Abstract
Context:
Pituitary antibodies have been measured mainly to identify patients whose disease is caused or sustained by pituitary-specific autoimmunity. Although reported in over 100 publications, they have yielded variable results and are thus considered of limited clinical utility.
Objectives:
Our objectives were to analyze all publications reporting pituitary antibodies by immunofluorescence for detecting the major sources of variability, to experimentally test these sources and devise an optimized immunofluorescence protocol, and to assess prevalence and significance of pituitary antibodies in patients with pituitary diseases.
Study Design and Outcome Measures:
We first evaluated the effect of pituitary gland species, section fixation, autofluorescence quenching, blockade of unwanted antibody binding, and use of purified IgG on the performance of this antibody assay. We then measured cross-sectionally the prevalence of pituitary antibodies in 390 pituitary cases and 60 healthy controls, expressing results as present or absent and according to the (granular, diffuse, perinuclear, or mixed) staining pattern.
Results:
Human pituitary was the best substrate to detect pituitary antibodies and yielded an optimal signal-to-noise ratio when treated with Sudan black B to reduce autofluorescence. Pituitary antibodies were more common in cases (95 of 390, 24%) than controls (3 of 60, 5%, P = .001) but did not discriminate among pituitary diseases when reported dichotomously. However, when expressed according to their cytosolic staining, a granular pattern was highly predictive of pituitary autoimmunity (P < .0001).
Conclusion:
We report a comprehensive study of pituitary antibodies by immunofluorescence and provide a method and an interpretation scheme that should be useful for identifying and monitoring patients with pituitary autoimmunity.
Pituitary autoimmunity can be defined as the presence of immune responses directed against the patient's pituitary gland (1). In the clinical arena, these responses are currently assessed in selected laboratories by measuring serum pituitary antibodies. Broadly speaking, antibodies can be detected by morphological or molecular approaches. The molecular approach is used when the targeted autoantigen is known. In this case, the autoantigen can be purified or synthesized and antibodies against it measured by quantitative techniques such as ELISA or in vitro transcription translation followed by immunoprecipitation. The pituitary gland, however, lags behind the other classic endocrine glands because its autoantigens remain to be identified or validated for clinical use (2). The detection of circulating pituitary antibodies thus relies on morphological approaches such as indirect immunofluorescence (IIF), which are generally considered less sensitive, less quantitative, more labor intensive, and more subjective in the reader's interpretation.
After an initial attempt (3), pituitary antibodies by IIF were reported successfully in 1975 by Bottazzo and colleagues (4) in patients with organ-specific (mainly endocrine) autoimmune diseases. The authors found that 19 of 287 patients (7%) had serum antibodies recognizing cytosolic antigens in the human anterior pituitary. Using 4 of the strongest sera, they noted that antibodies recognized antigens contained in granules of prolactin (PRL)-secreting cells but not PRL itself (4). Up to December 2013, a total of 122 articles have measured pituitary antibodies by IIF using the pituitary gland as substrate. They included 43 cohort studies (summarized in Supplemental Table 1) and 79 case reports (summarized in Supplemental Table 2) and analyzed a broad spectrum of diseases ranging from biopsy-proven hypophysitis to cryptorchidism. Our review of these articles showed large variability in the results that we attributed to the use of different pituitary gland species (human, baboon, rat, Macaca mulatta, bovine, guinea pig, sheep, or swine), different fixation methods (unfixed sections or fixed in acetone, ethanol, methanol, formaldehyde, or glutaraldehyde), and inconsistent disease definitions. We designed the present study to develop a robust and reproducible method for detecting pituitary antibodies by IIF and used this method to assess the prevalence and clinical significance of pituitary antibodies in a well-characterized cohort of patients with different pituitary diseases.
Patients and Methods
Patients and sera
This is a cross-sectional case-control study that analyzed sera from a total of 413 (390 pituitary and 23 thyroid) cases and 60 healthy controls.
Pituitary cases were chosen to represent a broad spectrum of surgical and medical diseases (Supplemental Table 3). They included 218 benign pituitary tumors (75 nonsecreting adenomas, 71 PRL-secreting adenomas, 52 GH-secreting adenomas, 10 ACTH-secreting adenomas, and 10 more rare tumors: 4 pituitary meningiomas, 1 chordoma, 1 spindle cell oncocytoma, 1 granular cell tumor, 1 pituicytoma, 1 TSH-secreting adenoma, and 1 FSH-secreting adenoma); 24 developmental lesions (12 Rathke's cleft cysts and 12 craniopharyngiomas); 44 isolated pituitary hormone deficiencies (21 of anterior pituitary hormones, 23 of ADH); 9 germinomas; 37 sellar masses of unknown pathology; and 58 hypophysitis cases (31 biopsy-proven and 27 clinically suspected). Patients derived from outpatient visits performed by R.S. (n = 242) or G.W. (n = 55) at the Johns Hopkins Pituitary Center during the period from October 2008 to October 2013 as well as from worldwide referrals to the Johns Hopkins Hypophysitis Research Center (n = 83). The study was approved by the Institutional Review Board, and patients signed a written informed consent.
Thyroid cases consisted of 23 patients with Hashimoto's thyroiditis, a disease where pituitary antibodies are reported to be 4-fold more common than in healthy controls (see Figure 2 in Ref. 5). Their sera were used as assay calibrators to initially select the pituitary gland species that performed best in IIF experiments.
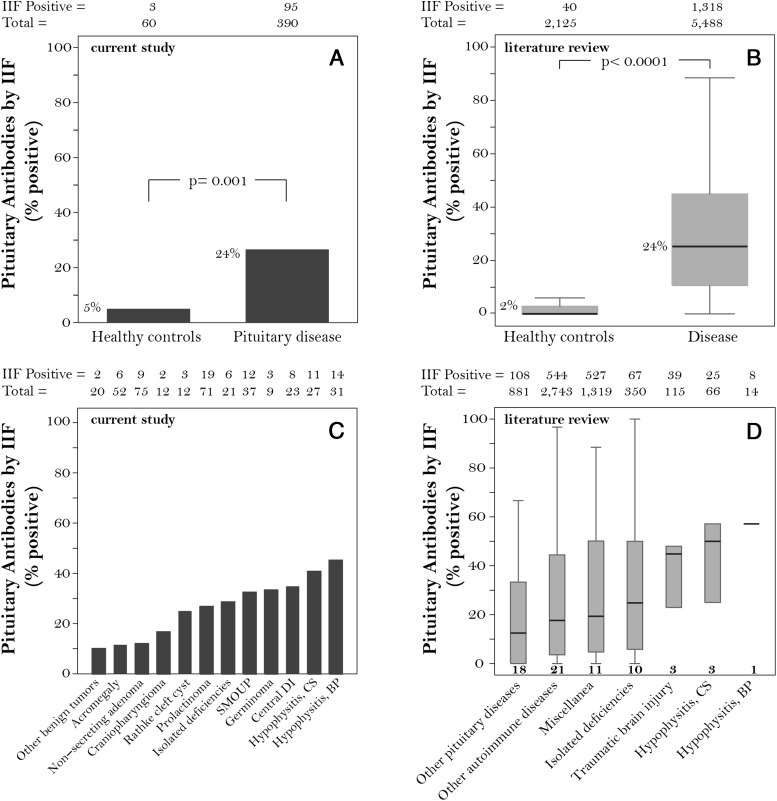
Figure 2.
Prevalence of pituitary antibodies reported dichotomously as present or absent in the current study (left panels) and in previously published studies (right panels). A, Prevalence in our cohort of 60 healthy controls and 390 patients. B, Summary of the studies published from 1969 to 2013 using pituitary gland as substrate for the immunofluorescence assays. C, Prevalence in our cohort of patients with pituitary diseases according to the diagnostic category. D, Results in published literature according to diagnostic categories. Bold numbers underneath each box in D indicate the number of studies. Numbers above the graphs indicate the total number of patients that were studied and how many had positive pituitary antibodies by IIF. Abbreviations: BP, biopsy proven; CS, clinically suspected; DI, diabetes insipidus; SMOUP, sellar masses of unknown pathology.
Healthy controls consisted of 60 blood donors working in the Johns Hopkins University research laboratories.
All sera were diluted 1:10 in PBS supplemented with 0.2% Tween 20 before addition to the pituitary sections.
Pituitary gland species, collection, and preparation
To identify the most suitable pituitary substrate for detection of pituitary antibodies in humans, we first tested 23 Hashimoto's thyroiditis cases and 20 healthy controls on human, cynomolgus monkey, dog, and mouse pituitaries collected at Johns Hopkins. Human pituitaries (n = 45) were obtained in a deidentified fashion from the autopsy laboratory of the Department of Pathology within 24 hours of death. Cynomolgus monkey (n = 2), dog (n = 1), and mouse (n = 7) pituitaries were obtained from the Johns Hopkins Comparative Medicine Department. Glands were embedded in optimal cutting temperature compound (Sakura Finetek USA, Inc), frozen in liquid nitrogen, and sectioned transversely with the cryostat to prepare 5-μm slices, which were then attached onto Superfrost Plus microscope slides (Fisher Scientific).
Analysis and attenuation of fluorescence emitted from the pituitary gland itself (autofluorescence) by laser scanning confocal microscopy
Because the pituitary gland was reported to emit a significant amount of autofluorescence (4), we quantified this feature by confocal microscopy in 4 species; human, cynomolgus monkey, mouse, and M. mulatta, the latter from a commercial kit kindly donated by Euroimmun (6). An argon laser producing excitation wavelength of 488 nm was used to collect emission spectra every 10 nm in the 500 to 640 range, using a Zeiss 710 NLO microscope equipped with a 25× multi-immersion objective. We compared 3 dyes that have been reported to dampen autofluorescence: Evans blue (0.05% solution in physiological saline) (7), Pontamine sky blue (0.05% solution in PBS containing 1% dimethylsulfoxide) (8), and Sudan black B (0.3% solution in 70% ethanol with overnight stirring at room temperature, followed by paper filtration) (9, 10).
Fixation of frozen sections
Fixation is known to improve morphology of frozen sections, likely by preventing further tissue breakdown during the staining procedure, but to have minimal effect on antigenicity (11). Several approaches have been used to prepare pituitary frozen sections for IIF, including no fixation at all and fixation with various fixatives. We compared sections fixed in alumina-filtered ice-cold acetone, 100% ethanol, 100% methanol (20 minutes at room temperature), or 4% paraformaldehyde (10 minutes at room temperature), and found no differences in morphology or antigenicity, choosing acetone for the final protocol based on personal preferences.
Blockade of unwanted antibody binding
The primary (that is, the patient's Igs) or secondary antibodies can theoretically attach to tissue targets independently of their specific (Fab-mediated) binding sites. This unwanted attachment, still the subject of considerable debate (12), is thought to occur via 2 mechanisms: adhesion of the antibody to tissue macromolecules via ionic and hydrophobic interactions (12) and binding of the antibody tail (crystallizable fragment, Fc) to Fc receptors (13). Various blocking solutions have been used to decrease this unwanted attachment. We compared pituitary sections blocked for 1 hour at room temperature in 1% BSA, 1% fetal bovine serum (Sigma), 1% normal goat serum (Sigma), 0.2% human IgG Fc fragment (Jackson Immunoresearch Laboratory), 0.2% human IgG Fab fragment (Jackson Immunoresearch Laboratory), or 0.25% casein (Dako). We found no differences among the various blocking solutions and chose 0.25% casein because it is available as a ready-to-use solution. To specifically assess the binding of antibodies to Fc receptors, we incubated sections for 1 hour at room temperature with a monoclonal antibody directed against Fc receptor II (CD16) and III (CD32) (0.5 μg per section; BioLegend).
IIF staining protocol and interpretation of the IIF results
The final optimized IIF staining protocol is presented in a detailed step-by-step document as Supplemental Material 1. Sections were read independently by A.R. and P.C. and considered positive when stained cells were present in at least 2 of 10 microscopic fields. Discrepant readings occurred rarely (15 of 473 sections, 3%) and were resolved by repeated experiments. Positive sections were scored based on area of involvement, subcellular location, staining intensity, and staining features (Supplemental Material 1). 1) Area of involvement included isolated or multiple patterns, to indicate the staining of either a few isolated noncontiguous cells within a microscopic field, or rather multiple confluent aggregated cells within that field. These 2 patterns were described since the early days of pituitary IIF and called type 1 (single cell) and type 2 (multiple cell) by Pouplard et al (14, 15). 2) Subcellular location included cytosolic, nuclear, plasma membrane, or nuclear membrane staining, although the cytosolic location was the most commonly observed. 3) Staining intensity was classified as weak, moderate, or strong. 4) Staining feature was our attempt to define the different visual aspects originating from the antibody binding. It comprised a diffuse (or homogeneous) pattern when the stain involved uniformly the entire cytosol, a perinuclear pattern when a tight ring of staining encircled the nucleus, a granular pattern when numerous small vesicles were seen in the cytosol, or a mixed pattern when a combination of the above patterns was seen.
Double IIF staining
One-third (n = 31) of the pituitary cases that tested positive for pituitary antibodies were randomly selected to identify the type of adenohypophyseal cells recognized by the patient's antibodies. The 3 healthy controls that tested positive were also analyzed. Acetone-fixed human pituitary sections were costained with the patient serum and a commercial rabbit polyclonal antibody directed against GH, PRL, ACTH, TSHβ, FSHβ, or LHβ (all purchased from Dr A. F. Parlow, National Hormone and Peptide Program). After proper washing, sections were costained with a fluorescein isothiocyanate-conjugated goat F(ab′)2 antihuman IgG (diluted as above) to reveal the patient antibody binding in the green channel, and a DyLight 649-conjugated goat antirabbit IgG (Jackson Immunoresearch Laboratory) diluted 1:1000 in PBS supplemented with 0.2% Tween 20 to reveal the antihormone binding in the red channel. 4′,6-Diamidino-2-phenylindole was included in the counterstaining step to reveal nuclei in the blue channel.
Purification of IgG from a subset of human sera
To assess whether purified IgG provided a clearer IIF signal-to-noise ratio than whole serum Igs, we evaluated 4 hypophysitis cases (2 granular and 2 diffuse pattern) and 3 healthy controls. Serum (1 mL) was mixed in a 1:1 ratio with binding buffer (50mM sodium acetate, pH 5.0), centrifuged at room temperature for 20 minutes at 10,000g, and applied to a pre-equilibrated protein G agarose column (Pierce Biotechnology), allowing it to flow completely into the agarose resin. After washing with binding buffer, patient Igs bound to the column were eluted with an acid buffer (0.1M glycine, pH 2.8), quantified by 280-nm spectrophotometry, and verified for integrity by 10% SDS-PAGE.
Literature review
We reviewed all articles that measured human pituitary antibodies by IIF on pituitary gland substrates, from the original report in 1969 (3) to December 2013. These articles have been collected through the years by P.C. using mainly PubMed and Google Scholar queries, and are available at the Johns Hopkins Hypophysitis Research Center (go to http://pathology2.jhu.edu/hypophysitis, and select the Literature link). The articles (n = 122) featured cohort studies (n = 43, Supplemental Table 1) and case reports (n = 79, Supplemental Table 2) and were written in English (n = 109), French (n = 6), Spanish (n = 4), German (n = 2), or Portuguese (n = 1). Articles in Asian languages were not included in this analysis. Data regarding diagnosis, number of patients and controls, number of IIF positives, pituitary substrate species, fixation, and double IIF staining for the various adenohypophyseal cell types were extracted from the articles and entered into a FileMaker database.
Statistical analysis
The main outcome measure was pituitary antibodies, expressed dichotomously as positive (present) or negative (absent). The outcome was modeled by logistic regression in a multivariate model that included diagnostic category, age, sex, pituitary species, and type of fixative. The area under the receiver operating characteristic (ROC) curve was used to assess how well the pituitary antibody assay performed depending upon 4 pituitary species substrates (human, monkey, dog, and mouse) and 4 serum dilutions (1:10, 1:20, 1:40, and 1:80). An assay with no value has an area of 0.5 or less, whereas a perfect assay has an area of 1. All statistical analyses were carried out using Stata software, release 13.
Results
The human pituitary is the best substrate to detect pituitary antibodies
Given the large variation in the pituitary species used as IIF substrate to detect pituitary antibodies (Supplemental Figure 1), we first compared the performance of human, cynomolgus monkey, dog, and mouse pituitary substrates using a set of 23 cases with Hashimoto's thyroiditis (a disease where pituitary antibodies are expected to be found) (5) and 20 healthy controls. The human substrate outperformed the other species, yielding an area under the ROC curve (0.767) that was significantly greater than that obtained with monkey (0.583), dog (0.517), or mouse (0.559) substrates (Figure 1A). In addition, the human substrate nicely displayed the typical adenohypophyseal architecture, with acini of endocrine cells embedded in a network of connective tissue strands, an architecture that was less clear or different in pituitaries from other species.
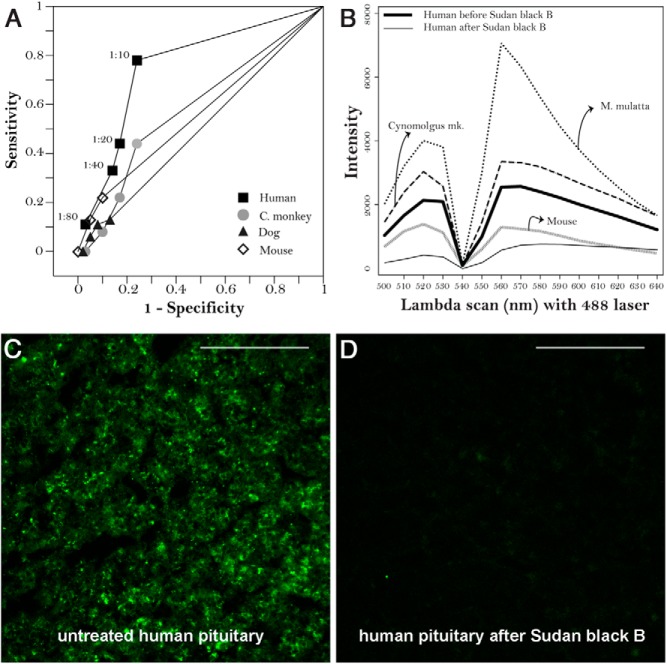
Figure 1.
Basic immunofluorescence characteristic of pituitary gland substrates. A, ROC curves comparing pituitary substrates from four species (human, cynomolgus monkey, dog, and mouse) for the ability to detect pituitary antibodies by indirect immunofluorescence. B, The λ-scans produced by a 488-nm laser excitation showing the intrinsic fluorescence emitted by pituitary glands of 4 species (M. mulatta, cynomolgus monkey, human, and mouse). The human substrate is shown before (thick line) and after (thin line) treatment with Sudan black B. C, Representative image of an unstained, untreated human pituitary gland section to show its autofluorescence. D, The same pituitary section after treatment with Sudan black B. In both panels, magnification, ×40; and scale bar, 100 μm.
The pituitary gland emitted a significant amount of autofluorescence (Figure 1B), originating from the acinar cells (Figure 1C). This autofluorescence confounded interpretation of the results by suggesting positivity for antibody binding in cells that were actually negative and by overall attenuating the signal-to-noise ratio. The commercial M. mulatta pituitary emitted the highest autofluorescence (Figure 1B, dotted line), whereas the in-house mouse (gray line) and human (thick black line) pituitaries were the lowest ones. Incubation of the sections with Sudan black B significantly reduced autofluorescence (Figure 1B, thin black line, and Figure 1D).
Based on ROC curve performance (Figure 1A) and excellent attenuation of autofluorescence after Sudan black B treatment (Figure 1B), the human pituitary was chosen as the substrate for all of the remaining experiments.
Pituitary antibodies are more common in pituitary diseases than healthy controls but do not differentiate among diseases when reported as present/absent
When pituitary antibodies were expressed dichotomously as present or absent and their prevalence compared between pituitary cases and healthy controls, they were significantly more common in disease (95 of 390, 24%) than health (3 of 60, 5%, P = .001, Figure 2A). Our findings were in agreement with those of published articles, where pituitary antibodies also showed greater prevalence in disease (1318 of 5488, 24%) than health (40 of 2125, 2%, P < .0001, Figure 2B). Nevertheless, reporting pituitary antibodies dichotomously, which is the most common reporting format, was not clinically useful because it did not discriminate among pituitary diseases (Figure 2C). In fact, although more common in diseases with an autoimmune pathogenesis, pituitary antibodies were also found at similar frequencies in germinoma, central diabetes insipidus, and isolated anterior hormone deficiencies, as well as in patients with sellar masses of unknown origin (Figure 2C). A similar trend emerged from the analysis of published articles, where the prevalence of pituitary antibodies also did not differ significantly among disease categories (Figure 2D).
When reported according to their cytosolic staining pattern, pituitary antibodies differentiate pituitary diseases of distinct pathogenesis
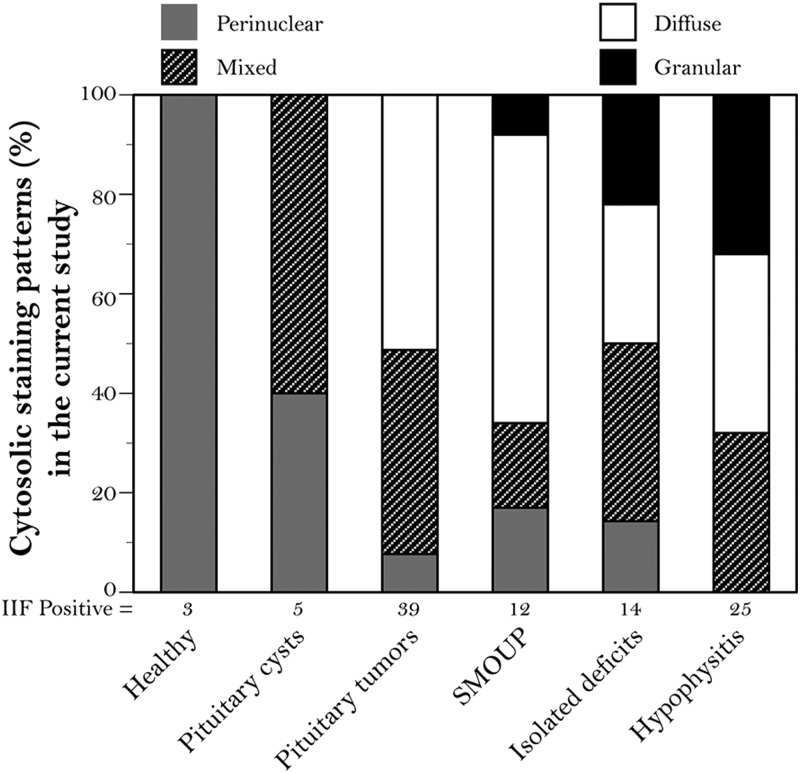
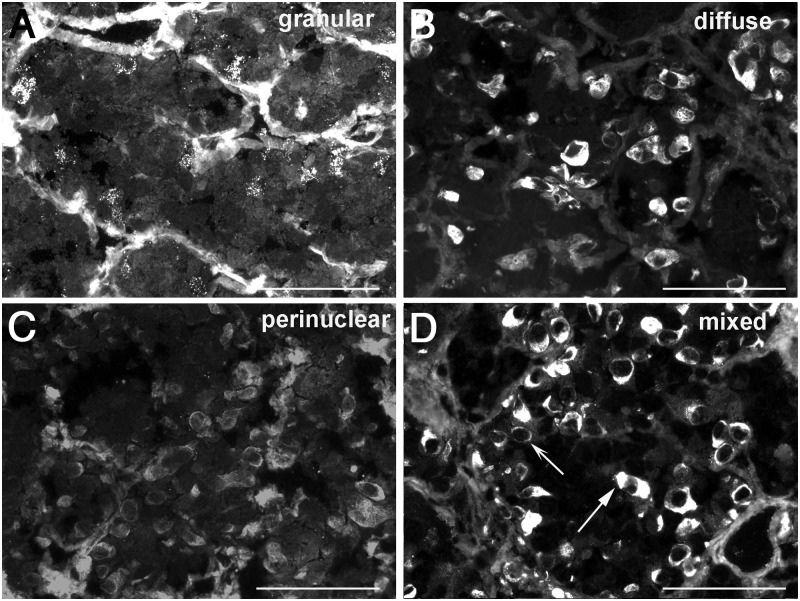
In the attempt to improve the clinical utility of pituitary antibodies, we expressed IIF results using a more comprehensive scheme that went beyond the positive/negative classification. We scored positive sera according to distribution area (isolated or multiple cells), staining intensity (weak, moderate, or strong), and cytosolic staining pattern (perinuclear, perinuclear and diffuse, diffuse, and granular). Staining intensity (P = .622) and distribution area (P = .157) did not distinguish disease types. On the contrary, distribution of the cytosolic staining patterns was markedly different among pituitary diseases (P < .0001, Figure 3). A granular cytosolic staining (Figure 4A) was highly predictive of pituitary autoimmunity because it was most commonly found in patients with hypophysitis, isolated hormone deficiencies, or a sellar mass of unknown pathology (Figure 3). The diffuse pattern (Figure 4B) was found in pituitary diseases with an autoimmune basis but also in pituitary tumors (Figure 3). The perinuclear pattern (Figure 4C), the only one observed in healthy controls, was present in most disease categories so that its inclusion in an overall positive/negative classification markedly reduced the diagnostic accuracy of pituitary antibodies. The mixed pattern (Figure 4D), similarly, did not associate with specific disease categories.
Figure 3.
Distribution (percentage) of cytosolic staining patterns in the 95 patients and 3 healthy controls that tested positive for pituitary antibodies by IIF in the current study. The 5 main diagnostic categories of pituitary diseases are classified according to 4 types of cytosolic recognition: perinuclear (gray shading), mixed (hatched shading), diffuse (white shading), or granular (black shading). Abbreviation: SMOUP, sellar masses of unknown pathology.
Figure 4.
Cytosolic staining patterns produced by pituitary antibodies. A, Granular pattern: several adenohypophyseal cells show distinct and fine granularity dispersed throughout their cytosol. B, Diffuse pattern: a homogeneous coloration is observed in the entire cytosol. C, Perinuclear pattern: a delicate ring of staining encircles the nucleus. D, Mixed pattern: the presence of cells featuring a combination of the diffuse (straight arrow) and perinuclear (concave arrow) pattern. Magnification, ×40; scale bars, 100 μm.
Use of purified IgG increases sensitivity and specificity of the assay
When used in patients who tested positive in the IIF assay based on whole serum, IgG yielded a clearer signal; stained cells stood out more promptly from the surrounding negative areas, overall conferring a starry sky appearance (Supplemental Figure 2). In particular, the granularity feature that we considered associated with pituitary autoimmunity became more prominent (Supplemental Figure 2, arrow). The increased sensitivity was not at the expense of decreased specificity because the healthy controls that tested negative remained negative or unchanged when using their Igs.
Blockade of Fc receptors can be useful in cases of ambiguous cytosolic positivity
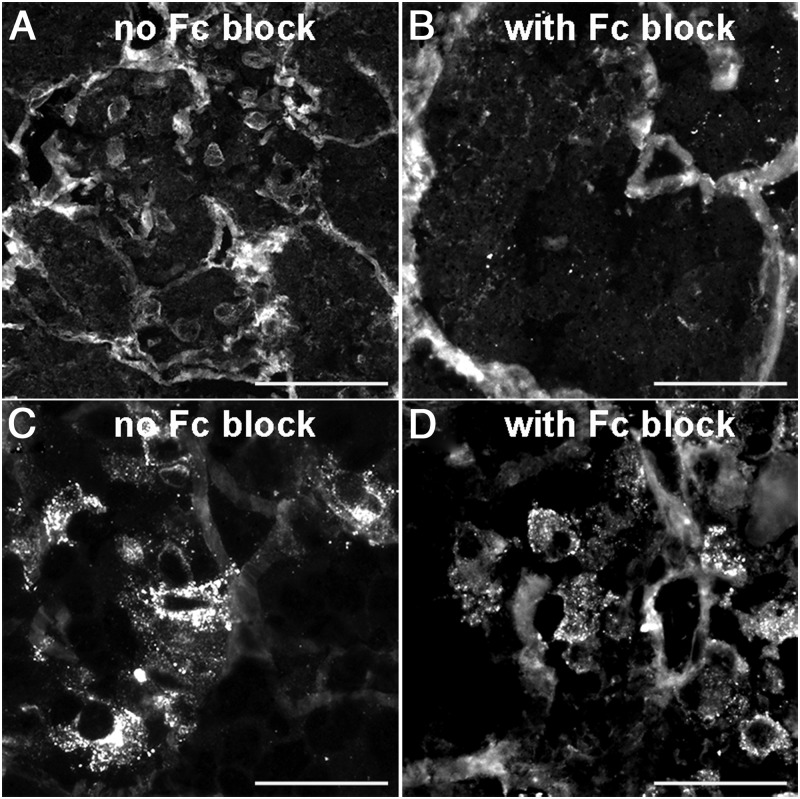
The perinuclear positivity observed in healthy controls (Figure 5A) was markedly decreased by treating the sections with an Fc blocking reagent (Figure 5B), suggesting that this type of antibody binding reflects a nonspecific type of reaction rather than a true pituitary autoimmune response. In contrast, the granular pattern observed in cases with pituitary autoimmunity (Figure 5C) was unaffected by the Fc block pretreatment (Figure 5D), indicating the existence of a true antipituitary antibody response.
Figure 5.
Influence of Fc blockade on the cytosolic staining patterns produced pituitary antibodies. A and B, Before and after effect of Fc blockade when using the serum from a healthy control with a perinuclear pattern: the perinuclear staining (A) disappears (B) when Fc receptors were blocked. C and D, Before and after effect using the serum from a hypophysitis patient with a granular pattern: the staining pattern is unaffected by Fc blockade. Magnification, ×40; scale bars, 100 μm.
Recognition of pituitary cytosolic antigens is broad
Analysis of a subset of IIF patients (n = 31) who tested positive for pituitary antibodies showed that FSH-secreting gonadotrophs were the most frequently recognized cells, independent of disease (Table 1), followed by TSH-, LH-, and ACTH-secreting cells, overall indicating a widespread recognition of pituitary cytosolic antigens. In contrast, GH- and PRL-secreting cells, which are the most abundant anterior pituitary cell type, were recognized only rarely in our series. Analysis of the 3 positive healthy controls, showed that ACTH- and FSH-secreting cells were the preferred targets, in keeping with the notion that ACTH-secreting cells have been postulated to express Fc receptors (13). Our literature review identified 22 studies where hormone double staining was performed, and of them, 18 tested more than one anterior pituitary hormone. Pituitary antibodies were similarly found to recognize numerous adenohypophyseal antigenic targets, although variability in the results was large and dependent upon the substrate used (Supplemental Figure 3) and the center performing the assay.
Table 1.
Double IIF on Human Pituitary Substrate to Identify the Type of Anterior Pituitary Cells Recognized by the Patient Antibodies
| Type of Cell, n (%)a |
||||||
|---|---|---|---|---|---|---|
| GH | PRL | ACTH | LH | TSH | FSH | |
| Cases (n = 31) | ||||||
| Pituitary adenomas (n = 13) | 0 (0) | 0 (0) | 3 (10) | 3 (10) | 11 (36) | 12 (39) |
| SMOUP (n = 2) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (3) | 2 (6) |
| Isolated deficiencies (n = 8) | 0 (0) | 1 (3) | 0 (0) | 2 (7) | 7 (23) | 6 (19) |
| Hypophysitis (n = 8) | 1 (3) | 0 (0) | 2 (6) | 3 (10) | 5 (16) | 7 (23) |
| Controls (n = 3) | 0 (0) | 0 (0) | 2 (67) | 0 (0) | 1 (33) | 2 (67) |
| Total positive | 1 | 1 | 8 | 8 | 25 | 29 |
Abbreviations: SMOUP, sellar mass of unknown pathology.
Results are shown as number of patient sera recognizing a particular pituitary cell type (n) and percentage of the total patient sera or healthy controls recognizing that cell type.
Discussion
This study reports a detailed optimization of the IIF technique to measure pituitary antibodies in human sera using human pituitary gland as substrate, and its application in the thus far largest cohort of patients with pituitary diseases.
Pituitary antibodies, although first described in 1965 (16), have yet to become an established diagnostic tool in the clinical arena and are currently measured only in a few specialized centers around the world. One of the reasons for this limited use is the notion that pituitary autoimmunity, the condition where these antibodies should be more useful, has been traditionally considered rare and the assay consequently of limited clinical significance. The widespread use of noninvasive imaging modalities of the sellar region, the increased awareness in the medical community of hypophysitis, and the development of novel cancer immunotherapies that cause hypophysitis as a side effect (17), however, indicate that pituitary autoimmunity is more common than previously thought and thus the need of a reliable antibody assay more pressing. Another reason relates to the detection technique itself. Antibodies to pituitary substrates and pituitary-specific or -related antigens have been measured by a variety of techniques (summarized in Supplemental Table 4), mainly featuring non–antigen-specific approaches such as IIF, given that true pathogenic pituitary autoantigens remain to be discovered and/or validated. Literature analysis reveals a large variability in the IIF results. For example, in Hashimoto's thyroiditis, serum pituitary antibodies measured by IIF using pituitary gland as the substrate were reported at a prevalence ranging from 0% (14) to 9% (5) or 56% (18). This study identifies the species of the pituitary gland substrate as the most important technical feature.
Species has a strong influence on antigenicity. Although autoantigens are evolutionarily conserved (19), they do contain species-specific epitopes that are often the ones with the greatest pathogenic significance, so that autoantibodies in humans are best detected using human autoantigens. In thyroglobulin, for example, evolutionarily conserved epitopes are recognized by antibodies found in patients with thyroid autoimmunity but also in apparently healthy controls, whereas human-specific epitopes are recognized predominantly by patients (20, 21). Similarly, to detect antibodies to double-stranded DNA in systemic lupus erythematosus, human DNA is the most effective autoantigen (22). This notion of species specificity is even truer in assays based on tissue substrates, rather than purified autoantigens, because the structural context in which autoantigens are found influences antibody recognition (23). For pituitary antibodies, the human pituitary substrate has been used rarely (22 of 122 publications, 18%; Supplemental Figure 1), likely because there are no commercial sources and availability currently depends upon a pathology laboratory with a rapid autopsy program. Human pituitary was used during the early days of pituitary IIF (4), when hypophysectomy represented a form of treatment for patients with advanced breast cancer receiving extensive hormone therapy, including estradiol and the synthetic nonsteroidal estrogen diethylstilbestrol. That original study also used rat and bovine pituitary gland substrates, reporting that “animal experiments were inconclusive owing to substantial nonspecific fluorescence” (4). Only one study formally analyzed pituitary gland species for their ability to detect pituitary antibodies. Glück and Scherbaum (24) compared the human pituitary substrate with that of 6 other species (baboon, cynomolgus monkey, pig, cow, sheep, and rat) using a set of 46 sera that had tested positive on the human substrate and 37 negative hospital controls. They found that the human substrate had superior sensitivity and specificity, concluding that the use of animal substrates “yields results that bear no clinical significance.” Our study confirms that of Glück and Scherbaum (24) and also shows that, when used in combination with a method to decrease autofluorescence, the human pituitary substrate allows determination of not only whether pituitary antibodies are present or absent but also the type of cytosolic staining. A granular type of staining turned out to be the best indicator of an autoimmune pituitary pathology and is therefore a finding that can be useful to clinicians in establishing a diagnosis of hypophysitis.
Fixation and blockade of unwanted antibody binding influenced minimally IIF performance, but a special mention should be reserved for the blockade of Fc receptors. In 1976, Pouplard and colleagues (13) reported that sera of 17 of 22 healthy controls (77%) and 361 of 408 patients (88%) with various diseases contained antibodies that bound to ACTH-secreting cells on either fixed or unfixed human pituitary sections. This binding occurred through the Fc portion of the antibody and gave rise to a “uniform smooth staining pattern involving the entire cytoplasm.” The authors hypothesized that ACTH cells expressed Fc receptors and could therefore bind any serum antibody, independently of whether it was directed against the pituitary or not, thus cautioning the researcher about “looking for autoantibodies to anterior pituitary gland in human patients.” Although we confirmed human ACTH cells can indeed bind human Igs, the binding was of low prevalence and, most importantly, did not affect the granular staining pattern. In this study, we used autopsy pituitaries collected within a relatively short interval postmortem, whereas Pouplard et al (13) used pituitaries collected at surgery from women with advanced breast cancer receiving estrogen therapy. Considering that estrogens markedly increase the expression of Fc receptors (25, 26), it is possible that the discrepancy between our findings and those of Pouplard et al (13) relates once again to the substrate. It is our impression that treating pituitary sections with a Fc receptor blocker is useful in cases of ambiguous cytosolic positivity but, like the purification of IgG, not necessarily needed as a routine protocol feature.
Much remains to be learned. We still have a limited understanding of pituitary autoimmunity and do not know whether pituitary antibodies play a role in disease pathogenesis. Despite attempts by numerous laboratories, including our own, we are still uncertain about the targets recognized by pituitary antibodies. It is our hope that this study will promote use and standardization of the pituitary antibody test; a greater use would lead to a deeper understanding of disease pathogenesis and increase the likelihood of discovering novel autoantigens.
In summary, by performing the largest determination of pituitary antibodies in patients with pituitary diseases, we show that a cytosolic granular staining pattern is the most indicative of pituitary autoimmunity. We also emphasize the importance of using a human pituitary gland substrate and provide protocol details that should be valuable for increasing use and standardization of this IIF assay.
Acknowledgments
We are grateful to Dr Shey-Cherng Tzou for reviewing the manuscript.
The work was supported by National Institutes of Health R21 Grant DK080351 (May 2009 to April 2011) to P.C. and by an Ipsen Pharmaceutical grant to R.S. The cryostat and fluorescence microscope were purchased through an administrative supplement (June 2009) to Grant DK080351.
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 1589
- IIF
- indirect immunofluorescence
- ROC
- receiver operating characteristic.
References
- 1. Caturegli P, Lupi I, Landek-Salgado MA, Kimura H, Rose NR. Pituitary autoimmunity: 30 years later. Autoimmun Rev. 2008; 7:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caturegli P. Autoimmune hypophysitis: an underestimated disease in search of its autoantigen(s). J Clin Endocrinol Metab. 2007;92:2038–2040 [DOI] [PubMed] [Google Scholar]
- 3. Nerup J, Lindholm J, Soborg M, Halberg P. Organ-specific antibodies in idiopathic panhypopituitarism, primary thyroid and adrenal insufficiency. Acta Med Scand. 1969;185:293–296 [DOI] [PubMed] [Google Scholar]
- 4. Bottazzo GF, Pouplard A, Florin-Christensen A, Doniach D. Autoantibodies to prolactin-secreting cells of human pituitary. Lancet. 1975;2:97–101 [DOI] [PubMed] [Google Scholar]
- 5. Guaraldi F, Landek-Salgado MA, Hutfless S, et al. Pituitary antibodies in women with Hashimoto's thyroiditis: prevalence in diagnostic and prediagnostic sera. Thyroid. 2012;22:509–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmermann T. Spectral imaging and linear unmixing in light microscopy. Adv Biochem Eng Biotechnol. 2005;95:245–265 [DOI] [PubMed] [Google Scholar]
- 7. De la Lande IS, Waterson JG. Modification of autofluorescence in the formaldehyde-treated rabbit ear artery by Evans blue. J Histochem Cytochem. 1968;16:281–282 [DOI] [PubMed] [Google Scholar]
- 8. Cowen T, Haven AJ, Burnstock G. Pontamine sky blue: a counterstain for background autofluorescence in fluorescence and immunofluorescence histochemistry. Histochemistry. 1985;82:205–208 [DOI] [PubMed] [Google Scholar]
- 9. Romijn HJ, van Uum JF, Breedijk I, Emmering J, Radu I, Pool CW. Double immunolabeling of neuropeptides in the human hypothalamus as analyzed by confocal laser scanning fluorescence microscopy. J Histochem Cytochem. 1999;47:229–236 [DOI] [PubMed] [Google Scholar]
- 10. Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47:719–730 [DOI] [PubMed] [Google Scholar]
- 11. Stumpf WE, Roth LJ. Thin sections cut at temperature of −70 degrees to −90 degree C. Nature. 1965;205:712–713 [DOI] [PubMed] [Google Scholar]
- 12. Buchwalow I, Samoilova V, Boecker W, Tiemann M. Non-specific binding of antibodies in immunohistochemistry: fallacies and facts. Sci Rep. 2011;1:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pouplard A, Bottazzo GF, Doniach D, Roitt IM. Binding of human immunoglobulins to pituitary ACTH cells. Nature. 1976;261:142–144 [DOI] [PubMed] [Google Scholar]
- 14. Pouplard A, Bigorgne JC, Chevalier JM, Rohmer V, Poron MF. Pituitary insufficiency and auto-immunity [in French]. Nouv Presse Med. 1980;9:1757–1760 [PubMed] [Google Scholar]
- 15. Pouplard-Barthelaix A, Lepinard V, Luxembourger L, Rohmer V, Berthelot J, Bigorgne JC. Circulating pituitary autoantibodies against cells secreting luteinising and follicle stimulating hormones in children with cryptorchidism. Lancet. 1984;2:631–632 [DOI] [PubMed] [Google Scholar]
- 16. Engelberth O, Jezkova Z. Autoantibodies in Sheehan's syndrome. Lancet. 1965;1:107514283762 [Google Scholar]
- 17. Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98:1361–1375 [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi I, Inukai T, Takahashi M, et al. Anterior pituitary cell antibodies detected in Hashimoto's thyroiditis and Graves' disease. Endocrinol Jpn. 1988;35:705–708 [DOI] [PubMed] [Google Scholar]
- 19. Backes C, Ludwig N, Leidinger P, et al. Immunogenicity of autoantigens. BMC Genomics. 2011;12:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown NK, McCormick DJ, Brusic V, David CS, Kong YC. A novel H2A-E+ transgenic model susceptible to human but not mouse thyroglobulin-induced autoimmune thyroiditis: identification of mouse pathogenic epitopes. Cell Immunol. 2008;251:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Latrofa F, Ricci D, Grasso L, et al. Characterization of thyroglobulin epitopes in patients with autoimmune and non-autoimmune thyroid diseases using recombinant human monoclonal thyroglobulin autoantibodies. J Clin Endocrinol Metab. 2008;93:591–596 [DOI] [PubMed] [Google Scholar]
- 22. Janyapoon K, Jivakanont P, Surbrsing R, Siriprapapan W, Tachawuttiwat T, Korbsrisate S. Detection of anti-dsDNA by ELISA using different sources of antigens. Pathology. 2005;37:63–68 [DOI] [PubMed] [Google Scholar]
- 23. McClain MT, Lutz CS, Kaufman KM, Faig OZ, Gross TF, James JA. Structural availability influences the capacity of autoantigenic epitopes to induce a widespread lupus-like autoimmune response. Proc Natl Acad Sci U S A. 2004;101:3551–3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glück M, Scherbaum WA. Substrate specificity for the detection of autoantibodies to anterior pituitary cells in human sera. Horm Metab Res. 1990;22:541–545 [DOI] [PubMed] [Google Scholar]
- 25. Gomez F, Ruiz P, Bernal JA, Escobar M, Garcia-Egido A, Lopez-Saez JJ. Enhancement of splenic-macrophage Fcgamma receptor expression by treatment with estrogens. Clin Diagn Lab Immunol. 2001;8:806–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsieh CH, Nickel EA, Chen J, et al. Mechanism of the salutary effects of estrogen on kupffer cell phagocytic capacity following trauma-hemorrhage: pivotal role of Akt activation. J Immunol. 2009;182:4406–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]