin in astrocytes is caveola- and Dab-1 dependent and is required for the synthesis of the neurotrophic factor oleic acid. J Neurochem 111: 49–60, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, Ghetie V, Ward ES. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol 10: 1289–1298, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Botos E, Klumperman J, Oorschot V, Ígyártó B, Magyar A, Oláh M, Kiss AL. Caveolin-1 is transported to multi-vesicular bodies after albumin-induced endocytosis of caveolae in HepG2 cells. J Cell Mol Med 12: 1632–1639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchäckert Y, Rummel S, Vohwinkel CU, Gabrielli NM, Grzesik BA, Mayer K, Herold S, Morty RE, Seeger W, Vadász I. Megalin mediates transepithelial albumin clearance from the alveolar space of intact rabbit lungs. J Physiol 590: 5167–5181, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med 197: 315–322, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Gaßner B, Börner S, Nikolaev VO, Schlegel N, Waschke J, Steinbronn N, Strasser R, Kuhn M. Atrial natriuretic peptide enhances microvascular albumin permeability by the caveolae-mediated transcellular pathway. Cardiovasc Res 93: 141–151, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrinskikh E, Giral H, Caldas Y, Levi M, Doctor RB. Shank2 redistributes with NaPiIIa during regulated endocytosis. Am J Physiol Cell Physiol 299: C1324–C1334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobrinskikh E, Giral H, Caldas YA, Levi M, Doctor RB. Shank2 redistributes with NaPilla during regulated endocytosis. Am J Physiol Cell Physiol 299: C1324–C1334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doctor RB, Bennett V, Mandel LJ. Degradation of spectrin and ankyrin in the ischemic rat kidney. Am J Physiol Cell Physiol 264: C1003–C1013, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Eddy AA, Kim H, Lopez-Guisa J, Oda T, Soloway PD, McCulloch L, Liu E, Wing D. Interstitial fibrosis in mice with overload proteinuria: deficiency of TIMP-1 is not protective. Kidney Int 58: 618–628, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Eyre J, Ioannou K, Grubb BD, Saleem MA, Mathieson PW, Brunskill NJ, Christensen EI, Topham PS. Statin-sensitive endocytosis of albumin by glomerular podocytes. Am J Physiol Renal Physiol 292: F674–F681, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Gagliardini E, Conti S, Benigni A, Remuzzi G, Remuzzi A. Imaging of the porous ultrastructure of the glomerular epithelial filtration slit. J Am Soc Nephrol 21: 2081–2089, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gekle M. Renal tubule albumin transport. Annu Rev Physiol 67: 573–594, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Haymann JP, Levraud JP, Bouet S, Kappes V, Hagège J, Nguyen G, Xu Y, Rondeau E, Sraer JD. Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol 11: 632–639, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Bronson CL, Hayton WL, Radmacher MD, Roopenian DC, Robinson JM, Anderson CL. Albumin turnover: FcRn-mediated recycling saves as much albumin from degradation as the liver produces. Am J Physiol Gastrointest Liver Physiol 290: G352–G360, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Kinugasa S, Tojo A, Sakai T, Tsumura H, Takahashi M, Hirata Y, Fujita T. Selective albuminuria via podocyte albumin transport in puromycin nephrotic rats is attenuated by an inhibitor of NADPH oxidase. Kidney Int 80: 1328–1338, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Kuo T, Baker K, Yoshida M, Qiao SW, Aveson V, Lencer W, Blumberg R. Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol 30: 777–789, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levidiotis V, Power DA. New insights into the molecular biology of the glomerular filtration barrier and associated disease. Nephrology 10: 157–166, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A syntaxin1, Ga and N-type Ca2+ channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci 24: 4070–4081, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miner JH. The glomerular basement membrane. Exp Cell Res 318: 973–978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mundel P, Heid HW, Mundel TM, Krüger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 139: 193–204, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagai J, Sato K, Yumoto R, Takano M. Megalin/cubilin-mediated uptake of FITC-labeled IgG by OK kidney epithelial cells. Drug Metab Pharmacokinet 26: 474–485, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Okamura K, Dummer P, Kopp J, Qiu L, Levi M, Faubel S, Blaine J. Endocytosis of albumin by podocytes elicits an inflammatory response and induces apoptotic cell death. PloS One 8: e54817, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olbricht CJ, Cannon JK, Garg LC, Tisher CC. Activities of cathepsins B and L in isolated nephron segments from proteinuric and nonproteinuric rats. Am J Physiol Renal Fluid Electrolyte Physiol 250: F1055–F1062, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Peti-Peterdi J. Independent two-photon measurements of albumin GSC give low values. Am J Physiol Renal Physiol 296: F1255–F1257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puri V, Watanabe R, Singh RD, Dominguez M, Brown JC, Wheatley CL, Marks DL, Pagano RE. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J Cell Biol 154: 535–548, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren Z, Liang W, Chen C, Yang H, Singhal PC, Ding G. Angiotensin II induces nephrin dephosphorylation and podocyte injury: role of caveolin-1. Cell Signalling 24: 443–450, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice WL, Van Hoek AN, Păunescu TG, Huynh C, Goetze B, Singh B, Scipioni L, Stern LA, Brown D. High resolution helium ion scanning microscopy of the rat kidney. PloS One 8: e57051, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Sakairi T, Abe Y, Kajiyama H, Bartlett LD, Howard LV, Jat PS, Kopp JB. Conditionally immortalized human podocyte cell lines established from urine. Am J Physiol Renal Physiol 298: F557–F567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Sandoval RM, Wagner MC, Patel M, Campos-Bilderback SB, Rhodes GJ, Wang E, Wean SE, Clendenon SS, Molitoris BA. Multiple factors influence glomerular albumin permeability in rats. J Am Soc Nephrol 23: 447–457, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarav M, Wang Y, Hack BK, Chang A, Jensen M, Bao L, Quigg RJ. Renal FcRn reclaims albumin but facilitates elimination of IgG. J Am Soc Nephrol 20: 1941–1952, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smithies O. Why the kidney glomerulus does not clog: a gel permeation/diffusion hypothesis of renal function. Proc Natl Acad Sci USA 100: 4108–4113, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sörensson J, Fierlbeck W, Heider T, Schwarz K, Park DS, Mundel P, Lisanti M, Ballermann BJ. Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol 13: 2639–2647, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi K, Morizane Y, Kamami-Levy C, Suzuki J, Kayama M, Cai W, Miller JW, Vavvas DG. AMPK inhibits oxidative stress induced caveolin-1 phosphorylation and endocytosis by suppressing the dissociation between c-Abl and prdx1 in endothelial cells. J Biol Chem 288: 20581–20591, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanner GA. Glomerular sieving coefficient of serum albumin in the rat: a two-photon microscopy study. Am J Physiol Renal Physiol 296: F1258–F1265, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol Renal Fluid Electrolyte Physiol 263: F601–F606, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Tojo A, Kinugasa S. Mechanisms of glomerular albumin filtration and tubular reabsorption. Int J Nephrol 2012: 9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tojo A, Onozato M, Ha H, Kurihara H, Sakai T, Goto A, Fujita T, Endou H. Reduced albumin reabsorption in the proximal tubule of early-stage diabetic rats. Histochem Cell Biol 116: 269–276, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Tojo A, Onozato M, Kitiyakara C, Kinugasa S, Fukuda S, Sakai T, Fujita T. Glomerular albumin filtration through podocyte cell body in puromycin aminonucleoside nephrotic rat. Medical Mol Morphol 41: 92–98, 2008 [DOI] [PubMed] [Google Scholar]
- 44.von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson Mark J, MacGregor Kylie A, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries Jens P, Saenger W, Kräusslich HG, Shupliakov O, Robinson Phillip J, McCluskey A, Haucke V. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146: 471–484, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest 116: 1284–1291, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yumoto R, Nishikawa H, Okamoto M, Katayama H, Nagai J, Takano M. Clathrin-mediated endocytosis of FITC-albumin in alveolar type II epithelial cell line RLE-6TN. Am J Physiol Lung Cell Mol Physiol 290: L946–L955, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Yumoto R, Suzuka S, Oda K, Nagai J, Takano M. Endocytic uptake of FITC-albumin by human alveolar epithelial cell line A549. Drug Metab Pharmacokinet 27: 336–343, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Zhai XY, Nielsen R, Birn H, Drumm K, Mildenberger S, Freudinger R, Moestrup SK, Verroust PJ, Christensen EI, Gekle M. Cubilin- and megalin-mediated uptake of albumin in cultured proximal tubule cells of opossum kidney. Kidney Int 58: 1523–1533, 2000 [DOI] [PubMed] [Google Scholar]