Abstract
Context:
Pazopanib is a small molecule inhibitor of kinases principally including vascular endothelial growth factor receptors-1, -2, and -3; platelet-derived growth factor receptors-α and -β; and c-Kit. We previously reported a tumor response rate of 49% in patients with advanced differentiated thyroid cancer and 0% in patients with advanced anaplastic thyroid cancer. The present report details results of pazopanib therapy in advanced medullary thyroid cancer (MTC).
Objective, Design, Setting, Patients, Intervention, and Outcome Measures:
Having noted preclinical activity of pazopanib in MTC, patients with advanced MTC who had disease progression within the preceding 6 months were accrued to this multiinstitutional phase II clinical trial to assess tumor response rate (by Response Evaluation Criteria In Solid Tumors criteria) and safety of pazopanib given orally once daily at 800 mg until disease progression or intolerability.
Results:
From September 22, 2008, to December 11, 2011, 35 individuals (80% males, median age 60 y) were enrolled. All patients have been followed up until treatment discontinuation or for a minimum of four cycles. Eight patients (23%) are still on the study treatment. The median number of therapy cycles was eight. Five patients attained partial Response Evaluation Criteria In Solid Tumors responses (14.3%; 90% confidence interval 5.8%–27.7%), with a median progression-free survival and overall survival of 9.4 and 19.9 months, respectively. Side effects included treatment-requiring (new) hypertension (33%), fatigue (14%), diarrhea (9%), and abnormal liver tests (6%); 3 of 35 patients (8.6%) discontinued therapy due to adverse events. There was one death of a study patient after withdrawal from the trial deemed potentially treatment related.
Conclusions:
Pazopanib has promising clinical activity in metastatic MTC with overall manageable toxicities.
Medullary thyroid cancer (MTC) is an uncommon malignancy of the thyroid gland that accounts for less than 5% of all thyroid cancers (1, 2). Most MTC patients respond well to locoregional therapies including surgery and/or radiation therapy, with 5-year survival rates after diagnosis greater than 80% (1, 2). However, those with progressive metastatic disease have historically been faced with largely ineffective systemic therapies. In particular, until recently, systemic therapy for metastatic MTC included principally cytotoxic chemotherapy; such therapy, however, produces tumor response rates between 10% and 20%, accompanied also by significant toxicities (1, 2).
The discovery that MTC is most often characterized by activating mutations of the rearranged during transfection (RET) kinase led to the more recent study of the therapeutic potential of small-molecule ATP-mimetic RET kinase inhibitors. Two such agents, vandetanib and cabozantinib, have now been approved by the US Food and Drug Administration for treating progressive, symptomatic, and metastatic MTC. In addition to the prolongation of progression-free survival relative to placebo, these agents yielded Response Evaluation Criteria In Solid Tumors (RECIST) response rates of 45% and 27%, respectively, in large randomized clinical trials (3, 4).
Examination of the potential therapeutic effects of primarily vascular endothelial growth factor receptor (VEGFR)-targeted kinase inhibitors such as sorafenib, sunitinib, and motesanib is also underway, yielding quite varied results (5–10). A two-stage phase II trial of sorafenib in metastatic MTC terminated enrollment after the first stage with only one partial tumor response (PR) among 15 patients enrolled, with a median progression-free survival (PFS) of 17.9 months (5). Two phase II studies of sunitinib included MTC patients; one is closed, reporting that three of six MTC patients attained a PR (8), and the other has completed enrollment, with results forthcoming (9). Motesanib was also studied in 91 MTC patients, with a PR rate of only 2% (10). A phase II study of imatinib, a primarily Bcl-Abl and c-Kit kinase inhibitor, resulted in no tumor responses among 15 MTC patients enrolled (11). Collectively, results of studies of these multiple kinase inhibitors have been overall disappointing.
In the present manuscript, we report results of examination of the clinical activity and adverse effects of pazopanib in MTC. Pazopanib is an orally bioavailable competitive (with respect to ACT) multitargeted kinase inhibitor, which most potently inhibits VEGFR1–3, platelet-derived growth factor-α/β, c-Kit, and FGFR1/3/4 (12). Pazopanib also inhibits other kinases, but much less potently; in particular, pazopanib inhibits VEGFR1/2 greater than 2 orders of magnitude more potently than RET in vitro (12). Having previously observed a high level of clinical responses from pazopanib in treating advanced and progressive follicular-cell derived (differentiated) thyroid cancer (DTC; 49% RECIST partial responses) (13), and upon noting similar effects of pazopanib on DTC and MTC cell proliferation in vitro, we undertook a multiinstitutional international phase 2 trial of pazopanib in patients with progressive metastatic MTC.
Patients and Methods
Preclinical investigations
The in vitro effects of continuous 5 μM pazopanib or dimethylsulfoxide diluent exposures on tumor cell proliferation were examined in BHP2–7 (differentiated) or TT (medullary) thyroid cancer cells via serially assessing cell proliferation. Briefly, subconfluent BHP2–7 or TT cells were plated on triplicate sets of 35-mm tissue culture dishes (1.675 × 105 or 3.45 × 105 cells/dish, respectively), allowed to adhere for 1 day, and then treated with diluent or pazopanib continuously as specified. At indicated times, cells from each plate in each triplicate set released using trypsinization were harvested at specified time points, with cells manually counted using a hemocytometer after staining with Trypan Blue to assure that only viable cells were counted. Means and SDs were determined and plotted graphically for each time point set; differences were examined statistically using the t statistic assessing the statistical significances of differences in sample means.
Study design
The primary end point of this phase II clinical trial was the tumor response rate defined as the percentage of patients whose disease burden met the RECIST 1.0 criteria for a partial or complete response on two consecutive evaluations at least 8 weeks apart among the patients who started pazopanib treatment. Secondary end points included safety, duration of response, PFS, and overall survival. Duration of response was defined as the time of registration to disease progression.
A three-outcome, one-stage, phase II clinical trial study design was chosen such that, at a 0.10 significance level, there would be a 90% chance of detecting a tumor response rate of at least 20% when the true response rate was at least 5%. If at least four patients had a tumor response (among the first 33 patients enrolled), we would conclude that the regimen was promising. If more than 33 eligible patients were enrolled due to timing of closure notice, only the first 33 eligible patients would be used to assess antitumor activity with the prespecified decision rule, but with all enrolled patient data are reported.
An interim analysis was prespecified after 14 patients had been followed up for at least 6 months. If none of these 14 patients had a tumor response, enrollment would be terminated and the regimen would be considered to have insufficient antitumor activity for further testing in this patient population. (Interim analysis revealed that there had been one partial tumor response among the first 14 patients enrolled and, as such, enrollment continued.)
Estimates of the distribution of progression-free and overall survival times were obtained using the Kaplan-Meier method. The log-rank test was used to assess where PFS differed with respect to changes in calcitonin or CEA (carcinoembryonic antibody).
Eligibility
Inclusion criteria included age of 18 years or older and histologically or cytologically confirmed advanced or metastasized medullary thyroid cancer; disease progression (documented by a measurable increase in tumor burden on successive imaging) 6 months or less prior to registration was also required. Assessment of RET mutational status was not required for study entry. According to Eastern Cooperative Oncology Group, performance status of 0, 1, or 2, at least one lesion that could be accurately measured in at least one dimension as 2.0 cm or greater by conventional imaging or 1.0 cm or greater by spiral computed tomography scan; adequate blood chemistries [absolute neutrophil count ≥1500/mm3, platelet count ≥100 000/mm3, leukocyte count ≥3000/mm3, total bilirubin ≤1.5 times the institutional upper limit of normal (ULN), serum creatinine ≤1.5 × ULN, aspartate aminotransferase ≤2.5 × ULN, proteinuria ≤1+ and INR (International Normalized Ratio) ≤1.2 × ULN], and enrollment systolic blood pressure (BP) less than 140 mm Hg and diastolic BP less than 90 mm Hg were also required.
Individuals were excluded from participation if they had more than two prior systemic therapeutic regimens; had discontinued radiation, or had surgery or systemic therapy 28 days or less prior to registration; had more than 1+ proteinuria on two consecutive dipsticks taken at least 1 week apart or QTc prolongation (>500 msec) or other significant electrocardiogram abnormalities; were receiving concomitant medications associated with a risk of QTc prolongation or Torsades de Pointes, cytochrome P450 drug interactions, or affected the activity or pharmacokinetics of pazopanib; were currently taking warfarin (low molecular weight heparin anticoagulation was allowed); had any condition precluding ability to swallow and/or retain pazopanib; had active or untreated brain metastases or brain metastases requiring ongoing therapy; had comorbid conditions such as a nonhealing wound, ulcer, and/or bone fracture(s); had poorly controlled depression or anxiety disorder; had active cerebrovascular accident, abdominal fistula, gastrointestinal (GI) perforation, diverticulitis, or GI bleeding 28 days or less prior to registration; had venous thrombosis less than 12 weeks prior to registration; or had a history of a bleeding disorder, New York Heart Association class III/IV heart failure, clinically significant active cardiac arrhythmia, or recent myocardial infarction.
Women of child-bearing potential also underwent a serum pregnancy test within 7 days of registration and agreed to use an effective contraception during treatment. Institutional review boards of all participating institutions approved this study, and informed consent was obtained from each patient prior to study entry.
Within 4 weeks of study entry and prior to each cycle of treatment, patients underwent a complete medical examination with blood chemistries and adverse event assessment. Blood pressure measurements were taken twice daily beginning on day 8 until treatment discontinuation. Tumor measurements (RECIST 1.0) were required before therapy and at 8-week intervals.
Treatment and dose modification
Pazopanib was administered orally once daily at 800 mg until disease progression or intolerability; dosage reductions were required in response to preset adverse event parameters. Cycle length was 28 ± 3 days. At most, three dose reductions were allowed for grade 3 or higher adverse events (excluding proteinuria, hypertension, hemorrhage/bleeding, perforation, thrombosis or thrombocytopenia, neutropenia, or anemia), and treatment could not be resumed if toxicity did not resolve to grade 1 or less within 3 weeks. Pazopanib dose reductions were as follows: 600 mg/d, then 400 mg/d, and finally 200 mg/d. Treatment was permanently discontinued for any GI perforation, grade 3 or 4 hemorrhage, or a second instance of grade 2 hemorrhage, symptomatic grade 4 thrombosis, or second instance of grade 2 or higher thrombosis; second instance of grade 3 or 4 thrombocytopenia, neutropenia, or anemia; or elevated BP not controlled with antihypertensives or urine protein greater than 1+.
Results
Preclinical studies of pazopanib in DTC and TT cells
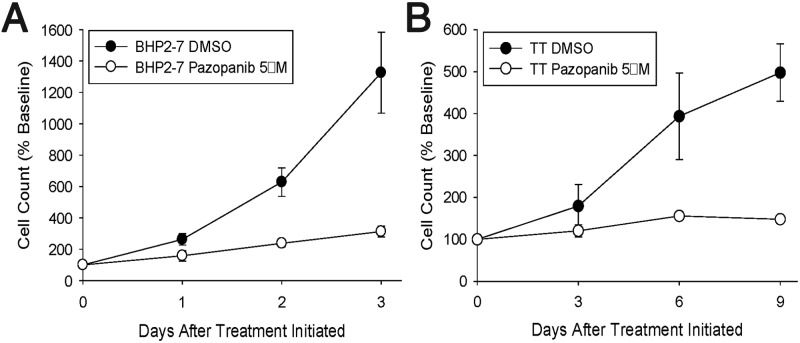
Having found pazopanib to be active in differentiated thyroid cancer both in the laboratory and in the clinic, we sought also to define the in vitro effects of pazopanib in a medullary thyroid cancer cell line relative to DTC lines. Importantly, however, we were aware that the recent clinical development of kinase inhibitors for MTC has focused primarily instead on RET inhibitors, with low activities otherwise reported to date for non-RET-directed agents. For example, motesanib, an agent putatively similarly targeted as pazopanib, produced a response rate of only 2% in a study of 91 MTC patients (10). We were spurred on, however, by encouraging preclinical effects of pazopanib in MTC. In particular, although the relative proliferative rates of BHP2–7 DTC and TT MTC cells varied considerably from one another (see Figure 1, A and B, respectively; filled circles denote proliferation of diluent treated cells), pazopanib similarly affected the proliferative rate in each cell line (Figure 1, see paired filled vs open circles in A and B). Proliferation was significantly reduced in response to pazopanib treatment in each line relative to dimethylsulfoxide controls (P < .001), but with pazopanib effects in TT vs BHP2–7 cells not significantly different (P = NS). This result suggested to us that pazopanib might have therapeutic promise in not only DTC but also MTC, thus heightening our enthusiasm for studying the clinical effects of pazopanib in advanced and progressive MTC.
Figure 1.
In vitro effects of pazopanib on proliferation of DTC (A; BHP2–7) compared with MTC (B; TT) tumor cells (continuous exposures). DMSO, dimethylsulfoxide.
Clinical trial
From September 22, 2008, to December 11, 2011, 35 individuals (28 males, seven females) aged 26–83 years (median 60 y) were enrolled into this trial from the United States, Singapore, Taiwan, Australia, and Hong Kong. None of these patients were found to be ineligible or cancelled participation prior to starting treatment. Patient and disease characteristics of these 35 patients are presented in Table 1. The most common sites of metastases were lymph nodes (80%), liver (57.1%), bone (51.4%), and lung (45.7%). Previous treatments included radiation therapy (42.8%) and systemic therapy (42.9%). Medications being taken at the time of registration included antihypertensives (40.0%), narcotics (25.7%), and octreotide (2.9%).
Table 1.
Patient Characteristics
| Characteristics | n = 35 |
|---|---|
| Median age, y (25th to 75th percentile) | 60 (48–70) |
| Male | 28 (80%) |
| Race | |
| Caucasian | 28 (80.%) |
| Asian | 7 (20.0%) |
| Ethnicity | |
| Non-Hispanic/non-Latino | 33 (94.3%) |
| Hispanic/Latino | 1 (2.9%) |
| Not reported | 1 (2.9%) |
| ECOG performance status | |
| 0 | 15 (42.9%) |
| 1 | 18 (51.4%) |
| 2 | 2 (5.7%) |
| Most common sites of disease | |
| Nodes | 28 (80.0%) |
| Liver | 20 (57.1%) |
| Bone | 18 (51.4%) |
| Lung | 16 (45.7%) |
| Prior radiation | 15 (42.9%) |
| Number of prior systematic therapies | |
| 0 | 20 (57.1%) |
| 1 | 10 (28.6%) |
| 2 | 5 (14.3%) |
| Prior systematic therapies | |
| Vandetanib | 6 (17.1%) |
| Cabozantinib | 3 (8.6%) |
| Sunitinib | 1 (2.9%) |
| Tanespimycin | 1 (2.9%) |
| ARRY-401 | 1 (2.9%) |
| Octreotide | 4 (11.4%) |
| Zoledronic acid | 1 (2.9%) |
| Capecitabine | 1 (2.9%) |
| Topotecan + lapatinib | 1 (2.9%) |
| Radioiodine | 1 (2.9%) |
| Symptoms/alterations at registration | |
| Grade 2 hypertension | 1 (2.9%) |
| Grade 1 hypertension | 4 (11.4%) |
| Grade 2 fatigue | 4 (11.4%) |
| Grade 1 fatigue | 10 (28.6%) |
| Grade 1 anorexia | 4 (11.4%) |
| Grade 1 anemia | 10 (28.6%) |
| Grade 1 AST | 4 (11.4%) |
| Narcotic use | 9 (25.7%) |
| Hypertension medication use | 14 (40.0%) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group; AST, aspartate aminotransferase.
The data were locked on May 21, 2013. All patients have been followed up until treatment discontinuation and/or for a minimum of four cycles. Eight patients (23%) are still on the study treatment. The median number of cycles administered thus far is eight (range: 1–43+; total: 393+).
During the course of treatment, 7 of the 21 patients (33%) not taking antihypertensives at study entry began taking them, and 4 of the 26 patients (15%) not taking narcotics at study entry began taking them. Fourteen patients (40%) had dose reductions due to fatigue (n = 5); abnormal liver function tests (n = 2); hand-foot syndrome (n = 2); radiation recall (n = 1); diverticulitis (n = 1); hypertension (n = 1), diarrhea (n = 1); and mood alterations (n = 1). The most Common Terminology Criteria for Adverse Events (CTCAE v3.0, grades 3–5) toxicities were fatigue (14%) and diarrhea (9%) (Table 2). Reasons for discontinuation of treatment included disease progression (n = 21), refusal (n = 1), adverse events (failure to thrive: n = 1; grade 4 depression: n = 1; bowel perforation: n = 1), comorbid conditions (n = 1), and transfer to a corresponding compassionate use protocol E10–5004 (n = 1).
Table 2.
All Grade 3–5 Adverse Events and All Grade 1–2 Adverse Events Reported by at Least Three Patients
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Abdominal pain | 2 (5.7%) | 0 | 1 (2.9%) | 0 | 0 |
| Agitation | 1 (2.9%) | 0 | 1 (2.9%) | 0 | 0 |
| Anorexia | 8 (22.9%) | 8 (22.9%) | 0 | 0 | 0 |
| Anxiety | 0 | 1 (2.9%) | 1 (2.9%) | 0 | 0 |
| Colonic fistula | 0 | 0 | 1 (2.9%) | 0 | 0 |
| Colonic perforation | 0 | 0 | 0 | 1 (2.9%) | 1 (2.9%) |
| Depression | 2 (5.7%) | 0 | 1 (2.9%) | 0 | 0 |
| Diarrhea | 13 (37.1%) | 11 (31.4%) | 3 (8.6%) | 0 | 0 |
| Dyspnea | 0 | 0 | 2 (5.7%) | 0 | 0 |
| Fatigue | 7 (20.0%) | 10 (28.6%) | 5 (14.3%) | 0 | 0 |
| Hand and foot syndrome/reaction | 0 | 1 (2.9%) | 2 (5.7%) | 0 | 0 |
| Hypertension | 7 (20.0%) | 10 (28.6%) | 1 (2.9%) | 0 | 0 |
| Infectious colitis (grade 0–2 ANC) | 0 | 0 | 1 (2.9%) | 0 | 0 |
| Joint pain | 1 (2.9%) | 1 (2.9%) | 1 (2.9%) | 0 | 0 |
| Myalgia | 3 (8.6%) | 0 | 0 | 0 | 0 |
| Nausea | 12 (34.3%) | 6 (17.1%) | 0 | 0 | 0 |
| Neutrophil count decreased | 4 (11.4%) | 1 (2.9%) | 1 (2.9%) | 0 | 0 |
| Peripheral sensory neuropathy | 6 (17.1%) | 0 | 0 | 0 | 0 |
| Pneumonitis | 0 | 0 | 1 (2.9%) | 0 | 0 |
| Skin hypopigmentation | 18 (51.4%) | 4 (11.4%) | 0 | 0 | 0 |
| Skin ulceration | 0 | 0 | 0 | 1 (2.9%) | 0 |
| Reproductive tract disorder | 0 | 0 | 1 (2.9%) | 0 | 0 |
| Taste alteration | 5 (14.3%) | 0 | 0 | 0 | 0 |
| Vomiting | 7 (20.0%) | 1 (2.9%) | 1 (2.9%) | 0 | 0 |
| Weight loss | 0 | 4 (11.4%) | 1 (2.9%) | 0 | 0 |
| Alanine aminotransferase increased | 3 (8.6%) | 1 (2.9%) | 0 | 0 | 0 |
| Aspartate aminotransferase increased | 10 (28.6%) | 3 (8.6%) | 1 (2.9%) | 0 | 0 |
| Bilirubin increased | 2 (5.7%) | 1 (2.9%) | 0 | 0 | 0 |
| Hemoglobin decreased | 7 (20.0%) | 2 (5.7%) | 0 | 0 | 0 |
| Leukocyte count decreased | 9 (25.7%) | 4 (11.4%) | 1 (2.9%) | 0 | 0 |
| Lipase increased | 0 | 0 | 0 | 1 (2.9%) | 0 |
| Lymphocyte count decreased | 0 | 1 (2.9%) | 1 (2.9%) | 0 | 0 |
| Platelet count decreased | 9 (25.7%) | 1 (2.9%) | 0 | 0 | 0 |
| Protein urine positive | 9 (25.7%) | 4 (11.4%) | 0 | 0 | 0 |
| Serum cholesterol increased | 3 (8.6%) | 0 | 0 | 0 | 0 |
Abbreviation: ANC, absolute neutrophil count.
Among the 35 enrolled patients, there have been five PRs (14.3%, 90% confidence interval 5.8°-27.7%), lasting 29 weeks, 29 weeks, 1.0 year, 1.8 years, and 4.0+ years, respectively (Figure 2). Moreover, these five PRs were observed among the first 33 patients enrolled. Thus, per the prespecified study design, we conclude that this regimen should be considered for further testing in patients with medullary thyroid cancer.
Figure 2.
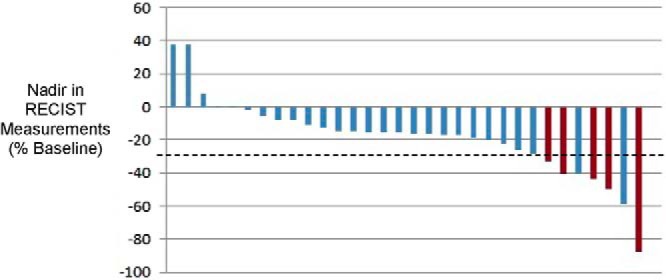
Change in tumor burden (nadir, RECIST criteria, percentage relative to enrollment/baseline measurements; first 24 treatment cycles). *, Three patients went off study without tumor burden reevaluated; RECIST (confirmed) PRs are highlighted in red.
At the time of the data lock, eight patients (23%) were alive without disease progression, five were alive with disease progression, and 22 have died of disease. One of these 22 deaths was considered to be related to pazopanib. A 28-year-old Asian woman who had a partial disease response went off study after 17 cycles of treatment and directly onto a compassionate-use protocol of 400 mg daily pazopanib. After four additional cycles of pazopanib therapy, the patient developed enteritis and colitis and then died of sepsis.
The median progression-free survival and overall survival times were estimated to be 9.4 months and 19.9 months, respectively; more than 70% of the participants remained on the study therapy more than 6 months (Figure 3).
Figure 3.
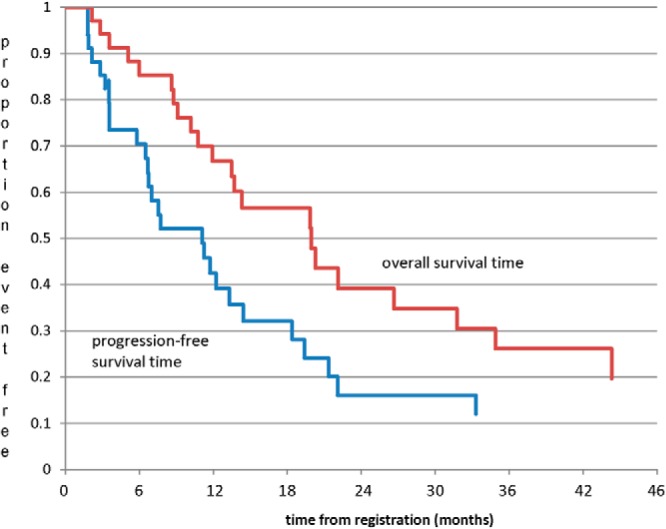
Progression-free and overall survival.
Exploratory studies assessed potential association between response to therapy and induced changes in calcitonin or CEA for all patients with at least one on-treatment value. The median percent change at CEA nadir from baseline levels was −23% (n = 31; range 55% to −90%) for CEA and −50% (n = 33; range 33% to −89%) for calcitonin. PFS was found to be significantly longer among those whose decrease in CEA was at least 25% relative to baseline (P = .0230), but no difference was found with respect to whether the percent decrease in calcitonin was 50% or greater relative to baseline (P = .3018).
Additional exploratory assessments were also undertaken to assess the response to pazopanib based on previously administered therapies; overall, it did not appear that pazopanib response varied based on prior therapies (Table 3), noting that this trial was not designed or statistically powered to address this issue definitively. Moreover, in examining whether the response to pazopanib might correlate with patient RET mutational status, we reviewed accessible patient charts after the trial closure. This interrogation demonstrated that 18 of 21 assessable patient charts were specifically evaluated with respect to RET germline mutational status; in all 18 analyzed samples, RET mutational status was wild type.
Table 3.
Response to Pazopanib Based on Prior Therapy
| Prior treatment | Response to Study (Pazopanib) Therapy |
||||
|---|---|---|---|---|---|
| Partial Response by RECIST Criteria 1.0 | Stable Disease | Progressed Before/at Second Disease Evaluation | Discontinued for Reasons Other Than Due to Disease Progression Without Two Disease Evaluations | Response Rate (90% CI) | |
| None (n = 20) | 3 | 11 | 4 | 2 | 15% (4.2%–34.4%) |
| Non-TKI regimens (n = 5) | 1 | 3 | 1 | 0 | 20% (1.0%–65.7%) |
| TKI-containing regimen (n = 10) | 1 | 6 | 1 | 2 | 10% (0.5%–39.4%) |
Abbreviations: CI, confidence interval; TKI, tyrosine kinase inhibitor.
Discussion
The present manuscript reporting the effects of pazopanib therapy in a cohort of 35 patients afflicted with progressive and metastatic MTC indicates that pazopanib appears to have promising clinical activity in MTC, with a confirmed RECIST tumor response rate of 14.3%, median PFS time of 9.4 months, and manageable toxicities. Collectively, presented data suggest that pazopanib may thus represent a drug also worthy of consideration in the therapeutic armamentarium for progressive metastatic MTC to supplement Food and Drug Administration-approved therapies (vandetanib and cabozantinib) (3, 4) as well as historical cytotoxic regimens (14, 15).
Although reported RECIST response rates for the more specific RET kinase inhibitors vandetanib and cabozantinib are higher than we observed in the case of pazopanib (3, 4), pazopanib response rates were higher than those otherwise reported for other primarily VEGFR-targeted kinase inhibitors including sorafenib, sunitinib, and motesanib as well as compared with that attained from imatinib therapy (5–11). Direct comparisons of competing therapeutics in randomized trials in various patient contexts, however, have not yet been accomplished and are required to further clarify the role of pazopanib relative to other competing agents in MTC. Moreover, the activities of various agents in the setting of progression through, or nonresponse to, other competing MTC therapies remain to be ascertained; this issue is, however, becoming increasing important with the availability of additional therapeutics. Direct comparative assessment of available active MTC therapies is particularly critical because some trials (eg, the vandetanib phase 3 trial) imposed no requirement for disease progression prior to enrollment, whereas the present trial enrolled only patients with rapidly progressive disease (requiring progressing in the <6 months prior to enrollment), thus making comparisons of response rates and time to progression data invalid based solely on available trial results.
The molecular mechanism(s) involved in the clinical activity of pazopanib in MTC also remain to be better clarified. Although pazopanib inhibits RET kinase, its potency in inhibiting other kinase targets is much greater. In particular, in vitro kinase assays indicate that pazopanib inhibits VEGFR1 kinase 280 times more potently than it does RET kinase (12). It is thus possible that effects on molecular targets other than and/or in addition to RET may perhaps in part mediate observed pazopanib activity in MTC. Also remaining to be clarified is whether the activity of pazopanib varies in differing clinical milieus, including in RET mutant vs RET wild-type MTC or in RET kinase inhibitor naïve vs RET kinase inhibitor pretreated MTC patients. Our experience suggests, however, that pazopanib can induce responses in patients who have previously been treated with, and progressed through, prior RET kinase inhibitors or other therapies (Table 3).
Also of potential interest is that our results suggest that induced changes in CEA, but not induced changes in calcitonin, are correlated with PFS among pazopanib-treated patients. The sample size of the present trial, however, precludes definitive assessment of the predictive value of changes in these tumor markers with regard to pazopanib response.
In summary, presented data suggest that pazopanib has both preclinical and clinical activity in rapidly progressive metastatic MTC, providing rationale for its further study in this patient population. Its effects relative to other competing therapeutics in various MTC patient contexts remain, however, to be further clarified by randomized comparison trials. Also in need of further clarity is the definition of the precise molecular targets of greatest therapeutic relevance in MTC because pazopanib overall represents a poor inhibitor of RET (relative to its ability to inhibit VEGFRs) and yet has encouraging clinical activity in progressive and metastatic MTC. This observation poses the intriguing possibility that RET may not necessarily represent the sole kinase target of therapeutic relevance in MTC.
Acknowledgments
We are deeply indebted to the patients who participated in this trial and to the support personnel at all participating trial sites required to facilitate patient care and data collection. We also acknowledge the able administrative assistance provided by Ms Candace Kostelec.
The trial registration number is NCT00625846 (clinicaltrials.gov).
This work was supported by National Cancer Institute Grants CA15083 and CM62205.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BP
- blood pressure
- DTC
- differentiated thyroid cancer
- GI
- gastrointestinal
- MTC
- medullary thyroid cancer
- PFS
- progression-free survival
- PR
- partial tumor response
- RECIST
- Response Evaluation Criteria In Solid Tumors
- RET
- rearranged during transfection
- ULN
- upper limit of normal
- VEGFR
- vascular endothelial growth factor receptor.
References
- 1. Griebeler ML, Gharib H, Thompson GB. Medullary thyroid carcinoma. Endocr Pract. 2013;19:1–31 [DOI] [PubMed] [Google Scholar]
- 2. Quayle FJ, Moley JF. Medullary thyroid carcinoma: including MEN 2A and MEN 2B syndromes. J Surg Oncol. 2005;89:122–129 [DOI] [PubMed] [Google Scholar]
- 3. Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schoffski P, Elisei R, Müller S, et al. An international, double-blind, randomized, placebo-controlled phase III trial (EXAM) of cabozantinib (XL184) in medullary thyroid carcinoma (MTC) patients (pts) with documented RECIST progression at baseline. J Clin Oncol. 2012;30(suppl May 20:5508 [Google Scholar]
- 5. Lam ET, Ringel MD, Kloos RT, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol. 2010;28:2323–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Capdevila J, Iglesias L, Halperin I, et al. Sorafenib in metastatic thyroid cancer. Endocr Relat Cancer. 2012;19:209–216 [DOI] [PubMed] [Google Scholar]
- 7. Ahmed M, Barbachano Y, Riddell A, et al. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in a UK based population. Eur J Endocrinol. 2011;165:315–322 [DOI] [PubMed] [Google Scholar]
- 8. Carr LL, Mankoff DA, Goulart BH, et al. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16:5260–5268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ravaud A, de la Fouchardière C, Asselineau J, et al. Efficacy of sunitinib in advanced medullary thyroid carcinoma: intermediate results of phase II THYSU. Oncologist. 2010;15:212–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schlumberger MJ, Elisei R, Bastholt L, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009;27:3794–3801 [DOI] [PubMed] [Google Scholar]
- 11. de Groot JW, Zonnenberg BA, van Ufford-Mannesse PQ, et al. A phase II trial of imatinib therapy for metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92:3466–3469 [DOI] [PubMed] [Google Scholar]
- 12. Kumar R, Knick VB, Rudolph SK, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6:2012–2021 [DOI] [PubMed] [Google Scholar]
- 13. Bible KC, Suman VJ, Molina JR, et al. Endocrine Malignancies Disease Oriented Group; Mayo Clinic Cancer Center; Mayo Phase 2 Consortium. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nocera M, Baudin E, Pellegriti G, Cailleux AF, Mechelany-Corone C, Schlumberger M. Treatment of advanced medullary thyroid cancer with an alternating combination of doxorubicin-streptozocin and 5 FU-dacarbazine. Groupe d'Etude des Tumeurs à Calcitonine (GETC). Br J Cancer. 2000;83:715–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlumberger M, Abdelmoumene N, Delisle MJ, Couette JE. Treatment of advanced medullary thyroid cancer with an alternating combination of 5 FU-streptozocin and 5 FU-dacarbazine. The Groupe d'Etude des Tumeurs a Calcitonine (GETC). Br J Cancer. 1995;71(2):363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]