Abstract
Context:
Assisted reproductive technology (ART) cycle cancelation rates are increased among overweight and obese women; however, the reasons for this are not completely clear. Premature luteinization due to inadequate endogenous gonadotropin suppression is a possibility for this higher risk of cancellation.
Objective:
The objective of the study was to investigate the impact of female obesity on the pharmacokinetics of cetrorelix (GnRH antagonist).
Design:
This was an interventional study.
Setting:
The study was conducted at a university clinical and translational research center.
Participants:
Regularly menstruating obese (n = 10) and normal-weight (n = 10) women participated in the study.
Interventions:
A frequent blood sampling study was performed after a GnRH antagonist was administered, followed by recombinant LH.
Main Outcomes Measured:
Pharmacokinetics of cetrorelix in obese vs normal weight women were measured.
Results:
Five of the obese women (50%) and none of the normal-weight women had a rebound of LH (defined as >50% increase in LH level from nadir) over the 14-hour postdose observation period. The obese group had a significantly decreased distributional half-life of cetrorelix compared with the normal-weight group (8.1 ± 1.6 vs 12.7 ± 6.2 hours, P = .02). The obese group exhibited increased clearance of cetrorelix compared with the normal-weight group (25.8 ± 6.8 vs 20.1 ± 8.3 L/h, P = .058).
Conclusions:
The altered pharmacokinetics of cetrorelix in obese women may lead to premature ovulation during ART, and this could be one of the mechanisms that results in increased cycle cancelation in this group of women. In accordance with the higher gonadotropin requirements for obese women undergoing ART, weight-based dosing of GnRH antagonists may be required.
Assisted reproductive technology (ART) cycle cancellation rates are increased among overweight and obese women (1); however, the reasons for this are not completely clear. According to the definitions used by the Society for Assisted Reproductive Technology (2), cycles can be cancelled due to poor response to exogenous gonadotropins (1) or to excessive gonadotropin response (2). Failure to obtain any viable embryos due to poor oocyte yield or failure of embryo development can also result in cycle cancellation (3, 4). Inadequate suppression of endogenous gonadotropins can result in premature ovulation and can also result in cycle cancellation (2). Although premature LH surge and ovulation have been described as a reason for poor oocyte yield during ovarian stimulation for GnRH antagonist ART cycles (5), no attempt was made to investigate the mechanisms involved in these responses.
Although poor response to exogenous gonadotropins is the most common reason for ART cycle cancellation in overweight and obese women (1), a significant portion of cancelled cycles in this group are unexplained. In one study of 1239 women undergoing in vitro fertilization (IVF), including 79 who were morbidly obese, the morbidly obese women were noted to have a higher cancellation rate than normal weight women, in some cases due to a poor response to exogenous gonadotropin stimulation (6). However, when a portion of these women underwent a subsequent cycle at a higher starting dose of gonadotropin, cancellation rates remained high (6). In another study of 3457 IVF cycles, women with a body mass index (BMI) greater than 30 kg/m2 had a cancellation rate that was 3 times higher than women with a normal BMI (7). Of these cancelled cycles, one third had an unspecified reason for cancellation (7).
Although obese women undergoing ART require higher doses of gonadotropins than normal-weight women (1, 3, 8), there are no published recommendations for weight-based dosing of GnRH antagonists, used to prevent premature ovulation (9).
Cetrorelix is a GnRH antagonist used to prevent premature LH surge and ovulation during ART (10) and is available in two dosing regimens: 0.25 mg daily dosing or 3 mg, lasting 4 days (reviewed in Reference 11). Compared with ART protocols using GnRH agonists, use of GnRH antagonists to prevent premature ovulation in ART results in overall equivalent pregnancy rates and significantly lower risk of ovarian hyperstimulation syndrome (9). The aim of this analysis was to investigate whether female obesity altered the pharmacokinetics of cetrorelix in a manner that could put obese women at risk for premature ovulation.
Materials and Methods
Participants
Regularly menstruating obese (n = 10) and normal-weight (n = 10) women were recruited from the community through campus-wide advertisements. Inclusion criteria were as follows: 1) age 18–40 years at the time of study; 2) obese (≥30 kg/m2) or normal (18–25 kg/m2) BMI; 3) history of regular menses every 25–40 days; and 4) normal baseline prolactin, TSH, and blood count. Participants were excluded if they had a chronic disease or used medication known to affect reproductive hormones, used exogenous sex steroids within the last 3 months, exercised more than 4 hours weekly, or were attempting pregnancy. All participants had a baseline physical examination by study personnel and underwent all blood tests at the Clinical and Translational Research Center of the University of Colorado School of Medicine's Clinical and Translational Sciences Institute.
The study was approved by the Colorado Multiple Institutional Review Board, and signed informed consent was obtained from each participant prior to participation.
Protocol
The participants underwent a luteal phase frequent blood sampling study investigating LH and FSH luteal phase dynamics, reported separately (12). The frequent blood sampling study was scheduled 6–10 days after a commercially available urinary LH kit indicated that an ovulatory LH surge was about to occur. The presence of a corpus luteum was confirmed in all participants by ultrasound on the day of their frequent sampling study.
This analysis focuses on the following: GnRH antagonist (cetrorelix 3 mg sc, Cetrotide; EMD Serono) was given at midnight and the participants slept undisturbed in the inpatient Clinical and Translational Research Center of the University of Colorado School of Medicine's Clinical and Translational Sciences Institute. At 8:00 am the following morning, a 6-hour frequent blood sampling study (blood drawn every 10 min) was commenced. Intravenous administration of a physiological dose of recombinant LH (lutropin-α 12.5 IU, Luveris; EMD Serono) was given after the first blood draw of the morning. Recombinant LH was given to evaluate the pharmacokinetics of exogenous LH in obese vs normal-weight ovulatory women and is reported separately (12).
Assays
Cetrorelix was measured in serum samples at 8, 10 and 14 hours after the administration using HPLC-tandem mass spectrometry (Applied Biosystems by Prolytic GmbH). Intraassay coefficient of variation (CV) ranged from 1.59% to 3.21%, and the interassay CV ranged from 1.94% to 3.94%. The assay's limit of detection is 0.065 ng/mL.
LH was measured with an immunofluorometric assay (DELFIA; PerkinElmer) that has been used previously in the authors' laboratory (13). The LH intraassay CV ranged from 2.86% to 4.05%, and the interassay CV ranged from 2.62% to 4.68%.
Data analysis
Calculation of pharmacokinetic variables was performed by noncompartmental analysis with Phoenix WinNonlin (Pharsight Corp; version 6.2.1). Groups were compared using t tests or Mann-Whitney tests as appropriate using SAS software (version 9.2 × 64 platform). Results of statistical analysis are reported as mean ± SD if a t test was used and as median (25th percentile, 75th percentile) if a Mann-Whitney test was used. P < .05 was considered statistically significant. This is a secondary analysis of a study investigating FSH and LH dynamics in the luteal phase of the menstrual cycle (12). The original analysis was powered for the outcome of LH pulsatility (12).
Results
The demographic information for the study groups are presented in Table 1. By design, the obese women had a significantly higher BMI than the normal-weight women (Table 1). The groups did not differ in terms of race and ethnicity, with most participants being Caucasian and non-Hispanic (Table 1). The obese women were significantly older than the normal-weight women (Table 1). Ovarian reserve parameters (FSH, anti-müllerian hormone, and antral follicle count) did not differ between the groups (Table 1). Menstrual cycle interval was similar between the groups (obese group 31.6 ± 4.2 vs normal weight group 29.6 ± 3.4 d, P = .4).
Table 1.
Demographic Information
| Obese (n = 10) | Normal Weight (n = 10) | P Value | |
|---|---|---|---|
| Age, y | 32.5 ± 4.7a | 27.3 ± 2.6 | .006 |
| Race | .08 | ||
| Caucasian | 4 (40)b | 9 (90) | |
| African American | 3 (30) | 0 (0) | |
| Other/not reported | 3 (30) | 1 (10) | |
| Ethnicity | 1.0 | ||
| Hispanic | 1 (10) | 2 (20) | |
| Non-Hispanic | 9 (90) | 8 (80) | |
| BMI, kg/m2 | 34.3 (31.8, 38.9)c | 22.3 (21.1, 22.8) | <.001 |
| FSH, U/L | 3.8 (2, 4.2) | 3.3 (3, 4.9) | .7 |
| Anti-Müllerian hormone, ng/dL | 1.6 (0.6, 6.2) | 5.4 (1.8, 10.3) | .1 |
| Antral follicle count | 16.5 (12, 41.4) | 23 (15.7, 50.7) | .2 |
Mean ± SD.
Frequency (percentage).
Median (25th percentile, 75th percentile).
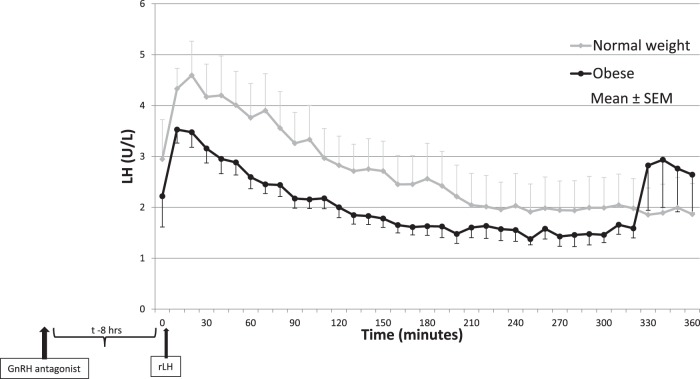
The composite mean LH values for each group are seen in Figure 1. Five of the obese women (50%) had rebound of LH (defined as >50% increase in LH level from nadir) over the 14-hour postdose observation period, with a percent range of 68%–498%. None of the normal-weight women had a rebound in LH. The obese women with LH rebound were compared with the obese women with no LH rebound, and no differences were identified with respect to age, BMI, waist and hip circumference, anti-müllerian hormone, or visceral fat.
Figure 1.
Composite mean LH values after suppression with GnRH antagonist and administration of recombinant LH (rLH) in the normal-weight (gray) and obese (black) groups.
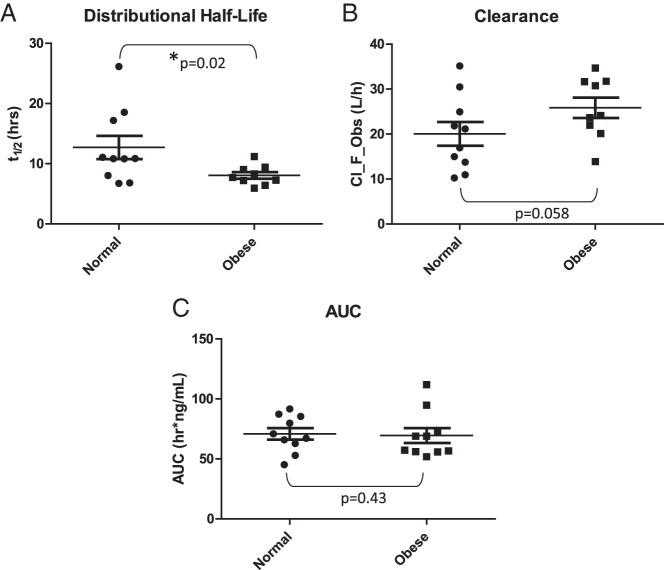
The obese group had a significantly decreased distributional half-life of cetrorelix compared with the normal-weight group (8.1 ± 1.6 vs 12.7 ± 6.2 h, P = .02) (Figure 2A). It is important to note that the calculated half-life presented here does not represent the elimination half-life of cetrorelix because the data available for calculation were limited and did not extend beyond 14 hours. The obese group exhibited increased clearance of cetrorelix (although not significantly so) compared with the normal-weight group (25.8 ± 6.8 vs 20.1 ± 8.3 L/h, P = .058) (Figure 2B). The groups did not differ with respect to area under the curve (AUC) (obese: 69.4 ± 19.6, normal weight: 70.9 ± 15.2 h/ng·mL, P = .8) (Figure 2C) or volume of distribution (obese: 297.6 ± 71.5, normal weight: 318.2 ± 77.7 L, P = .5). No differences were seen between the obese women with LH rebound compared with the obese women with no LH rebound when comparing distributional half-life of cetrorelix (rebound 7.6 ± 1.4 vs no rebound 8.7 ± 1.9 h, P = .09) or clearance of cetrorelix (rebound 27.8 ± 6.4 vs no rebound 20.1 ± 9.7 L/h, P = .2). Age was not a significant covariate for half-life, clearance, or volume of distribution.
Figure 2.
Cetrorelix pharmacokinetic parameters in normal-weight and obese patients. A, Whisker plot showing individual half-life values for each participant in the normal-weight (left) and obese (right) groups. The half-life of cetrorelix is significantly decreased in the obese group compared with the normal-weight group (P = .02). B, Whisker plot showing the individual clearance values for each participant in the normal-weight (left) and obese (right) groups. The clearance of cetrorelix is increased in the obese group compared with the normal-weight group (P = .058). C, Whisker plot showing the individual AUC values for each participant in the normal-weight (left) and obese (right) groups. The AUC of cetrorelix does not differ significantly between the obese group and the normal-weight group (P = .43). Cl_F_Obs, Clearance.
There were significant correlations between BMI, cetrorelix clearance, and half-life (Table 2). There was not a significant correlation between BMI and volume of distribution (Table 2).
Table 2.
Correlation Between BMI and GnRH Antagonist Clearance, GnRH Antagonist Half-Life, and GnRH Antagonist Volume of Distribution
| BMI, kg/m2 | Clearance, L/h | Half-Life, h | Volume of Distribution, L |
|---|---|---|---|
| R | 0.43 | −0.44 | −0.12 |
| P value | .03 | .03 | .3 |
Discussion
Obese women have a significantly decreased distributional half-life and an increased clearance of cetrorelix compared with the normal-weight women. Nearly half of the obese women that we studied had premature recovery of LH after GnRH antagonist suppression, less than 14 hours after cetrorelix administration. This is remarkable in that a single sc dose of 3 mg of cetrorelix is expected to suppress LH for 96 hours (14). The phase 1 trial for cetrorelix illustrated that LH levels return to baseline after a median of 100 hours (4.2 d) with a range of 39–401 hours (15). The altered pharmacokinetics of the GnRH antagonist that we observed in obese women may help explain their increased ART cancelation rate.
The phase 1 trial included 48 healthy premenopausal women who received cetrorelix on cycle day 8, with 12 participants receiving a 3-mg dose (15). The analysis is not broken down by weight, but the mean weight for the entire study population was 66.1 kg (145 lb) (15). Using the average female height in the United States of 1.62 m (16), the average BMI in the phase 1 study population was 25 kg/m2. Despite the expected 96-hour duration of the LH suppression (15), our study found that half of the obese participants had premature recovery of LH after GnRH antagonist suppression less than 14 hours after the cetrorelix dose was administered. This premature escape from suppression in a subset of obese women is a novel finding and raises concern that some obese women may need weight-based dosing of GnRH antagonists to prevent premature ovulation. Weight-based dosing is required for a variety of medications (17), including some used in ART.
This premature recovery of LH after GnRH antagonist suppression after cetrorelix was an unexpected finding and was noted as part of a study investigating FSH and LH dynamics in the luteal phase [reported separately (12)] and was powered accordingly for the former study. Therefore, the limitations of this study include small sample size and nonideal pharmacokinetic sampling times. Our relatively small sample of 20 women adds significantly to the existing literature on the pharmacokinetics of cetrorelix because the phase 1 trial of cetrorelix (15) included only 12 participants, presumably all of average weight, who received a 3-mg dose. This preliminary analysis suggests that obese women may have altered cetrorelix pharmacokinetics, and further investigation with a specifically designed trial is warranted. An additional area of concern is the difference in age between the normal-weight and obese group. Age was not a significant covariate for the pharmacokinetic variables in our study. Additionally, the known age-related pharmacokinetic changes that may be applicable to our data are slower drug elimination and decreased renal drug excretion (one route for cetrorelix excretion) with increasing age (18). If age did impact our data, our results would be even more significant because the older group (obese women) had a significantly decreased half-life and a (nonsignificantly) increased clearance.
The findings of this study may be highly relevant to ART protocols because obese women had significantly increased clearance of certrorelix compared with the normal-weight women, resulting in a premature recovery of LH after GnRH antagonist suppression less than 14 hours after administration. This is clinically important because GnRH antagonists (including cetrorelix) are commonly used in ART protocols, and assumptions about the duration of effectiveness of a single dose of cetrorelix may be overestimated for obese women. More than one third of reproductive-age women in the United States are obese (19), and overweight and obese women make up a large percentage of those undergoing ART (1). The altered pharmacokinetics of cetrorelix in obese women may lead to premature ovulation, and this could be one of the mechanisms that results in increased cycle cancelation in this group of women. In accordance with the higher gonadotropin requirements for obese women undergoing ART, weight-based dosing of GnRH antagonists may be required.
Acknowledgments
Clinical trial registration number for this study was NCT01457703 (www.clinicaltrials.gov).
The contents are the authors' sole responsibility and do not necessarily represent the official views of the National Institutes of Health.
This work was supported by National Institutes of Health (NIH) Grant U54HD058155; the Center for the Study of Reproductive Biology (to N.S.); NIH/National Center for Research Resources Colorado Clinical and Translational Science Institute Grant UL1 RR025780 (to N.S.); and the University of Colorado Cancer Center Grant P30 CA046934 (to E.L.B.-P.).
Disclosure Summary: L.W.R. received the Clinical Research Fellowship and Mentor Award supported by Pfizer, Inc for research presented at the 94th Annual Meeting of The Endocrine Society, 2012, and an American Society for Reproductive Medicine Corporate Member Council In-training Travel Award for the International Federation of Fertility Societies/American Society for Reproductive Medicine 2013. A.J.P. receives investigator-initiated grant support. N.S. has stock options in MenoGeniX and receives investigator-initiated grant support. The other authors have nothing to declare.
Footnotes
- ART
- assisted reproductive technology
- AUC
- area under the curve
- BMI
- body mass index
- CV
- coefficient of variation.
References
- 1. Luke B, Brown MB, Missmer SA, Bukulmez O, Leach R, Stern JE. The effect of increasing obesity on the response to and outcome of assisted reproductive technology: a national study. Fertil Steril. 2011;96:820–825 [DOI] [PubMed] [Google Scholar]
- 2. Technology SfAR. A patient's guide to assisted reproductive technology: cycle cancellation. http://www.sart.org/detail.aspx?id=1892
- 3. Zander-Fox DL, Henshaw R, Hamilton H, Lane M. Does obesity really matter? The impact of BMI on embryo quality and pregnancy outcomes after IVF in women aged </=38 years. Aust N Z J Obstet Gynaecol. 2012;52:270–276 [DOI] [PubMed] [Google Scholar]
- 4. Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15:532–538 [DOI] [PubMed] [Google Scholar]
- 5. Tavaniotou A, Albano C, Van Steirteghem A, Devroey P. The impact of LH serum concentration on the clinical outcome of IVF cycles in patients receiving two regimens of clomiphene citrate/gonadotrophin/0.25 mg cetrorelix. Reprod Biomed Online. 2003;6:421–426 [DOI] [PubMed] [Google Scholar]
- 6. Dokras A, Baredziak L, Blaine J, Syrop C, VanVoorhis BJ, Sparks A. Obstetric outcomes after in vitro fertilization in obese and morbidly obese women. Obstet Gynecol. 2006;108:61–69 [DOI] [PubMed] [Google Scholar]
- 7. Fedorcsak P, Dale PO, Storeng R, et al. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod. 2004;19:2523–2528 [DOI] [PubMed] [Google Scholar]
- 8. Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98:102–108 [DOI] [PubMed] [Google Scholar]
- 9. Al-Inany HG, Youssef MA, Aboulghar M, et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2011:CD001750. [DOI] [PubMed] [Google Scholar]
- 10. Diedrich K, Diedrich C, Santos E, et al. Suppression of the endogenous luteinizing hormone surge by the gonadotrophin-releasing hormone antagonist Cetrorelix during ovarian stimulation. Hum Reprod. 1994;9:788–791 [DOI] [PubMed] [Google Scholar]
- 11. Depalo R, Jayakrishan K, Garruti G, et al. GnRH agonist versus GnRH antagonist in in vitro fertilization and embryo transfer (IVF/ET). Reprod Biol Endocrinol. 2012;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roth LW, Allshouse AA, Bradshaw-Pierce EL, et al. Luteal phase dynamics of follicle-stimulating and luteinizing hormones in obese and normal weight women [published online February 28, 2014]. Clin Endocrinol (Oxf). doi:10.1111/cen.12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92:2468–2473 [DOI] [PubMed] [Google Scholar]
- 14. EMD-Serono Inc. Cetrotide package insert. http://www.emdserono.com/cmg.emdserono_us/en/images/Cetrotide_tcm115_19346.pdf Accessed September 12, 2013
- 15. Erb K, Klipping C, Duijkers I, Pechstein B, Schueler A, Hermann R. Pharmacodynamic effects and plasma pharmacokinetics of single doses of cetrorelix acetate in healthy premenopausal women. Fertil Steril. 2001;75:316–323 [DOI] [PubMed] [Google Scholar]
- 16. Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007–2010. National Center for Health Statistics. Vital Health Stat. 2012;11. [PubMed] [Google Scholar]
- 17. Pai MP. Drug dosing based on weight and body surface area: mathematical assumptions and limitations in obese adults. Pharmacotherapy. 2012;32:856–868 [DOI] [PubMed] [Google Scholar]
- 18. Cusack BJ. Pharmacokinetics in older persons. Am J Geriatr Pharmacother. 2004;2:274–302 [DOI] [PubMed] [Google Scholar]
- 19. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497 [DOI] [PubMed] [Google Scholar]