Abstract
Context:
Uterine leiomyomas are highly prevalent benign tumors of premenopausal women and the most common indication for hysterectomy. However, the exact etiology of this tumor is not fully understood.
Objective:
The objective of the study was to evaluate the role of activin-A and myostatin and their signaling pathways in human myometrial and leiomyoma cells.
Design:
This was a laboratory study.
Setting:
Myometrial and leiomyoma cells (primary and cell lines) were cultured in vitro.
Patients:
The study included premenopausal women who were admitted to the hospital for myomectomy or hysterectomy.
Interventions:
Primary myometrial and leiomyoma cells and/or cell lines were treated with activin-A (4 nM) and myostatin (4 nM) for different days of interval (to measure proliferation rate) or 30 minutes (to measure signaling molecules) or 48 hours to measure proliferating markers, extracellular matrix mRNA, and/or protein expression by real-time PCR, Western blot, and/or immunocytochemistry.
Results:
We found that activin-A and myostatin significantly reduce cell proliferation in primary myometrial cells but not in leiomyoma cells as measured by a CyQUANT cell proliferation assay kit. Reduced expression of proliferating cell nuclear antigen and Ki-67 were also observed in myometrial cells in response to activin-A and myostatin treatment. Activin-A also significantly increased mRNA expression of fibronectin, collagen1A1, and versican in primary leiomyoma cells. Finally, we found that activin-A and myostatin activate Smad-2/3 signaling but do not affect ERK or p38 signaling in both myometrial and leiomyoma cells.
Conclusions:
This study results suggest that activin-A and myostatin can exert antiproliferative and/or fibrotic effects on these cell types via Smad-2/3 signaling.
Uterine leiomyomas (fibroids or myomas) are the most common benign tumors of uterus in reproductive-aged women (1, 2). Uterine leiomyomas affect approximately 77% of women, and approximately 25% of Caucasians have clinically significant symptoms (3, 4). However, clinical symptoms are more severe in African-American women with high prevalence (5–7). So far, uterine leiomyomas are the most common indication for hysterectomy in the world.
Despite the high prevalence and distressing effect on women's health, the pathogenesis of uterine leiomyomas is not fully understood. Extensive studies established that ovarian steroids (estrogen and progesterone) have tremendous effect on leiomyoma growth, and their actions are partly mediated by local production of growth factors (8, 9). Growth factors are essential elements in controlling the cellular proliferation rate and extracellular matrix (ECM) deposition; therefore, overexpression of either growth factors or their cognate receptors may contribute to tumorigenesis (8).
Uterine leiomyomas are characterized by quantitative and qualitative abnormalities in ECM components primarily collagens, fibronectin, and proteoglycans (9–15). Moreover, ECM may serve as a reservoir for growth factors, cytokines, chemokines, angiogenic and inflammatory response mediators (1, 16).
Activin-A and myostatin are members of the TGF-β superfamily and have a wide variety of biological functions such as the regulation of cellular differentiation, apoptosis, and carcinogenesis including cell growth inhibition of different cell types (8, 17–19). They also have a stimulatory effect on ECM production in different cell types (20–22). In most cases, similar to TGF-β, activin-A and myostatin exert their biological effects through the Smad signaling pathway. However, TGF-β family members can also activate non-Smad signaling pathways such as c-Jun N-terminal kinase, p38 MAPK, ERK, RhoA, and protein phosphatase 2A/p70S6K (23).
Activin-A and myostatin initiate Smad signaling by binding to a type II receptor [ActRIIA (for activin-A) or ActRIIB (for activin-A and myostatin)]. Upon ligand binding, the type II receptor phosphorylates and activates the type I receptor [activin receptor-like kinase (ALK)-4/ActRIB (for activin-A and myostatin) or ALK5/TGF-βRI (for myostatin)]. The activated type I receptor then phosphorylates receptor-regulated Smads (Smad2 and Smad3), which can bind to the common partner Smad (Smad4). Receptor-regulated Smads and the common partner Smad complex migrate into the nucleus, in which they regulate the transcription of target genes (24, 25).
We have previously shown that both activin-A and myostatin have a cytostatic effect on myometrial cell proliferation using a cell line model [pregnant human myometrial 1 (PHM1)] (26, 27). In addition, we recently investigated the expression levels of activin-A and myostatin in human leiomyoma and adjacent healthy myometrial tissue and found increased expression levels of both activin-A and myostatin in leiomyoma (28).
We hypothesized that activin-A and myostatin play a role in regulating leiomyoma growth. Because fibroid growth is driven by cell proliferation and ECM deposition, our aim was to investigate the proliferative/antiproliferative and fibrotic/antifibrotic effect of activin-A and myostatin and their signaling pathway in myometrial and leiomyoma cells.
Materials and Methods
Materials
Recombinant human activin-A was generated using a stable activin-expressing cell line generously provided by Dr J. Mather (Genentech, Inc) and was purified by Wolfgang Fischer (Peptide Biology Laboratory, The Salk Institute). Myostatin was produced as previously shown (27). SB431542 was purchased from Sigma-Aldrich .
Myometrial and leiomyoma tissue collection
The study included premenopausal women who were admitted to the hospital for myomectomy or hysterectomy. All patients gave their informed consent and the permission of the Human Investigation Committee was granted.
Samples of fibroid and adjacent normal myometrium were excised from women undergoing hysterectomy for fibroids. Fibroid tissue was defined based on well-established histopathological criteria. Considering the high variability that could occur with a different age, race, hormonal milieu, tumor size, and location of tumors, we included in the study the most homogenous sample possible. All patients were Caucasian (age range 41–49 y). The location of the fibroids was intramural, and their size range was 3–10 cm in diameter. The tissue samples were taken only from women who had not received exogenous hormones for the previous 3 months.
Primary myometrial and leiomyoma cell culture
Myometrial and leiomyoma samples were placed into Hanks' balanced salt solution (Euroclone) after surgery and immediately transported to the laboratory for necessary actions. Samples were washed several times with Dulbecco's PBS (Invitrogen, Life Technologies) to remove excess blood. After cutting myometrial and leiomyoma tissue into small pieces, the samples were mixed in 0.1% type 2 (Invitrogen, Life Technologies) or type 8 collagenase (Serva Electrophoresis GmbH) in serum free DMEM (Lonza) and incubated at 37°C for 5–6 hours in a water bath with manual shaking. Digested cell suspensions were then centrifuged at 1200 rpm for 10 minutes and washed several times. Finally, the cell pellet was dispersed in DMEM containing 10% fetal bovine serum (FBS) (Gibco, Life Technologies), 1% penicillin-streptomycin (EuroClone), 50 mg/L gentamicin (Lonza), and 1% Amphotericin B (Lonza). Cells were plated in plastic dishes and maintained using same media at 37°C in 95% air-5% CO2. The growth medium was changed after 24 hours or 48 hours to remove unattached cells and subsequently twice a week. The purity of cells was assessed by staining with specific smooth muscle cell marker (anti-α-smooth muscle actin). The lower passage number (≤4) of cells was used for the experiments to avoid changes in phenotype and gene expressions.
Myometrial and leiomyoma cell line culture
The primary cultures of myometrium and leiomyoma were immortalized using HPV-16 following the protocol of Rhim (29) with minor modifications as previously described (30). Briefly, primary cells were infected at 40%–50% confluence on first passage with retrovirus stock (pSLXN virus with geneticin selection gene was a gift from Dr Rhim, Center for Prostrate Disease Research, Bethesda, Maryland). To enhance infection by the retroviral vector, polybrene (5 μg/mL) was added to each flask. After incubation at 37°C for 24 hours, the cells were washed once with PBS, heated to 37°C, and cultured in fresh DMEM-F12 supplemented with 10% FBS (Gibco, Life Technologies). The cells were maintained at 37°C and 5% CO2 for 48 hours before adding fresh media containing 100 μg/mL of geneticin (Sigma-Aldrich). The cells were grown for 4 days in selection media before fresh media were added. Both myometrial and leiomyoma cells were cultured in fresh DMEM-F12 supplemented with 10% FBS, 1% antibiotic (penicillin-streptomycin; EuroClone), 1% Fungizone (Amphotericin B; Lonza), and 1% glutamine (Gibco, Life Technologies) at 37°C in 95% air-5% CO2.
Cell proliferation assay
Cellular growth curves were measured using the CyQUANT cell proliferation assay kit (Invitrogen, Life Technologies) as previously described (26, 27). Briefly, myometrial and leiomyoma cells were seeded in 96-well plates at initial densities of 1000 cells/well in a total volume of 100 μL DMEM supplemented with 10% FBS. Cells were treated with 4 nM of activin-A, 4 nM myostatin, and tranilast (300 μM) (positive control) (31, 32) (Invitrogen, Life Technologies) and allowed to divide for the number of days indicated (days 0, 5, 10, and 15). The doses for activin-A and myostatin were chosen based on our previous studies in which we established dose-response relationships of these two compounds for cell proliferation (26, 27). Cellular growth media were replaced after every 3 days, maintaining original serum concentrations with activin-A and myostatin doses. At the indicated times, media were discarded, and plates were frozen at −80°C. At the day of the assay, plates were thawed, cells were lysed, and total cellular nucleic acid was measured using florescence at 520 nm emission after excitation at 480 nm.
Immunocytochemistry
For the detection of proliferating cell nuclear antigen (PCNA), Ki-67, and fibronectin, immunocytochemistry was performed as previously described (33). Myometrial and leiomyoma cells were seeded in chamber tissue culture slides and allowed to divide. Cells were treated with 4 nM of activin-A or myostatin for 48 hours. At the indicated times, cells were rinsed with PBS, fixed for 20 minutes in cooled (−20°C) methanol (for PCNA) or 4% paraformaldehyde (for Ki-67 and fibronectin). Cells were washed three times with PBS, treated with 0.2% Triton X-100 in PBS for 5 minutes and washed three times with PBS. To inhibit endogenous peroxidase activity, cells were incubated for 10 minutes with 3% hydrogen peroxide in deionized water. Cells were washed three times with PBS and to block nonspecific background, cells were incubated for 20 minutes at room temperature with normal horse serum (for PCNA and fibronectin) or normal goat serum (for Ki-67) diluted 1:75 in 1% BSA in PBS. Cells were then incubated with PCNA monoclonal mouse antibody at 1:300 dilutions, Ki-67 monoclonal rabbit antibody at 1:1000 dilutions, or fibronectin mouse antibody at 1:600 for 1 hour at room temperature. After washing with PBS, cells were incubated with biotinylated antimouse IgG made in horse (for PCNA and fibronectin) or biotinylated antirabbit IgG made in goat (for Ki-67) diluted 1:200 (Vector Laboratories). The peroxidase avidin biotin complex method (Vector Laboratories) was performed for 1 hour at room temperature using 3′, 3′ diaminobenzidine tetrahydrochloride (Sigma-Aldrich) as chromogen. The immunocytochemistry morphological analysis has been done by two investigators in a blinded fashion without knowledge of the experimental group. The PCNA and Ki-67 positive rate was determined by observing more than 1000 nuclei for each experimental sample using a morphological imaging system (LUCIA, version 4.5; Nikon Instruments).
Western blotting
Primary myometrial and leiomyoma cells were treated with activin-A (4 nM) and myostatin (4 nM) for 30 minutes or 48 hours and left untreated for measuring protein expression. For the inhibition of Smad2/3 signaling, we performed a pretreatment of 1 hour with 10 μM SB-431542 before treatment with activin-A (1 nM).
Cells were rinsed in PBS and solubilized in TRIzol reagent (Invitrogen, Life Technologies). Proteins were extracted following the manufacturer's instructions. Soluble protein was quantified using a Bradford protein assay (Bio-Rad Laboratories), and equal amounts of proteins were loaded onto 4%–12% NuPAGE gels (Invitrogen, Life Technologies) and resolved by SDS-PAGE under reducing conditions. Proteins were transferred to 0.2-μm nitrocellulose membranes in an X-cell II apparatus (Invitrogen, Life Technologies) according to the manufacturer's instructions. Ponceau S solution (Euroclone) was used for the detection of proteins on nitrocellulose membranes. After blocking the membrane with 5% (wt/vol) nonfat milk powder in Tris-buffered saline with Tween 20 (TBST) [50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 0.05% Tween 20], membranes were incubated overnight with a primary antibody as 1:750 dilutions for polyclonal rabbit phosphorylated (p)-Smad2 or monoclonal rabbit Smad2 (Cell Signaling Technology), 1:1000 dilutions for polyclonal rabbit p-Smad3 (Santa Cruz Biotechnology), 1:1000 dilutions for polyclonal rabbit Smad3 (Santa Cruz Biotechnology), 1:1000 dilutions for monoclonal mouse p-p38 MAPK (Cell Signaling Technology), monoclonal mouse 1:3000 dilutions for p-ERK 1 and 2 (Sigma-Aldrich), 1:1000 dilutions for monoclonal mouse PCNA (Dako), 1:10 000 dilutions for monoclonal mouse antihuman fibronectin (Sigma-Aldrich), or 1:3000 dilutions for monoclonal mouse tubulin (Sigma-Aldrich). Membranes were washed four times in TBST and incubated with 1:10 000 dilutions of horseradish peroxidase-conjugated antirabbit IgG (Thermo Scientific Pierce Protein Biology Products) against p-Smad2, Smad2, p-Smad3, and Smad3 for 2 hours. Similarly, membranes were incubated with 1:1000 dilutions of horseradish peroxidase-linked antimouse IgG (GE Healthcare) against p-p38 MAPK, p-ERK 1 and 2, PCNA, antihuman fibronectin, and tubulin for 2 hours. Membranes were washed four times in TBST, and immunoreactive proteins were visualized using Super Signal West Pico chemiluminescent substrate (Thermo Scientific Pierce Protein Biology Products). The bands were quantified by scanning and analysis using Quantity One software (Bio-Rad Laboratories).
Real-time PCR
Cells were lysed using TRIzol reagent (Invitrogen, Life Technologies) and stored at −80°C. Total RNA (colorless upper aqueous phase) was isolated using chloroform according to the manufacturer's instructions. The ReliaPrep RNA cell miniprep system was used to purify and concentrate RNA (Promega Corp). We performed the reverse transcriptase (RT) using the high-capacity cDNA RT kit (Applied Biosystems, Life Technologies) with 1 μg RNA. The real-time PCR was performed with the thermal cycle protocol of initial denaturation at 95°C for 20 seconds, followed by 40 cycles of 95°C for 1 second, and 60°C for 20 seconds using 50 ng cDNA in a final reaction volume of 15 μL with the following TaqMan gene expression assays (Applied Biosystems, Life Technologies): Hs00365052_m1 (fibronectin), Hs00164004_m1 (collagen1A1), Hs00171642_m1 (versican), and Hs99999909_m1 (hypoxanthine-guanine phosphoribosyl transferase). The blank for each reaction, consisting of amplifications performed in the absences of the RT enzyme, was performed.
Data analysis
Statistical analyses were performed using GraphPad Prism version 4.01 for Windows (GraphPad Inc). The data were analyzed using Kruskal-Wallis test, followed by a post hoc Dunn's multiple comparison test. Results are expressed as median, first quartiles, or 25th percentiles, third quartiles, or 75th percentiles, and minimum and maximum values. Differences were considered significant when P < .05. All experiments were done in triplicate, except for the cell proliferation assay in which n = 6.
Results
Effect of activin-A and myostatin on myometrial and leiomyoma cell proliferation
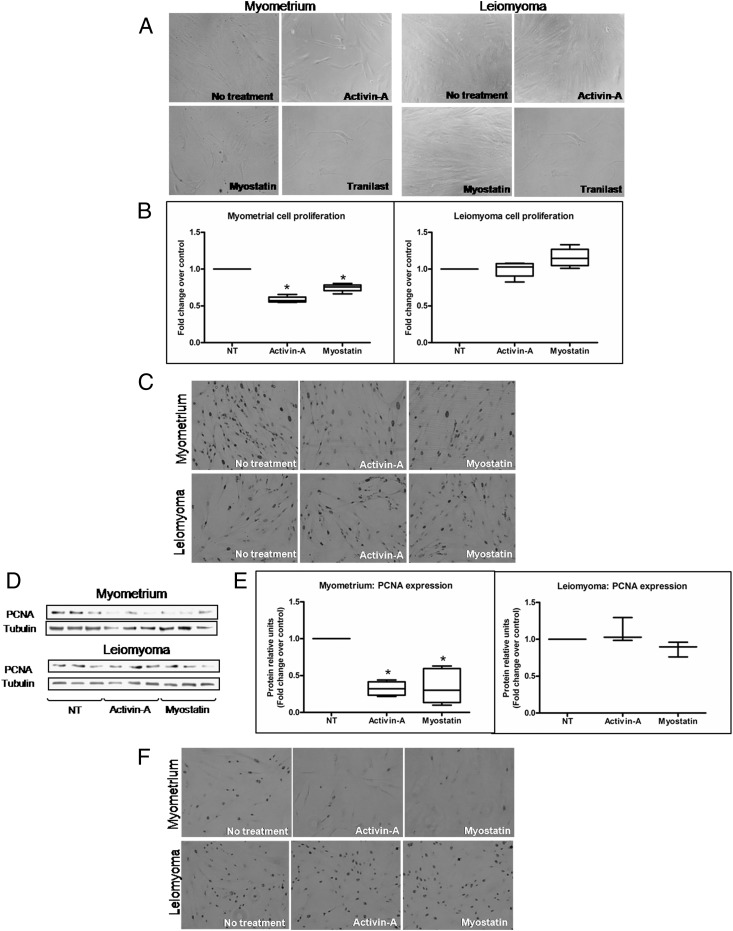
To quantify the cell proliferation effect, we used three different approaches: 1) cell proliferation assay, 2) detection of PCNA, and 3) detection of Ki-67. Cells were treated with activin-A, myostatin, and tranilast, and proliferation was measured after various intervals (days 0, 5, 10, and 15). After 5 days of treatment with activin-A, myostatin, or tranilast, the number of myometrial cells, observed with the phase-contrast microscopy, was clearly reduced compared with untreated cells (Figure 1A). By contrast, the leiomyoma cells appeared reduced in number only by treatment with tranilast (Figure 1A). Tranilast is an antiallergic drug that was developed by Kissei Pharmaceuticals. It has an antiproliferative effect on both myometrial (32) and leiomyoma cells (31, 32). The average of results obtained by CyQUANT cell proliferation kit (Invitrogen, Life Technologies) of myometrial and leiomyoma cells isolated from four different patients are shown in Figure 1B. After 10 days of treatment, activin-A and myostatin showed antiproliferative effect only in myometrial cells (Figure 1B). We detected the PCNA expression by immunocytochemistry and Western blotting (Figure 1, C–E). At microscopic observation, we found that the positive nuclei in the treated myometrial cells were clearly reduced in number compared with untreated cells (Figure 1C). However, activin-A and myostatin were unable to reduce PCNA expression in leiomyoma cells compared with the control (Figure 1C). Western blotting also showed a significant reduction of PCNA expression in myometrial cells but not in leiomyoma cells after activin-A and myostatin treatment (Figure 1, D and E). Finally, we checked Ki-67 expression by immunocytochemistry and obtained results consistent with the other approaches. In myometrial cells, the number of Ki-67 positive nuclei was clearly reduced, whereas the number of Ki-67 positive nuclei was unchanged in leiomyoma cells relative to the control after activin-A and myostatin treatment (Figure 1F).
Figure 1.
A–F, Effect of activin-A and myostatin in primary myometrial and leiomyoma cell proliferation. A, Microscopic observation of cell cultures. Representative photograph shows the effect of activin-A, myostatin, and tranilast (used as positive control) on cells after 5 days of treatment. The observation was performed with the phase microscopy with the objective ×10. B, Effect of activin-A and myostatin on myometrial and leiomyoma cell proliferation after 10 days (n = 4). NT, no treatment. C, Immunocytochemistry shows the effect of activin-A and myostatin on PCNA expression in myometrial and leiomyoma cells. D, Representative Western blotting showing the effect of activin-A and myostatin on PCNA expression in myometrial and leiomyoma cells. E, PCNA expression fold changes in myometrial and leiomyoma cells after activin-A and myostatin treatments (n = 4). Data are presented as median, first quartiles, third quartiles, and minimum and maximum values. *, P < .05. F, Immunocytochemistry shows the effect of activin-A and myostatin on Ki-67 expression in myometrial and leiomyoma cells.
Effects of activin-A and myostatin on extracellular matrix expression in myometrial and leiomyoma cells
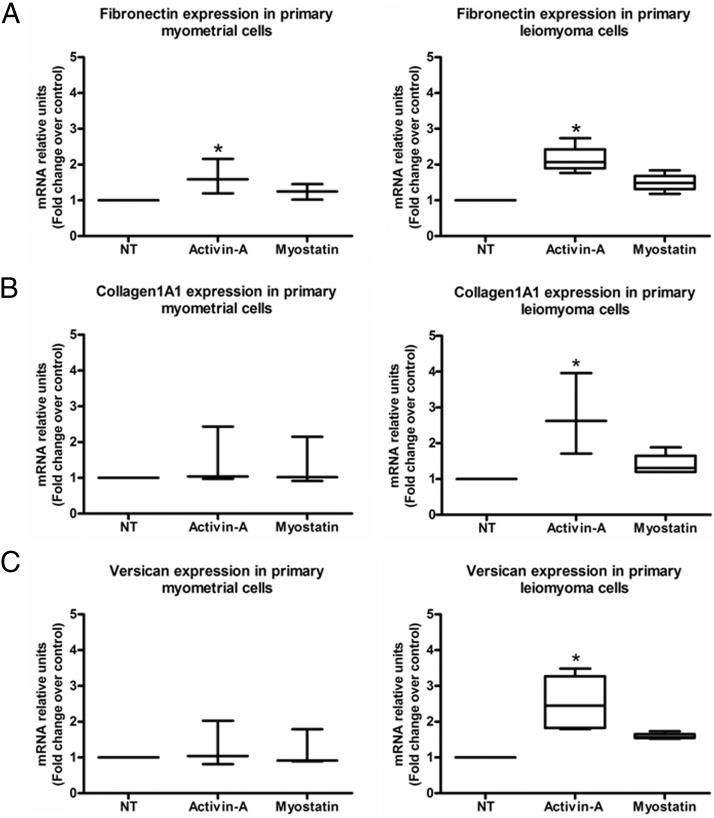
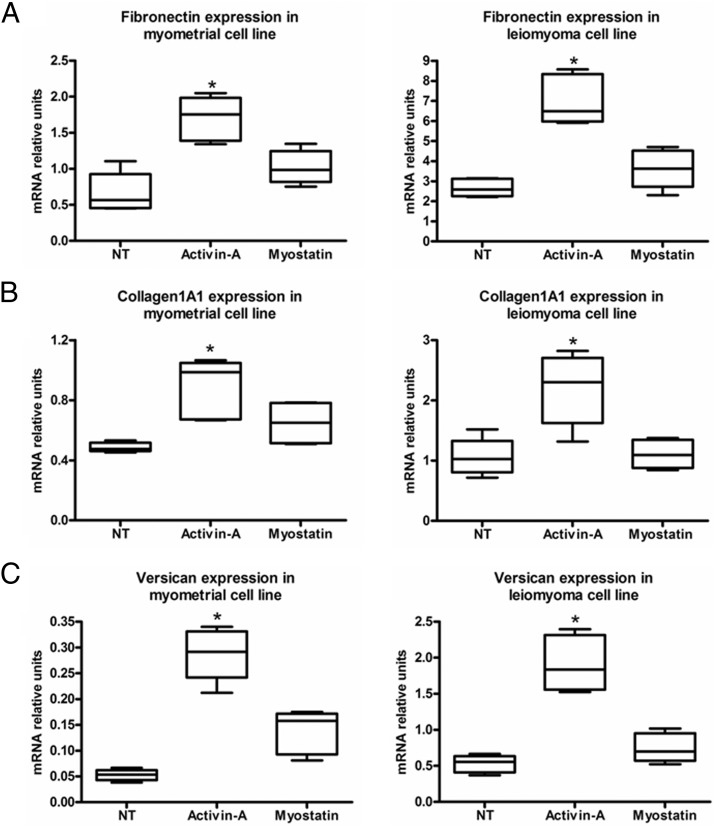
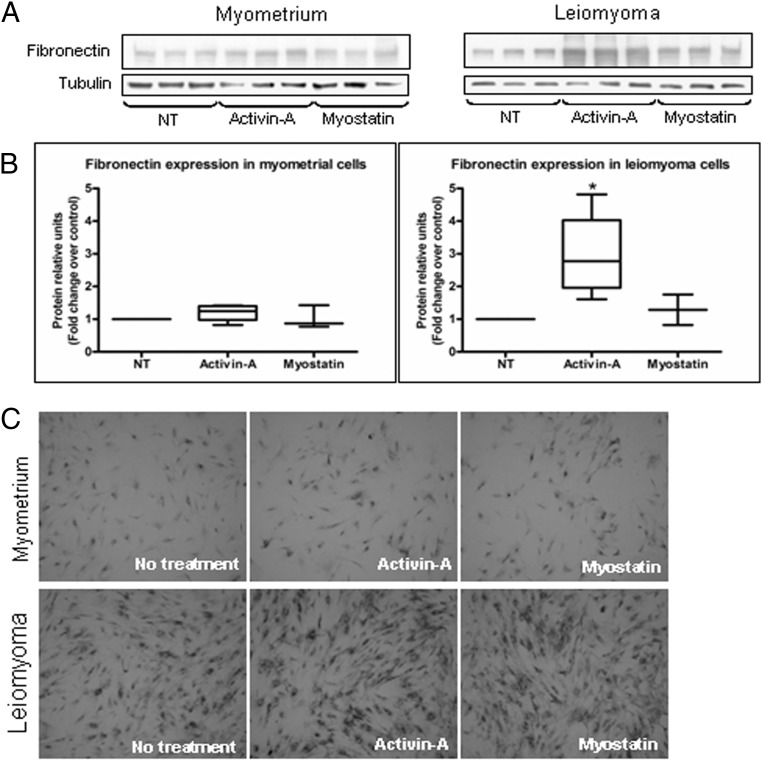
Considering that leiomyomas are characterized by increased extracellular matrix deposition, our next step was to investigate the effects of activin-A and myostatin on the expression of the extracellular matrix fibronectin, collagen1A1, and versican (10, 12, 15). Real-time PCR showed that of the two growth factors, activin-A significantly increased fibronectin, collagen1A1, and versican expression mainly in primary leiomyoma cells (Figure 2, A–C). The increased expression observed for all genes was approximately 2-fold. Activin-A also significantly increased the fibronectin expression in the primary myometrial cells. We found similar results in the leiomyoma cell line. Activin-A also significantly increased fibronectin, but also collagen and versican expression in the myometrial cell line (Figure 3, A–C). Western blot and immunocytochemistry gave results that were similar to those found with real-time PCR in primary cells (Figure 4, A–C). We found that activin-A significantly increased fibronectin protein expression in leiomyoma cells as measured by Western blot (Figure 4, A and B). Similarly, the increase expression of fibronectin by activin-A was evident also by immunocytochemistry (Figure 4C).
Figure 2.
A–C, Real-time PCR shows the effect of activin-A and myostatin on ECM mRNA expression in human primary myometrial and leiomyoma cells (n = 3). NT, no treatment. Results are presented as median, first quartiles, third quartiles, and minimum and maximum values. *, P < .05.
Figure 3.
A–C, Real-time PCR shows the effect of activin-A and myostatin on ECM mRNA expression in human myometrial and leiomyoma cell lines. NT, no treatment. Data are expressed as median, first quartiles, third quartiles, and minimum and maximum values. *, P < .05.
Figure 4.
A–C, A, Representative Western blotting of fibronectin for myometrial and leiomyoma cells. B, Effect of activin-A and myostatin on fibronectin expression in myometrial and leiomyoma cells (n = 4). NT, no treatment. Results are expressed as median, first quartiles, third quartiles, and minimum and maximum values. *, P < .05. C, Immunocytochemistry showing fibronectin induction in myometrial and leiomyoma cells after activin-A and myostatin treatment.
Activin-A and myostatin signaling in myometrial and leiomyoma cells
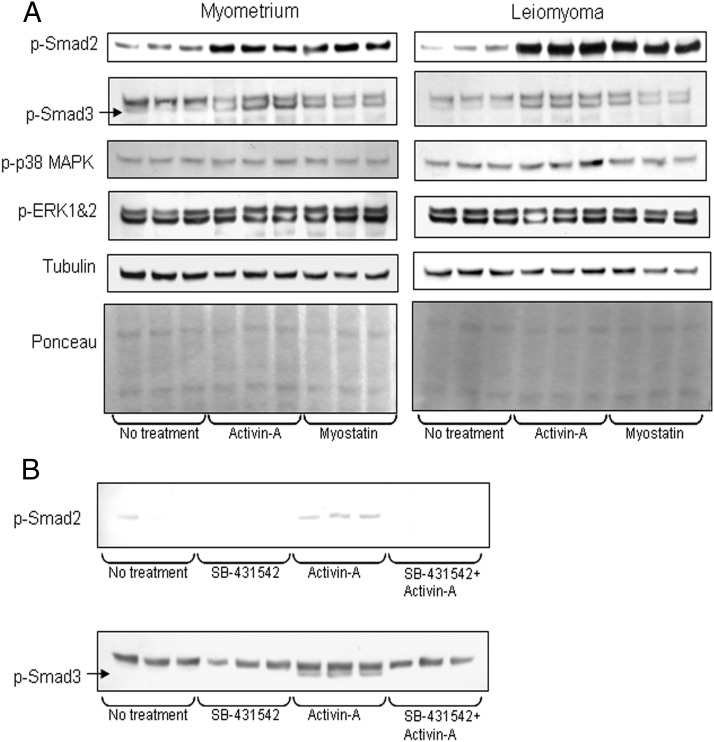
Considering the cytostatic and/or fibrotic effects of activin-A and myostatin, we investigated which signaling pathways are activated by these ligands. We found that activin-A and myostatin increased p-Smad2 and -3 levels in both myometrial and leiomyoma cells (Figure 5A) without altering total Smad levels (data not shown). Our results also showed that activin-A and myostatin were unable to alter the expression of p-p38 MAPK and p-ERK 1 and 2 in both myometrial and leiomyoma cells (Figure 5A).
Figure 5.
A and B, A, Effect of activin-A and myostatin on Smad and non-Smad signaling molecules expression in myometrial and leiomyoma cells. B, Western blotting of p-smad2 and -3 after activin-A induction with and without pretreatment with SB431542.
Our p-Smad3 Western blot showed two bands. The upper band was present in the absence of activin-A/myostatin treatment and its intensity unchanged after activin-A/myostatin treatment, suggesting it is not p-Smad3 but rather a nonspecific band. To verify this supposition and to demonstrate that the lower band is the specific one, we used the inhibitor SB-431542, a potent and specific inhibitor of the kinase activities of the TGF-β superfamily type I receptors ALK4, ALK5, and ALK7 (34). Pretreatment with SB-431542 blocked the activin-A induced phosphorylation of smad2 and also blocked detection of the lower but not the upper band in p-Smad3 Western blots, confirming the lower but not the upper band is indeed p-smad3 (Figure 5B).
Discussion
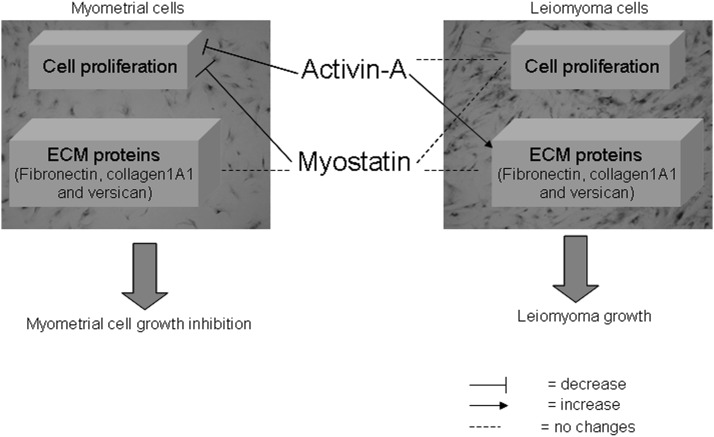
In the present study, we demonstrate that activin-A and myostatin act on both myometrial and leiomyoma cells through the Smad signaling pathway. However, their functions are different in the two cell types because they have cytostatic effect on healthy myometrial cells but only fibrotic effects on leiomyoma cells (Figure 6). These data provide a deeper understanding of the alterations acquired in leiomyoma.
Figure 6.
Role of activin-A and myostatin and their signaling pathway in human myometrial and leiomyoma cell functions.
In the past, the role of activin in myometrium was controversial. Binding of iodinated activin-A was reported in rat myometrium (35), and expression of activin receptors was detected in one study (36) but not detected in another (37). However, we subsequently characterized the actions of activin-A in uterine tissue of rats and myometrial cell lines (PHM1) (26) as well as in human myometrial and leiomyoma tissue explants (28). PHM1 cells express all of the activin receptors in addition to activin-A and follistatin (26). Activin-A inhibits PHM1 cell proliferation (26), and in vivo experiments showed that activin mRNA expression is regulated in the rodent uterus during the estrous cycle (38) and in response to steroid deficiency and replacement (26). Estrogen treatment reduces activin-A mRNA levels in human myometrial tissue explants (28). It was also found that activin-A mRNA levels were higher in menopausal than in fertile myometrial specimens (28). The high expression levels of activin-A in leiomyoma, the regulation of myometrial cell proliferation by activin-A, and its regulation by steroids suggest its potential role in myometrial pathophysiology.
The expression of myostatin and its signaling components has also been documented in rat uterine explants, the myometrial cell line PHM1 (27), and in human myometrial and leiomyoma explants (28). Myostatin treatment decreases PHM1 cell proliferation, and it can induce Smad signaling in PHM1 cells as well as in rat uterine explants (27). In human tissue explants, the expression levels of myostatin are higher in leiomyoma relative to normal adjacent tissue (28). Steroid regulation of myostatin has been documented in rat uterus (27) and human tissue explants (28). It has been shown that ovariectomized rats have higher myostatin expression than normal cycling rats and that estrogen treatment abrogates myostatin expression (27). In addition, myostatin mRNA levels in tissue explants were reduced after estrogen treatment, and the expression levels were higher in menopausal than in fertile myometrial specimens (28). In general, our previous findings suggest the potential role of myostatin in myometrial pathophysiology.
Overall, the above findings indicate that both activin-A and myostatin can act on rat uterus and myometrial cells (PHM1 cell lines). In particular, activin-A and myostatin can down-regulate PHM1 cell proliferation (26, 27), but their expression levels are higher in leiomyoma tissue explants (28).
In the present study, we expected to observe similar effects on human primary myometrial cells, and we sought to determine the effects of activin-A and myostatin on leiomyoma cells. In line with our previous findings, we found that both activin-A and myostatin can down-regulate primary myometrial cell proliferation. On the contrary, they were both unable to reduce leiomyoma cell proliferation, indicating that the antiproliferative effects of activin-A and myostatin are present only on myometrial cells and not on leiomyoma cells.
To date, several growth factors such as epidermal growth factor, heparin-binding epidermal growth factor, platelet-derived growth factor, IGF, TGF-α, TGF-β, vascular endothelial growth factor, acidic fibroblast growth factor, and basic fibroblast growth factor and their respective receptors have been reported to play a role in leiomyoma growth (8). Among these growth factors, the TGF-β superfamily has been extensively studied. TGF-β ligands have been shown to exert bimodal effects on cell proliferation (10, 39–42), to induce elevated production of ECM-related genes (10, 43), and to decrease production of ECM degradation-related genes (43). In addition, TGF-β can activate the MAPK/ERK/Smad pathways and regulate the expression of different types of genes whose products may, at least in part, influence the leiomyoma growth and regression (8, 43–49).
Activin-A and myostatin are members of the TGF-β super family that have a wide range of biological functions including cytostatic effects on multiple cell types. Activin has potent antiproliferative effects on different types of healthy and pathological cells including liver cells (50), breast cancer cells (51), and vascular endothelial cells (52). Myostatin also inhibits the proliferation of C2C12 muscle cells (53) and rhabdomyosarcoma cells (54). In the present report, we found that activin-A and myostatin inhibit the proliferation of myometrial cells but, interestingly, not their pathological leiomyoma counterparts.
The ECM synthesis is an important event in leiomyoma growth. Recent studies suggest that alterations in ECM can modify mechanical stress on cells, which leads to activation of internal mechanical signaling contributing to leiomyoma (55–57). Primarily collagens, fibronectin, and proteoglycans have been found in leiomyoma with altered expression compared with normal myometrium. In the present study, we examined whether activin-A and myostatin increase ECM in myometrial and leiomyoma cells (primary and cell lines). It is notable that we used primary myometrial and leiomyoma cells as a reliable cell culture model for all the experiments of our present study, and we confirmed the smooth muscle nature of primary myometrial and leiomyoma cells by positive immunocytochemical staining for smooth muscle actin. In parallel with primary cells, we also used myometrial and leiomyoma cell lines (immortalized human uterine leiomyoma and patient matched myometrial cells) as additional cell culture models to further confirm the validity of our cell culture system.
We found that activin-A significantly increased fibronectin, collagen1A1, and versican mRNA expression in primary leiomyoma cells, and it significantly increased fibronectin mRNA expression but not collagen1A1 and versican expression in primary myometrial cells. The increased fibronectin expression by activin-A in leiomyoma cells was seen also by Western blot and immunocytochemistry.
In a cell line model, we found that activin-A significantly increased fibronectin, collagen1A1, and versican mRNA expression in both immortalized leiomyoma and patient-matched myometrial cells. Here it is notable that the increased expression of collagen1A1 and versican by activin-A was found in myometrial cell line, but this phenomenon was not present in primary myometrial cells. The different ECM expression in response to activin-A treatment from the two models (primary and cell lines) could be due to the fact that primary cells may have high variability due to the variability of the tissue of origin.
It has been shown that activin-A up-regulates collagen and fibronectin in normal and keloid fibroblasts (21). Keloid is a fibrotic disorder that shares similar molecular and epidemiological features with uterine leiomyoma (14, 58, 59). Furthermore, similar to activin-A, Arici and Sozen (10) reported that TGF-β3 induces fibronectin expression in leiomyoma cells and concomitantly stimulates myometrial and leiomyoma cell proliferation in culture.
Overall, the above results suggest that activin-A and myostatin lack cytostatic or significant proproliferative effect on leiomyoma cells. However, the ability of activin-A to increase fibronectin, collagen1A1, and versican expression in leiomyoma demonstrates its potentially profibrotic role in leiomyoma growth.
Finally, our study addresses the signaling mechanisms of activin-A and myostatin underlying cytostatic and/or fibrotic effects in myometrial and leiomyoma cells. We tested several possible signaling pathways (Smad and non-Smad) in this regard. Although activin-A and myostatin were unable to induce non-Smad signaling pathways (p-p38 MAPK and p-ERK 1 and 2), they both activated the Smad signaling pathway (induction of p-Smad2 and -3) in both cell types. Regarding the induction of Smad signaling in leiomyoma, we previously saw that in fibroid tissue explants, activin-A was not able to induce the expression of Smad7 mRNA (28). On the other hand, we found that activin-A induces the expression of Smad7 in isolated leiomyoma cells in culture (Ciarmela P, unpublished data). This difference could be due to a limitation of the exogenous protein to reach the internal part of the tissue in fibroid explants or modification of genetic expression that could occur in cells when placed in culture in vitro (ie, we observed that Cripto/teratocarcinoma-derived growth factor 1 mRNA is expressed in tissue, whereas its expression is lost in cultured cells in vitro (Ciarmela P, unpublished data).
In conclusion, our results indicate that activin-A and myostatin signal via the Smad pathway and have antiproliferative and/or fibrotic effects, depending on the cell type (myometrial or leiomyoma). Together with our previous findings (26–28), these data strongly suggest that activin-A and myostatin are emerging as important growth factors in the field of leiomyoma research.
Acknowledgments
This study is dedicated to the memory of Wylie Vale, formerly a professor at the Salk Institute (La Jolla, California). Wylie was an outstanding scientist who made fundamental contributions to our present understanding of the biology of the TGF-β superfamily of growth factors. He was an inspiring teacher and a dear friend. This work is a prosecution of previous works done under Wylie's supervision in his laboratory, and he had a fundamental role in the conception and design of this study.
We thank Jörg Müller (Bayer Pharma AG) for his significant input in both the experimental design and discussions on experimental results. M.S.I. was a recipient of a fellowship from Polytechnic University of Marche, reserved for a PhD student from a non-European Union country. O.P. and M.J. are recipients of a fellowship from Polytechnic University of Marche, reserved for a PhD student coming from universities of the UNIADRION, a network of universities established with the purpose of creating a permanent connection among universities and research centers from the Adriatic-Ionian Region (Italy). The university network was born within the framework of the Adriatic-Ionian Initiative. We also thank Dr Rosaria Gesuita (Department of Epidemiology, Biostatistics, and Medical Information Technology, Polytechnic University of Marche, Ancona, Italy) for her valuable help to perform statistical data analysis.
This work was supported by a grant from the Grants for Targets (G4T) program of Bayer Pharma AG (to P.C.); a grant from the “Fondazione Cassa di Risparmio di Fabriano e Cupramontana” (to P.L., M.C., and P.C.); Italian Ministry of the University and Research, PRIN 2010–2011, Grant 20102CHST5_007 (to S.R.G.); the Clayton Medical Research Foundation, Inc; and the Cancer Center Core Grant P30 CA014195–38 (to P.C.G).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ActRII
- activin type II receptor
- ALK
- activin receptor-like kinase
- ECM
- extracellular matrix
- FBS
- fetal bovine serum
- p
- phosphorylated
- PCNA
- proliferating cell nuclear antigen
- PHM1
- pregnant human myometrial 1
- RT
- reverse transcriptase
- TBST
- Tris-buffered saline with Tween 20.
References
- 1. Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592 [DOI] [PubMed] [Google Scholar]
- 2. Islam MS, Protic O, Toti P, et al. Uterine leiomyoma: available medical treatments and new possible therapeutic options. J Clin Endocrinol Metab. 2013a;98:921–934 [DOI] [PubMed] [Google Scholar]
- 3. Buttram VC, Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36:433–445 [DOI] [PubMed] [Google Scholar]
- 4. Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94:435–438 [DOI] [PubMed] [Google Scholar]
- 5. Segars JH, Akopians AL. The two health disparities of uterine fibroids. Fertil Steril. 2013;99:1851–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:19887–19892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound screening study. Obstet Gynecol. 2009;113:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ciarmela P, Islam MS, Reis FM, et al. Growth factors and myometrium: biological effects in uterine fibroid and possible clinical implications. Hum Reprod Update. 2011;17:772–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Islam MS, Protic O, Stortoni P, et al. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil Steril. 2013;100:178–193 [DOI] [PubMed] [Google Scholar]
- 10. Arici A, Sozen I. Transforming growth factor-β3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73:1006–1011 [DOI] [PubMed] [Google Scholar]
- 11. Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900–906 [DOI] [PubMed] [Google Scholar]
- 12. Norian JM, Malik M, Parker CY, et al. Transforming growth factor β3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci. 2009;16:1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril. 2004;82(suppl 3):1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Catherino WH, Leppert PC, Stenmark MH, et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40:204–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malik M, Catherino WH. Development and validation of a three-dimensional in vitro model for uterine leiomyoma and patient-matched myometrium. Fertil Steril. 2012;97:1287–1293 [DOI] [PubMed] [Google Scholar]
- 16. Chegini N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med. 2010;28:180–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen YG, Lui HM, Lin SL, Lee JM, Ying SY. Regulation of cell proliferation, apoptosis, and carcinogenesis by activin. Exp Biol Med. 2002;227:75–87 [DOI] [PubMed] [Google Scholar]
- 18. Luisi S, Florio P, Reis FM, Petraglia F. Expression and secretion of activin A: possible physiological and clinical implications. Eur J Endocrinol. 2001;145:225–236 [DOI] [PubMed] [Google Scholar]
- 19. Tsuchida K, Nakatani M, Hitachi K, et al. Activin signaling as an emerging target for therapeutic interventions. Cell Commun Signaling. 2009;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem. 2008;283:19371–19378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mukhopadhyay A, Chan SY, Lim IJ, Phillips DJ, Phan TT. The role of the activin system in keloid pathogenesis. Am J Physiol Cell Physiol. 2007;292:C1331–C1338 [DOI] [PubMed] [Google Scholar]
- 22. Date M, Matsuzaki K, Matsushita M, Tahashi Y, Sakitani K, Inoue K. Differential regulation of activin A for hepatocyte growth and fibronectin synthesis in rat liver injury. J Hepatol. 2000;32:251–260 [DOI] [PubMed] [Google Scholar]
- 23. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584 [DOI] [PubMed] [Google Scholar]
- 24. Heldin CH, Miyazono K, Ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;465–471 [DOI] [PubMed] [Google Scholar]
- 25. Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700 [DOI] [PubMed] [Google Scholar]
- 26. Ciarmela P, Wiater E, Vale W. Activin-A in myometrium: characterization of the actions on myometrial cells. Endocrinology. 2008;149:2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ciarmela P, Wiater E, Smith SM, Vale W. Presence, actions, and regulation of myostatin in rat uterus and myometrial cells. Endocrinology. 2009;150:906–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ciarmela P, Bloise E, Gray PC, et al. Activin-A and myostatin response and steroid regulation in human myometrium: disruption of their signalling in uterine fibroid. J Clin Endocrinol Metab. 2011a;96:755–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rhim JS. Generation of immortal human prostate cell lines for the study of prostate cancer. In: Russell P, Jackson P, Kingsley EA, eds., Prostate Cancer Methods and Protocols. Springer, 2003:69–77 [DOI] [PubMed] [Google Scholar]
- 30. Malik M, Webb J, Catherino WH. Retinoic acid treatment of human leiomyoma cells transformed the cell phenotype to one strongly resembling myometrial cells. Clin Endocrinol (Oxf). 2008;69:462–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shime H, Kariya M, Orii A, et al. Tranilast inhibits the proliferation of uterine leiomyoma cells in vitro through G1 arrest associated with the induction of p21waf1 and p53. J Clin Endocrinol Metab. 2002;87:5610–5617 [DOI] [PubMed] [Google Scholar]
- 32. Islam MS, Protic O, Stortoni P, et al. Antiproliferative effect of tranilast on human myometrial and leiomyoma cells. Biol Biomed Rep. 2012;2:321–327 [Google Scholar]
- 33. Ciarmela P, Marzioni D, Islam MS, et al. Possible role of RKIP in cytotrophoblast migration: immunohistochemical and in vitro studies. J Cell Physiol. 2012;227:1821–1828 [DOI] [PubMed] [Google Scholar]
- 34. Inman GJ, Nicolas FJ, Callahan JF, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74 [DOI] [PubMed] [Google Scholar]
- 35. Draper LB, Chong H, Wang E, Woodruff TK. The uterine myometrium is a target for increased levels of activin A during pregnancy. Endocrinology. 1997;138:3042–3046 [DOI] [PubMed] [Google Scholar]
- 36. Hayashi K, Carpenter KD, Gray CA, Spencer TE. The activin-follistatin system in the neonatal ovine uterus. Biol Reprod. 2003;69:843–850 [DOI] [PubMed] [Google Scholar]
- 37. Schneider-Kolsky M, Manuelpillai U, Gargett C, Wallace EM. Activin βA-subunit and activin receptors in human myometrium at term and during labour. BJOG. 2001;108:869–874 [DOI] [PubMed] [Google Scholar]
- 38. Jones RL, Kaitu'u-Lino TJ, Nie G, Sanchez-Partida LG, Findlay JK, Salamonsen LA. Complex expression patterns support potential roles for maternally derived activins in the establishment of pregnancy in mouse. Reproduction. 2006;132:799–810 [DOI] [PubMed] [Google Scholar]
- 39. Arici A, Sozen I. Expression, menstrual cycle-dependent activation, and bimodal mitogenic effect of transforming growth factor-β1 in human myometrium and leiomyoma. Am J Obstet Gynecol. 2003;188:76–83 [DOI] [PubMed] [Google Scholar]
- 40. Tang XM, Dou Q, Zhao Y, McLean F, Davis J, Chegini N. The expression of transforming growth factor-βs and TGF-β receptor mRNA and protein and the effect of TGF-βs on human myometrial smooth muscle cells in vitro. Mol Hum Reprod. 1997;3:233–240 [DOI] [PubMed] [Google Scholar]
- 41. Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-β 3 (TGF β3) and altered responses to the antiproliferative effects of TGF β. J Clin Endocrinol Metab. 2001;86:913–920 [DOI] [PubMed] [Google Scholar]
- 42. Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, Ross R. TGF-β induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990;63:515–524 [DOI] [PubMed] [Google Scholar]
- 43. Joseph DS, Malik M, Nurudeen S, Catherino WH. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor β-3. Fertil Steril. 2010;93:1500–1508 [DOI] [PubMed] [Google Scholar]
- 44. Wolanska M, Bankowski E. Transforming growth factor β and platelet-derived growth factor in human myometrium and in uterine leiomyomas at various stages of tumour growth. Eur J Obstet Gynecol Reprod Biol. 2007;130:238–244 [DOI] [PubMed] [Google Scholar]
- 45. Chegini N, Luo X, Ding L, Ripley D. The expression of Smads and transforming growth factor β receptors in leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Mol Cell Endocrinol. 2003;209:9–16 [DOI] [PubMed] [Google Scholar]
- 46. Ding L, Xu J, Luo X, Chegini N. Gonadotropin releasing hormone and transforming growth factor β activate mitogen-activated protein kinase/extracellularly regulated kinase and differentially regulate fibronectin, type I collagen, and plasminogen activator inhibitor-1 expression in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab. 2004;89:5549–5557 [DOI] [PubMed] [Google Scholar]
- 47. Levens E, Luo X, Ding L, Williams RS, Chegini N. Fibromodulin is expressed in leiomyoma and myometrium and regulated by gonadotropin-releasing hormone analogue therapy and TGF-β through Smad and MAPK-mediated signalling. Mol Hum Reprod. 2005;11:489–494 [DOI] [PubMed] [Google Scholar]
- 48. Luo X, Ding L, Chegini N. CCNs, fibulin-1C and S100A4 expression in leiomyoma and myometrium: inverse association with TGF-β and regulation by TGF-β in leiomyoma and myometrial smooth muscle cells. Mol Hum Reprod. 2006;12:245–256 [DOI] [PubMed] [Google Scholar]
- 49. Luo X, Ding L, Xu J, Chegini N. Gene expression profiling of leiomyoma and myometrial smooth muscle cells in response to transforming growth factor-β. Endocrinology. 2005;146:1097–1118 [DOI] [PubMed] [Google Scholar]
- 50. Ho J, de Guise C, Kim C, Lemay S, Wang XF, Lebrun JJ. Activin induces hepatocyte cell growth arrest through induction of the cyclin-dependent kinase inhibitor p15INK4B and Sp1. Cell Signal. 2004;16:693–701 [DOI] [PubMed] [Google Scholar]
- 51. Burdette JE, Jeruss JS, Kurley SJ, Lee EJ, Woodruff TK. Activin A mediates growth inhibition and cell cycle arrest through Smads in human breast cancer cells. Cancer Res. 2005;65:7968–7975 [DOI] [PubMed] [Google Scholar]
- 52. Kaneda H, Arao T, Matsumoto K, et al. Activin A inhibits vascular endothelial cell growth and suppresses tumour angiogenesis in gastric cancer. Br J Cancer. 2011;105:1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Taylor WE, Bhasin S, Artaza J, et al. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol Endocrinol Metab. 2001;280:E221–E228 [DOI] [PubMed] [Google Scholar]
- 54. Langley B, Thomas M, McFarlane C, Gilmour S, Sharma M, Kambadur R. Myostatin inhibits rhabdomyosarcoma cell proliferation through an Rb-independent pathway. Oncogene. 2004;23:524–534 [DOI] [PubMed] [Google Scholar]
- 55. Rogers R, Norian J, Malik M, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198:474.e1–474.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Norian JM, Owen CM, Taboas J, et al. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Malik M, Segars J, Catherino WH. Integrin β1 regulates leiomyoma cytoskeletal integrity and growth. Matrix Biol. 2012;31:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. David AC, Sam M, Nichole MB, William WH, Arnold IC. Proteoglycans of uterine fibroids and keloid scars: similarity in their proteoglycan composition. Biochem J. 2012;443:361–368 [DOI] [PubMed] [Google Scholar]
- 59. Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195:415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]