Abstract
Context:
Current guidelines for parathyroidectomy in primary hyperparathyroidism (PHPT) include an estimated glomerular filtration rate (eGFR) less than 60 mL/min per 1.73 m2. Although the biochemical abnormalities associated with PHPT could impair renal function, there are currently no data examining whether more severe hypercalcemia, hypercalciuria, or nephrolithiasis are associated with chronic kidney disease (CKD) in mild PHPT.
Objective:
This cross-sectional study evaluated predictors of renal function in PHPT.
Design:
This is a case series of PHPT patients with (eGFR < 60 mL/min per 1.73 m2) and without (eGFR ≥ 60 mL/min per 1.73 m2) CKD.
Settings and Participants:
We studied 114 PHPT patients in a university hospital setting.
Outcome Measures:
We identified predictors of renal function using multiple linear regression.
Results:
eGFR was associated with age, hypertension, antihypertensive medication use, fasting glucose, and 25-hydroxyvitamin D. eGFR was positively rather than negatively associated with several PHPT disease severity indices including history of nephrolithiasis, 24-hour urinary calcium excretion, and 1,25-dihydroxyvitamin D but not serum calcium or PTH levels. An eGFR less than 60 mL/min per 1.73 m2 was observed in 15% (n = 17), all of whom had stage 3 CKD (eGFR 30–59 mL/min per 1.73 m2). Those with CKD were older, had higher 25-hydroxyvitamin D levels and lower 1,25-dihydroxyvitamin D levels, and were more likely to be hypertensive than those without CKD. There were no between-group (<60 vs ≥60 mL/min per 1.73 m2) differences in serum calcium, PTH, nephrolithiasis, or meeting surgical criteria other than eGFR. Multiple linear regression indicated that age and diastolic blood pressure were negatively associated with eGFR, whereas serum calcium, kidney stones, and alcohol use were positive predictors. Calculation of eGFR using either the Modification of Diet in Renal Disease or Chronic Kidney Disease Epidemiology Collaboration equation yielded similar results.
Conclusions:
PHPT patients with stage 3 CKD do not have biochemical or clinical evidence of more severe hyperparathyroidism compared with those without CKD. Traditional risk factors, rather than clinical or biochemical indices of PHPT, are associated with lower eGFR in mild PHPT.
Primary hyperparathyroidism (PHPT), characterized by hypercalcemia and elevated PTH, is a relatively common endocrine disorder (estimated prevalence 1:1000) most frequently identified among postmenopausal women (female-male 3:1) (1). An important potential comorbidity in PHPT is chronic kidney disease (CKD), with or without nephrolithiasis. Estimates of the prevalence of CKD in PHPT vary, but most studies indicate rates of approximately 16%–17% when an estimated glomerular filtration rate (eGFR) threshold of less than 60 mL/min per 1.73 m2 is used (2–4). This percentage may increase as the prevalence of CKD rises with the aging of the population (5). In 2008, the third International Workshop for the Management of Asymptomatic PHPT recommended parathyroidectomy (PTX) when PHPT patients have CKD, defined as an eGFR less than 60 mL/min per 1.73 m2 (6, 7). The guidelines recommended using the Modification of Diet in Renal Disease equation (MDRD) to estimate renal function because it was thought to be more accurate than the Cockcroft-Gault method (8).
The third International Workshop CKD guideline is based on the theoretical concern that CKD-associated increases in PTH could worsen the hyperparathyroid state in PHPT, whereas biochemical abnormalities associated with PHPT might hasten the progression of CKD. Although PHPT has the potential to cause CKD by a number of mechanisms including hypercalcemia-induced diuresis, nephrocalcinosis, and nephrolithiasis among others, the contribution of PHPT to the pathogenesis of CKD in modern PHPT is unclear (9). Although the third International Workshop guideline raises interesting questions about how PHPT and CKD affect each another, it is based on very limited empirical evidence. Furthermore, it preferentially impacts elderly individuals with PHPT. These older patients are more likely to meet this guideline due to age-related declines in renal function, but their age may place them at higher risk for complications of surgery. Taken together, these issues make a compelling case for the collection of data to indicate whether this specific eGFR level is associated with increasing levels of PTH in PHPT or worse skeletal health or whether PHPT increases the risk of progressive renal insufficiency.
Since the publication of the last set of guidelines for the Management of Asymptomatic PHPT, newer methods for estimating renal function, such as the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation have been developed. It is unclear whether one method is superior for estimating renal function in PHPT. The purpose of this analysis was 2-fold: 1) to evaluate whether the biochemical abnormalities or clinical correlates of PHPT were associated with worse renal function and 2) to assess whether there were differences in the former analysis when different methods for estimating eGFR were used. We hypothesized that clinical indicators of disease severity, such as the level of hypercalcemia and nephrolithiasis, would be associated with worse renal function in patients with PHPT.
Materials and Methods
This is a case series of PHPT patients with and without reduced renal function. All patients gave written, informed consent, and this investigation was approved by the Institutional Review Board of Columbia University Medical Center.
Subjects
Participants in this analysis represent consecutive patients with PHPT who agreed to participate in our prior studies of PHPT and in whom a creatinine level was available (114 of 119 patients) (10, 11). As previously described, participants were recruited from the Metabolic Bone Diseases Unit as well as the Endocrine Surgery and General Endocrinology Clinics at Columbia University Medical Center. Fifty-five consecutive PHPT patients were enrolled between 2005 and 2008 (10) and 59 between June 2011 and January 2013. All participants had PHPT, diagnosed by the presence of hypercalcemia (calcium > 10.2 mg/dL) and an elevated or inappropriately normal PTH level. None had thiazide-induced hyperparathyroidism or familial hypocalciuric hypercalcemia (excluded on the basis of history and a fractional excretion of calcium > 0.01). Exclusion criteria included current use of cinacalcet, malignancy within 5 years other than nonmelanomatous skin cancer, granulomatous diseases, HIV, liver disease; gastrointestinal diseases affecting calcium metabolism; and pregnancy. Both symptomatic (ie, those with nephrolithiasis) and asymptomatic PHPT were enrolled, regardless of meeting 2008 guidelines for parathyroidectomy (6).
Clinical evaluation
Demographic data, medical history, and medication use were obtained from participants as previously described using the Northern Manhattan Study (NOMAS) questionnaire (10–12, 14), including race/ethnicity by self-identification; history of myocardial infarction (MI); stroke; hypercholesterolemia: a physician's report of elevated lipid levels or being on a lipid-lowering medication; hypertension (a patient's self-report of hypertension or antihypertensive medication use); diabetes mellitus (a patient's self-report of diabetes or use of insulin or other hypoglycemic medications); cigarette smoking (categorized as nonsmoker, current smoker, or past-smoker); and information regarding kidney stones and osteoporosis (obtained by history).
Biochemical evaluation
Fasting samples for serum calcium, phosphate, albumin, glucose, and creatinine were measured by an automated chemistry analyzer. PTH was measured by an immunochemilumometric assay for intact PTH, which detects PTH (1–84) and PTH (7–84). Creatinine values were measured in a Clinical Laboratory Improvement Amendments-certified laboratory that complies with national standards and used methods that produce results with an acceptable bias (15). Serum 25-hydroxyvitamin D (25OHD) was measured by liquid chromatography/tandem mass spectroscopy, and serum 1,25-dihydroxyvitamin D was measured by a RIA. C-terminal fibroblast growth factor-23 (FGF-23) was measured by an ELISA [Immutopics; published normal mean 43.6 ± 4.3 RU/mL (16)]. eGFR was calculated from a single creatinine level (n = 55) or the average of two creatinine values (mean ± SD 4.4 ± 7.2 months apart on average) when available (n = 59) using the MDRD equation as recommended by the third International Workshop on Asymptomatic PHPT (7, 8). Because there is uncertainty regarding the best method for estimating eGFR, we also used the CKD-EPI equation (17).
Statistical analysis
Between-group differences were evaluated by an independent two-sided t test, a χ2 test, or a Fisher's exact test as appropriate. Critical test values were adjusted for unequal variances when appropriate (SAS Stat; SAS Institute). Relationships between eGFR and continuous variables were assessed with Spearman correlation. Stepwise multiple regression was used to evaluate independent predictors [serum PTH; serum calcium; nephrolithiasis; duration of PHPT; age; gender; body mass index (BMI); blood pressure (BP); fasting serum glucose; smoking; alcohol consumption; diagnoses of hypertension, hypercholesterolemia, and diabetes; and antihypertensive medication use] of eGFR as a continuous variable. Both systolic and diastolic BP (SBP and DBP) measurements and history of hypertension were assessed as potential covariates because a single BP measurement may or may not be reflective of average values and therefore may or may not be related to end-organ effects. Urinary calcium excretion was not considered in multiple regression models because it was available on only a subset of participants. The stepwise selection process criterion for entry to the model was a univariate P < .3, and the criterion for retention in the model was a multivariate P < .10. Regression diagnostics were performed to assess normality and other model assumptions. Our analysis indicates that residuals were normally distributed (Shapiro-Wilk test, P = .18). Agreement between the methods for estimating glomerular filtration rate was assessed with a Bland-Altman plot. For all analyses, a two-tailed P < .05 was considered to indicate statistical significance. Statistical analysis was performed using SAS, version 9.3.
Results
Participants were predominantly female (83.3%) and had evidence of mild hypercalcemia (mean ± SD: serum calcium 10.6 ± 0.6 mg/dL; serum PTH 90 ± 54 pg/mL).
Analyses of eGFR using the MDRD equation
Mean eGFR rate in all participants was 82 ± 19 mL/min per 1.73 m2 (range 40–130 mL/min per 1.73 m2). eGFR was negatively associated with age (r = −0.40, P < .0001), 25OHD (r = −0.30, P = .001), fasting serum glucose (r = −0.22, P = .02), and a diagnosis of hypertension (r = −0.20, P = .04) as well as antihypertensive medication use (r = −0.21, P = .02). Several biochemical and clinical indices of PHPT were positively correlated with eGFR: 1,25-dihydroxyvitamin D (r = 0.23, P = .02), 24-hour urine calcium (r = 0.42, P = .01), and a history of nephrolithiasis (r = 0.19, P = .045). No associations were found with any other PHPT indices, including disease duration (r = 0.03, P = .78), serum PTH (r = 0.03, P = .72), serum calcium (r = 0.15, P = .11), phosphate (r = −0.09, P = .33), or serum FGF-23 (r = −0.25, P = .09). The eGFR also had no relationship with SBP (r = −0.08, P = .40), DBP (r = −0.07, P = .50), weight (r = 0.07, P = .47), BMI (r = −0.01, P = .92), alcohol intake (r = 0.17, P = .09), or smoking (r = −0.07, P = .47) in univariate analyses. Other relevant associations included a positive correlation between 24-hour urine calcium and nephrolithiasis (r = 0.34, P = .03) and a trend toward higher calcium excretion in those with higher 1,25-dihydroxyvitamin D levels (r = 0.28, P = .08).
Seventeen participants (15%) had an eGFR less than 60 mL/min per 1.73 m2 (Table 1): 14 with CKD stage 3A (45–59 mL/min per 1.73 m2) and three with CKD stage 3B (30–44 mL/min per 1.73 m2). Those with an eGFR less than 60 (mean 52 ± 6 mL/min per 1.73 m2) were older than those with eGFR of 60 or greater (mean 88 ± 14 mL/min per 1.73 m2) but did not differ by gender, race/ethnicity, or BMI. With regard to their PHPT, this threshold did not distinguish groups by PHPT duration, nephrolithiasis rates or meeting surgical criteria other than the eGFR threshold. Those with CKD did not have evidence of more severe PHPT (higher PTH or calcium) but did have higher 25OHD and lower 1,25-dihydroxyvitamin D levels. Those with low eGFR had a tendency toward greater vitamin D supplementation (2047 ± 3257 vs 419 ± 650 IU/d, P = .06), although there was notable interindividual variability, particularly among those with CKD. Additionally, after adjusting for differences in 25OHD, the differences in PTH levels were further attenuated (least squares means ± SE 90 ± 5 vs 87 ± 13, P = .83). FGF-23 level was elevated compared with published normal means but did not differ between those with and without CKD. There were no other biochemical differences between the groups, nor were there differences in comorbidities, lifestyle factors, or medication use except for a higher frequency of hypertension among those with an eGFR less than 60 mL/min per 1.73 m2, which was of borderline significance (Table 2).
Table 1.
Demographic, Anthropometric, and Biochemical Characteristics
| eGFR ≥ 60 mL/min (n = 97) | eGFR < 60 mL/min (n = 17) | P Value | |
|---|---|---|---|
| Age, y | 61.2 ± 9.4 | 68.0 ± 8.1 | .006 |
| Female, % | 84.5% | 76.5% | .65 |
| Postmenopausal, % | 90.2% | 76.9% | .30 |
| Height, in. | 64.2 ± 3.6 | 64.5 ± 2.9 | .77 |
| Weight, lb | 153 ± 39 | 158 ± 30 | .67 |
| BMI, kg/m2 | 25.8 ± 5.2 | 26.6 ± 4.7 | .56 |
| Race | |||
| White, % | 90.7 | 100.0 | .22 |
| Black, % | 7.2 | 0.0 | |
| Asian, % | 2.0 | 0.0 | |
| Ethnicity | |||
| Hispanic, % | 10.3 | 5.9 | 1.0 |
| PHPT characteristics | |||
| PHPT duration, y | 4.3 ± 5.7 | 3.3 ± 3.1 | .49 |
| Kidney stones, % | 18.8 | 25.0 | .72 |
| Osteoporosis, % | 40.6 | 53.3 | .54 |
| History of fracture, % | 16.7 | 12.5 | 1.0 |
| Serum calcium ≥1 mg/dL above limit, % | 26.0 | 12.5 | .48 |
| Age <50 y, % | 8.3 | 0.0 | .86 |
| Meets surgical guidelines, % | 66.0 | 100.0 | .003 |
| Biochemical evaluation | |||
| 25OHD, ng/mL | 34 ± 11 | 40 ± 12 | .04 |
| 1,25-dihydroxyvitamin D, pg/mL | 71 ± 21 | 51 ± 20 | .0004 |
| Serum calcium, mg/dL | 10.6 ± 0.6 | 10.5 ± 0.5 | .34 |
| Serum PTH, pg/mL | 92 ± 57 | 80 ± 31 | .43 |
| Phosphate, mg/dL | 3.0 ± 0.5 | 3.0 ± 0.4 | .98 |
| Albumin, mg/dL | 4.5 ± 0.3 | 4.5 ± 0.3 | .77 |
| Glucose, mg/dL | 92 ± 15 | 96 ± 15 | .33 |
| FGF-23, RU/mLa | 105 ± 61 | 110 ± 49 | .84 |
| Urine calcium, mg per 24 hb | 285 ± 154 | 182 ± 98 | .12 |
| eGFR, mL/min per 1.73 m2 | 88 ± 14 | 52 ± 6 | N/A |
Abbreviation: N/A, not applicable. Results represent mean ± SD or percentage.
Available for 49 subjects; normal published mean FGF-23 43.6 ± 14.3 RU/mL (16).
Available for 41 subjects.
Table 2.
Comorbidities, Medications, and Lifestyle Factors
| eGFR ≥ 60 mL/min | eGFR < 60 ng/mL | P Value | |
|---|---|---|---|
| Hypertension, % | 35.0 | 58.8 | .06 |
| SBP, mm Hg | 125 ± 16 | 125 ± 18 | .95 |
| DBP, mm Hg | 75 ± 11 | 76 ± 11 | .83 |
| History of myocardial infarction, % | 2.1 | 6.3 | .68 |
| History of CVA, % | 4.1 | 5.9 | .96 |
| History of CHF, % | 0 | 0 | 1.0 |
| Diabetes, % | 4.1 | 0 | 1.0 |
| Hypercholesterolemia, % | 47.4 | 35.3 | .35 |
| Tobacco ever-use, % | 45.3 | 52.9 | .56 |
| Pack-years | 3.6 ± 10.1 | 5.6 ± 10.2 | .46 |
| Current tobacco use, % | 3.2 | 0.0 | 1.0 |
| Alcohol use, % | 38.1 | 29.4 | .49 |
| Antihypertensive medication use, %a | 37.1 | 58.8 | .09 |
| Cholesterol medication use, % | 37.2 | 29.4 | .54 |
| Bisphosphonate use, % | 5.2 | 5.9 | 1.0 |
Abbreviations: CHF, congestive heart failure; CVA, cerebrovascular accident. Results represent mean ± SD or percentage.
Some participants were taking antihypertensive medications for indications other than hypertension.
As shown in Table 3, in a stepwise multiple linear regression model with potential risk factors for CKD [including general risk factors (age; gender; BMI; BP; fasting serum glucose; smoking; alcohol use; and diagnoses of hypertension, hypercholesterolemia, and diabetes; and antihypertensive medication use) as well as those specific to PHPT (serum PTH; serum calcium; nephrolithiasis; duration of PHPT)], age, and DBP were negatively associated with eGFR. The parameter estimates are interpreted to mean that each 10-year increase in age was associated with a 6.5-mL/min per 1.73 m2 lower eGFR, whereas each 10-mm Hg increase in DBP was associated with a 4.2-mL/min per 1.73 m2 lower eGFR. In contrast, serum calcium, a history of nephrolithiasis, and alcohol intake were positively associated with eGFR (ie, higher calcium, greater alcohol intake, and a history of kidney stones were associated with better renal function).
Table 3.
Multiple Linear Regression Model of eGFRa
| Parameter | β | SE | Partial R2 | P Value | Model R2 |
|---|---|---|---|---|---|
| eGFR calculated by MDRD | |||||
| Age (per 10 y increase) | −6.5 | 2.0 | 0.103 | .002 | 0.285 |
| Kidney stones | 10.1 | 4.5 | 0.058 | .03 | |
| Serum calcium (per 1 mg/dL increase) | 7.4 | 3.1 | 0.040 | .02 | |
| DBP (per 10 mm Hg increase) | −4.2 | 1.9 | 0.044 | .03 | |
| Alcohol consumption (per weekly drink) | 1.4 | 0.7 | 0.040 | .049 | |
| eGFR calculated by CKD-EPI | |||||
| Age (per 10 y increase) | −9.2 | 1.8 | 0.212 | <.0001 | 0.334 |
| Serum calcium (per 1 mg/dL increase) | 5.2 | 2.8 | 0.031 | .07 | |
| DBP (per 10 mm Hg increase) | −4.5 | 1.7 | 0.041 | .01 | |
| Alcohol consumption (per weekly drink) | 1.5 | 0.6 | 0.049 | .02 |
Abbreviations: β, parameter estimate; R2, coefficient of determination.
eGFR was analyzed as a continuous measure.
Estimated eGFR using the CKD-EPI equation
There was a very strong association (r = 0.96, P < .0001) between eGFR calculated using the two methods in PHPT. As shown in Figure 1, the Bland-Altman plot indicated good agreement in the clinically relevant range between eGFR estimated by the MDRD and CKD-EPI with no evidence of systemic bias. Disagreement increased at eGFR greater than 100 mL/min per 1.73 m2. In the entire cohort, mean eGFR (using the CKD-EPI equation) was 82 ± 18 mL/min per 1.73 m2. Eighteen participants were categorized as having an eGFR less than 60 mL/min per 1.73 m2: 15 with CKD stage 3A and three with CKD stage 3B. Two individuals who had previously been categorized in the non-CKD group using the MDRD equation were recategorized as having CKD, whereas one individual who had been categorized as has having CKD using the MDRD equation now had an eGFR greater than 60 mL/min per 1.73 m2. All three individuals had eGFRs ranging from 59 to 63 mL/min per 1.73 m2 using the MDRD method.
Figure 1.
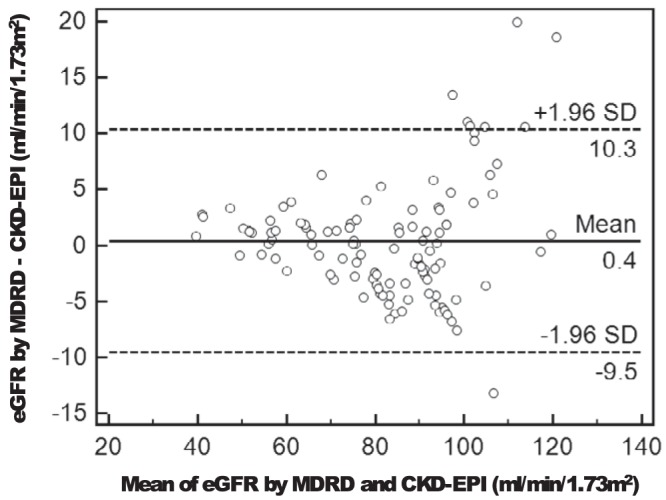
Bland-Altman plot of the agreement between the eGFR by the MDRD and the CKD-EPI equations. The solid horizontal line represents the mean difference, and the dashed lines represent the limits of agreement (mean ± 1.96 SD).
Associations between eGFR and demographics and biochemistries were similar, regardless of the method used to estimate glomerular filtration rate: in the entire cohort, more severe kidney dysfunction (eGFR calculated by CKD-EPI) was associated with older age (r = −0.53, P < .0001), lower levels of 25OHD (r = −0.28, P = .003), higher levels of fasting glucose (r = −0.21, P = .03), and a history of hypertension (r = −0.25, P = .007) as well as antihypertensive medication use (r = −0.23, P = .01). In contrast, higher levels of urinary calcium (r = 0.45, P = .004) and 1,25-dihydroxyvitamin D (r = 0.21, P = .03) were associated with higher eGFR. eGFR was no longer significantly associated with nephrolithiasis (r = 0.15, P = .09). As when the MDRD estimation was used, there was no association between eGFR and PHPT such as the duration of disease (r = −0.04, P = .64), serum PTH (r = −0.005, P = .96), serum calcium (r = 0.11, P = .26), phosphate (r = −0.07, P = .43), or serum FGF-23 (r = −0.25, P = .09). In addition, eGFR did not have a relationship with SBP (r = −0.11, P = .25), DBP (r = −0.06, P = .55), weight (r = 0.05, P = .59), BMI (r = −0.05, P = .63), alcohol intake (r = 0.12, P = .23), or smoking (r = −0.10, P = .32) in univariate analyses.
Between-group differences were similar, regardless of the eGFR estimation method. As when the MDRD method was used, analysis with CKD-EPI demonstrated that those with an eGFR less than 60 (mean ± SD 53 ± 6 mL/min per 1.73 m2) were older (69.3 ± 7.6 vs 60.9 ± 7.6 y, P = .0004) than those with eGFR of 60 or greater (mean eGFR 88 ± 14 mL/min per 1.73 m2). Likewise, those with CKD were more likely to have a history of hypertension (58.8 vs 35.1%, P = .06), had higher 25OHD (40 ± 11 vs 33 ± 11 ng/mL, P = .03), and had lower 1,25-dihydroxyvitamin D (55 ± 17 vs 70 ± 22, P = .008). Additionally, those with CKD had lower urinary calcium excretion (145 ± 54 vs 291 ± 152 mg per 24 h, P = .0003) and were more likely to be taking antihypertensive medications (61.1 vs 36.5%, P = .05). There were no other between-group differences in biochemistries, comorbidities, lifestyle factors, or bisphosphonate use (11.1% vs 4.2%, P = .43). Predictors of eGFR (using the CKD-EPI method) were similar (Table 3), although nephrolithiasis was no longer significantly associated with eGFR (P = .14), and the association with serum calcium was of borderline significance (P = .07).
Discussion
In this study we assessed associations between clinical and biochemical indices of disease severity and renal function in mild PHPT. We did not find that higher serum calcium or PTH or nephrolithiasis were associated with worse renal function. In fact, counter to our hypothesis, one of our multiple regression models indicated that higher serum calcium and nephrolithiasis were associated with better rather than worse renal function. Instead, traditional risk factors for renal failure in the nonhyperparathyroid population, such as age and DBP, were associated with worse renal function in PHPT. Our data do not support the hypotheses that the biochemical or clinical abnormalities of mild PHPT predispose to CKD or that the hyperparathyroid process (as demonstrated by higher serum PTH) is exacerbated by stage 3 CKD. We can not, however, exclude the possibility that severe PHPT, which is rarely seen in the United States today, could be detrimental to renal function. Likewise, we can not rule out the possibility and indeed would expect that more severe CKD could be characterized by higher PTH levels.
The fact that biochemical indices of disease severity were not associated with worse renal function in PHPT is consistent with most longitudinal data in PHPT, which suggests renal function remains stable when the disease is monitored without surgical intervention over many years (18, 19). One recent epidemiological study in mild PHPT (mean calcium 10.5 mg/dL), however, indicated a 13.8-fold increased risk for developing renal failure in those with vs without PHPT (20). It is unclear how generalizable these data are to the vast majority of PHPT patients, however, given the high rates of comorbidities and mortality in this study. Additionally, it is unclear whether the relationship is causal. Although data are limited, PTX has not been shown to improve renal function, and data in severe PHPT actually suggest CKD may worsen after PTX, although one small study (n = 19) indicated that concentrating capacity improves after PTX (9, 21).
We did not detect the increase in PTH from secondary hyperparathyroidism that has been observed in CKD patients without PHPT and that was anticipated by the third International Workshop's Guideline. These findings are consistent with our prior published findings (4). Likewise, Tassone et al (3) found secondary increases in PTH in PHPT only when the glomerular filtration rate was less than 30 mL/min per 1.73 m2, a lower threshold than that identified by current International PHPT guidelines. Other data are inconsistent: a glomerular filtration rate limit less than 70 mL/min per 1.73 m2 was associated with a further elevation of PTH in PHPT in one investigation but not in another (22, 23). Differences in the number of participants with severe CKD between studies (3, 22, 23) may account for these discrepancies.
Alternatively, these inconsistencies may be explained by differences in the 25OHD level, which is likely to be an important effect modifier in determining the threshold of glomerular filtration rate at which an increase in PTH occurs. In the three studies that did not find increases in PTH with a glomerular filtration rate of less than 60–70 mL/min per 1.73 m2, the 25OHD level was 24 ng/mL or greater, whereas the mean level was clearly deficient (15 ng/mL) in the only study that reported an increase in PTH. Our current cohort differs from those previously reported by having robust 25OHD levels (cohort mean 34 ng/mL). Moreover, those with CKD had a trend toward a higher vitamin D intake, likely accounting for the higher 25OHD levels in this group.
Several indices of PHPT disease activity were unexpectedly worse in those with better renal function. The positive association between serum calcium and eGFR can be explained by higher 1,25-dihydroxyvitamin D levels or lower phosphate (within the normal range) in those with more functioning renal mass (higher eGFR). We did find lower urine calcium excretion in those with CKD (when eGFR was estimated using the CKD-EPI equation). This finding has been reported in CKD without concomitant PHPT (18). The reason for the positive association between eGFR and history of nephrolithiasis observed in our study is unclear but could be related to higher urine calcium excretion due to higher 1,25-dihydroxyvitamin D production in those with better renal function and PHPT (which also stimulates production of 1,25-dihydroxyvitamin D). Indeed, we did find a positive association between urine calcium excretion and kidney stones as well as a trend toward higher calcium excretion in those with higher 1,25-dihydroxyvitamin D levels. Although an association between urine calcium and nephrolithiasis has been demonstrated in the general population (24), it has not been consistently shown in those with PHPT. In fact, because of the inconsistent relationship, elevated urine calcium (>400 mg per 24 h) is no longer considered to be an indication for PTX (4). The mechanism underlying this somewhat unexpected positive association between eGFR and nephrolithiasis, however, requires further investigation.
In contrast to some prior work, we did not observe higher serum phosphate (within the normal range) in those with an eGFR less than 60 mL/min per 1.73 m2, although frankly elevated phosphate levels would not be expected until an eGFR reached less than 30 mL/min per 1.73 m2 (4). Additionally, we did not find an increase in FGF-23 levels in those with CKD. This finding was unexpected because FGF-23 is typically identifiable as an early indicator of CKD in those without PHPT (25). However, in PHPT, FGF-23 is elevated compared with normal ranges, which could make it a less sensitive indicator of CKD (26, 27). Alternatively, the lack of association in our study could be due to the fact that FGF-23 levels were available on only a minority of patients (n = 41; only six of whom had an eGFR less than 60 mL/min per 1.73 m2). Thus, these values may not be reflective of a larger group of PHPT patients with CKD. The positive association between alcohol consumption and eGFR observed in our study is consistent with the findings from a recent Italian study (13).
Because newer methods of calculating eGFR have become available since the 2008 guidelines suggested the use of MDRD, we analyzed our data using two calculation tools. Importantly, differences among the results were quite limited. A few individuals with an eGFR close to the threshold of 60 mL/min per 1.73 m2 were reclassified from stage 2 to stage 3 CKD or vice versa. Several studies suggest that the CKD-EPI equation may improve risk stratification for mortality and renal outcomes compared with the MDRD equation (20–22), but the former is thought to classify a larger proportion of older individuals as having CKD, which could lead to an overdiagnosis of CKD in the elderly. The Kidney Disease Improving Global Outcomes guidelines do not unequivocally recommend one equation over the other. Because our data suggest that individuals who have eGFR levels close to the threshold of 60 mL/min per 1.73 m2 may or may not meet criteria for parathyroidectomy, depending on which equation is used, other information may be useful in those cases. These could include repeat serum creatinine determinations and/or serum cystatin levels among others.
Our study has several limitations, most notably, its cross-sectional design and the fact that few participants had reduced renal function. Furthermore, those with CKD had very mild renal dysfunction. We noted a CKD prevalence rate of approximately 15%–16% in our PHPT cohort. This is consistent with rates in most prior studies (2, 17, 18) and suggests that CKD is not seen in most patients with mild PHPT. However, it is also possible that calcium could normalize due to lower 1,25-dihydroxyvitamin and higher serum phosphate in those with late-stage CKD, leading to PHPT underdiagnosis. Although the size of our cohort was robust by PHPT cohort standards, the small numbers with CKD in our study could have impaired our ability to detect between-group differences. For example, with the current sample, we had the ability to detect differences in PTH of 0.76 SD or greater. For these reasons we conducted both continuous and categorical analyses.
Second, a single creatinine level (as was available for about half our participants) may not be reflective of chronic renal function. It is also important to note that our analysis was performed on a convenience sample of PHPT patients enrolled in investigations designed for other purposes. For this reason, more accurate methods for assessing renal function such as inulin clearance, creatinine clearance, cystatin C, or isolution dilution mass spectrometry were not available, and we can not determine from this analysis which method of estimating glomerular filtration rate is better in patients with PHPT, given the lack of a gold standard. Our intention, however, was to test the renal cut point added to the International PHPT Guidelines for Surgery in 2008 and to determine whether the two methods of measuring eGFR were generally consistent, which our data suggest. Similarly, we could not diagnose CKD stages 1 and 2 because we did not have information regarding type of renal disease or proteinuria, both of which would be important to collect in future prospective investigations. Further investigation of renal function in PHPT is clearly warranted.
Given that this was a convenience sample, information regarding nephrolithiasis was based on history and FGF-23, and urinary calcium excretion values were available on only a subset of participants and could not be included in linear regression models. However, urine calcium excretion was positively rather than negatively associated with eGFR in our univariate analyses, probably reflecting the reduced filtration of calcium in those with renal impairment. Lastly, our models accounted for only 28%–33% of the variance in eGFR, which highlights the need for further evaluation of the pathophysiology of kidney dysfunction in PHPT.
Despite these limitations, we believe that these data provide new insight regarding clinical risk factors for renal dysfunction in mild PHPT and also add to the very limited data available specifically assessing the eGFR cut point identified as a guideline for parathyroidectomy. In summary, our findings indicate that traditional risk factors, rather than clinical or biochemical indices of PHPT disease activity, are associated with worse renal function in those with PHPT. These results do not support the theoretical basis for surgical intervention in PHPT patients with mildly impaired renal function. Larger studies that include adequate numbers of PHPT patients with both mild and more severe CKD are needed to determine the appropriate eGFR at which PHPT may be exacerbated by CKD or vice versa. Likewise, longitudinal data are needed to further elucidate the etiology of renal dysfunction in PHPT and to determine whether a cure of PHPT alters or reverses the course of CKD in affected patients.
Acknowledgments
E-mail addresses include the following: Marcella D. Walker, mad2037@columbia.edu; Thomas Nickolas, tln2001@cumc.columbia.edu; Anna Kepley, alk2186@columbia.edu; James A. Lee, jal74@columbia.edu; Chiyuan Zhang, cz2168@columbia.edu; Donald J. McMahon, djm6@columbia.edu; and Shonni J. Silverberg, sjs5@columbia.edu
This work was supported by National Institutes of Health Grants UL1 TR000040, K24 DK074457, R01 DK084986, R01 DK066329 as well as the Joseph Weintraub Family Foundation.
Disclosure Summary: The authors have no conflict of interest.
Footnotes
- BMI
- body mass index
- BP
- blood pressure
- CKD
- chronic kidney disease
- CKD-EPI
- Chronic Kidney Disease Epidemiology Collaboration
- DBP
- diastolic BP
- eGFR
- estimated glomerular filtration rate
- FGF-23
- fibroblast growth factor-23
- MDRD
- Modification of Diet in Renal Disease equation
- 25OHD
- 25-hydroxyvitamin D
- PHPT
- primary hyperparathyroidism
- PTX
- parathyroidectomy
- SBP
- systolic BP.
References
- 1. Marcocci C, Cetani F. Clinical practice. Primary hyperparathyroidism. N Engl J Med. 2011;365:2389–2397 [DOI] [PubMed] [Google Scholar]
- 2. Rejnmark L, Vestergaard P, Mosekilde L. Nephrolithiasis and renal calcifications in primary hyperparathyroidism. J Clin Endocrinol Metab. 2011;96:2377–2385 [DOI] [PubMed] [Google Scholar]
- 3. Tassone F, Gianotti L, Emmolo I, Ghio M, Borretta G. Glomerular filtration rate and parathyroid hormone secretion in primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94:4458–4461 [DOI] [PubMed] [Google Scholar]
- 4. Walker MD, Dempster D, McMahon DJ, et al. Effect of renal function on skeletal health in primary hyperparathyroidism. J Clin Endocrinol Metab. 2012;97(5):1501–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nickolas TL, Leonard MB, Shane E. Chronic kidney disease and bone fracture: a growing concern. Kidney Int. 2008;74:721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bilezikian JP, Khan AA, Potts JT., Jr Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94:335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 8. Eastell R, Arnold A, Brandi ML, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94:340–350 [DOI] [PubMed] [Google Scholar]
- 9. Kristoffersson A, Backman C, Granqvist K, Jarhult J. Pre- and postoperative evaluation of renal function with five different tests in patients with primary hyperparathyroidism. J Intern Med. 1990;227:317–324 [DOI] [PubMed] [Google Scholar]
- 10. Walker MD, Fleischer JB, Di Tullio MR, et al. Cardiac structure and diastolic function in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2010;95:2172–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walker MD, Cong E, Kepley A, et al. Association between serum 25-hydroxyvitamin D level and subclinical cardiovascular disease in primary hyperparathyroidism. J Clin Endocrinol Metab. 2014;99(2):671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gan R, Sacco RL, Kargman DE, Roberts JK, Boden-Albala B, Gu Q. Testing the validity of the lacunar hypothesis: the Northern Manhattan Stroke Study experience. Neurology. 1997;48:1204–1211 [DOI] [PubMed] [Google Scholar]
- 13. Buja A, Scafato E, Baggio B, et al. Renal impairment and moderate alcohol consumption in the elderly. Results from the Italian Longitudinal Study on Aging (ILSA). Public Health Nutr. 2011;14:1907–1918 [DOI] [PubMed] [Google Scholar]
- 14. Walker MD, Fleischer J, Rundek T, McMahon DJ, Homma S, Sacco R, Silverberg SJ. Carotid vascular abnormalities in primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94:3849–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18 [DOI] [PubMed] [Google Scholar]
- 16. Laroche M, Boyer JF, Jahafar H, Allard J, Tack I. Normal FGF23 levels in adult idiopathic phosphate diabetes. Calcif Tissue Int. 2009;84:112–117 [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rao DS, Wilson RJ, Kleerekoper M, Parfitt AM. Lack of biochemical progression or continuation of accelerated bone loss in mild asymptomatic primary hyperparathyroidism: evidence for biphasic disease course. J Clin Endocrinol Metab. 1988;67:1294–1298 [DOI] [PubMed] [Google Scholar]
- 19. Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–1255 [DOI] [PubMed] [Google Scholar]
- 20. Yu N, Donnan PT, Leese GP. A record linkage study of outcomes in patients with mild primary hyperparathyroidism: the Parathyroid Epidemiology and Audit Research Study (PEARS). Clin Endocrinol (Oxf). 2010;73(1):30–34 [DOI] [PubMed] [Google Scholar]
- 21. Peacock M. Primary hyperparathyroidism and the kidney: biochemical and clinical spectrum. J Bone Miner Res. 2002;2(suppl 17):N87–N94 [PubMed] [Google Scholar]
- 22. Gianotti L, Tassone F, Cesario F, et al. A slight decrease in renal function further impairs bone mineral density in primary hyperparathyroidism. J Clin Endocrinol Metab. 2006;91:3011–3016 [DOI] [PubMed] [Google Scholar]
- 23. Yamashita H, Noguchi S, Uchino S, et al. Influence of renal function on clinico-pathological features of primary hyperparathyroidism. Eur J Endocrinol. 2003;148:597–602 [DOI] [PubMed] [Google Scholar]
- 24. Lemann J, Jr, Worcester EM, Gray RW. Hypercalciuria and stones. Am J Kidney Dis. 1991;17:386–391 [DOI] [PubMed] [Google Scholar]
- 25. Evenepoel P, Meijers B, Viaene L, et al. Fibroblast growth factor-23 in early chronic kidney disease: additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2010;5:1268–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamashita H, Yamashita T, Miyamoto M, et al. Fibroblast growth factor (FGF)-23 in patients with primary hyperparathyroidism. Eur J Endocrinol. 2004;151:55–60 [DOI] [PubMed] [Google Scholar]
- 27. Witteveen JE, van Lierop AH, Papapoulos SE, Hamdy NA. Increased circulating levels of FGF23: an adaptive response in primary hyperparathyroidism? Eur J Endocrinol. 2012;166:55–60 [DOI] [PubMed] [Google Scholar]


