Abstract
Context:
The mechanisms causing excessive aldosterone production and hypertension in primary aldosteronism (PA) are complex and often incompletely recognized. Autoantibodies to the angiotensin AT1 receptor (AT1R) have been reported in some PA patients with an aldosterone-producing adenoma but not with idiopathic adrenal hyperplasia.
Objective:
We investigated whether these autoantibodies will activate AT1R and thereby potentially contribute to the pathophysiology of PA.
Design:
AT1R autoantibody activity in sera and/or IgG purified from 13 biochemically confirmed PA patients was measured using AT1R-transfected cells, and their contractile effects were assayed using perfused rat cremaster arterioles. Aldosterone stimulation was measured in vitro using isolated human adrenal carcinoma (HAC15) adrenal cells. These data were compared with sera obtained from a group of normotensive control subjects who were expected to have negligible AT1R autoantibodies.
Results:
Sera from each of the 13 PA patients significantly increased AT1R activation in AT1R-transfected cells compared with 20 control subjects, and this activity was inhibited by the selective AT1R blocker losartan. Sera and IgG purified from AT1R autoantibody-positive sera demonstrated significant vasoconstrictive effects in isolated rat cremaster arterioles and were blocked by losartan. Moreover, the AT1R autoantibody-positive IgG directly stimulated aldosterone production in the cultured adrenal cells and enhanced angiotensin-induced aldosterone production in these cells, and these effects were blocked by candesartan.
Conclusions:
These data support a probable pathophysiological role for AT1R autoantibodies in PA and thereby raise important etiological and therapeutic implications.
The pathophysiological mechanisms causing excessive aldosterone production and hypertension in primary aldosteronism (PA) have challenged investigators since the first description of an aldosterone-producing adrenal adenoma (APA) (1). Extensive follow-up studies after successful resection of the tumors demonstrated residual hypertension in up to 50% of these patients (2–5). This question was sharpened when a large number of patients with a similar phenotype and biochemical PA were demonstrated to have idiopathic adrenal hyperplasia (IAH) rather than an adenoma (6, 7); and when their adrenals were removed in three small studies, almost all retained some degree of residual hypertension (8–10). Thus, aldosterone excess alone does not account for the complex hypertensive pathophysiology in patients with PA.
Autoantibodies that activate the G protein-coupled angiotensin II type 1 receptor (AT1R) have been described in patients during acute circumstances such as preeclampsia (11), in some circumstances involving acute renal transplant rejection (12); and in a few screened hypertensive subjects (13). We hypothesized the presence of such autoantibodies would cause or contribute to the hypertension and hyperaldosteronism normally associated with IAH and also in those with an APA, irrespective of the presence or absence of an identifiable etiology such as associated mutations of the KCNJ5 channels (14). Rossitto et al (15) recently reported the presence of autoantibodies to the AT1R, as measured by an ELISA, in subjects with an APA but not in those with IAH. They proposed use of this assay as a diagnostic tool to differentiate the two different types of PA. The ELISA technique, however, has limitations with regard to sensitivity when linear peptide targets are used. Because this type of assay inherently does not measure the ability of these autoantibodies to activate the AT1R, there is need for further studies to examine the hypertensive mechanisms by which their presence might alter or participate in the pathophysiology of PA.
In the present study, we have examined the mechanism(s) by which these autoantibodies might interact with and affect important target tissues including arterial smooth muscle and the adrenal cortex in patients with biochemically confirmed PA. We retrospectively had available sera from 13 subjects with documented PA and observed all harbored some level of autoantibodies that activated AT1R-transfected Chinese hamster ovary (CHO) cells in vitro. We have demonstrated these autoantibodies increase vascular contractility in vitro similar to angiotensin II (Ang II) in the presence of a concurrently suppressed classical renin-angiotensin system. Like native Ang II, this autoantibody contractile effect appears to be largely inhibited by direct AT1R blockade. These autoantibodies also increase and/or enhance Ang II-mediated aldosterone production in vitro. These data support the possibility these activating autoantibodies, when present, may contribute to and/or modulate known pathophysiological mechanism(s) identified in patients with PA and in other subjects expressing this same AT1R-oriented autoantibody.
Materials and Methods
Study subjects
We had accumulated sera from 13 subjects with a variable history of hypertension, hypokalemia, and a plasma aldosterone to plasma renin activity (PRA) ratio of greater than 30 (Table 1). The diagnosis of PA was confirmed by a plasma aldosterone to PRA value greater than 80 and/or by an abnormal captopril suppression test [2 h after captopril plasma aldosterone ≥12 ng/dL and/or plasma aldosterone to PRA ratio ≥12 (16)]; an 8:00 am plasma aldosterone of 6 ng/dL or greater after a high Na (>250 mEq Na/d) diet for 3 days (two subjects) or a plasma aldosterone concentration of 6 ng/dL or greater after a saline infusion test of 2 L over 4 hours in one subject (17). All underwent a serial 2-mm section computed tomography (CT) of the adrenals to identify existing nodules. Patients identified as willing operative candidates underwent adrenal vein sampling to confirm lateralization (ipsilateral/contralateral adrenal vein plasma aldosterone/cortisol > 3:1). Two subjects had a visible adenoma on CT and lateralized on venous sampling (Table 1). These two subjects and a third 32-year-old patient with marked elevation of plasma aldosterone and a single 2.2-cm nodule on CT were considered to have an APA and underwent unilateral adrenal surgical resection. Each had an early morning plasma aldosterone value of 5 ng/dL or less in the week after resection, and their surgery was considered successful. Two other subjects failed to lateralize on adrenal venous sampling and were considered to have IAH. Two subjects with a left adrenal mass underwent adrenal venous sampling, but the radiologist was unable to locate and sample the right adrenal vein, so comparative values from the right side were not available.
Table 1.
Clinical Characteristics of the 13 Studied PA Subjects
| Dx | Age/Sex | HTN, y | K/Na | PA/PRA | CT | Adrenal Vein A/C (I/C) | Sx/1 wk After Sx PA, ng/dL | FU # | ELISA Titer |
|---|---|---|---|---|---|---|---|---|---|
| APA | 56/F | 20 | 2.7/142 | 21/0.2 p-Sal | L 2 cm | L 23, R 2.0 (11.5) | L/5 | NT 3 | 1:1600 |
| APA | 32/M | >10 | 2.9/142 | 27/1.6 p-CEI | L 2.5 cm | Not done | L/≤1.0 | NT 3 | 1:1600 |
| APA | 53/M | >10 | 3.2/139 | 13.5/1.14 p-CEI | L 2.2 cm | L 3.9, R 0.57 (6.8) | L/5 | NT 1 | 1:1600 |
| IAH | 79/M | 40 | 2.9/132 | 11.8/0.15 p-CEI | L 0.8 cm | L 3.1, R 1.3 (2.4) | L (L nod hyper) | NT 4 | 1:6400 |
| IAH | 61/M | >10 | 3.4/144 | 39.8/0.15 | L 1.5 cm | L 1.3, R 3.3 (2.5) | No (no lateralization) | NT 4 | 1:3200 |
| IPA | 76/F | 30 | 3.8/138 | 32.9/0.1 p-HS | Normal | Not done | No (Pt declined) | D | 1:3200 |
| IPA | 63/F | 30 | 3.4/144 | 24.7/1.05 p-CEI | L mass | L 3.9, no R | No (Pt declined) | D | 1:6400 |
| IPA | 65/M | 35 | 3.1/134 | 32.9/0.19 p-HS | L mass | Not done | No (renal failure) | NT 3 | 1:1600 |
| IPA | 66/M | >10 | 4.0/141 | 11.5/0.5 p-CEI | Normal | Not done | No (not needed) | NT 2 | 1:6400 |
| IPA | 64/M | 30 | 3.2/141 | 17.6/0.30 p-CEI | L mass | L 7.6, no R | No (Pt declined) | NT 2 | 1:6400 |
| IPA | 62/M | 30 | 3.3/134 | 33.7/0.31 | Normal | Not done | No (not needed) | NT 2 | 1:3200 |
| IPA | 73/M | 30 | 3.2/144 | 21/0.15 p-CEI | Nod hyper | Not done | No (not needed) | NT 3 | 1:1600 |
| IPA | 61/M | >10 | 2.9/136 | 23.6/0.15 | Normal | Not done | No (not needed) | NT 2 | 1:3200 |
Abbreviations: A/C, aldosterone to cortisol ratio; cm, size of adrenal adenoma; CT, computerized axial tomography scan of adrenal; D, deceased; Dx, diagnosis; F, female; FU, follow-up; HTN, hypertension; I/C, ratio of the ipsilateral and contralateral adrenal vein A to C ratios; K/Na, initial K/Na; L, left; M. male; nod hyper, nodular hyperplasia; not needed, the patient's BP was already controlled, so further studies and surgery were not needed; NT, normotensive (BP < 140/90 mm Hg); PA/PRA, plasma aldosterone to plasma renin activity ratio; p-CEI, after converting enzyme inhibitor; p-HS, after high salt diet; p-Sal, postsaline infusion; Pt declined, the patient declined to consider surgical resection of a tumor, even if it was confirmed by adrenal venous sampling, so the procedure was not performed; R, right; Sx, surgery; #, number of medications required.
Each subject subsequently declined a repeat study and decided to proceed with successful medical therapy. Six other subjects who were not operative candidates or who refused consideration for surgery irrespective of their diagnosis did not undergo lateralization studies and were treated medically with satisfactory blood pressure (BP) control. These last eight (two + six) subjects with an unconfirmed pathology for their PA comprise a group we have called indeterminate primary aldosteronism (IPA) group. Twenty normotensive subjects, 11 males (aged 20–55 y) and nine females (aged 22–45 y) provided control sera. Inasmuch as these subtype groups are very small, we have proceeded to examine the mechanism(s) by which these autoantibodies, irrespective of their underlying subtype, possess activity that would potentially contribute to the elevated BP observed in PA.
Enzyme-linked immunosorbent assay
ELISA assays to screen for AT1R autoantibodies were performed as previously described (13). Briefly, microtiter plates were coated with a multiple antigenic peptide (Pi Proteomics) containing the amino acids AFHYESQ, a sequence from the second extracellular loop (ECL2) of AT1R identified as the functional epitope of AT1R-activating autoantibodies (11). To determine antibody titer, sera were diluted 1:100 in 1% BSA in PBS and thereafter diluted 2-fold. Goat antihuman IgG conjugated with alkaline phosphatase and its substrate, paranitrophenyl-phosphate 104, were used to detect antibody binding. The titers were determined as the highest dilution with an OD value of 0.10 at 60 minutes.
IgG purification
IgG was purified from the patient or control sera for some studies using the NAb Protein A/G spin kit (Pierce Biotechnology). This column fails to retain bound or free aldosterone and medications.
Cell-based AT1R assay
Ang II-like autoantibody activity was measured in AT1R-transfected CHO cells using β-arrestin activation as the parameter (DiscoveRx). Negative (buffer) and positive Ang II controls were included in each assay. Samples also were run in the presence of the orthosteric AT1R blocker losartan (10 μM) or candesartan (10 μM). Three positive samples were also tested using the converting enzyme inhibitor lisinopril (10 μM). The β-arrestin recruitment levels by the sera/IgG were expressed as Ang II equivalent values. These were obtained by running a concurrent Ang II standard curve and comparing patient sample relative luminescence units to those on the standard curve. The intraassay coefficient of variation for values with a nominal value of 94 pM of Ang II was 5.4% (n = 9) and for values with a mean value of 191 pM was 6.8% (n = 9). The interassay values were 8.2% (n = 9) and 11.3% (n = 9), respectively.
Contractility assay
Autoantibody pressor activity was assayed in vitro using a perfused rat cremaster arteriole assay as previously described (18). For pressor activity, sera (1:100) and/or serum IgG (0.1 mg/mL) were tested. After a contractile response was observed, losartan (10 μM) was added to block the antibody effect. When the losartan blockade was maximal but not 100%, prazosin (10 μM) was added to determine whether α1-adrenergic activating autoantibodies also were present. Pooled normal human IgG (Sigma) was examined as a negative control. Each set of assays used a separate cremaster arteriole with inherently different basal contractility. We therefore normalized each response to its baseline diameter and expressed the data as change from baseline to minimize this variability. The intraassay coefficient of variation for values with a 5.9% mean change of arterial diameter was 7.8% (n = 5) and for values with a 19.9% mean diameter change was 6.7% (n = 5). The interassay values were 12.0% (n = 5) and 8.7% (n = 5), respectively.
Aldosterone assay
The human adrenocortical carcinoma cell line (HAC15) was provided by W. E. Rainey (University of Michigan, Ann Arbor, Michigan) (19). The cells were cultured in DMEM-F12 (1:1) supplemented with pyruvate, glutamax, insulin/transferrin/selenium, nonessential amino acids, antibiotics, and 10% cosmic calf serum at 37°C under an atmosphere of 5% CO2. When 80% confluent, the cells were transferred into 96-well plates and expanded until 80% confluent. After overnight incubation in DMEM/DMF12 with 0.1% cosmic serum, different dilutions of IgG or Ang II were added and incubated at 37°C for 24 hours. The incubation was stopped by placing the cells at 4°C. Aldosterone production was measured in cell culture supernatants by time-resolved fluorescence. The samples were assayed using primary antibodies as previously described (20). A dosage response curve from IgG was constructed ranging from 1:50 to 1:1000 (equivalent to 0.6–0.03 mg/mL). Cells were incubated for 24 hours prior to assay for aldosterone production. IgG purified from the sera of five of our control subjects and a commercial pooled normal human IgG were used for control purposes. Candesartan 5 and 10 μM was used to inhibit activation of the AT1R. The intraassay coefficient of variation for samples with a mean production of 3.14 μg of aldosterone/well was 8.3% (n = 24). The interassay coefficient of variation for a specimen with a mean of 28 μg/well was 9.2% (n = 12).
Statistics
Data are expressed as mean ± SD. AT1R autoantibody bioactivity values in the β-arrestin bioassays were expressed in Ang II-equivalent units. The positivity of bioactive autoantibodies was defined as values above the mean +2 SD from the control group. Comparison between patient and normotensive control groups was performed using the nonparametric Mann-Whitney test. Differences in dose effects and vasoconstriction were assessed by a paired or unpaired Student's t test as appropriate. A value of P < .05 was considered statistically significant.
Study approval
The study was approved by the University of Oklahoma Health Sciences Center and Veterans Affairs Medical Center Institutional Review Board and Institutional Animal Care and Use Committees. All subjects provided informed consent.
Results
The ELISA titers for the control subjects ranged from less than 1:800 to 1:3200. Only four of the patients with PA had significantly elevated ELISA titers (1:6400 or higher) directed to the targeted AFHYESQ peptide of the ECL2 of AT1R (Table 1). The remainder had values no different from the control subjects. There was a higher percentage of male patients with PA, but this likely reflected the primary source of subjects from our Veterans Affairs Medical Center hospital endocrinology clinic. These subjects tended to be older than the controls; however, we found no significant rise in AT1R autoantibodies with age within this group.
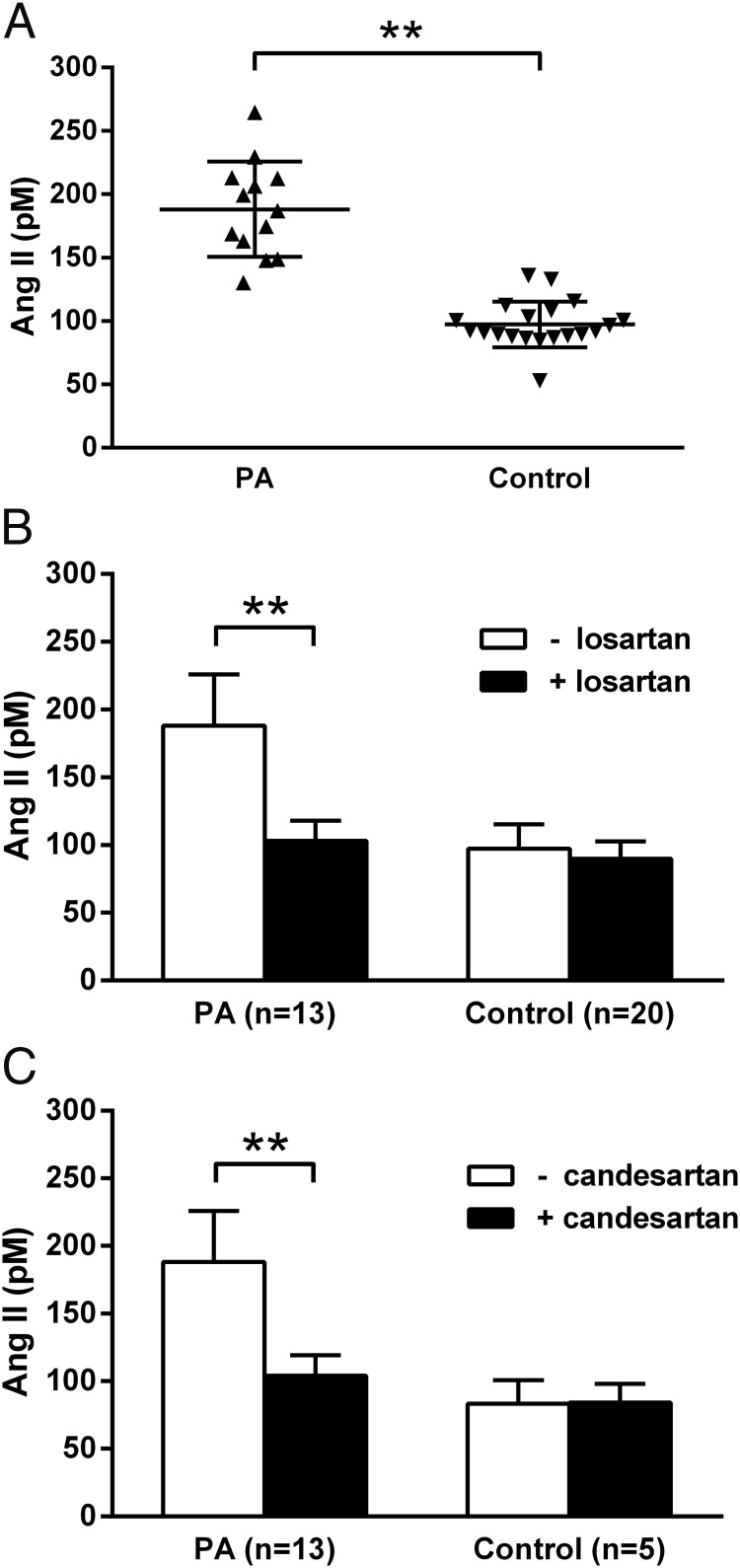
Sera from individual subjects with PA led to a highly significant activation of AT1R in transfected CHO cells in vitro (Figure 1A). These data are expressed as activation equivalent to the Ang II standard curve used for the assay. The mean activity was elevated compared with the 20 control subjects (PA: 188 ± 37 vs control: 97 ± 18 in pM Ang II equivalent activity; P < .001). Commercially available pooled human control IgG (Sigma) had no significant effect on the AT1R activity in these transfected cells compared with the buffer controls (data not shown). The AT1R biological activity was observed in patient samples that were ELISA negative for autoantibodies. This activity was largely but not completely reversed by losartan (10 μM) and by candesartan (10 μM) (Figure 1, B and C). There was no significant effect of losartan (n = 20) or candesartan (n = 5) on the activity observed in the controls. Lisinopril failed to suppress the activity in three positive samples tested in vitro (data not shown).
Figure 1.
A, The effect of sera from PA patients and healthy controls on AT1R activation in transfected CHO cells. B and C, The effect of 10 μM losartan and 10 μM candesartan on autoantibody activation of transfected AT1R using sera from PA and control subjects. Values are expressed in Ang II equivalent dosages (mean ± SD). Sera from the PA group led to significant activation of AT1R, which was suppressed by AT1R blockers losartan and candesartan. **, P < .001.
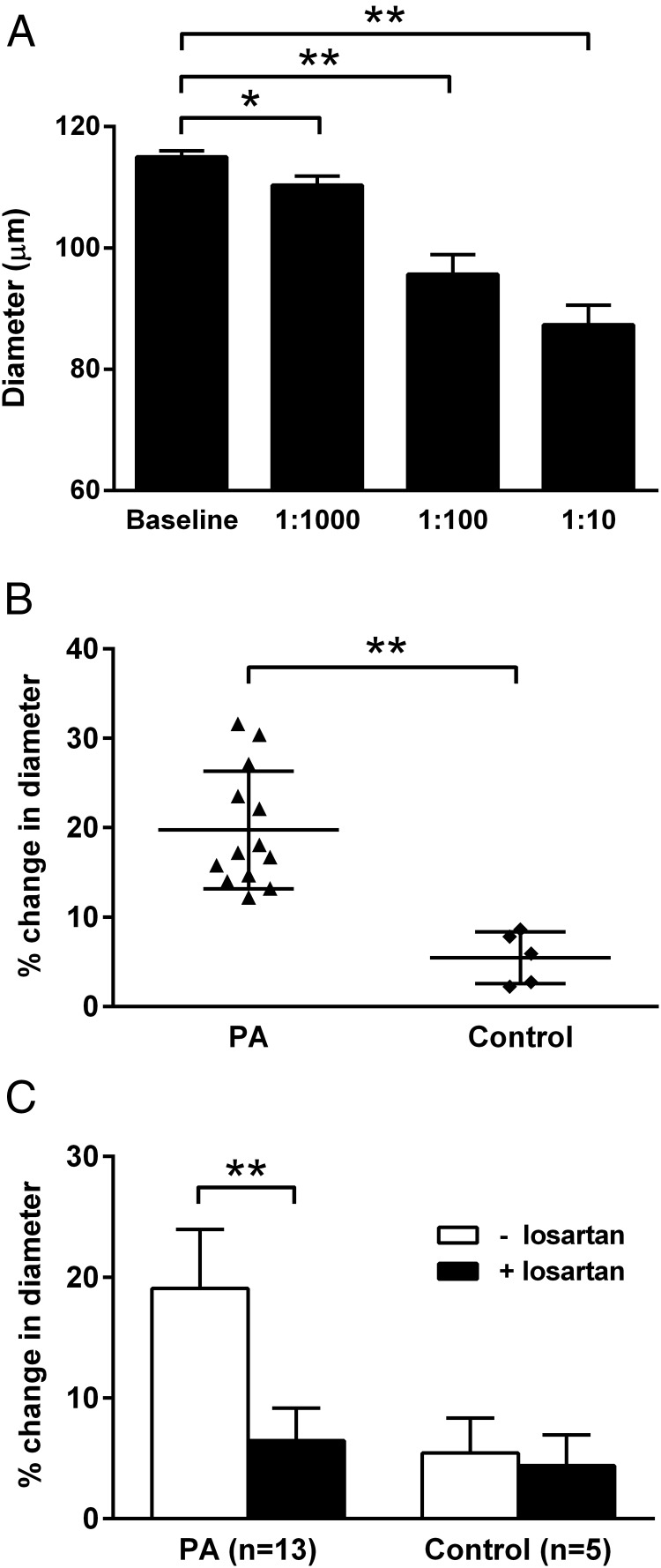
Three PA subjects with positive activity from the receptor assay were initially studied for AT1R contractility activity using the rat cremaster arteriole assay. IgG purified from each sera was tested and demonstrated a dosage-response effect with the mean maximal activity at a 1:10 dilution (Figure 2A). Significant contractile activity was observed at dilutions ranging from 1:10 to 1:100. Figure 2B shows cremaster arteriole contractility for sera (1:100) from individual subjects with PA, all of which were higher than the control subjects. Contractility in the PA group was greater than for the control group (PA: 19% ± 5% vs control: 5% ± 3% contraction from baseline; P < .001). Losartan (10 μM) largely suppressed IgG-mediated arteriole contractility in this group (Figure 2C). No significant effect of losartan (n = 5) on the contractile activity was observed in the controls.
Figure 2.
A, The dosage effect of serum IgG purified from three AT1R autoantibody-positive PA patients on cremaster arteriole diameter (vasoconstriction). Data are expressed as mean ± SD. B and C, Vasoconstriction induced by sera (1:100) from individual PA patients and the effect of 10 μM losartan on PA sera-induced vasoconstriction (mean ± SD) in the rat cremaster arteriole assay. Values are expressed as percentage decrease in the diameter of the arterioles. There was a greater contractility in the PA group compared with the control group, and losartan effectively blocked this contractile effect. The effect of the AT1R blocker on arterial contractility for the control subjects was negligible. *, P < .05; **, P < .001.
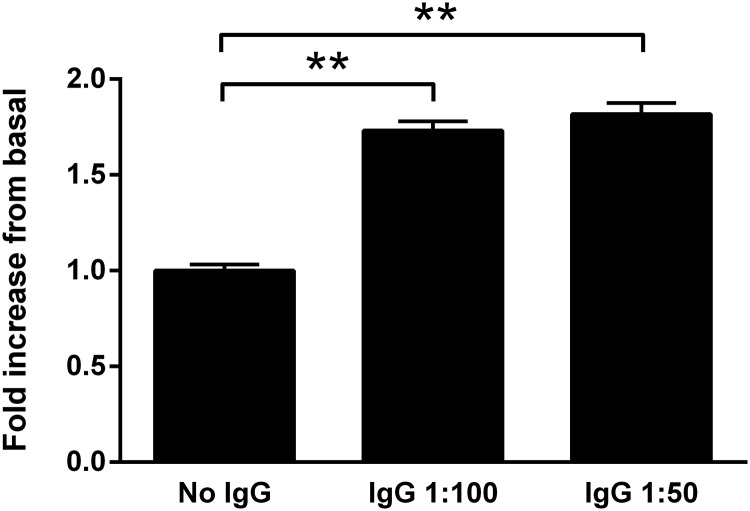
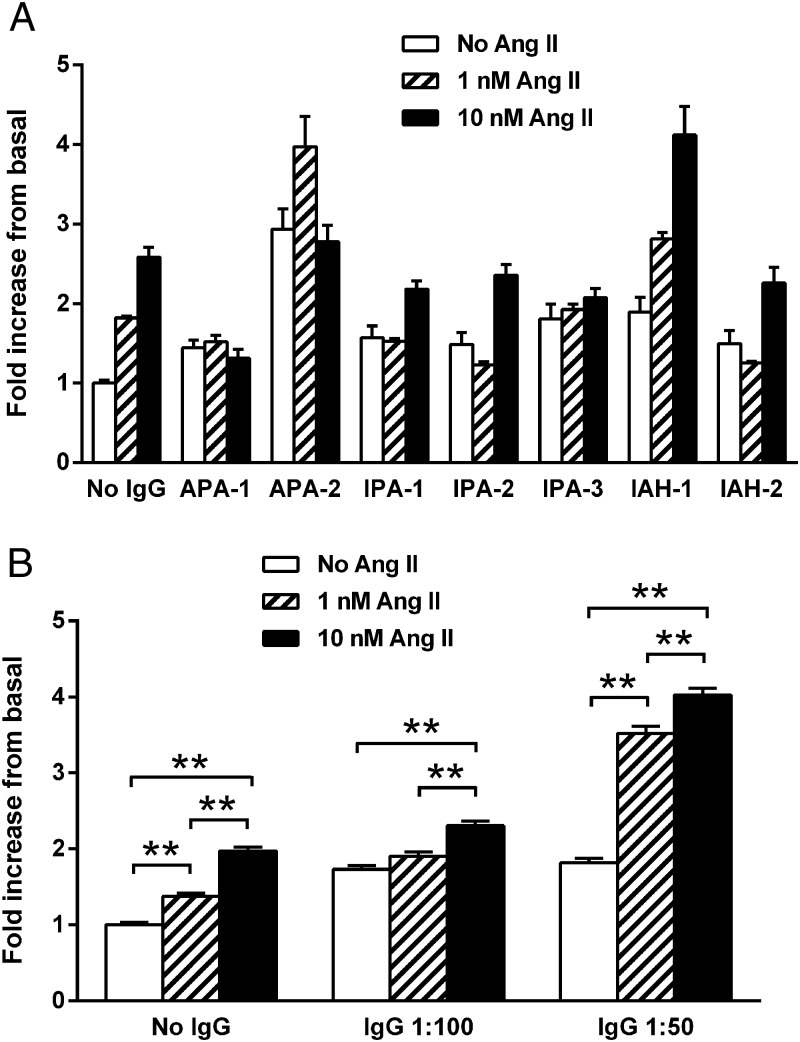
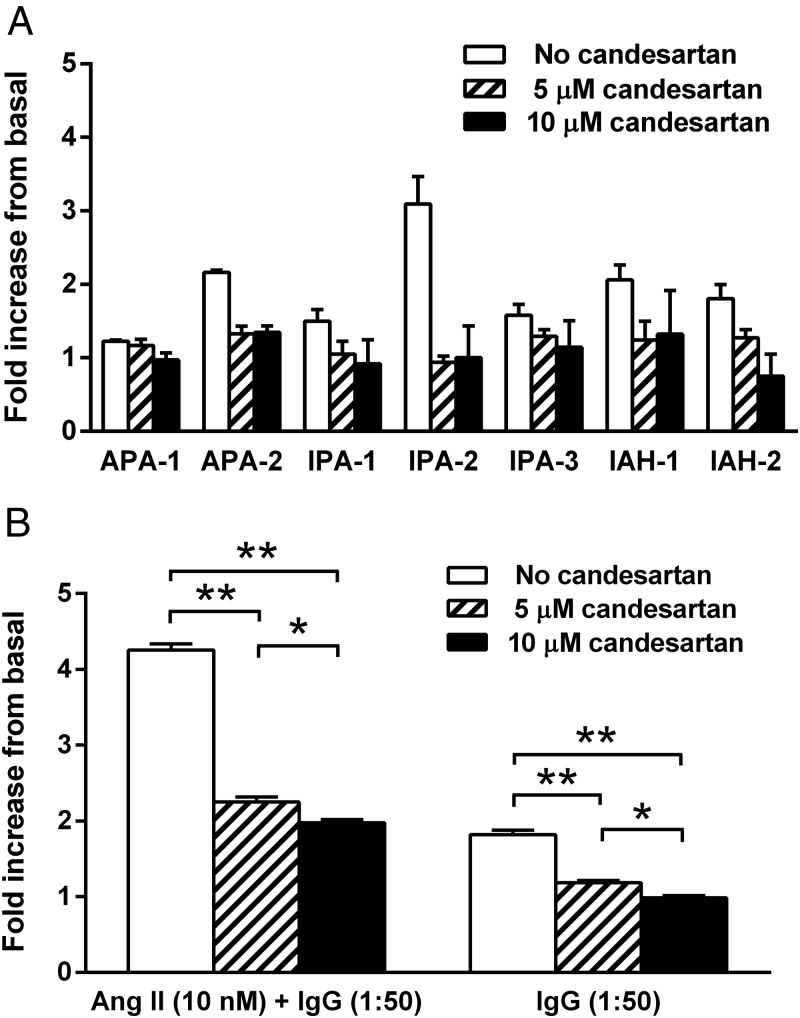
IgG purified from the sera of seven subjects with PA, including two with an APA (positive lateralization with adrenal venous sampling, surgical resection, and postoperative suppression of aldosterone), two with IAH (failure to lateralize on adrenal venous sampling), and three with IPA, were studied to determine whether IgG purified from their sera would stimulate aldosterone secretion from HAC15 cells in vitro. These data (Figure 3) demonstrate IgG alone produced 1.7-fold and 1.8-fold increases in aldosterone production over the buffer baseline for 1:50 and 1:100 dilutions, respectively (P < .001). Ang II at low dosage (1 and 10 nM) has a predictable stimulatory effect on aldosterone production compared with the buffer baseline (Figure 4A). When the IgG at 1:50 dilutions from these subjects was added along with the Ang II for overnight incubation, there was a marked increase (P < .001) in mean aldosterone production of 2.5- and 2.0-fold, respectively, over the 1 and 10 nM Ang II concentrations alone (Figure 4B). This enhanced response was less prominent at the 1:100 IgG dilutions and at higher Ang II concentrations of 100 and 1000 nM (data not shown). No direct effect of human control IgG was observed. The candesartan dosages of 5 and 10 μM were effective in blocking aldosterone production from the HAC15 cells exposed to IgG at 1:50 dilutions in the absence and presence of Ang II (10 nM) (Figure 5, A and B).
Figure 3.
PA patient IgG-induced aldosterone secretion in HAC15 cells. IgG (n = 7) was diluted 1:50 (0.6 mg/mL) and 1:100 (0.3 mg/mL). Values are expressed as fold increase over the buffer baseline aldosterone production for each assay (mean ± SEM). There was a highly significant increase in aldosterone production by the IgG from these patients. **, P < .001.
Figure 4.
A, The effect of low dosages of Ang II (1 and 10 nM) on aldosterone production in HAC15 cells in the presence of a 1:50 dilution of IgG from seven subjects with PA (two APA, three IPA, and two IAH). Aldosterone production was increased by IgG alone and markedly so in the presence of low dosages of Ang II when compared with buffer baseline or to Ang II alone for each subject. B, The mean values for the same seven subjects using both 1:50 and 1:100 dilution of IgG. The 1:50 dilution demonstrated a significant increase in Ang II stimulation of aldosterone compared with Ang II alone. The 1:100 dilutions demonstrated a highly significant effect of IgG alone but exerted less effect on Ang II activity. **, P < .001.
Figure 5.
A, The effect of AT1R blockade with increasing dosages of candesartan on aldosterone production in HAC15 cells stimulated by a 1:50 dilution (0.6 mg/mL) of IgG from each of seven PA subjects. B, The mean effect of candesartan on IgG-stimulated aldosterone production in the presence and absence of Ang II (10 nM). Candesartan at 5 and 10 μM suppressed aldosterone stimulation. *, P < .05; **, P < .001.
Discussion
The mechanisms underlying the pathophysiology of aldosterone overproduction and abnormal cellular growth in APA and IAH have been eagerly sought by many investigators since these conditions were first reported. Current views relate a complex interaction of aldosterone on renal Na retention and a central nervous system effect, leading to increased autonomic outflow, increased peripheral and renal artery vasoconstriction, and upward resetting of the pressure natriuresis curve. Other factors have been proposed, but none have achieved convincing documentation or confirmation. Recently there is evidence up to 50% of APA harbor a somatic mutant KCNJ5 channel gene(s) capable of producing both aldosterone overproduction and cellular growth (14, 21, 22). A different form of hyperplasia has been previously reported (familial hyperplasia type 2) but is much less prevalent than IAH (23). An uncommon variant with diffuse adrenal hyperplasia (familial hyperplasia type 3) has been attributed to a similar germ line mutation but is clearly different from IAH (14).
In the present study, sera from 13 subjects with confirmed PA demonstrated increased activity from an antibody directed to a pentapeptide located within the ECL2 of the AT1R (11). This antibody produced a contractile effect that was blocked by the angiotensin receptor blocker losartan in an in vitro assay using a representative peripheral resistance artery. Furthermore, this antibody was capable of activating AT1R in transfected CHO cells in vitro, and this too was blocked by losartan and candesartan but not by the converting enzyme inhibitor lisinopril. Evidence for activity in a second Ang II target organ was provided by the ability of serum IgG from seven of these PA subjects (including two with documented APA, two with IAH, and three with IPA) to stimulate aldosterone production in HAC15 cells in vitro. This effect also was largely blocked by candesartan. Notably, this IgG, when incubated with Ang II, enhanced HAC15 aldosterone production above that of using Ang II alone. The use of purified IgG derived from the same subjects and activity measurements using marked dilutions of the sera largely eliminate possible cross-reactivity with circulating components of the endogenous renin-angiotensin-aldosterone system, potential nonimmunological agonists, and medications.
Activating autoantibodies such as thyroid-stimulating immunoglobulin are now recognized to be directed toward an extracellular domain of the G protein-coupled receptors (24). The AT1R-activating autoantibodies have been demonstrated to target the AFHYESQ domain residing on the ECL2 (11). These autoantibodies are thought to act as allosteric agonists and fail to activate the normal desensitization of the receptor that would otherwise mitigate their activity (25). These allosteric effects by themselves appear to activate the target receptor; however, they variably enhance or decrease the concurrent activation by the orthosteric ligand normally associated with the receptor. We have reported α1-adrenergic autoantibodies act as a partial antagonist to the effect of phenylephrine on cremaster arteriole contractility, whereas at the same time, autoantibodies to the β1/2-adrenergic receptors facilitate activity of isoproterenol on cAMP production and arteriole dilation (26). In the present study, we report AT1R autoantibodies in vitro activate AT1R and stimulate arteriole contractility and adrenal cell aldosterone production. Their presumed alteration of the allosteric configuration of the receptor facilitates Ang II-mediated adrenal cell production of aldosterone. Thus, even relatively low levels of such autoantibodies would produce a variable and potentially significant activation of their specific targeted receptor and transduced cellular functions. The specific allosteric changes in the molecular structure of the various G protein-coupled receptors are not known at present but are under investigation in our and in other laboratories. There is precedent in this differential activity in as much as low and high sodium dietary intakes in humans and animals create contrasting changes in vascular and adrenal sensitivity to infused and to endogenous Ang II. These studies, however, demonstrate the effects of these autoantibodies may not be identical with that of native Ang II on vascular activity and aldosterone secretion.
Although the classical renin-angiotensin system generally is suppressed in PA, Wisgerhof et al (27) demonstrated aldosterone levels hyperresponded to infused Ang II in subjects with IAH compared with APA and normal subjects. These studies are not at variance with our proposal that activating autoantibodies are capable of producing some if not all of the pathophysiology in antibody-positive subjects. The observation that plasma aldosterone frequently rises with prolonged upright posture in IAH would not be incompatible with the observation that measured small changes in the classical renin-angiotensin aldosterone pathway would still be operative and be enhanced by the positive allosteric effects on adrenal aldosterone production in autoantibody-positive subjects. This also may explain the observation of Rossitto et al (15) that captopril, which would likely lower the already suppressed circulating Ang II in these subjects, was associated with a greater drop in plasma aldosterone concentrations in their PA subjects harboring ELISA-positive AT1R autoantibodies than in those who did not.
The impact of these autoantibodies on cellular hypertrophy is less clear. Similar activating autoantibodies directed toward other target organs such as thyroid-stimulating immunoglobulin on the thyroid may be associated with a mixed picture of hypertrophy and micronodularity. Pathophysiological autoantibodies directed toward β1-adrenergic receptor are associated with cardiac hypertrophy after 6 months of exposure in an animal model (28), but the targeted cardiac tissues may express this hypertrophy secondary to accompanying hypertension and/or persistent tachycardia as well as from the autoimmune stimulus. It is of interest that reexamination of the adrenal in many cases of PA with a dominant APA reveals coexistent hypertrophic changes in the adrenal cortex not dissimilar to IAH (29). We have no information that would directly tie the presence of AT1R-activating autoantibodies to the presence of APA macroadenomas or to their underlying genetic structure.
Rossitto et al (15) observed elevated ELISA titers primarily in their subjects with an APA and not with IAH and proposed their use as a diagnostic aid in differentiating the two entities. Restraints on the application of adrenal venous sampling in our small group of patients limit our ability to examine the prevalence of these activating autoantibodies in subgroups of PA. However, we used three different biological assays to assess arterial and adrenal AT1R activation using both sera and IgG and used selective AT1R blockers to pharmacologically establish this activity. We found sera from all 13 of our subjects with biochemically confirmed PA had significant autoimmune activation of the AT1R. This activation was shown to produce contraction in a cremaster resistance arteriole bioassay as well as to directly activate aldosterone production in HAC15 cells in vitro. The failure of our ELISA to identify all of the subjects with increased biological activation of the AT1R is not surprising. The synthetic peptide, containing the putative AT1R ECL2 target AFHYESQ sequence, may have been too short for optimal antibody binding or may not have included potential other interactive sites. ELISA studies, however, by their nature do not provide an assessment of the biological activity that is necessary for the assessment of the pathophysiological significance of these autoantibodies.
Our study was designed to examine the potential of these autoantibodies contributing to the pathophysiology of the hypertension in PA. It was not designed to determine the actual prevalence of these active agents in patients with either APA or IAH because this would require a much larger number of subjects whose adrenal pathology was characterized by adrenal venous lateralization, a procedure that has lost favor in our institution owing to a decreased need for surgical intervention to establish effective BP control. We have initiated a study in collaboration with a group that has access to a large number of carefully characterized PA subjects. We have no information as to the underlying causes for the production of these activating autoantibodies; but our colleague (M.W.C) has reported molecular mimicry is involved in the production of activating autoantibodies to the β1-adrenergic receptor in a myosin-induced rat model of myocarditis (30). The presence of these autoantibodies after surgery to remove the APA could explain in part the persistence of hypertension. We have observed in two subjects significant improvement in hypertension and decreased aldosterone levels with the use of losartan. It is not yet clear whether these autoantibodies represent the elusive X factor that has been sought by many laboratories, but they are a plausible candidate for additional attention and study.
Acknowledgments
This work was supported by a Veterans Affairs Merit Review grant (to D.C.K. and X.Y.); National Institutes of Health Grants HL056267 (to M.W.C. and D.C.K) and HL27255 (to C.E.G.-S.); an AHA Postdoctoral Fellowship (to H.L.); and individual grant support from Will and Helen Webster.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ang II
- angiotensin II
- APA
- aldosterone-producing adrenal adenoma
- AT1R
- angiotensin AT1 receptor
- BP
- blood pressure
- CHO
- Chinese hamster ovary
- CT
- computed tomography
- ECL2
- second extracellular loop
- HAC15
- human adrenocortical carcinoma cell line
- IAH
- idiopathic adrenal hyperplasia
- IPA
- indeterminate PA
- PA
- primary aldosteronism
- PRA
- plasma renin activity.
References
- 1. Conn JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955;45:3–17 [PubMed] [Google Scholar]
- 2. Weinberger MH, Grim CE, Hollifield JW, et al. Primary aldosteronism: diagnosis, localization, and treatment. Ann Intern Med. 1979;90:386–395 [DOI] [PubMed] [Google Scholar]
- 3. Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med. 2001;135:258–261 [DOI] [PubMed] [Google Scholar]
- 4. Ferriss JB, Brown JJ, Fraser R, et al. Results of adrenal surgery in patients with hypertension, aldosterone excess, and low plasma renin concentration. Br Med J. 1975;1:135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weigel RJ, Wells SA, Gunnells JC, Leight GS. Surgical treatment of primary hyperaldosteronism. Ann Surg. 1994;219:347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banks WA, Kastin AJ, Biglieri EG, Ruiz AE. Primary adrenal hyperplasia: a new subset of primary hyperaldosteronism. J Clin Endocrinol Metab. 1984;58:783–785 [DOI] [PubMed] [Google Scholar]
- 7. Katz FH. Primary aldosteronism with suppressed plasma renin activity due to bilateral nodular adrenocortical hyperplasia. Ann Intern Med. 1967;67:1035–1042 [DOI] [PubMed] [Google Scholar]
- 8. Longo DL, Esterly JA, Grim CE, Keitzer WF. Pathology of the adrenal gland in refractory low-renin hypertension. Arch Pathol Lab Med. 1978;102:322–327 [PubMed] [Google Scholar]
- 9. Biglieri EG, Schambelan M, Slaton PE, Stockigt JR. The intercurrent hypertension of primary aldosteronism. Circ Res. 1970;27:195–202 [PubMed] [Google Scholar]
- 10. Baer L, Sommers SC, Krakoff LR, Newton MA, Laragh JH. Pseudo-primary aldosteronism. An entity distinct from true primary aldosteronism. Circ Res. 1970;27:203–220 [PubMed] [Google Scholar]
- 11. Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569 [DOI] [PubMed] [Google Scholar]
- 13. Fu ML, Herlitz H, Schulze W, et al. Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. J Hypertens. 2000;18:945–953 [DOI] [PubMed] [Google Scholar]
- 14. Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rossitto G, Regolisti G, Rossi E, et al. Elevation of angiotensin-II type-1-receptor autoantibodies titer in primary aldosteronism as a result of aldosterone-producing adenoma. Hypertension. 2013;61:526–533 [DOI] [PubMed] [Google Scholar]
- 16. Lyons DF, Kem DC, Brown RD, Hanson CS, Carollo ML. Single dose captopril as a diagnostic test for primary aldosteronism. J Clin Endocrinol Metab. 1983;57:892–896 [DOI] [PubMed] [Google Scholar]
- 17. Kem DC, Weinberger MH, Mayes DM, Nugent CA. Saline suppression of plasma aldosterone in hypertension. Arch Intern Med. 1971;128:380–386 [PubMed] [Google Scholar]
- 18. Li H, Kem DC, Reim S, Vanderlinde-Wood M, et al. Agonistic autoantibodies as vasodilators in orthostatic hypotension: a new mechanism. Hypertension. 2012;59:402–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang T, Rowland JG, Parmar J, Nesterova M, Seki T, Rainey WE. Comparison of aldosterone production among human adrenocortical cell lines. Horm Metab Res. 2012;44:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oki K, Kopf PG, Campbell WB, Luis Lam M, Yamazaki T, Gomez-Sanchez CE, Gomez-Sanchez EP. Angiotensin II and III metabolism and effects on steroid production in the HAC15 human adrenocortical cell line. Endocrinology. 2013;154:214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Azizan EA, Murthy M, Stowasser M, et al. Somatic mutations affecting the selectivity filter of KCNJ5 are frequent in 2 large unselected collections of adrenal aldosteronomas. Hypertension. 2012;59:587–591 [DOI] [PubMed] [Google Scholar]
- 22. Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology. 2012;153:1774–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Torpy DJ, Gordon RD, Lin JP, et al. Familial hyperaldosteronism type II: description of a large kindred and exclusion of the aldosterone synthase (CYP11B2) gene. J Clin Endocrinol Metab. 1998;83:3214–3218 [DOI] [PubMed] [Google Scholar]
- 24. Dragun D, Philippe A, Catar R, Hegner B. Autoimmune mediated G-protein receptor activation in cardiovascular and renal pathologies. Thromb Haemost. 2009;101:643–648 [PubMed] [Google Scholar]
- 25. May LT, Leach K, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2007;47:1–51 [DOI] [PubMed] [Google Scholar]
- 26. Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3:e000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wisgerhof M, Brown RD, Hogan MJ, Carpenter PC, Edis AJ. The plasma aldosterone response to angiotensin II infusion in aldosterone-producing adenoma and idiopathic hyperaldosteronism. J Clin Endocrinol Metab. 1981;52:195–198 [DOI] [PubMed] [Google Scholar]
- 28. Iwata M, Yoshikawa T, Baba A, et al. Autoimmunity against the second extracellular loop of β(1)-adrenergic receptors induces β-adrenergic receptor desensitization and myocardial hypertrophy in vivo. Circ Res. 2001;88:578–586 [DOI] [PubMed] [Google Scholar]
- 29. Tunny TJ, Gordon RD, Klemm SA, Cohn D. Histological and biochemical distinctiveness of atypical aldosterone-producing adenomas responsive to upright posture and angiotensin. Clin Endocrinol (Oxf). 1991;34:363–369 [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Heuser JS, Cunningham LC, Kosanke SD, Cunningham MW. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006;177:8234–8240 [DOI] [PubMed] [Google Scholar]