Abstract
Context:
A challenge for automated glycemic control in type 1 diabetes (T1D) is the large variation in insulin needs between individuals and within individuals at different times in their lives.
Objectives:
The objectives of the study was to test the ability of a third-generation bihormonal bionic pancreas algorithm, initialized with only subject weight; to adapt automatically to the different insulin needs of adults and adolescents; and to evaluate the impact of optional, automatically adaptive meal-priming boluses.
Design:
This was a randomized controlled trial.
Setting:
The study was conducted at an inpatient clinical research center.
Patients:
Twelve adults and 12 adolescents with T1D participated in the study.
Interventions:
Subjects in each age group were randomized to automated glycemic control for 48 hours with or without automatically adaptive meal-priming boluses.
Main Outcome Measures:
Mean plasma glucose (PG), time with PG less than 60 mg/dL, and insulin total daily dose were measured.
Results:
The 48-hour mean PG values with and without adaptive meal-priming boluses were 132 ± 9 vs 146 ± 9 mg/dL (P = .03) in adults and 162 ± 6 vs 175 ± 9 mg/dL (P = .01) in adolescents. Adaptive meal-priming boluses improved mean PG without increasing time spent with PG less than 60 mg/dL: 1.4% vs 2.3% (P = .6) in adults and 0.1% vs 0.1% (P = 1.0) in adolescents. Large increases in adaptive meal-priming boluses and shifts in the timing and size of automatic insulin doses occurred in adolescents. Much less adaptation occurred in adults. There was nearly a 4-fold variation in the total daily insulin dose across all cohorts (0.36–1.41 U/kg · d).
Conclusions:
A single control algorithm, initialized only with subject weight, can quickly adapt to regulate glycemia in patients with TID and highly variable insulin requirements.
The Diabetes Control and Complications Trial and its long-term follow-up showed that maintaining mean blood glucose (BG) concentration close to the nondiabetic range prevents development of and slows progression of both microvascular and macrovascular complications in individuals with type 1 diabetes (T1D) (1–3). However, the therapy required to maintain near-normal BG values is extremely demanding for patients, requiring frequent BG checks, an estimation of carbohydrate intake with each meal, calculation of insulin requirements to treat meals, and multiple daily insulin injections or use of an insulin pump. Despite these efforts, hyperglycemic excursions and episodic hypoglycemia are common in most patients with T1D (4–7). Most patients do not meet targets for glucose control, leaving them at increased risk for complications (8–10). In addition, hypoglycemia can result in life-threatening consequences and limits intensification of insulin treatment (11). Integrating continuous glucose monitoring (CGM) technology with insulin pumps and a control algorithm to create a bionic endocrine pancreas has the potential to improve glycemic control, reduce hypoglycemia, and reduce the burden of care in TID.
A bionic pancreas must achieve safe and effective glycemic control in patients of all ages in the face of exercise and meals. One of the main challenges in achieving this goal stems from the large differences in interindividual insulin requirements, in particular between preadolescent children and adults, with generally modest insulin requirements, and pubescent adolescents, who typically have much higher requirements (12, 13). A single system capable of automatically adapting to the individual needs across all three age populations would obviate the need to repeatedly tune the system manually throughout the transition from childhood to adolescence to adulthood. The adaptation should be sufficiently robust and quick enough to manage not only the changes in insulin requirements over months during development but also changes occurring over hours, days, and weeks in response to intercurrent illness, emotional stress, and physical activity.
We have previously built and tested two generations of bihormonal bionic endocrine pancreas systems in adults with T1D. The first-generation system used venous plasma glucose (PG) readings every 5 minutes as the sole input to the control algorithm, which in turn autonomously determined doses of insulin and/or glucagon that were administered sc (14). The second-generation system used a similar control algorithm but responded to data from a CGM, rather than venous PG (15). Although both systems performed well in adults, we found that our second-generation system, when set for adults, could not provide sufficient insulin dosing in many adolescents because their average insulin requirements were 2–3 times those of adults. When the system was set for adolescents, it dosed insulin too aggressively for most adults (data not shown). Most clinical trials of closed-loop BG control systems have focused on either adult or adolescent populations. All other control systems were either preconditioned for each subject with information from their insulin regimen under usual care, required a training period to customize the algorithm to the subject's insulin needs, or both (16–40). Such approaches may overconstrain the control system, rendering it unable to cope with the long-term variability in insulin demand.
Our third-generation bihormonal bionic endocrine pancreas has been designed to respond to changes in glycemia on multiple time scales, cope with large variations in inter- and intrasubject insulin requirements, and requires only the subject's weight for initialization. Within the shortest time frame, corresponding to the temporal resolution of the system (5 min interval), the instantaneous basal and bolus doses are responsive to the ambient glucose level. However, the system responds to longer-term changes in a subject's insulin requirements, as might occur over periods of hours to years, by using time-averaged glucose data over a moving window of the last 24 hours.
A completely automated closed-loop control system would not require meal announcements and would simply react to changes in BG, mimicking the pancreas. However, delays in sc insulin absorption have led many investigators to explore the use of meal announcements. These meal announcements have required accurate carbohydrate estimations, as resulting insulin dosing for meals is based on preset carbohydrate to insulin ratios (16, 18, 26, 27, 31–34, 36–38), just as in the current standard of care. Our second-generation system used meal announcements, but the size of the meal-priming insulin bolus administered by the system at the start of a meal was based entirely on subject weight and was intended to provide less than 50% of the average insulin that would be required for meals. The resulting fraction of insulin varied among volunteers, but inter- and intrasubject variability in the meal insulin requirement presents another opportunity for automated adaptation. Our third-generation system includes an adaptive meal-priming insulin bolus capability that automatically adjusts the size of breakfast, lunch, and dinner doses by administering 75% of the average prandial insulin provided for previous meals at that time of day.
We hypothesized that our third-generation bihormonal bionic endocrine pancreas, initialized only with subject weight, could automatically regulate BG safely and effectively in both adults and adolescents with T1D and could automatically adjust insulin dosing in a period of approximately 24 hours to meet individual insulin demands. Furthermore, we hypothesized that use of adaptive meal-priming boluses would improve glycemic control relative to an entirely reactive system with no meal-priming boluses. Here we report results of a study testing these hypotheses in experiments involving 24 adult and adolescent subjects with T1D.
Materials and Methods
The protocol was approved by the Massachusetts General Hospital and Boston University Human Research Committees.
Subjects
Twelve adult and 12 adolescent subjects with T1D participated. All subjects provided written informed consent or assent forms. At baseline, adolescent subjects were 12 years old or older and 21 years old or younger; adult subjects were required to be older than 21 years. Other requirements included glycosylated hemoglobin (HbA1c) of 9.0% or less, body mass index of 20–35 kg/m2, daily insulin requirement of 1 U/kg · d or less for adults and 2 U/kg · d or less for adolescents, and peak stimulated C-peptide level of 0.1 nmol/L or less in response to a mixed meal. Other criteria are detailed in the Supplemental Material.
Closed-loop glucose control system
Insulin and glucagon were administered by a fully automated system (Supplemental Figure 1) using a revised version of control algorithms previously described (14, 15) (Supplemental Material). The only input signal was data from the Freestyle Navigator (Abbott Diabetes Care), a Food and Drug Administration-approved interstitial-fluid CGM. Insulin dosing was controlled by an augmented model-predictive control algorithm incorporating a pharmacokinetic model for insulin lispro that assumed a peak time (tmax) of 65 minutes. Glucagon dosing was controlled by a proportional-derivative algorithm. Insulin lispro and glucagon (Eli Lilly) were administered sc by OmniPod patch pumps (Insulet). All of the components were worn by the subject except for the CGM receiver and a computer, which were mounted on an iv pole to allow freedom of movement.
The control system came directly online with no learning period, and no information about the subject's usual insulin regimen was provided to the algorithm. The control system only received CGM glucose (CGMG) data and commanded dosing of insulin and/or glucagon every 5 minutes. Half of the adults and adolescents were randomly assigned to receive meal-priming insulin boluses administered at the beginning of each meal. The first meal-priming bolus was solely based on weight (0.05 U/kg), after which meal-priming boluses were automatically adapted by the control system online. In the first 24 hours, adaptation was based on CGMG data and dosing responses of any preceding meals, and in the second 24 hours, adaptation was based on CGMG data and dosing responses of the corresponding meal in the first 24 hours. The other half of the subjects in each age group received no meal-priming boluses.
Automated glucose control experiments
Subjects were admitted to the Massachusetts General Hospital Clinical Research Center and received basal insulin from their own pump until initiation of closed-loop control. The Navigator CGM, inserted the day before, was linked wirelessly to the system and calibrated strictly according to the manufacturer's instructions, except that venous PG values were used for calibration. Venous PG levels, from which the primary outcomes were derived, were measured every 15 minutes with the GlucoScout (International Biomedical) and were confirmed hourly with a YSI 2300 STAT Plus analyzer (YSI Life Sciences). Only CGMG values were available to the control system.
Closed-loop control was initiated at 3:00 pm. Six meals (each consumed in 30 min) were provided over 51 hours, with 50% or more of calories from carbohydrate (Supplemental Material). The mean carbohydrate consumption was 98 ± 17 (range 63–144) g per meal [mean carbohydrate consumptions for the adolescent and adult groups were, respectively, 97 ± 16 (range 63–133) and 100 ± 18 (range 68–144) g per meal, which averaged 4.9 ± 0.6 and 3.9 ± 0.6 g/kg · d, respectively]. Exercise on a stationary bicycle began at 4:00 pm on the second day and lasted approximately 30 minutes with a target heart rate of 120–140 beats/min until a total of 4000 heartbeats was reached. The 51-hour closed-loop experiment ended at 6:00 pm on the third day.
Hypoglycemia was defined as venous PG of less than 70 mg/dL and was treated with fruit juice if the PG remained of less than 70 mg/dL for three consecutive 15-minute measurements, less than 60 mg/dL for two consecutive measurements, less than 50 mg/dL once, or if subjects had symptoms of hypoglycemia concurrent with PG of less than 70 mg/dL (Supplemental Material).
Laboratory and pharmacokinetic analyses
Samples for insulin and glucagon measurements were obtained at 30-minute intervals from 7:00 am to 4:00 pm and at 60-minute intervals otherwise. Insulin, glucagon, and HbA1c were assayed and plasma tmax for lispro was derived as previously described (6).
Statistical analyses
The prespecified primary outcomes were mean PG, percentage of PG values less than 60, less than70, 70–120, 70–180, greater than 180, and greater than 250 mg/dL, and number of hypoglycemic events that were carbohydrate treated according to protocol. Outcomes were calculated for the last 48 hours of each experiment to reduce the influence of preexperimental conditions. Nighttime was defined as 11:00 pm to 7:00 am.
Other than pump replacements when failure was clinically suspected, no additional interventions were made; the control system was allowed to recover and manage glycemic consequences autonomously.
Statistical analyses between the two age groups (12 subjects each) and among the four subgroups (six subjects each) were performed with the unpaired-sample Student's t test.
Results
At baseline, adults 22–66 years old had a mean body mass of 78 ± 14 kg and a mean HbA1c of 7.3% ± 0.9% with a daily insulin dose of 39 ± 13 U. In contrast, adolescents 12–18 years old had a mean body mass of 58 ± 9 kg and a mean HbA1c of 7.9% ± 0.4%, with a daily insulin dose of 57 ± 12 U (Table 1).
Table 1.
Baseline Characteristics of Subjects
| Adult | Pediatric | |
|---|---|---|
| Number | 12 | 12 |
| Sex | 6 M/6 F | 3 M/9 F |
| Age, y | 45 ± 14 (26–66) | 15 ± 2 (12–18) |
| BM, kg | 78 ± 11 (66–101) | 58 ± 9 (47–76) |
| BMI, kg/m2 | 26 ± 3 (22–32) | 22 ± 3 (18–28) |
| Diabetes duration, y | 27 ± 15 (7–54) | 7 ± 4 (2–13) |
| Daily insulin dose, U/kg | 0.49 ± 0.13 (0.31–0.77) | 0.94 ± 0.17 (0.68–1.25) |
| HbA1c, % | 7.3 ± 0.9 (5.8–8.5) | 7.9 ± 0.4 (7.1–8.3) |
| Stimulated C-peptide, nmol/La | <0.1 | <0.1 |
Abbreviations: BM, body mass; BMI, body mass index; F, female; M, male.
All subjects had fasting and stimulated C-peptide less than the assay limit (<0.1 nmol/L).
Glycemic control: adult cohort
The 48-hour mean PG was 132 ± 9 mg/dL in the subgroup receiving an adaptive meal-priming bolus (AMB) and 146 ± 9 mg/dL with no meal-priming bolus (NMB) (P = .03), with 80% and 70% of PG values within 70–180 mg/dL (P = .04), respectively (Table 2 and Figure 1, A and C). There was no difference in PG values less than 70 mg/dL (5.1% vs 3.6%, P = .7) or less than 60 mg/dL (1.4% vs 2.3%, P = .6). Mean PG during nighttime hours was 112 ± 14 mg/dL vs 110 ± 14 mg/dL (P = .9) with 92% and 94% of PG values 70–180 mg/dL, respectively. The overall average area under the curve (AUC) in the CGMG trace over the 4-hour postprandial periods for all six meals was modestly reduced in the AMB subgroup by 12% relative to the NMB subgroup (Figure 2B, P = .01). There were 22 episodes of treatment-requiring hypoglycemia in five subjects, which corresponds to an average of 0.92 episodes per day.
Table 2.
Summary Results of All 48-Hour Closed-Loop Experiments
| ID | CGM, mg/dL | BG, mg/dL | Projected HbA1c, % | Percentage in Range |
Insulin, U/kg · d | Glucagon, μg/kg · d | Insulin tmax, min | ||
|---|---|---|---|---|---|---|---|---|---|
| <60 mg/dL | <70 mg/dL | 70–180 mg/dL | |||||||
| 202, A, AMB | 127 | 142 | 6.6 | 0.0 | 0.0 | 81.8 | 0.49 | 3.63 | 61 |
| 282, A, AMB | 142 | 125 | 6.0 | 9.3 | 16.6 | 68.9 | 0.99 | 8.98 | 73 |
| 290, A, AMB | 125 | 132 | 6.2 | 0.0 | 1.0 | 85.8 | 0.57 | 6.26 | 57 |
| 305, A, AMB | 120 | 121 | 5.8 | 4.6 | 9.7 | 82.6 | 0.50 | 10.06 | 73 |
| 312, A, AMB | 124 | 131 | 6.2 | 0.0 | 2.6 | 79.0 | 0.36 | 7.35 | 65 |
| 323, A, AMB | 133 | 143 | 6.6 | 0.0 | 0.5 | 82.4 | 0.71 | 4.31 | 35 |
| Mean, n = 6 | 129 | 132 | 6.2 | 2.3 | 5.1 | 80 | 0.6 | 6.8 | 61 |
| SD, n = 6 | 8 | 9 | 0.3 | 3.9 | 6.7 | 6 | 0.2 | 2.5 | 14 |
| 283, A, NMB | 147 | 156 | 7.1 | 0.0 | 3.6 | 56.2 | 0.83 | 6.43 | 134 |
| 303, A, NMB | 132 | 140 | 6.5 | 1.6 | 6.4 | 71.8 | 0.55 | 6.36 | 53 |
| 316, A, NMB | 130 | 134 | 6.3 | 6.7 | 11.3 | 63.1 | 0.51 | 12.81 | 114 |
| 324, A, NMB | 136 | 142 | 6.6 | 0.0 | 0.0 | 80.8 | 0.62 | 5.97 | 72 |
| 325, A, NMB | 148 | 145 | 6.7 | 0.0 | 0.0 | 74.1 | 0.85 | 1.96 | 61 |
| 327, A, NMB | 147 | 157 | 7.1 | 0.0 | 0.5 | 71.6 | 0.85 | 6.14 | 36 |
| Mean, n = 6 | 140 | 146 | 6.7 | 1.4 | 3.6 | 70 | 0.7 | 6.6 | 78 |
| SD, n = 6 | 8 | 9 | 0.3 | 2.7 | 4.5 | 9 | 0.2 | 3.5 | 38 |
| P value, A, AMB vs NMB | .03 | .03 | .03 | .64 | .67 | .04 | .40 | .93 | .32 |
| 228, P, AMB | 148 | 153 | 7.0 | 0.0 | 0.0 | 78.6 | 0.86 | 1.69 | 42 |
| 242, P, AMB | 156 | 166 | 7.4 | 0.0 | 1.0 | 63.1 | 1.39 | 5.73 | 69 |
| 275, P, AMB | 154 | 163 | 7.3 | 0.0 | 0.0 | 69.7 | 1.15 | 1.82 | 53 |
| 299, P, AMB | 151 | 159 | 7.2 | 0.0 | 0.0 | 69.0 | 1.09 | 2.77 | 118 |
| 300, P, AMB | 156 | 159 | 7.2 | 0.0 | 0.0 | 75.0 | 1.08 | 1.47 | 53 |
| 326, P, AMB | 170 | 171 | 7.6 | 0.5 | 1.0 | 55.2 | 1.08 | 1.47 | 73 |
| Mean, n = 6 | 156 | 162 | 7.3 | 0.1 | 0.3 | 68 | 1.1 | 2.5 | 68 |
| SD, n = 6 | 8 | 6 | 0.2 | 0.2 | 0.5 | 8 | 0.2 | 1.7 | 27 |
| 263, P, NMB | 173 | 179 | 7.9 | 0.0 | 0.0 | 59.0 | 1.38 | 2.27 | 57 |
| 301, P, NMB | 157 | 164 | 7.3 | 0.0 | 0.0 | 62.9 | 0.93 | 0.56 | 57 |
| 308, P, NMB | 157 | 174 | 7.7 | 0.0 | 0.0 | 63.9 | 0.98 | 3.31 | 69 |
| 318, P, NMB | 161 | 174 | 7.7 | 0.0 | 0.0 | 62.5 | 1.02 | 1.83 | 61 |
| 319, P, NMB | 170 | 190 | 8.2 | 0.5 | 1.6 | 52.3 | 1.26 | 5.29 | 49 |
| 331, P, NMB | 172 | 171 | 7.6 | 0.0 | 1.0 | 57.1 | 1.41 | 4.00 | 65 |
| Mean, n = 6 | 165 | 175 | 7.7 | 0.1 | 0.4 | 60 | 1.2 | 2.9 | 60 |
| SD, n = 6 | 8 | 9 | 0.3 | 0.2 | 0.7 | 4 | 0.2 | 1.7 | 7 |
| P value, P, AMB vs NMB | .06 | .01 | .01 | 1.00 | .78 | .05 | .63 | .70 | .49 |
| P value, A vs P, AMB | <.01 | <.01 | <.01 | .218 | .144 | .021 | <.01 | <.01 | .573 |
| P value, A vs P, NMB | <.01 | <.01 | <.01 | .289 | .145 | .038 | <.01 | .049 | .284 |
Results are broken out across the four subgroups: adults (A) receiving automatically AMB, adults receiving NMB, adolescents (P) receiving AMB, adolescents receiving NMB.
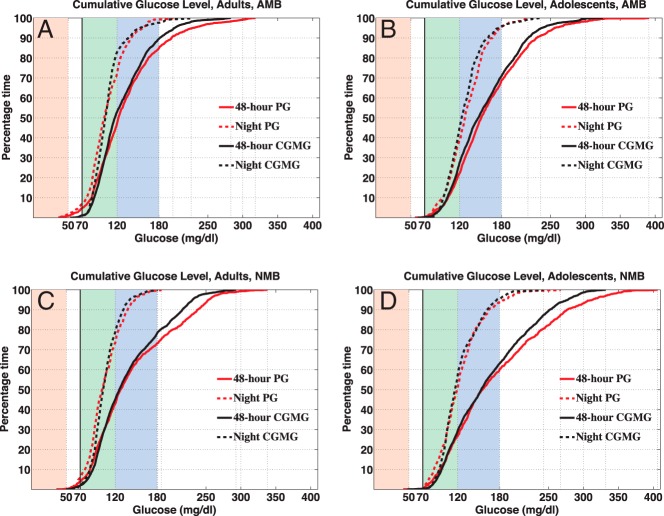
Figure 1.
Each panel shows cumulative plots of 48-hour and night PG and 48-hour and night CGMG. A, Cumulative plots for adults with AMB, in which 4.8% of the 48-hour PG was 70 mg/dL or less, 80.1% within 70–80 mg/dL (1.2% and 88.4% for CGMG), and 7.1% of night PG was 70 mg/dL or less, 92.1% within 70–80 mg/dL (0.9% and 96.7% for CGMG). B, Cumulative plots for adolescent subjects with AMB, in which 0.3% of the 48-hour PG was 70 mg/dL or less, 68.4% within 70–80 mg/dL (0.2% and 70.7% for CGMG), and 0.5% 0% of night PG 70 mg/dL or less, 95.2% within 70–80 mg/dL (0% and 95.7% for CGMG). C, Cumulative plots for adults with NMB, in which 4% of the 48-hour PG was 70 mg/dL or less, 69.1% within 70–80 mg/dL (2% and 76.1% for CGMG), and 5.8% of night PG 70 mg/dL or less, 93.9% within 70–80 mg/dL (2% and 97.9% for CGMG). D, Cumulative plots for adolescent subjects with NMB, in which 0.4% of the 48-hour PG was 70 mg/dL or less, 59.6% within 70–80 mg/dL (0.3%, 62.7% for CGMG), and 0.3% of night PG 70 mg/dL or less, 93.9% within 70–80 mg/dL (0.3% and 95.2% for CGMG).
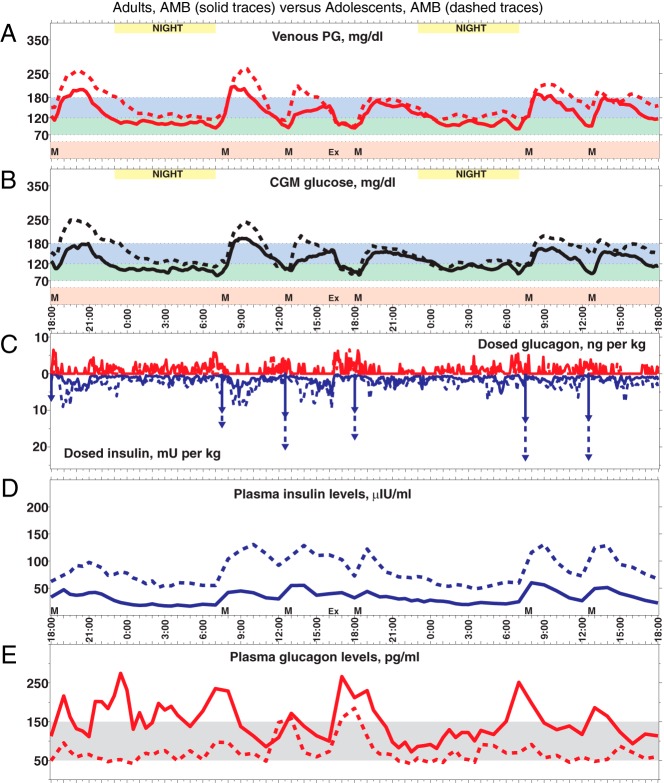
Figure 2.
A, Forty-eight-hour mean PG trace (PG sampled every 15 min) for adults with AMB (solid line) superimposed on a trace for adolescent subjects with AMB (dashed line). The six meals are indicated by black triangles. B, Forty-eight -hour mean CGMG trace (CGMG sampled every 5 min) that provided the input for the closed-loop control algorithm. C, Superposition of the means of sc insulin-glucagon doses (including priming insulin doses, indicated by downward arrows) administered by the closed-loop system in response to CGMG in panel B. D and E, Superposition of mean plasma insulin and glucagon levels, respectively. Notice the higher PG and CGMG for adolescent subjects relative to adults in both panels A and B, despite a higher insulin dosing (C) and corresponding higher plasma insulin levels (D) as well as lower glucagon dosing (C) and corresponding lower plasma glucagon levels (E). Although the system's dosing per kilogram of body mass was the same at initialization, it automatically increased its insulin dosing more for adolescent subjects than adults (B) and achieved better control by the second day in both cases. These plots broken out across the adult and adolescent AMB subgroups are shown are Supplemental Figures 26 and 28.
Glycemic control: adolescent cohort
In adolescent subjects, 48-hour mean PG was 162 ± 6 mg/dL vs 175 ± 9 mg/dl (P = .01) in the AMB and NMB subgroups, respectively, with 68% vs 60% of PG values within 70–180 mg/dL (P = .05) (Table 2 and Figure 1, B and D). There was no difference in PG less than 70 mg/dL (0.3% vs 0.4%, P = .8) or less than 60 mg/dL (0.1% vs 0.1%, P = 1.0). Mean PG values during nighttime hours were 137 ± 14 mg/dL vs 132 ± 12 mg/dL (P = .5) with 95% and 94% of PG values within 70–180 mg/dL, respectively. The overall average AUC in the CGMG trace over the 4-hour postprandial periods for all six meals was not significantly different between the AMB and NMB subgroups (Figure 3B, P = .28). There was one treatment-requiring episode of hypoglycemia, which corresponds to an average of 0.04 episodes per day.
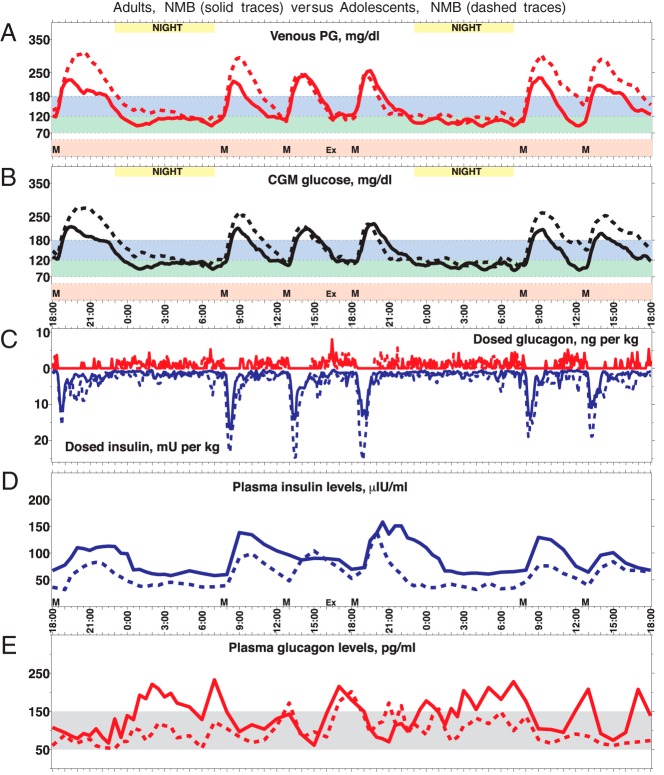
Figure 3.
A, Forty-eight -hour mean PG trace (PG sampled every 15 min) for adults with NMB (solid line) superimposed on a trace for adolescent subjects with NMB (dashed line). The six meals are indicated by black triangles. B, Forty-eight -hour mean CGMG trace (CGMG sampled every 5 min) that provided the input for the closed-loop control algorithm. C, Superposition of the means of sc insulin-glucagon doses (including priming insulin doses, indicated by downward arrows) administered by the closed-loop system in response to CGMG in panel B. D and E, Superposition of mean plasma insulin and glucagon levels, respectively. Notice the higher PG and CGMG for adolescent subjects relative to adults in both panels A and B, despite higher insulin dosing (C) and corresponding higher plasma insulin levels (D) as well as lower glucagon dosing (C) and corresponding lower plasma glucagon levels (E). Although the system's dosing per kilogram of body mass was the same at initialization, it automatically increased its insulin dosing more for adolescent subjects than adults (B) and achieved better control by the second day in both cases. These plots broken out across the adult and adolescent NMB subgroups are shown are Supplemental Figures 27 and 29. Note that subject 283 (Supplemental Figure 8) has very high antiinsulin antibody levels, which appear to interfere with the insulin immunoassay. This results in extremely high measured insulin levels and shifts the overall mean for the adult NMB group upward, explaining why the mean insulin levels in the adult NMB cohort are higher than in the adolescent NMB cohort despite higher dosing in the adolescent cohort.
Adaptation of the meal-priming bolus
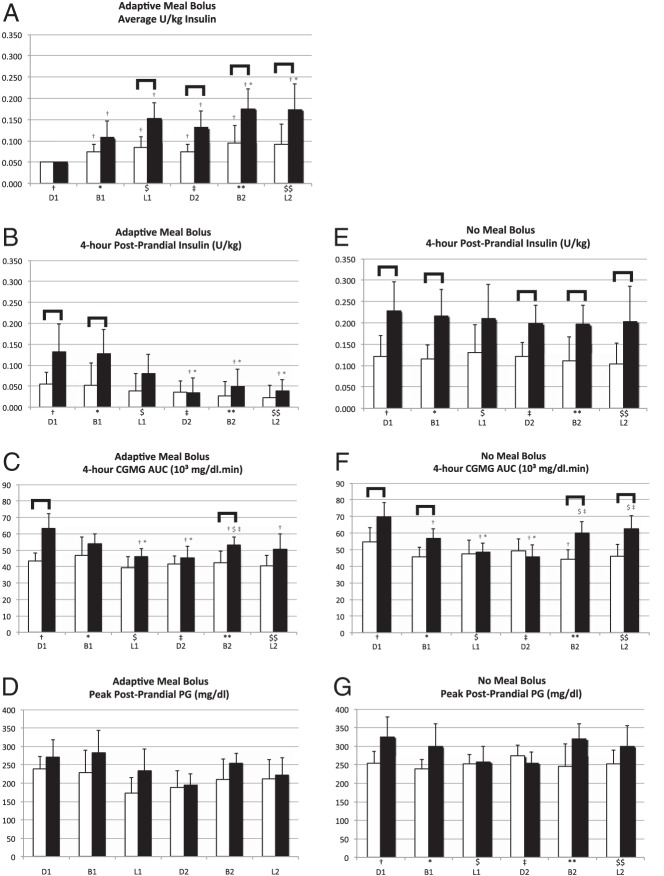
The size of the meal-priming bolus for the first meal was set at 0.05 U/kg (see Figures 2C and 4A). In both the adult and adolescent AMB subgroups, the average meal-priming bolus size automatically increased for all subsequent meals (Figure 4A), more so in adolescents with the average bolus size for the last four meals being approximately 2.5–3.5 times larger than the first meal (P < .05). The average meal-priming boluses for each of the last four meals were significantly larger in adolescents than in adults (P < .05) (Figure 4A), but the differences in the automatic controller-dosed postprandial insulin were consequently reduced (Figures 2C and 4B). Correspondingly, the significant difference in the AUC of CGMG between adults and adolescents for the first meal (P = .002) was no longer significant for the second through fourth and sixth meals (Figure 4C).
Figure 4.
Glycemic control quantities relating to the times around the six meals, ordered as first dinner (D1), first breakfast (B1), first lunch (L1), second dinner (D2), second breakfast (B2), and second lunch (L2). For each meal, the bar on the left relates to adults and the one on the right relates to adolescent subjects. Each of the six meals is assigned a reference symbol: †, D1; *, B1; $, L1; ‡, to D2; **, B2; and $$, L2. The appearance of any meal symbol above a bar pertaining to any of the other five meals for the same age group indicates a statistically significant difference (P < .5) in the values of the represented quantity for those two meals. The appearance of a square brace above any pair of bars indicates a statistically significant difference in the values of the represented quantities for that meal between the adult and adolescent cohorts. A, Average size of adaptive meal boluses. B and E, Four-hour postprandial insulin with AMB and NMB, respectively. C and F, Four-hour CGMG postprandial AUC with AMB and NMB, respectively. D and G, Peak postprandial PG with AMB and NMB, respectively.
Adaptation of the algorithm in the absence of meal-priming boluses
In the adult NMB subgroup, there was no difference between any of the meals in the automatic controller-dosed 4-hour postprandial insulin (Figure 4E) and minimal differences in the mean postprandial AUC between meals. Thus, there was very little net adaptation required by the control algorithm.
In adolescents, there was no difference between meals in the automatic controller-dosed 4-hour postprandial insulin (Figure 4E), but dosing was concentrated earlier in the postprandial period of the last five meals vs the first meal (Figure 3). The mean values of the postprandial AUC for the second through fourth meals were significantly lower than for the first meal (Figure 4F), and the peak postprandial PG levels of the third and fourth meals were lower than that of the first meal (Figure 4G, all P < .05).
Adolescents received significantly more automatic controller-dosed, 4-hour postprandial insulin than adults for every meal except the third (Figure 4E). Despite this, the AUC for adolescents was significantly larger than in adults for the first and second meals (P < .05), although not for the third and fourth meals (Figure 4F). Likewise, the peak postprandial PG was significantly higher in adolescents for the first meal (P < .05) but not the second through fourth meals (Figure 4G).
A significant difference between adults and adolescents reappeared in meals five and six for the AUC (Figure 4F) and for meal five for peak postprandial PG (Figure 4G), consistent with a partial relaxation of adaptation overnight (see Discussion).
Insulin administration
There was nearly a 4-fold variation in the total daily insulin dose between the adult and adolescent cohorts (0.36–1.41 U/kg · d). Adolescents received more insulin than adults in experiments with a meal-priming bolus (1.1 ± 0.2 vs 0.6 ± 0.2 U/kg · d, P = .001) and without (1.2 ± 0.2 vs 0.7 ± 0.2 U/kg · d, P = .002). The portion of the prandial insulin that came from the meal-priming bolus was similar in adolescents and adults (64% ± 5% vs 71% ± 5%, P = .06). There was no difference between the total insulin delivered with or without an adaptive meal-priming bolus (P = .4 for adults, P = .6 for adolescents).
Insulin pharmacokinetics and glucagon administration
As we found previously (14, 15), there was a large intersubject variation in insulin lispro pharmacokinetic parameters (Table 2) with tmax ranging 35–134 minutes (mean 70 ± 31 min) in adults and 42–118 minutes (mean 64 ± 23 min) in adolescents (P = .6).
The system delivered less glucagon in adolescents than in adults, both in the AMB subgroups (2.5 ± 1.7 vs 6.8 ± 2.5 μg/kg · d, P = .008) and the NMB subgroups (2.9 ± 1.7 vs 6.6 ± 3.5 μg/kg · d, P = .05). Glucagon dosing was not significantly different between the AMB and NMB subgoups for adolescents (P = .7) or for adults (P = .9). Mean plasma glucagon levels were in the normal fasted range (50–150 pg/mL) 74%, 61%, 91%, and 61% of the time for the adolescent and adult AMB and adolescent and adult NMB groups, respectively (Figures 2D and 3D).
Performance of the CGM
Overall, the Navigator CGM performed well relative to PG measurements, with a mean absolute relative difference (MARD) of 12.3% ± 4.7% (range 6%–29%). However, the experiment with the MARD of 29% (Supplemental Figure 3) accounted for 8 of the 12 episodes of hypoglycemia in the adult AMB subgroup and two of the experiments with MARDs of 15% or greater (Supplemental Figures 9 and 10) accounted for 9 of the 10 episodes of hypoglycemia in the adult NMB subgroup. None of the experiments in the adolescent cohort had a MARD greater than 11.7%.
Adverse events other than hypoglycemia
There was nausea with or without headache that may have been associated with glucagon administration in 2 of the 24 subjects (one adult and one adolescent). The peak glucagon levels were not unusually high in these subjects (see Supplemental Methods for further details).
Technical failures
All data from all subjects were included when calculating outcome metrics, regardless of technical failures. There were 10 glucagon pod failures and three insulin pod failures with alarms and three cases of clinically suspected failure of insulin delivery (all later affirmed by plasma insulin deficits relative to predictions, which resolved after pod replacement). In each case the only intervention was to replace the pod. There were four periods during which the Navigator software was transiently offline and the control algorithm continued automatic basal insulin delivery, based on the previous history (see Supplemental Material for further details).
Discussion
Insulin needs vary greatly among individuals, presenting a challenge to the design of bionic pancreas control algorithms. Many algorithms use individual usual-care insulin regimen information and/or require a training period (16–40). If the insulin requirements change (with changes in health, physical activity, body fat, and pubertal development), then the control system may be too constrained by its initial parameters to provide effective and safe glucose control. Although at least one other group has tested an adaptation capability (31), our approach is unique in that the only information provided to the algorithm is body mass. The insulin dosing adapts without constraints based on the usual-care insulin regimen. The experiments reported here are a stringent test of the adaptive capabilities of the algorithm.
Delays in the absorption of sc insulin and in CGM sensing to track changes in PG (10, 11, 14, 15, 41–43) have also led us and many others (14–16, 18, 26, 27, 31–34, 36–38) to experiment with meal-priming insulin doses. Other control algorithms require the user to calculate the carbohydrate content of the meal just as they do in current usual care (16, 18, 26, 27, 31–34, 36–38). This approach dramatically limits the reduction in burden of care that can be achieved with a bionic pancreas and is associated with a risk of hypoglycemia if too much insulin is provided (16, 26, 27, 34–38). Our approach is less burdensome to patients because no quantification of carbohydrate consumption is required.
After allowing 24 hours of adaptation, the mean PG under bionic pancreas control in both adult cohorts were consistent with meeting American Diabetes Association glycemic control guidelines (average PG <154 mg/dL) (44, 45). The amount of adaptation required by the algorithm for adults is modest because its initial parameters were conservatively set for average adult insulin requirements.
Consistent with our expectation that significant adaptation would be required in adolescents, the overall mean PG was higher in both adolescent subgroups than in adults and the adaptive meal-priming bolus was necessary to a achieve American Diabetes Association glycemic control guidelines for adolescents (average PG ≤168 mg/dL). Adolescents received 75% more insulin per kilogram than adults, and the average size of the adaptive meal-priming boluses on the second day was 1.8 larger than in adults. Likewise, the 4-hour postprandial insulin was 63% higher in the adolescent vs adult NMB subgroups. The exact same algorithm and initialization parameters were used in both adults and adolescents, demonstrating robust adaptation over only 24 hours. The adaptation rate and magnitude suggests the system is sufficiently robust to manage rapid changes in individual insulin requirement due to changes in activity level or illness.
The inherently higher insulin requirements of adolescents, coupled with their relatively lower body mass (which, in turn, relatively reduced the algorithm's dose scaling initially) required more time in adolescents than in adults for the system to adapt to the levels necessary to achieve effective insulin dosing. This contributed to the higher mean PG levels in adolescents because the study spans a relatively short period.
This study revealed that even more rapid adaptation may further improve performance. The adults in both the AMB and NMB subgroups with the lowest mean PG values accounted for a large fraction of recorded hypoglycemia. Therefore, we have refined the fourth-generation algorithm so that it will more strongly reduce insulin dosing as the mean CGMG is reduced. On the other hand, hypoglycemia was very low in both the adolescent NMB and AMB subgroups, and the mean PG was greater than the glycemic targets for some individuals. Consequently, we have refined the algorithm to be more aggressive when the mean CGMG is high. We also found that adaptation relaxed somewhat due to low mean glucose levels overnight and refined the algorithm to prevent that.
Our system is dependent on the CGM accurately estimating PG. The adult subject with the most time with PG less than 70 mg/dL also had the worst CGM performance. When the CGMG level was higher than the PG, the bionic pancreas dosed more insulin and less glucagon than would have been the case with an accurate estimation of PG, increasing the risk for hypoglycemia. More accurate CGM sensors and, ideally, sensors that do not require calibration, would improve system performance. A weakness of the Navigator CGM is that it underestimates PG in the hyperglycemic range (46). Because the algorithm adapts in response to mean CGMG, the system would have adapted more aggressively if this bias had not been present. The DexCom G4 Platinum CGM (Dexcom) does not exhibit such bias and has similar or superior accuracy (47). Substitution with the DexCom G4 CGM in future studies is expected to lower the mean PG achieved by the system and reduce the difference in mean PG between adults and adolescents.
Some hypoglycemia that occurred required treatment with extra carbohydrates according to our protocol. Although the strictly limited microdoses of glucagon were not sufficient in every case to prevent hypoglycemia from occurring, they may have been able to reduce the severity and duration of hypoglycemia by rebounding PG out of the hypoglycemic range. We were unable to directly test this hypothesis because the study protocol was designed conservatively with a premium on subject safety, which mandated relatively early treatment of hypoglycemia with oral carbohydrates. Indeed, the duration of hypoglycemia reported here likely represents a lower bound on what might be expected in clinical practice because those episodes that mandated carbohydrate treatment likely would have increased the duration of hypoglycemia had carbohydrates not been administered.
To our knowledge, this is the first demonstration that a single bionic pancreas system, initialized with a single objective parameter (body mass), can provide safe and effective glycemic control in both adolescents and adults with T1D with widely varying insulin requirements. The time scale of adaptation is less than 24 hours, which is sufficiently fast for any physiological change in insulin requirement that individuals with T1D are likely to experience. This adaptation rate is sufficient to manage intercurrent illness and changes in physical activity and provides a universal bionic pancreas device for all individuals with T1D from initial treatment at diagnosis and through developmental stages of life. Outpatient studies in adults and children will follow based on the strengths of the results presented here.
Acknowledgments
We thank the volunteers for their time and enthusiasm; the diabetes care providers who referred potential participants for study; the nurses and laboratory staff of the Massachusetts General Hospital Clinical Research Center, especially K. Hall and K. Grinke; the study staff at the Diabetes Research Center, including K. Grennan, C. Beauharnais, L. Macey, and M. Hillard for their dedicated effort and careful execution of the experimental protocol; John Jiang for technical support; M. Larkin, C. Collings, and N. Kingori of the Diabetes Research Center, Massachusetts General Hospital, for organizational and logistical support; J. Moy and P. Sluss of the Massachusetts General Hospital Reproductive Endocrine Laboratory for performing insulin and glucagon assays; L. Levitsky and N. Sherry for their assistance with protocol design and subject recruitment; T. Goodnow, M. Taub, and E. Budiman of Abbott Diabetes Care for providing Navigator hardware and software support and technical advice; R. Campbell and S. Gemmell of Insulet Corp for providing Insulet OmniPod hardware and software support and technical advice; J. Segars and J. Isenberg of International Biomedical for providing GlucoScout monitors and technical assistance in their use; J. Godine, D. Wexler, and C. Rosow for serving on the data safety and monitoring board for the study; the members of the Partners Human Research Committee and the Boston University Medical Campus Institutional Review Board for their oversight of the study; and C. Zimliki, K. Marin, and P. Beaston of the Office of Device Evaluation, US Food and Drug Administration, for their helpful suggestions during the process of obtaining the investigational device exemption for this study.
This study was registered with a clinical trial number of NCT01161862.
This study was supported by National Institutes of Health Grant R01-DK085633 (to E.R.D.); Juvenile Diabetes Research Foundation Grant 22-2010-771 (to E.R.D.) from The Leona M. and Harry B. Helmsley Charitable Trust; Grants M01-RR01066 and UL1-RR025758 from the National Institutes of Health National Center for Research Resources through the General Clinical Research Center and Clinical and Translational Science Center programs; and a grant from the Charlton Fund for Innovative Research in Diabetes (to D.M.N.).
Disclosure Summary: F.H.E.K. and E.R.D. have patents and patents pending, and S.J.R. has a patent pending on aspects of the bionic pancreas. E.R.D. and S.J.R. have received travel expenses and an honorarium for a lecture delivered to Eli Lilly employees in 2012. S.J.R. received travel expenses from Abbott Diabetes Care for a lecture at BIO in 2010 and received grant support of an investigator-initiated study ending in 2010. Abbott Diabetes Care, Insulet, and International Biomedical loaned equipment for this study and provided technical support for its use. The other authors have nothing to declare.
Footnotes
- AMB
- adaptive meal-priming bolus
- AUC
- area under the curve
- BG
- blood glucose
- CGM
- continuous glucose monitoring
- CGMG
- CGM glucose
- HbA1c
- glycosylated hemoglobin
- MARD
- mean absolute relative difference
- NMB
- no meal-priming bolus
- PG
- plasma glucose
- T1D
- type 1 diabetes
- tmax
- peak time.
References
- 1. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–25694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46:271–286 [PubMed] [Google Scholar]
- 5. Leese GP, Wang J, Broomhall J. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26:1176–1180 [DOI] [PubMed] [Google Scholar]
- 6. Cengiz E, Xing D, Wong JC, et al. T1D Exchange Clinic Network. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange clinic registry. Pediatr Diabetes. 2013;14:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weinstock RS, Xing D, Maahs DM, et al. T1D Exchange Clinic Network. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab. 2013;98:3411–3419 [DOI] [PubMed] [Google Scholar]
- 8. Petitti DB, Klingensmith J, Bell RA, et al. SEARCH for Diabetes in Youth Study Group. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr. 2009;155:668–72.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tamborlane RW, Bergenstal RM, Miller KM, DuBose SN, Hall CA. T1D Exchange Clinic Network. The T1D Exchange Clinic Registry. J Clin Endocrinol Metab. 2012;97:4383–4389 [DOI] [PubMed] [Google Scholar]
- 10. Wood JR, Miller KM, Maahs DM, et al. T1D Exchange Clinic Network. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36:2035–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912 [DOI] [PubMed] [Google Scholar]
- 12. Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–219 [DOI] [PubMed] [Google Scholar]
- 13. Kerouz N, el-Hayek R, Langhough R, MacDonald MJ. Insulin doses in children using conventional therapy for insulin dependent diabetes. Diabetes Res Clin Pract. 1995;29:113–120 [DOI] [PubMed] [Google Scholar]
- 14. El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Trans Med. 2010;2:27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care. 2012;35:2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elleri D, Allen JM, Kumareswaran K, et al. Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care. 2013;36:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dassau E, Zisser H, Harvey RA, et al. Clinical evaluation of a personalized artificial pancreas. Diabetes Care. 2013;36:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haidar H, Legault L, Dallaire M, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. Can Med Assoc J. 2013;185:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368:824–833 [DOI] [PubMed] [Google Scholar]
- 20. O'Grady MJ, Retterath AJ, Keenan DB, et al. The use of an automated, portable glucose control system for overnight glucose control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012;35:2182–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruiz JL, Sherr JL, Cengiz E, et al. Effect of insulin feedback on closed-loop glucose control: a crossover study. J Diabetes Sci Technol. 2012;6:1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Bon AC, Jonker LD, Koebrugge R, Koops R, Hoekstra JB, DeVries JH. Feasibility of a bihormonal closed-loop system to control postexercise and postprandial glucose excursions. J Diabetes Sci Technol. 2012;6:1114–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weinzimer SA, Sherr JL, Cengiz E, et al. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012;35:1994–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elleri D, Allen JM, Biagioni M, et al. Evaluation of a portable ambulatory prototype for automated overnight closed-loop insulin delivery in young people with type 1 diabetes. Pediatr Diabetes. 2012;13:449–453 [DOI] [PubMed] [Google Scholar]
- 25. Nimri R, Atlas E, Ajzensztejn M, Miller S, Oron T, Phillip M. Feasibility study of automated overnight closed-loop glucose control under MD-logic artificial pancreas in patients with type 1 diabetes: the DREAM Project. Diabetes Technol Ther. 2012;14:728–735 [DOI] [PubMed] [Google Scholar]
- 26. Breton M, Farret A, Bruttomesso D, et al. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61:2230–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy HR, Kumareswaran K, Elleri D, et al. Safety and efficacy of 24-h closed-loop insulin delivery in well-controlled pregnant women with type 1 diabetes: a randomized crossover case series. Diabetes Care. 2011;34:2527–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steil GM, Palerm CC, Kurtz N, et al. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab. 2011;96:1402–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomized controlled studies. BMJ. 2011;342:d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elleri D, Allen JM, Nodale M, et al. Automated overnight closed-loop glucose control in young children with type 1 diabetes. Diabetes Technol Ther. 2011;13:419–424 [DOI] [PubMed] [Google Scholar]
- 31. El Youssef J, Castle JR, Branigan DL, et al. A controlled study of the effectiveness of an adaptive closed-loop algorithm to minimize corticosteroid-induced stress hyperglycemia in type 1 diabetes. J Diabetes Sci Technol. 2011;5:1312–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Bon AC, Hermanides J, Koops R, Hoekstra JB, DeVries JH. Postprandial glycemic excursions with the use of a closed-loop platform in subjects with type 1 diabetes: a pilot study. J Diabetes Sci Technol. 2010;4:923–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33:1282–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care. 2010;33:1072–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hovorka R, Allen JM, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375:743–751 [DOI] [PubMed] [Google Scholar]
- 36. Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas. Diabetes Care. 2010;33:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clarke WL, Anderson S, Breton M, Patek S, Kashmer L, Kovatchev B. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: the Virginia experience. J Diabetes Sci Technol. 2009;3:1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bruttomesso D, Farret A, Costa S, et al. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: preliminary studies in Padova and Montpellier. J Diabetes Sci Technol. 2009;3:1014–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 40. Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55:3344–3350 [DOI] [PubMed] [Google Scholar]
- 41. Garg SK, Voelmle MM, Gottlieb PA. Time lag characterization of two continuous glucose monitoring systems. Diabetes Res Clin Pract. 2010;87:348–353 [DOI] [PubMed] [Google Scholar]
- 42. Davey RJ, Low C, Jones TW, Fournier PA. Contribution of an intrinsic lag of continuous glucose monitoring systems to differences in measured and actual glucose concentrations changing at variable rates in vitro. J Diabetes Sci Technol. 2010;4:1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mazze RS, Strock E, Borgman S, Wesley D, Stout P, Racchini J. Evaluating the accuracy, reliability, and clinical applicability of continuous glucose monitoring (CGM): is CGM ready for real time? Diabetes Technol Ther. 2009;11:11–18 [DOI] [PubMed] [Google Scholar]
- 44. American Diabetes Association. Standard of medical care in diabetes. Diabetes Care. 2013;36(suppl 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Damiano ER, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A comparative effectiveness analysis of three continuous glucose monitors. Diabetes Care. 2013;36:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Damiano ER, McKeon K, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A comparative effectiveness analysis of three continuous glucose monitors. Diabetes. 2013;62 (Supplement 1):A44. [DOI] [PMC free article] [PubMed] [Google Scholar]