Abstract
Context:
Somatostatin (SST) receptors (SSTRs) are expressed in a number of tissues, including the adrenal cortex, but their role in cortisol secretion has not been well characterized.
Objectives:
The objective of the study was to investigate the expression of SSTRs in the adrenal cortex and cultured adrenocortical cells from primary pigmented nodular adrenocortical disease (PPNAD) tissues and to test the effect of a single injection of 100 μg of the SST analog octreotide on cortisol secretion in patients with PPNAD.
Setting and Design:
The study was conducted at an academic research laboratory and clinical research center. Expression of SSTRs was examined in 26 PPNAD tissues and the immortalized PPNAD cell line CAR47. Ten subjects with PPNAD underwent a randomized, single-blind, crossover study of their cortisol secretion every 30 minutes over 12 hours (6:00 pm to 6:00 am) before and after the midnight administration of octreotide 100 μg sc.
Methods:
SSTRs expression was investigated by quantitative PCR and immunohistochemistry. The CAR47 and primary cell lines were studied in vitro. The data of the 10 patients were analyzed before and after the administration of octreotide.
Results:
All SSTRs, especially SSTR1–3, were expressed in PPNAD at significantly higher levels than in normal adrenal. SST was found to differentially regulate expression of its own receptors in the CAR47 cell line. However, the administration of octreotide to patients with PPNAD did not significantly affect cortisol secretion.
Conclusions:
SSTRs are overexpressed in PPNAD tissues in comparison with normal adrenal cortex. Octreotide did not exert any significant effect on cortisol secretion in a short clinical pilot study in a small number of patients with PPNAD, but long-acting SST analogs targeting multiple SSTRs may be worth investigating in this condition.
Somatostatin (SST) is expressed in a wide range of tissues throughout the body in which it plays a pivotal role in the regulation of secretion of multiple hormones and bioactive peptides (1). SST exerts its physiological actions through several G protein-coupled receptor subtypes [SST receptor (SSTR)-1, SSTR2A, SSTR2B, and SSTR3–5] whose activation leads to inhibition of adenylate cyclase and calcium channels as well as stimulation of potassium channels and phosphotyrosine phosphatase (2, 3). It has been observed that the adrenal cortex expresses all five receptor subtypes, SSTR2 and SSTR4 being the predominant subtypes (4, 5). All SSTR immunoreactivities were mainly visualized in zona reticularis (ZR), whereas the adrenal medulla was found to mostly express SSTR3 (6). Among adrenocortical lesions, SSTR2 and SSTR3 have been found to be expressed in cortisol-secreting adenomas, SSTR2, SSTR3 and SSTR4 in aldosterone-secreting adenomas, and SSTR2 and SSTR3 (without any detectable expression of SSTR4) in nonfunctioning tumors (5, 7). In pheochromocytomas, SSTR3 is the predominantly expressed receptor subtype, although the other receptors may also be occasionally present (6).
Primary pigmented nodular adrenocortical disease (PPNAD) is a pigmented form of primary bilateral adrenocortical hyperplasia that often leads to ACTH-independent Cushing syndrome (AICS) (8). It is most frequently diagnosed in the context of Carney complex (CNC), a multiple neoplasia syndrome characterized by the association of multiple myxomas, spotty skin pigmentation and endocrine overactivity (9). Both isolated PPNAD and CNC are caused, in the vast majority of the cases, by mutations of the PRKAR1A gene encoding the regulatory subunit type 1A (R1A) of protein kinase A (PKA) (9–11). PPNAD generally leads to mild, insidious and atypical forms of AICS and would thus be a great candidate disease for medical therapy if pharmacological agents, devoid of the adverse effects of ketoconazole and other currently used adrenolytic compounds (12), would be available for clinical use. In addition, PPNAD exhibits a pattern of unusual functional and histological features suggesting the occurrence of aberrant regulatory processes, which may be regarded as valuable therapeutic targets for the clinical management of PPNAD-associated hypercortisolism. First, PPNAD is characterized by the so called paradoxical increase of cortisol secretion in response to dexamethasone (13, 14), a phenomenon that is reproducible in vitro in PPNAD cell cultures and is mediated by both PKA catalytic subunits and increased expression of the glucocorticoid receptor (13, 15). These observations indicate that cortisol may positively regulate its own secretion in PPNAD tissues through an intracrine/autocrine regulatory loop. As a matter of fact, the glucocorticoid receptor antagonist mifepristone reduces cortisol secretion by cultured PPNAD cells and could therefore be used as an inhibitor of cortisol production in PPNAD patients (15).
Second, PPNAD tissues express neuroendocrine markers, such as synaptophysin, at both the RNA and protein levels (16, 17). This abnormal neuroendocrine differentiation may lead to expression of neurotransmitters and their receptors that may participate in the maintenance of cortisol hypersecretion through paracrine stimulatory mechanisms, as previously shown in ACTH-independent macronodular adrenal hyperplasia (18).
Third, PPNAD appears to develop from the corticomedullary junction or the ZR as early histopathological studies had suggested in humans (17, 19, 20), and mouse models of Prkar1a down-regulation and adrenal-specific Prkar1a ablation have recently demonstrated (21, 22). Given the anatomical and possible developmental relationship between PPNAD and ZR, and the higher expression of SSTRs in ZR, it seems conceivable that SSTRs may be expressed in PPNAD tissues and that somatostatin analogs (SSAs) may be able to decrease cortisol secretion in this condition. Indeed, although PRKAR1A mutations are thought to stimulate PKA activity independently of cAMP through an increase in the intracellular rate of free catalytic PKA subunits, several findings indicate that, in PPNAD cells, PKA and consequently cortisol secretion can be still stimulated by cAMP. For instance, cAMP analogs and/or phosphodiesterase inhibitors like IBMX, which increase cAMP intracellular concentration, activate cortisol production by cultured PPNAD cells (15). This effect may be mediated by regulatory subunits type RII (RII) of PKA. SST analogs like octreotide, which reduce intracellular cAMP production may therefore be able to decrease cortisol secretion in patients with PPNAD and AICS, as previously noticed in patients with macronodular bilateral adrenocortical hyperplasia and AICS due to food-dependent cortisol secretion (23, 24). However, no clinical and/or in vitro studies have been performed so far to evaluate SST and its receptors in PPNAD tissues as well as the effect of SSAs in PPNAD patients (25).
In the present study, we have investigated the expression of SSTRs by RT-PCR and immunohistochemical approaches in both PPNAD tissues and the PPNAD cell line CAR47. The action of the short-acting SSA octreotide on cortisol secretion in PPNAD patients was also examined in a short pilot proof-of-concept clinical study.
Subjects and Methods
Real-time RT-PCR of SSTR1–5 gene expression
Expression of SSTR mRNAs was investigated in the samples from the subjects of the clinical study described in details below and other patients with PPNAD, as well as for comparison purposes, in normal adrenals and specimens from patients with isolated micronodular adrenocortical disease (iMAD) or massive macronodular adrenocortical disease with or without mutations in genes related to these conditions (Table 1). In all patients, the diagnosis was made as recently established (8). Normal adrenal tissues were removed from seven patients with renal carcinoma and used as controls. The immortalized CAR47 cell line (derived from primary cells from patient CAR47.01 with PPNAD and CNC) (26) was used to investigate SSTR1–5 mRNA expression after incubation with somatostatin-14 (SST14; reference SC088; PolyPeptide Group) for 2 and 8 hours, and the somatostatin analog octreotide (PolyPeptide Group) for 8 hours. All experiments were performed three times with three different housekeeping genes, ie, glyceraldehyde-3-phosphate dehydrogenase, cyclophilin A, and porphobilinogendeaminase. Total RNA was extracted using the Tri-Reagent (Sigma-Aldrich) and further purified on RNeasy minispin columns (QIAGEN). RNAs were converted into cDNA by using ImProm-II reverse transcription system (Promega). PCR amplification was performed in duplicate using the SYBR Green I master mix buffer (Applied Biosystems) in an ABI PRISM 7900 sequence detector (Applied Biosystems) with specific primers (27) (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Quantification of cDNAs in each tissue-derived sample was normalized to cyclophilin cDNAs by using both standard curve and δ-cycle threshold (Ct) methods. Standard curves were established by using dilution series of cDNAs derived from normal adrenals. Gene expression was also normalized using the averageδ-Ct value of the normal adrenal cortex (δ-Ct). The final fold expression changes were calculated using the equation 2ΔΔCt. Data are reported as mean values ± SEM. Statistical analysis of the data was performed by use of the nonparametric Mann-Whitney t test (Prism; GraphPad Software). Probability values of P < .05 were considered significant.
Table 1.
Clinical and Genetic Characteristics of the Patients Tested
| In Vitro Study | Gender | Age | Disease | Gene | Mutation | Clinical Study | In Vitro Investigations |
|
|---|---|---|---|---|---|---|---|---|
| RT-PCR | IHC | |||||||
| P1 | Female | 42 y | PPNAD | PRKAR1A | c.439A>G/p.Ser147Gly | — | X | — |
| P2 | Female | 5 y | PPNAD | PRKAR1A | c.865G>T /p.G289W | — | X | — |
| P3 | Female | 4 mo | MAD | GNAS | R201H | — | X | — |
| P4 | Female | 4 y | iMAD | None | — | X | — | |
| P5 | Female | 21 y | PPNAD | PRKAR1A | c.709 (−5–107)del | — | X | — |
| P6 | Female | 7 y | iMAD | PDE11A | 171delTfs41X | — | X | — |
| P7 | Female | 5 y | iMAD/iPPNAD | None | — | X | — | |
| P8 | Male | 53 y | PPNAD | PRKAR1A | c.891 + 3A>G/ | X | X | X |
| P9 | Female | 14 y | PPNAD | PRKAR1A | c.101_105del/p.S34fsX9 | X | X | X |
| P10 | Female | 7 y | iMAD | None | — | X | — | |
| P11 | Female | 27 y | PPNAD | PRKAR1A | c.177 + 1G>A | — | X | — |
| P12 | Female | 16 y | PPNAD | PRKAR1A | c.43_58del/p.L15SfsX104 | — | — | X |
| P13 | Female | 22 y | PPNAD | PRKAR1A | c.491_492del/ p.V164DfsX4 | — | X | — |
| P14 | Female | 27 y | PPNAD | PRKAR1A | C709–7del6(TTTTTA) | — | — | X |
| P15 | Female | 43 y | PPNAD | None | X | X | X | |
| P16 | Female | 16 y | PPNAD | PRKAR1A | c.440 + 1insG | X | X | X |
| P17 | Male | 19 y | PPNAD | None | X | — | X | |
| P18 | Female | 33 y | PPNAD | PRKAR1A | c.178–22A>G | X | — | X |
| P19 | Female | 45 y | PPNAD | PRKAR1A | c.353_365del/p.I118TfsX6 | X | X | X |
| P20 | Male | 26 y | PPNAD | PRKAR1A | c.491_492delTG/p.Val164fsX4 | X | — | — |
| P21 | Male | 12 y | PPNAD | PRKAR1A | c.54_55 dup TG/ | X | — | — |
| P22 | Female | 23 y | PPNAD | PRKAR1A | c.709- (5_107)del 103 | X | — | — |
Abbreviations: IHC, immunohistochemistry; iPPNAD, isolated PPNAD; —, not included.
Immunohistochemistry
Sections from adrenal and PPNAD tissues were immunostained using the following commercially available rabbit polyclonal antisera raised against carboxyl-terminal fragments of human somatostatin receptor subtypes (GRAMSCH Laboratories, references SS-840 for SSTR1 antibodies, SS-860 for SSTR2B, SS-850 for SSTR3, and SS-890 for SSTR5; Sigma-Aldrich, reference S0945 for SSTR4) and rabbit monoclonal antibody (3582; Eoitomics Laboratories) for SSTR2A. The immunohistochemical procedures were performed as previously described. Antibodies were diluted in antibody diluents reagent solution (Invitrogen) at the following working dilutions: SSTR1 (1:2000), SSTR2A (1:200), SSTR2B (1:2000), SSTR3 (1:1500), SSTR4 (1:500), and SSTR5 (1:1500). Human duodenum and normal adrenal tissues were used as positive control (28).
Immunoreactivities for receptor proteins were quantified by using the score previously published by Minas et al (29). This score integrates both the percentage of positive cells (graded as 0, no staining; 1, <10%; 2, 11%–50%; 3, 51%–80%; 4, >80% of the cells) and staining intensity (graded as 0, no staining; 1, weak; 2, moderate; and 3, strong staining).
Immunohistofluorescence coupled with confocal laser scanning microscope analysis was used to investigate expression and localization of SST and 17α-hydroxylase (as a marker of adrenocortical cells) in paraffin-embedded adrenal tissues from nine patients with PPNAD (Table 1) and in four normal adrenals. Deparaffinized sections of PPNAD tissues were incubated with polyoclonal goat antibodies to somatostatin (1:500; Santa Cruz Biotechnologies), polyclonal rabbit antibodies to 17α-hydroxylase (1:100; provided by Drs V. Luu-The and G. Pelletier, Laval University Medical Center, Québec, Canada). For double labeling of tissue slices with antibodies against SST and 17α-hydroxylase, the sections were stained with both fluorescein secondary antibodies Alexa 488-conjugated donkey antigoat IgG and Alexa 594-conjugated donkey antirabbit at 1:300 (Invitrogen) and examined on a confocal laser scanning microscope (Leica Corp). Images were obtained on PRIMACEN, the Cell Imaging Platform of Normandy University, University of Rouen, France.
Expression of SSTRs was also examined by means of the same immunohistofluorescence technique in the CAR47 cell line both in basal conditions and after incubation with native SST14 and octreotide (PolyPeptide Group) for 2, 24, and 48 hours. Untreated cells were used as controls. Specificity controls of the immunohistochemical reactions were performed by substituting the primary or secondary antibodies with PBS.
SSTR immunoreactivities were quantified in the CAR47 cell line according to the method previously published by Briand et al (30) using the computer software Image J developed by Wayne Rasband (National Institutes of Health, Bethesda, Maryland). Data were analyzed by simple descriptive statistics and are presented as mean ± SD.
Clinical protocol
Patients were studied at the National Institutes of Health Clinical Center under protocol 95-CH-0059 that was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Informed consent was obtained from each patient prior to participation in the study. The characteristics of the studied subjects are listed in Table 1.
AICS was confirmed on the basis of elevated midnight serum cortisol values and/or elevated 24-hour urine cortisol or 17-hydroxysteroid excretion, as previously described (8, 9, 14). All patients had to have suppressed ACTH and were to undergo bilateral adrenalectomy for the treatment of their disease. Tissues were collected from patients at the time of adrenalectomy, snap frozen, and stored in liquid nitrogen for later analyses or paraffin-embedded and processed for routine histology and diagnosis. All patients were diagnosed with PPNAD after surgery and on the basis of their histopathology. DNA from all patients was sequenced for PRKAR1A as previously described (10, 11).
The administration of octreotide and its effects on cortisol secretion were studied in a randomized, single-blind, crossover study. Briefly, blood samples were collected via a catheter placed in the forearm vein of each patient. Patients were tested on 2 separate days, with random assignment to treatment or placebo. Testing began at 6:00 pm and continued for 12 hours, until 6:00 am the following morning. At midnight, each patient was given an injection of either octreotide (100 μg) or placebo sc. The serial cortisol measurements were taken from each patient every 30 minutes throughout the observation period. The patients were tested a second time within a 1-month span using the same protocol but different treatment arm (ie, if octreotide was administered the first time, they were switched to placebo the second time) to serve as their own control.
Serum cortisol was measured by fluorescence polarization immunoassay (Abbott Laboratories) with an intra- and interassay coefficient of variation of 2.1% and 4.1%, respectively; the functional detection limit was 1–2 μg/dL.
For statistical analysis of the data, the area under the curve (AUC) for the pretreatment period (6:00 pm to 11:59 pm) and posttreatment period (12:00 am to 6:00 am) for each treatment arm (octreotide and placebo) was computed using the trapezoidal rule and was compared between treatment arms and periods by paired t tests. Mixed modeling was used to compare posttreatment cortisol data and AUCs between octreotide and placebo, taking into consideration the crossover sequence and repeated measures and adjusting for the effect of sex. Due to the small patient sample size and the dispersion of basal (pretreatment) cortisol values identified in some patients, the posttreatment means expressed as a percentage of pretreatment means for each patient and the percentage change from pretreatment mean to posttreatment mean per patient were also calculated. Percentage change data were compared between treatment arms using the t test. A separate comparison of posttreatment cortisol data for the intervals of 4 am to 6 am between the two groups for individual time points was performed. Pretreatment and posttreatment cortisol values were averaged for each period and categorized to represent either low or high cortisol levels based on an arbitrary cutoff of 7.5 μg/dL and compared by McNemar's test.
Results
SSTR mRNA expression in PPNAD tissues
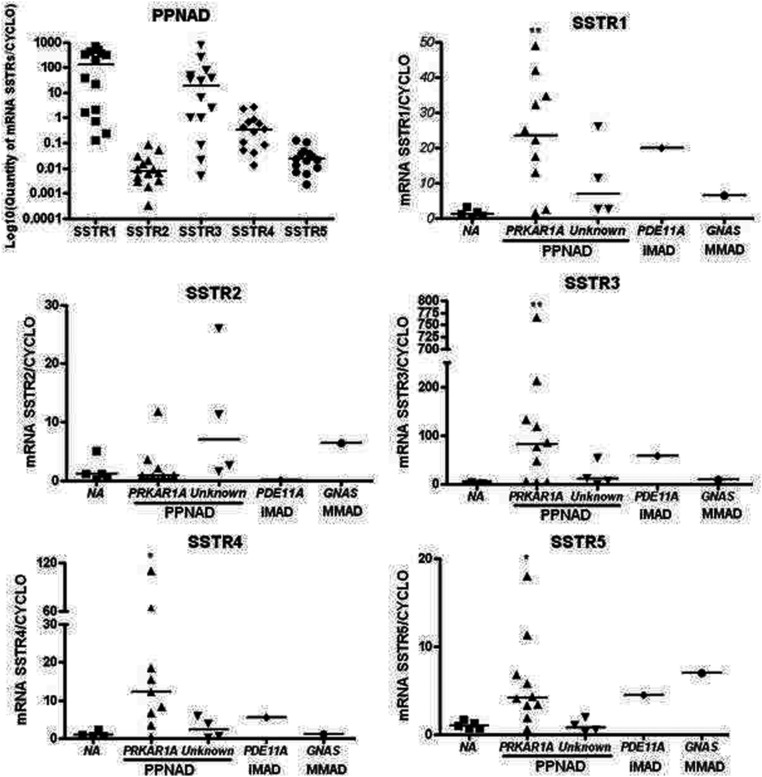
Variable amounts of mRNA encoding the five SSTR subtypes were detected in PPNAD tissues (Figure 1). SSTR1 and SSTR3 mRNAs were found to be the predominant SST receptor messages expressed in PPNAD tissues, whereas SSTR2, SSTR4, and SSTR5 mRNAs were expressed at lower levels. Interestingly, PRKAR1A-mutated PPNAD tissues were found to overexpress all SSTRs but SSTR2 mRNAs in comparison with normal adrenals (Figure 1). The amounts of cyclophilin, glyceraldehyde-3-phosphate dehydrogenase, and porphobilinogendeaminase mRNAs were comparable in all PPNAD samples tested.
Figure 1.
SSTR mRNA expression in normal adrenal gland and in PPNAD tissues. The expression levels of SSTR1–5 mRNAs were quantified by real-time PCR and expressed relative to the housekeeping gene cyclophilin A. For comparison with normal adrenals, expression levels of SSTRs mRNAs were expressed in adrenal disease samples by using the 2-ΔΔCt method. PRKAR1A-mutated tissues were found to overexpress SSTR1 and SSTR3–5 receptors when compared with normal adrenals (NA).
Immunohistochemical localization of SST receptors in PPNAD tissues
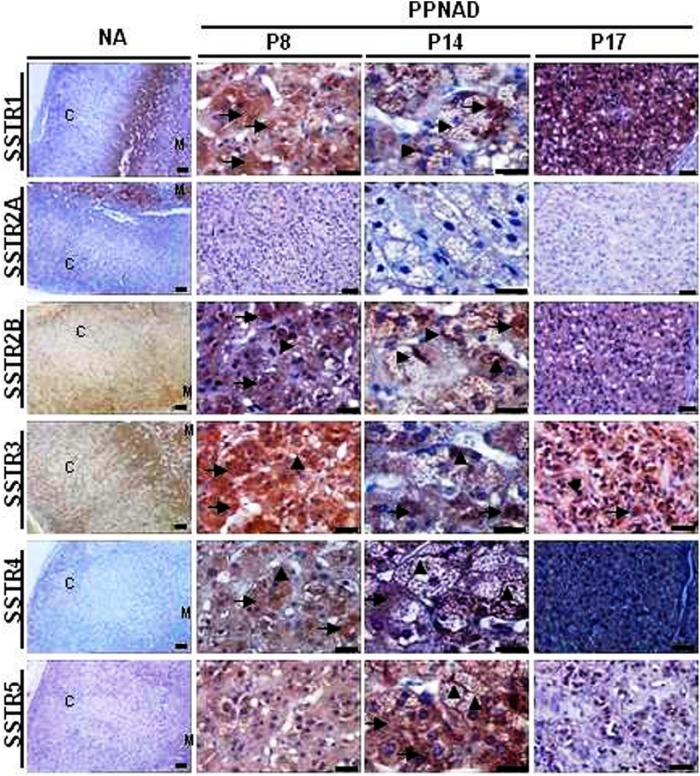
In normal adrenals, no significant staining was observed in zona glomerulosa and zona fasciculata for somatostatin receptors SSTR2B, SSTR3, and SSTR5. A weak and diffuse SSTR1 staining was detected in zona glomerulosa in one of four normal adrenals. In ZR and in the medulla, a weak signal was detected for SSTR1, SSTR2B, SSTR3, and SSTR5 stainings with moderate (SSTR1, SSTR2B, SSTR5) and strong (SSTR3) densities. SSTR2A was observed in the medulla with strong staining and density (Figure 2). Conversely, normal adrenal tissue was found to be negative for SSTR4 (Figure 2). In agreement with the pattern of distribution of SSTR mRNAs, each PPNAD tissue studied was found to be immunopositive for multiple SSTR subtypes (Supplemental Table 1). Microphotographs illustrating the distribution of SSTR immunoreactivities in PPNAD tissues are shown in Figure 2. Intense SSTR1 staining was observed both in adrenocortical micronodules and medulla in adrenal glands from PPNAD patients (Figure 2 and Supplemental Table 2). SSTR2A staining was detected in tissues with weak and moderate to strong intensities in adrenocortical micronodules and medulla, respectively. SSTR2B, SSTR3, SSTR4, and SSTR5 immunoreactivities could be visualized in adrenocortical micronodules with weak to moderate (SSTR4 and SSTR5) and strong (SSTR2B and SSTR3) intensities (Figure 2 and Supplemental Table 2). The adrenal medulla of PPNAD tissues also exhibited weak immunoreactivity for SSTR3 (Figure 2 and Supplemental Table 2). At high magnification, SSTR1, SSTR2A, and SSTR2B labelings were observed both in the cytoplasm and plasma membrane of adrenocortical cells, whereas SSTR3, SSTR4, and SSTR5 immunoreactivities were exclusively cytoplasmic (Figure 2).
Figure 2.
Immunohistochemical analysis of somatostatin receptors expression in PPNAD tissues and normal adrenal (NA). A, SSTR staining in NA and PPNAD tissues. From left to right columns: Low-magnification microphotographs showing SSTR stainings in normal adrenal gland and PPNAD tissues removed from patients P8 and P17. High-magnification microphotographs showing SSTR labeling in the PPNAD tissue from patient P14. SSTR immunoreactivities were observed at both cytoplasmic (arrows) and plasma membrane (arrowheads) levels. Scale bars, 50 μm.
Expression of SSTRs in the CAR47 cell line
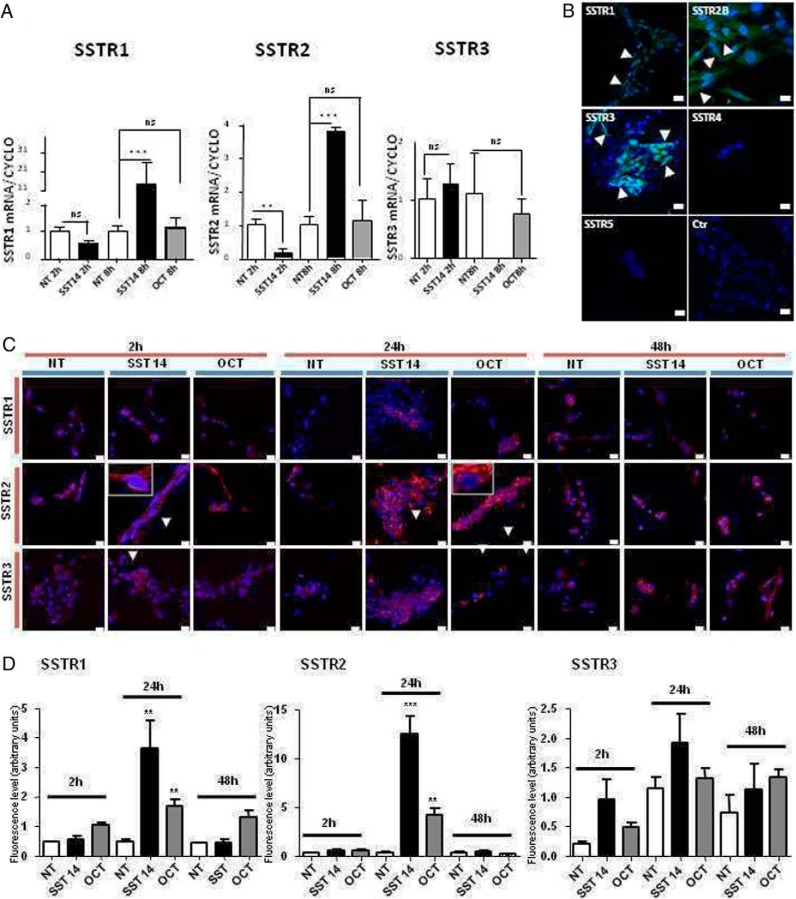
The CAR47 cell line was found to express the SSTR1, SSTR2, and SSTR3 mRNAs (Figure 3A). SST14 (10−8 M) increased the SSTR1 mRNA rate after 8 hours (+1306% ± 267% vs controls, P < .005). SST14 (10−8 M) exerted a biphasic action on SSTR2 expression by decreasing the mRNA level after 2 hours (−79% ± 8% vs controls, P < .01) and increasing the mRNA rate after 8hours (+264% ± 48% vs controls, P < .005). In contrast, SST14 had no effect on SSTR3 mRNA expression in CAR47 cells. In the same way, octreotide, which principally activates SSTR2, failed to influence SSTR1, SSTR2, and SSTR3 mRNA levels (Figure 3A).
Figure 3.
Expression of SSTRs in CAR47 cells. A, Expression levels of SSTR1–3 mRNAs in basal conditions and after treatment of CAR47 cells with SST14 (10−8 M) and octreotide (10−8 M). SSTR1–3 mRNA levels were quantified by real-time PCR and expressed relative to the housekeeping gene cyclophilin A. Data are presented as mean ± SEM. *, P < .05 or **, P < .01. NT, not treated cells (controls); oct, octreotide. B, Immunocytochemical localization of SSTR1–5 in the CAR47 cell line. CAR47 cells were found to be positive for SSTR1–3, whereas no labeling could be observed after incubation of the cells with antibodies to SSTR4 and SSTR5. SSTR immunoreactivity was visualized in the cytoplasm and at the periphery of the cells (green fluorescence, arrowheads). No staining could be observed when the primary antibodies were omitted. Ctr, Control experiment. C, Immunocytochemical analysis of the effect of SST and octreotide on expression of SSTR1–3 by the CAR47 cell line. SSTR immunoreactivities (red) were detected by use of the indirect immunocytofluorescence technique. Cell nuclei were stained with Hoescht 33342 (blue). CAR47 cells were treated for 2, 24, and 48 hours with SST14 (10−8 M) and octreotide (10−8 M). SST14 and octreotide weakly enhanced the intensity of SSTR1 labeling after 24 hours of incubation. In contrast, the two test substances favored SSTR2 translocation to the membrane and increased cytoplasmic labeling with a maximum effect at 24 hours of incubation (insets). SST14 and octreotide only mildly enhanced SSTR3 staining at 24 and 48 hours of incubation. D, Effect of SST and octreotide on SSTR labeling intensities in the CAR47 cell line. Data are presented as the mean ± SEM of fluorescence levels expressed as arbitrary units. Statistical significance was evaluated by one-way ANOVA followed by Dunnett's post hoc test and is displayed as *, P < .05; **, P < .01; ***, P < .005.
The presence of SSTRs was investigated in CAR47 cells by immunohistochemistry. CAR47 cells were immunopositive for SSTR1, SSTR2B, and SSTR3 at both the cytoplasmic and membrane levels (Figure 3B). In contrast, no immunoreactive material could be detected with antibodies against SSTR2A, SSTR4, and SSTR5. Similarly, no immunostaining was observed when primary antibodies were substituted with incubation buffer (Figure 3B).
Immunohistochemical analysis of SSTR expression was repeated after incubation of CAR47 cells with SST14 and octreotide for 2, 24, and 48 hours. No clear effect of SST14 and octreotide on SSTR1 expression was observed except for 24 hours of incubation, which significantly enhanced the labeling (Figure 3, C and D). Conversely, both SST14 and octreotide induced SSTR2B translocation to cell membrane as soon as 2 hours of incubation. Maximum staining was observed after 24 hours of incubation with SST14 and octreotide when SSTR2B immunoreactivity was observed at both the membrane and cytoplasmic levels (Figure 3, C and D). Then, the intensity of labeling decreased as shown by the pattern observed after 48 hours of incubation of cells with the two test substances (Figure 3, C and D). SST14 and octreotide had no effect on SSTR3 labeling at 2 hours of treatment, whereas a mild action of SST14 and octreotide was noticed at 24 and 48 hours.
Localization of SST in normal adrenal and PPNAD tissues
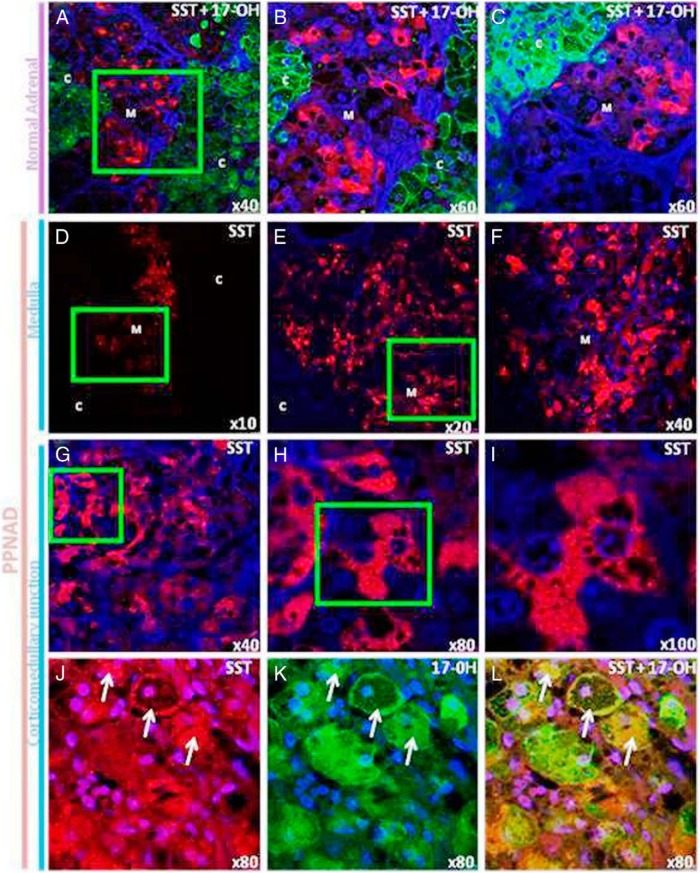
The occurrence and distribution of SST in normal adrenal and PPNAD tissues was investigated by means of colabeling experiments with SST and 17α-hydroxylase antibodies. In the normal adrenal gland (Figure 4, A–C), SST immunoreactivity was visualized in a subpopulation of medulla cells. SST-positive cells presented as isolated cells or arranged in small clusters (red fluorescence). No SST immunoreactive cells were observed in the cortex. In contrast, PPNAD tissues exhibited diffuse SST labeling of the medulla (red fluorescence, Figure 4, D–F). SST immunoreactivity was also observed in groups of adrenocortical steroidogenic cells, ie, positive for 17α-hydroxylase and containing numerous lipid droplets, close to the corticomedullary junction (Figure 4, G–L).
Figure 4.
Immunohistochemical localization of SST in normal adrenal gland and PPNAD tissues. A–C, Confocal laser scanning microscope photomicrographs showing the distribution of SST and 17α-hydroxylase immunoreactivities in the normal adrenal gland. The SST and 17α-hydroxylase immunoreactivities were revealed with Alexa 488-conjugated antibodies (red) and Alexa 594-conjugated antibodies (green), respectively. A, Anti-17α-hydroxylase antibodies produced diffuse labeling of the cortex, whereas SST-like immunoreactivity was exclusively detected in the medulla. B, High-magnification view of the field delimited by the green square in A. Medullary SST-positive cells presented as isolated cells or arranged in small clusters. C, High-magnification view of the corticomedullary junction showing that no SST immunoreactive cells could be observed in the inner cortex. D–I, Dual-channel confocal laser-scanning microscope analysis of the distribution of SST in PPNAD tissues. D–F, Different views at increasing magnifications showing diffuse SST labeling of the medulla in the adrenal gland removed from patient P1. G–I, Serial microphotographs at increasing magnification showing that SST labeling was also observed in groups of adrenocortical cells close to the corticomedullary junction containing numerous unstained lipid droplets (patient P2). J–L, Confocal laser-scanning microscope analysis of the corticomedullary junction in the PPNAD tissue removed from patient P5. J, Group of SST-positive cells (arrows) in the inner cortex close to the corticomedullary junction. K, The same SST-positive cells were also labeled by antibodies to 17α-hydroxylase. L, Merged microphotograph. C, cortex; M, medulla.
Clinical study
The mean age of patients included was 31.0 ± 13.9 years (range 18–59 y). All 10 subjects were diagnosed with PPNAD confirmed at surgery; eight patients harbored a mutation in PRKAR1A, whereas two did not. The cortisol levels of each patient with and without octreotide administration throughout the observation period are shown in Supplemental Figure 1. Simple descriptive statistics of cortisol levels before and after treatment, as well as the percentage change from pretreatment mean to posttreatment mean of each patient, for each treatment arm are shown in Table 2. Briefly, no significant differences were observed in pretreatment and posttreatment cortisol AUCs between the two treatment arms (P = .4260 and P = .1824, respectively). When we compared the percentage of change from pretreatment to posttreatment means, no statistically significant difference was found between treatments (P = .2541). Interestingly, there was a decrease in cortisol levels after the administration of octreotide that was noted in the aggregate data between 4 am and 6 am. A separate analysis for this particular time period was performed, but again the difference was not statistically significant. Paired analysis of high and low cortisol values showed also no statistically significant differences for the pre- and posttreatment measurements between the two treatment arms.
Table 2.
Cortisol Levels Before and After Treatment of Each Patient, for Each Treatment Arm (Octreotide and Placebo)
| Treatment Arm | Patient | Pretreatment Period |
Posttreatment Period |
Posttreatment Means Expressed as Percentage of Pretreatment Means | Change From Pretreatment Mean to Posttreatment Mean, % | ||
|---|---|---|---|---|---|---|---|
| Cortisol Mean | AUC | Cortisol Mean | AUC | ||||
| Octreotide | 1 | 15.0 | 176.5 | 2.1 | 22.7 | 14.1 | −85.9 |
| 2 | 8.8 | 105.1 | 8.4 | 87.2 | 95.0 | −5.0 | |
| 3 | 33.5 | 401.4 | 28.3 | 239.4 | 84.6 | −15.4 | |
| 4 | 4.2 | 50.2 | 4.3 | 45.0 | 102.3 | 2.3 | |
| 5 | 4.4 | 50.6 | 5.0 | 50.5 | 114.0 | 14.0 | |
| 6 | 26.2 | 315.9 | 25.4 | 280.7 | 96.9 | −3.1 | |
| 7 | 5.3 | 64.8 | 5.0 | 55.6 | 94.3 | −5.7 | |
| 8 | 9.2 | 78.6 | 8.8 | 95.7 | 94.7 | −5.3 | |
| 9 | 30.4 | 365.5 | 31.1 | 201.6 | 102.0 | 2.0 | |
| 10 | 8.2 | 90.5 | 8.2 | 90.7 | 99.8 | −0.2 | |
| Total | 14.5 (11.3) | 169.9 (138.1) | 12.6 (11.0) | 116.9 (90.2) | 89.8 (27.6) | −10.2 (27.6) | |
| Placebo | 1 | 49.1 | 593.7 | 19.3 | 206.1 | 39.4 | −60.6 |
| 2 | 12.0 | 145.3 | 11.1 | 116.2 | 92.0 | −8.0 | |
| 3 | 29.1 | 350.9 | 25.0 | 263.0 | 85.9 | −14.1 | |
| 4 | 4.3 | 52.1 | 6.1 | 64.6 | 140.6 | 40.6 | |
| 5 | 3.0 | 36.7 | 5.5 | 57.4 | 181.1 | 81.1 | |
| 6 | 25.1 | 300.7 | 22.1 | 241.4 | 88.1 | −11.9 | |
| 7 | 4.8 | 57.6 | 4.9 | 55.1 | 102.9 | 2.9 | |
| 8 | 6.7 | 79.2 | 10.5 | 115.9 | 157.1 | 57.1 | |
| 9 | 28.2 | 337.4 | 28.4 | 313.5 | 100.6 | 0.6 | |
| 10 | 8.7 | 104.2 | 8.2 | 89.0 | 93.7 | −6.3 | |
| Total | 17.1 (15.2) | 205.8 (183.2) | 14.1 (8.8) | 152.2 (95.4) | 108.1 (40.8) | 8.1 (40.8) | |
P = .2541 comparing percentage of change from before treatment to after treatment. P = .4260 comparing octreotide with placebo cortisol AUC, pretreatment period. P = .1824 comparing octreotide with placebo cortisol AUC, posttreatment period. P < .0001 comparing before treatment with after treatment using repeated measures, octreotide (same adjusted for sex). P < .0001 comparing before treatment with after treatment using repeated measures, placebo (same adjusted for sex).
However, it should be noted that the aggregate pre- vs posttreatment cortisol values for each of the octreotide and placebo treatment arms were highly statistically significantly different (P < .0001 for both groups), with lower levels after the treatment, even after adjusting for the effect of sex.
Discussion
SST and its synthetic analogs have been used in the treatment of a variety of neuroendocrine tumors and primary adrenal hypercortisolism with various rates of success (23, 24, 31). In the present study, we have investigated SST and SSTRs in PPNAD tissues and examined whether administration of octreotide could affect the cortisol levels in PPNAD patients. Our data show unequivocally high expression of SSTRs mRNAs, especially SSTR1 and SSTR3 mRNAs, in PPNAD tissues compared with normal adrenal and tissues from other adrenal lesions associated with AICS, such as iMAD or massive macronodular adrenocortical disease harboring PDE11A and GNAS mutations, respectively. Using the CAR47 cell line, developed from a patient with PPNAD and CNC, we then looked for the localization and expression of SSTR1–3 after treatment with SST14 and octreotide at different time points. Treatment with SST14 and octreotide did not have any significant effect on SSTR3 mRNA at any time point, whereas a significant effect was seen on SSTR1 and SSTR2 mRNAs but only with SST14, indicating that SST14 up-regulates these two receptors independently of the SSTR2 in CAR47 cells. Interestingly, SST14 was found to have a biphasic action on SSTR2 expression, ie, with a decrease of SSTR2 after 2 hours incubation followed by an increase at 8 hours of cell incubation. Such a pattern is in agreement with other reports showing that, in GH3 pituitary cells, SST induces complex effects on expression of its receptors including biphasic action of SSTR2 mRNA synthesis contrasting with an increase in SSTR1, SSTR3, SSTR4, and SSTR5 mRNA expression (32). Collectively, these data indicate that agonist-induced down-regulation and/or up-regulation of SSTR expression is time dependent and cell type specific, as previously described in neuroendocrine tumors (33).
We have also examined the action of SST14 and OCT on the expression of SSTR1–3 proteins in the CAR47 cell line by fluorescence microscopy. Interestingly, both SST14 and octreotide rapidly favored translocation of SSTR2 to the plasma membrane and increased SSTR2 expression, which reached a maximum at 24 hours of incubation before decreasing to basal levels or below at 48 hours. A similar action of SST14 was seen on SSTR1 expression. Conversely, the effects of the two compounds were less potent or nonsignificant on SSTR3 expression. These results show that SST exerts its initial effects mainly via SSTR2 but principally activates SSTR3 and to a lesser extent SSTR1 for its long-term actions on PPNAD cells.
In ACTH-independent macronodular adrenal hyperplasia tissues, adrenocortical cells abnormally express neuroendocrine regulatory factors, which play a role in the maintenance of cortisol secretion despite suppressed plasma ACTH levels (18). We have then verified whether the occurrence of SSTRs could be associated with expression of SST in PPNAD tissues. As in the normal adrenal gland, adrenomedullary cells in PPNAD were found to contain SST immunoreactivity. However, the adrenal medulla was diffusely labeled by the SST antibodies, whereas in the normal adrenal, SST immunoreactivity in PPNAD was restricted to some cells, showing that PPNAD may have significant impact on adrenomedullary cell function. Interestingly, in PPNAD tissues, SST labeling was also detected in groups of cells located in the ZR, which harbored a steroidogenic phenotype, ie, loaded with numerous lipid droplets and immunopositive for 17α-hydroxylase. These cells seem therefore to constitute a subpopulation of steroidogenic cells with some degree of neuroendocrine differentiation. Aberrant expression of SST in adrenocortical cells may be the result of the combined action of increased cortisol production and activation of PKA activity in PPNAD tissues because glucocorticoids and cAMP signaling pathway have been shown to cooperatively activate transcription of the SST gene in neuroendocrine cell lines (34). It is also physiopathologically relevant to notice that SST-positive adrenocortical cells are found close to the corticomedullary junction, a site from which histopathological micronodules seem to arise, as shown by histopathological studies of both human PPNAD tissues (17, 19, 20) and adrenal sections derived from murine PPNAD models (21, 22). Taken together, these data indicate that SST, released by chromaffin cells (35) and some ZR cells, may be able to modulate corticosteroid secretion in an autocrine/paracrine fashion.
The short clinical pilot study presented in this report did not show any consistent or significant effect of short-acting octreotide sc injections on cortisol secretion. Patients who received octreotide did exhibit a slight decrease in cortisol levels over time; however, this decrease was not high enough to be statistically significant neither within the group nor between groups. There might be several explanations on why the clinical investigation failed to show a suppressive effect on cortisol secretion by octreotide. First, the dose used was standard but possibly not sufficient. Second, due to the rarity of disease, the population of patients studied was necessarily small. Third, when we compared pre- and posttreatment cortisol levels within each group, a significant difference was found (P < .001) for both groups, with the late-night cortisol levels being higher than the early morning levels. This finding confirms the presence of an inverted diurnal rhythmicity of cortisol secretion in PPNAD patients, an observation that is not unusual in AICS (36), and indicates that the inhibitory action of octreotide may have been blunted by the spontaneous decline of plasma cortisol levels. It also suggests that administration of octreotide early in the morning, a time period during which cortisol concentrations appear more stable, may have produced more significant effects. Finally, the fact that prolonged incubation of PPNAD cells with SST and octreotide leads to down-regulation of SSTR2 in vitro suggests that treatments of PPNAD patients with long-acting SST analogs like pasireotide, which is able to bind all SSTRs but SSTR4, may exert a more significant action than the short-acting compound octreotide, which principally acts as a SSTR2 agonist.
In conclusion, we demonstrated that SST and SSTRs were present in PPNAD that was caused mostly by PRKAR1A mutations. SST drove the expression of SSTR2 and both SST and SSTRs were more highly expressed in PPNAD cells than in normal adrenal cortex or in other diseases associated with AICS. Although a limited proof-of-concept clinical trial with the short-acting analog octreotide did not show any significant effect on cortisol levels, interpretation of the laboratory data points to the need for exploring the clinical use of SSA in patients with PPNAD, with a different design, more potent long-acting compounds, at higher doses and in a larger number of subjects.
Acknowledgments
This work was supported by the National Institutes of Health Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Z.B. was the recipient of grants from Agence Nationale de la Recherche and Société Française d'Endocrinologie.
Disclosure Summary: E.L. and H.L. have received travel grants from Novartis. The other authors have nothing to disclose.
Footnotes
- AICS
- ACTH-independent Cushing syndrome
- AUC
- area under the curve
- CNC
- Carney complex
- Ct
- cycle threshold
- iMAD
- isolated micronodular adrenocortical disease
- PKA
- protein kinase A
- PPNAD
- primary pigmented nodular adrenocortical disease
- R1A
- regulatory subunit type 1A
- SSA
- somatostatin analog
- SST
- somatostatin
- SST14
- somatostatin-14
- SSTR
- SST receptor
- ZR
- zona reticularis.
References
- 1. Warren TG, Shields D. Expression of preprosomatostatin in heterologous cells: biosynthesis, posttranslational processing, and secretion of mature somatostatin. Cell. 1984;39:547–555 [DOI] [PubMed] [Google Scholar]
- 2. Hoyer D, Bell GI, Berelowitz M, et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995;16:86–88 [DOI] [PubMed] [Google Scholar]
- 3. Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2:999–1017 [DOI] [PubMed] [Google Scholar]
- 4. Epelbaum J, Bertherat J, Prevost G, et al. Molecular and pharmacological characterization of somatostatin receptor subtypes in adrenal, extraadrenal, and malignant pheochromocytomas. J Clin Endocrinol Metab. 1995;80:1837–1844 [DOI] [PubMed] [Google Scholar]
- 5. Ueberberg B, Tourne H, Redmann A, et al. Differential expression of the human somatostatin receptor subtypes sst1 to sst5 in various adrenal tumors and normal adrenal gland. Horm Metab Res. 2005;37:722–728 [DOI] [PubMed] [Google Scholar]
- 6. Unger N, Serdiuk I, Sheu SY, et al. Immunohistochemical localization of somatostatin receptor subtypes in benign and malignant adrenal tumours. Clin Endocrinol (Oxf). 2008;68:850–857 [DOI] [PubMed] [Google Scholar]
- 7. Kennedy JW, Dluhy RG. The biology and clinical relevance of somatostatin receptor scintigraphy in adrenal tumor management. Yale J Biol Med. 1997;70:565–575 [PMC free article] [PubMed] [Google Scholar]
- 8. Stratakis CA. Cushing syndrome caused by adrenocortical tumors and hyperplasias (corticotropin-independent Cushing syndrome). Endocr Dev. 2008;13:117–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pack SD, Kirschner LS, Pak E, Zhuang Z, Carney JA, Stratakis CA. Genetic and histologic studies of somatomammotropic pituitary tumors in patients with the “complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas” (Carney complex). J Clin Endocrinol Metab. 2000;85:3860–3865 [DOI] [PubMed] [Google Scholar]
- 10. Groussin L, Jullian E, Perlemoine K, et al. Mutations of the PRKAR1A gene in Cushing's syndrome due to sporadic primary pigmented nodular adrenocortical disease. J Clin Endocrinol Metab. 2002;87:4324–4329 [DOI] [PubMed] [Google Scholar]
- 11. Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92 [DOI] [PubMed] [Google Scholar]
- 12. Feelders RA, Hofland LJ. Medical treatment of Cushing's disease. J Clin Endocrinol Metab. 2013;98:425–438 [DOI] [PubMed] [Google Scholar]
- 13. Bourdeau I, Lacroix A, Schurch W, Caron P, Antakly T, Stratakis CA. Primary pigmented nodular adrenocortical disease: paradoxical responses of cortisol secretion to dexamethasone occur in vitro and are associated with increased expression of the glucocorticoid receptor. J Clin Endocrinol Metab. 2003;88:3931–3937 [DOI] [PubMed] [Google Scholar]
- 14. Stratakis CA, Sarlis N, Kirschner LS, et al. Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann Intern Med. 1999;131:585–591 [DOI] [PubMed] [Google Scholar]
- 15. Louiset E, Stratakis CA, Perraudin V, et al. The paradoxical increase in cortisol secretion induced by dexamethasone in primary pigmented nodular adrenocortical disease involves a glucocorticoid receptor-mediated effect of dexamethasone on protein kinase A catalytic subunits. J Clin Endocrinol Metab. 2009;94:2406–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horvath A, Mathyakina L, Vong Q, et al. Serial analysis of gene expression in adrenocortical hyperplasia caused by a germline PRKAR1A mutation. J Clin Endocrinol Metab. 2006;91:584–596 [DOI] [PubMed] [Google Scholar]
- 17. Stratakis CA, Carney JA, Kirschner LS, et al. Synaptophysin immunoreactivity in primary pigmented nodular adrenocortical disease: neuroendocrine properties of tumors associated with Carney complex. J Clin Endocrinol Metab. 1999;84:1122–1128 [DOI] [PubMed] [Google Scholar]
- 18. Bertherat J, Contesse V, Louiset E, et al. In vivo and in vitro screening for illegitimate receptors in adrenocorticotropin-independent macronodular adrenal hyperplasia causing Cushing's syndrome: identification of two cases of gonadotropin/gastric inhibitory polypeptide-dependent hypercortisolism. J Clin Endocrinol Metab. 2005;90:1302–1310 [DOI] [PubMed] [Google Scholar]
- 19. Aiba M, Hirayama A, Iri H, et al. Primary adrenocortical micronodular dysplasia: enzyme histochemical and ultrastructural studies of two cases with a review of the literature. Hum Pathol. 1990;21:503–511 [DOI] [PubMed] [Google Scholar]
- 20. Iseli BE, Hedinger CE. Histopathology and ultrastructure of primary adrenocortical nodular dysplasia with Cushing's syndrome. Histopathology. 1985;9:1171–1194 [DOI] [PubMed] [Google Scholar]
- 21. Griffin KJ, Kirschner LS, Matyakhina L, et al. A transgenic mouse bearing an antisense construct of regulatory subunit type 1A of protein kinase A develops endocrine and other tumours: comparison with Carney complex and other PRKAR1A induced lesions. J Med Genet. 2004;41:923–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sahut-Barnola I, de Joussineau C, Val P, et al. Cushing's syndrome and fetal features resurgence in adrenal cortex-specific Prkar1a knockout mice. PLoS Genet. 2010;6:e1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lacroix A, Bolte E, Tremblay J, et al. Gastric inhibitory polypeptide-dependent cortisol hypersecretion—a new cause of Cushing's syndrome. N Engl J Med. 1992;327:974–980 [DOI] [PubMed] [Google Scholar]
- 24. Reznik Y, Allali-Zerah V, Chayvialle JA, et al. Food-dependent Cushing's syndrome mediated by aberrant adrenal sensitivity to gastric inhibitory polypeptide. N Engl J Med. 1992;327:981–986 [DOI] [PubMed] [Google Scholar]
- 25. de Bruin C, Feelders RA, Lamberts SW, Hofland LJ. Somatostatin and dopamine receptors as targets for medical treatment of Cushing's syndrome. Rev Endocr Metab Disord. 2009;10:91–102 [DOI] [PubMed] [Google Scholar]
- 26. Nesterova M, Bossis I, Wen F, Horvath A, Matyakhina L, Stratakis CA. An immortalized human cell line bearing a PRKAR1A-inactivating mutation: effects of overexpression of the wild-type allele and other protein kinase A subunits. J Clin Endocrinol Metab. 2008;93:565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taboada GF, Luque RM, Bastos W, et al. Quantitative analysis of somatostatin receptor subtype (SSTR1–5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur J Endocrinol. 2007;156:65–74 [DOI] [PubMed] [Google Scholar]
- 28. Taniyama Y, Suzuki T, Mikami Y, Moriya T, Satomi S, Sasano H. Systemic distribution of somatostatin receptor subtypes in human: an immunohistochemical study. Endocr J. 2005;52:605–611 [DOI] [PubMed] [Google Scholar]
- 29. Minas V, Rolaki A, Kalantaridou SN, et al. Intratumoral CRH modulates immuno-escape of ovarian cancer cells through FasL regulation. Br J Cancer. 2007;97:637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Briand O, Helleboid-Chapman A, Ploton M, et al. The nuclear orphan receptor Nur77 is a lipotoxicity sensor regulating glucose-induced insulin secretion in pancreatic β-cells. Mol Endocrinol. 2012;26:399–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Colao A, Faggiano A, Pivonello R. Somatostatin analogues: treatment of pituitary and neuroendocrine tumors. Prog Brain Res. 2010;182:281–294 [DOI] [PubMed] [Google Scholar]
- 32. Hofland LJ, Lamberts SW. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev. 2003;24:28–47 [DOI] [PubMed] [Google Scholar]
- 33. Grozinsky-Glasberg S, Shimon I, Korbonits M, Grossman AB. Somatostatin analogues in the control of neuroendocrine tumours: efficacy and mechanisms. Endocr Relat Cancer. 2008;15:701–720 [DOI] [PubMed] [Google Scholar]
- 34. Liu JL, Papachristou DN, Patel YC. Glucocorticoids activate somatostatin gene transcription through co-operative interaction with the cyclic AMP signalling pathway. Biochem J. 1994;301(Pt 3):863–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haase M, Willenberg HS, Bornstein SR. Update on the corticomedullary interaction in the adrenal gland. Endocr Dev. 2011;20:28–37 [DOI] [PubMed] [Google Scholar]
- 36. van Aken MO, Pereira AM, van Thiel SW, et al. Irregular and frequent cortisol secretory episodes with preserved diurnal rhythmicity in primary adrenal Cushing's syndrome. J Clin Endocrinol Metab. 2005;90:1570–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]