Abstract
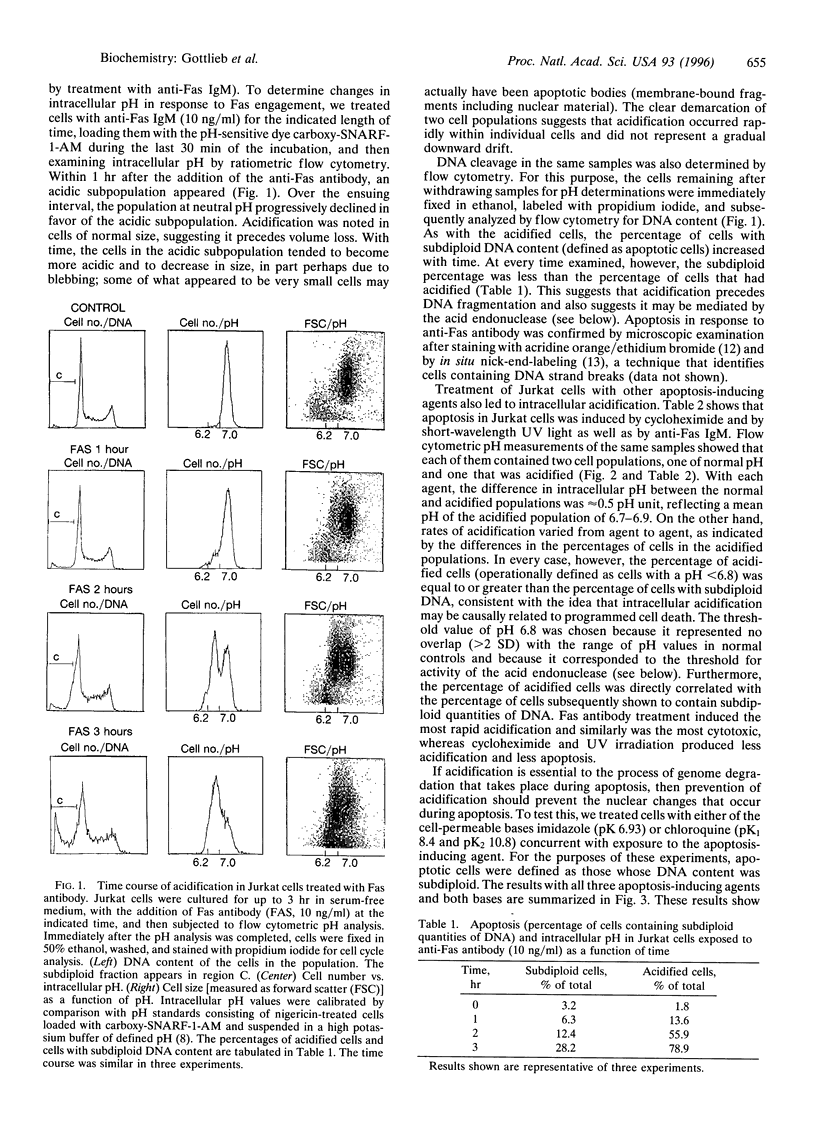
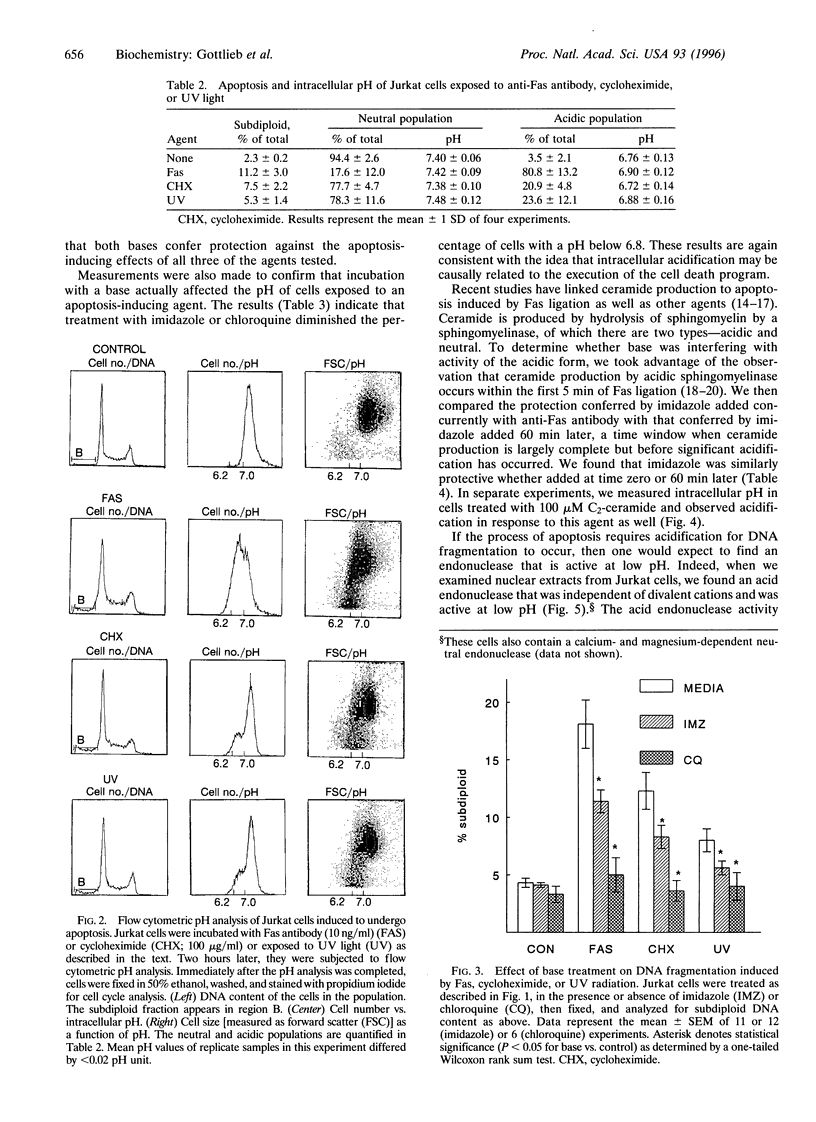
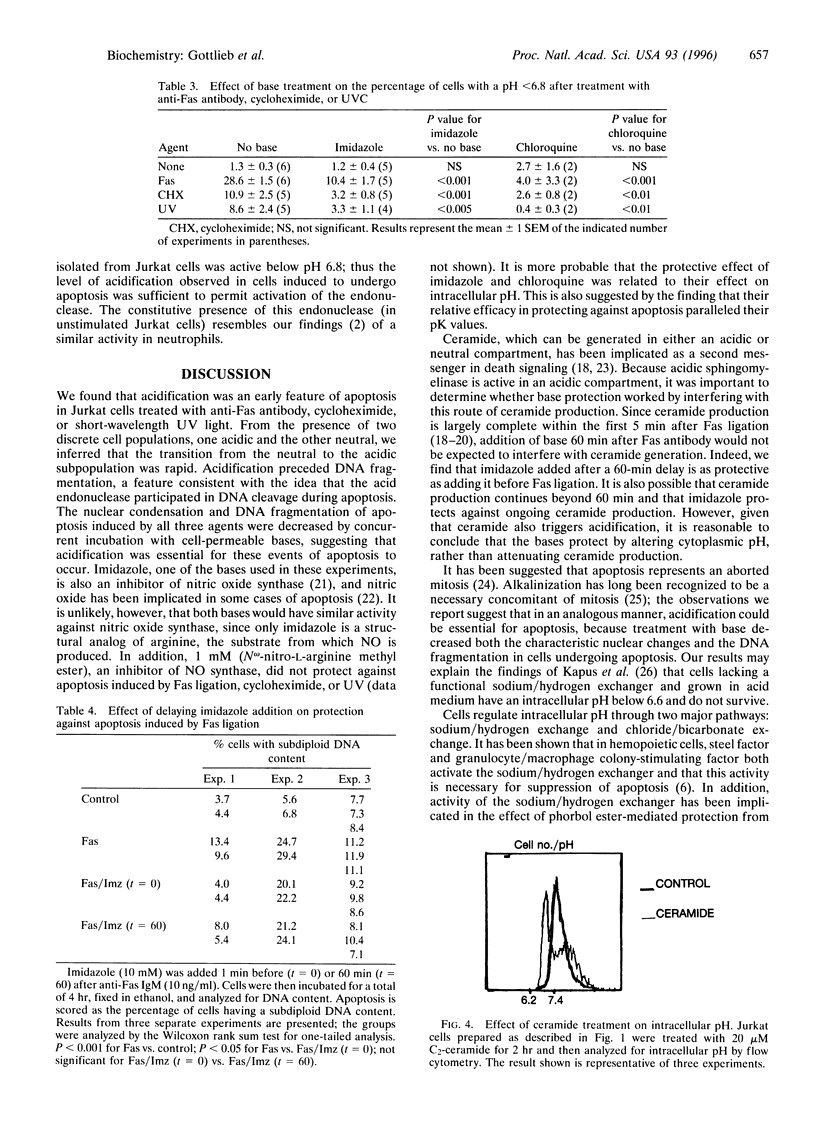
We have previously shown that in neutrophils deprived of granulocyte colony-stimulating factor, apoptosis is preceded by acidification and that the protection against apoptosis conferred on neutrophils by granulocyte colony-stimulating factor is dependent upon delay of this acidification. To test the hypothesis that acidification could be a general feature of apoptosis, we examined intracellular pH changes in another cell line. Jurkat cells, a T-lymphoblastoid line, were induced to undergo apoptosis with anti-Fas IgM, cycloheximide, or exposure to short-wavelength UV light. We found that acidification occurred in response to treatment with these agents and that acidification preceded DNA fragmentation. Jurkat cells were also found to possess an acid endonuclease that is active below pH 6.8, compatible with a possible role for this enzyme in chromatin digestion during apoptosis. Incubation of the cells with the bases imidazole or chloroquine during treatment with anti-Fas antibody or cycloheximide or after UV exposure decreased apoptosis as assessed by nuclear morphology and DNA content. The alkalinizing effect of imidazole and chloroquine was shown by the demonstration that the percentage of cells with an intracellular pH below 6.8 after treatment with anti-Fas antibody, cycloheximide, or UV was diminished in the presence of base as compared with similarly treated cells incubated in the absence of base. We conclude that acidification is an early event in programmed cell death and may be essential for genome destruction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry M. A., Eastman A. Identification of deoxyribonuclease II as an endonuclease involved in apoptosis. Arch Biochem Biophys. 1993 Jan;300(1):440–450. doi: 10.1006/abbi.1993.1060. [DOI] [PubMed] [Google Scholar]
- Barry M. A., Reynolds J. E., Eastman A. Etoposide-induced apoptosis in human HL-60 cells is associated with intracellular acidification. Cancer Res. 1993 May 15;53(10 Suppl):2349–2357. [PubMed] [Google Scholar]
- Bassnett S., Reinisch L., Beebe D. C. Intracellular pH measurement using single excitation-dual emission fluorescence ratios. Am J Physiol. 1990 Jan;258(1 Pt 1):C171–C178. doi: 10.1152/ajpcell.1990.258.1.C171. [DOI] [PubMed] [Google Scholar]
- Cifone M. G., De Maria R., Roncaioli P., Rippo M. R., Azuma M., Lanier L. L., Santoni A., Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J Exp Med. 1994 Oct 1;180(4):1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S., Reichner J. S., Mateo R. B., Albina J. E. Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or -independent mechanisms. Cancer Res. 1994 May 1;54(9):2462–2467. [PubMed] [Google Scholar]
- Cáceres-Cortés J., Rajotte D., Dumouchel J., Haddad P., Hoang T. Product of the steel locus suppresses apoptosis in hemopoietic cells. Comparison with pathways activated by granulocyte macrophage colony-stimulating factor. J Biol Chem. 1994 Apr 22;269(16):12084–12091. [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Cole P. W. Transglutaminase: mechanistic features of the active site as determined by kinetic and inhibitor studies. Biochim Biophys Acta. 1966 Aug 10;122(2):244–264. doi: 10.1016/0926-6593(66)90066-x. [DOI] [PubMed] [Google Scholar]
- Gottlieb R. A., Giesing H. A., Engler R. L., Babior B. M. The acid deoxyribonuclease of neutrophils: a possible participant in apoptosis-associated genome destruction. Blood. 1995 Sep 15;86(6):2414–2418. [PubMed] [Google Scholar]
- Gottlieb R. A., Giesing H. A., Zhu J. Y., Engler R. L., Babior B. M. Cell acidification in apoptosis: granulocyte colony-stimulating factor delays programmed cell death in neutrophils by up-regulating the vacuolar H(+)-ATPase. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):5965–5968. doi: 10.1073/pnas.92.13.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbins E., Bissonnette R., Mahboubi A., Martin S., Nishioka W., Brunner T., Baier G., Baier-Bitterlich G., Byrd C., Lang F. FAS-induced apoptosis is mediated via a ceramide-initiated RAS signaling pathway. Immunity. 1995 Apr;2(4):341–351. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Haimovitz-Friedman A., Kan C. C., Ehleiter D., Persaud R. S., McLoughlin M., Fuks Z., Kolesnick R. N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994 Aug 1;180(2):525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L., Zhang G., Uematsu S., Akahori Y., Hirabayashi Y. Induction of apoptotic DNA fragmentation and cell death by natural ceramide. FEBS Lett. 1995 Jan 23;358(2):211–214. doi: 10.1016/0014-5793(94)01428-4. [DOI] [PubMed] [Google Scholar]
- Kapus A., Grinstein S., Wasan S., Kandasamy R., Orlowski J. Functional characterization of three isoforms of the Na+/H+ exchanger stably expressed in Chinese hamster ovary cells. ATP dependence, osmotic sensitivity, and role in cell proliferation. J Biol Chem. 1994 Sep 23;269(38):23544–23552. [PubMed] [Google Scholar]
- Lamb J. A., Allen P. G., Tuan B. Y., Janmey P. A. Modulation of gelsolin function. Activation at low pH overrides Ca2+ requirement. J Biol Chem. 1993 Apr 25;268(12):8999–9004. [PubMed] [Google Scholar]
- Li J., Eastman A. Apoptosis in an interleukin-2-dependent cytotoxic T lymphocyte cell line is associated with intracellular acidification. Role of the Na(+)/H(+)-antiport. J Biol Chem. 1995 Feb 17;270(7):3203–3211. doi: 10.1074/jbc.270.7.3203. [DOI] [PubMed] [Google Scholar]
- Melino G., Annicchiarico-Petruzzelli M., Piredda L., Candi E., Gentile V., Davies P. J., Piacentini M. Tissue transglutaminase and apoptosis: sense and antisense transfection studies with human neuroblastoma cells. Mol Cell Biol. 1994 Oct;14(10):6584–6596. doi: 10.1128/mcb.14.10.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove E. A., Hedley D. W. Measurement of intracellular pH. Methods Cell Biol. 1990;33:59–69. doi: 10.1016/s0091-679x(08)60511-7. [DOI] [PubMed] [Google Scholar]
- Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. Programmed cell death induced by ceramide. Science. 1993 Mar 19;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Piacentini M., Annicchiarico-Petruzzelli M., Oliverio S., Piredda L., Biedler J. L., Melino E. Phenotype-specific "tissue" transglutaminase regulation in human neuroblastoma cells in response to retinoic acid: correlation with cell death by apoptosis. Int J Cancer. 1992 Sep 9;52(2):271–278. doi: 10.1002/ijc.2910520220. [DOI] [PubMed] [Google Scholar]
- Pérez-Sala D., Collado-Escobar D., Mollinedo F. Intracellular alkalinization suppresses lovastatin-induced apoptosis in HL-60 cells through the inactivation of a pH-dependent endonuclease. J Biol Chem. 1995 Mar 17;270(11):6235–6242. doi: 10.1074/jbc.270.11.6235. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Beyaert R., Vandevoorde V., Haegeman G., Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993 Aug;12(8):3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taresa E., Kedei N., Thomazy V., Fesus L. An involucrin-like protein in hepatocytes serves as a substrate for tissue transglutaminase during apoptosis. J Biol Chem. 1992 Dec 25;267(36):25648–25651. [PubMed] [Google Scholar]
- Telford W. G., King L. E., Fraker P. J. Comparative evaluation of several DNA binding dyes in the detection of apoptosis-associated chromatin degradation by flow cytometry. Cytometry. 1992;13(2):137–143. doi: 10.1002/cyto.990130205. [DOI] [PubMed] [Google Scholar]
- Tepper C. G., Jayadev S., Liu B., Bielawska A., Wolff R., Yonehara S., Hannun Y. A., Seldin M. F. Role for ceramide as an endogenous mediator of Fas-induced cytotoxicity. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8443–8447. doi: 10.1073/pnas.92.18.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper C. G., Jayadev S., Liu B., Bielawska A., Wolff R., Yonehara S., Hannun Y. A., Seldin M. F. Role for ceramide as an endogenous mediator of Fas-induced cytotoxicity. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8443–8447. doi: 10.1073/pnas.92.18.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegmann K., Schütze S., Machleidt T., Witte D., Krönke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994 Sep 23;78(6):1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Wijsman J. H., Jonker R. R., Keijzer R., van de Velde C. J., Cornelisse C. J., van Dierendonck J. H. A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem. 1993 Jan;41(1):7–12. doi: 10.1177/41.1.7678025. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Datto G. A., Samatovicz R. A., Tempsick R. A. Calmodulin-dependent nitric-oxide synthase. Mechanism of inhibition by imidazole and phenylimidazoles. J Biol Chem. 1993 May 5;268(13):9425–9429. [PubMed] [Google Scholar]
- Wolvetang E. J., Johnson K. L., Krauer K., Ralph S. J., Linnane A. W. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994 Feb 14;339(1-2):40–44. doi: 10.1016/0014-5793(94)80380-3. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- el Alaoui S., Mian S., Lawry J., Quash G., Griffin M. Cell cycle kinetics, tissue transglutaminase and programmed cell death (apoptosis). FEBS Lett. 1992 Oct 19;311(2):174–178. doi: 10.1016/0014-5793(92)81392-y. [DOI] [PubMed] [Google Scholar]




