Abstract
Context:
The ketogenic diet is increasingly used in refractory epilepsy and is associated with clinically significant effects on bone and mineral metabolism. Although hypercalciuria and loss of bone mineral density are common in patients on the ketogenic diet, hypercalcemia has not previously been described.
Objective:
The aim of the study was to describe three children who developed hypercalcemia while on the ketogenic diet.
Design:
A retrospective chart review of three children on the ketogenic with severe hypercalcemia was conducted.
Results:
We describe three children on the ketogenic diet for refractory seizures who presented with hypercalcemia. Case 1 was a 5.5-year-old male with an undiagnosed, rapidly progressive seizure disorder associated with developmental regression. Case 2 was a 2.5-year-old male with a chromosomal deletion of 2q24.3, and case 3 was a 4.6-year-old male with cerebral cortex dysplasia. Patients had been on a ketogenic diet for 6 to 12 months before presentation. Daily intake of calcium and vitamin D was not excessive, and all three patients were not acidotic because they were taking supplemental bicarbonate. Each child had elevated serum levels of calcium and normal serum phosphate levels, moderately elevated urinary calcium excretion, and low levels of serum alkaline phosphatase, PTH, and 1,25-dihydroxyvitamin D. All patients responded to calcitonin.
Conclusions:
Hypercalcemia is an uncommon complication of the ketogenic diet, and these children may represent the severe end of a clinical spectrum of disordered mineral metabolism. The mechanism for hypercalcemia is unknown but is consistent with excess bone resorption and impaired calcium excretion.
Patients with epilepsy have significant impairments to bone health, with a 2- to 3-fold increased risk of fractures (1). Antiepileptic medications that induce hepatic P450-metabolizing enzymes can increase osteoclast activation, inactivate vitamin D metabolites, and enhance degradation of estrogen, all of which have negative effects on bone mineral density (BMD) (2). This is of particular concern in childhood, when accrual of peak BMD is ongoing, and hence disruption of this process can have significant lifelong consequences (3). Moreover, up to one-third of epilepsy cases are classified as intractable, and these patients may have additional risk factors for low bone mass, including immobility, reduced muscle mass, and use of multiple anticonvulsant medications.
The use of a ketogenic diet in epilepsy was first described in the early 20th century (4) after initial observations that starvation was beneficial in seizure prevention. This diet achieves a state of ketosis through incorporation of fats as a large proportion of dietary calories, utilizing a ratio of 3 or 4 g of fat to every gram of protein and carbohydrates. Reduced carbohydrate intake suppressing circulating insulin levels is central to the diet, and fluid restriction may also play a role (5). The underlying mechanism of seizure prevention is not known, but it may include increased synthesis of γ-aminobutyric acid and neuronal uncoupling proteins, as well as reduced reactive oxygen species generation. Glucose restriction may also play a role in stabilizing synapses (6). Regardless of the exact mechanism, this diet is increasingly used in the management of pediatric epilepsy (7) and can be effective in half of patients, with one-third experiencing a significant reduction in seizure frequency (8).
The ketogenic diet is associated with a number of adverse effects on bone and mineral metabolism. Hypercalciuria, urine acidification, and hypocitraturia, all known risk factors for kidney stones, are common consequences of the ketogenic diet. Nephrolithiasis occurs in 1–6% of patients who are on this diet (9–11). Use of potassium citrate can ameliorate the urinary acidification and reduce the risk of calcium-containing renal stones and is now recommended for all patients on this diet (11). Nevertheless, hypercalciuria persists, and serum bicarbonate is not affected by usual doses of potassium citrate (11). Reduced BMD is also very common, but the specific role of the ketogenic diet in excess of the other risk factors for osteopenia in these patients is not clear. Serum levels of 25-hydroxyvitamin D [25(OH)D] and calcium are reduced, and bone resorption is increased in many patients on anticonvulsant therapy whether or not they are using a ketogenic diet. Several lines of evidence suggest that the ketogenic diet per se is damaging to the skeleton. For example, despite similar levels of serum 25(OH)D, children on the ketogenic diet have a greater reduction in bone mass than children who are taking anticonvulsant drugs alone (12). Moreover, reducing or eliminating antiepileptic medications and commencing the ketogenic diet, even when calcium and 25(OH)D supplementation is provided, is associated with a progressive decline in whole-body BMD (13). Nonambulatory children with low body mass index (BMI) are at the highest risk of osteopenia and osteoporosis (13).
In the context of altered bone metabolism and increasing osteopenia associated with the ketogenic diet, hypercalcemia has not previously been described as a potentially associated risk. We describe three children who developed hypercalcemia after 6 to 12 months of treatment with the ketogenic diet.
Patients and Methods
We performed a retrospective chart review of three children with hypercalcemia who were on the ketogenic diet for seizure control at The Children's Hospital of Philadelphia. The diagnosis of hypercalcemia was based on albumin-adjusted total serum calcium concentrations. Consistent with previous studies (14), we defined hypercalciuria as a fasting urine calcium:creatinine ratio of > 0.2 (mg/mg) for patients 1 year of age or older, >0.6 for patients between 6 months and 1 year, and > 0.8 for patients under 6 months. This study was performed in accordance with the policies and procedures of the Institutional Review Board of the Children's Hospital of Philadelphia as a retrospective review of a limited case series.
Case Reports
Case 1
Case 1 is a 5-year-old male with a history of an undiagnosed neurodegenerative disorder and refractory seizures. He had one seizure at 10 months of age and was developing appropriately seizure-free until 2 years of age. He subsequently experienced recurrent seizures, which were controlled on oxcarbazepine until he was 5 years old. His seizures then became refractory to therapy with lacosamide, depakote, ethosuximide, topiramate, and levetiracetam. He was commenced on a ketogenic diet with supplemental potassium citrate 5 months before presentation with hypercalcemia. His calcium was 9 mg/dL (reference [ref], 8.8–10.1) before starting this diet.
At presentation, he weighed 20.7 kg, his height was 120.5 cm, and his BMI was 14.3 kg/m2. Laboratory evaluation showed serum calcium of 12.3 mg/dL (ref, 8.8–10.1) with undetectable serum PTH (Table 1). The urinary calcium:creatinine ratio was elevated at 0.32 (ref, <0.2) (15). His renal function was normal. Before commencing the ketogenic diet, his alkaline phosphatase was 197 U/L (ref, 150–380), creatinine was 0.32 mg/dL (ref, 0.1–0.4), and serum urea nitrate (SUN) was 3 U/L (ref, 5–17). He was treated with iv saline hyperhydration and sc calcitonin at 6 U/kg/d divided twice daily. His serum calcium concentration normalized to 9.5 mg/dL within 2 weeks (Figure 1).
Table 1.
Clinical and Biochemical Characteristics
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age | 5 y 6 mo | 2 y 7 mo | 4 y 8 mo |
| Duration of ketogenic diet | 5 mo | 1 y | 8 mo |
| Diagnosis | Undiagnosed neurodegenerative disorder | Chromosomal 2q24.3 deletion | Cortical dysplasia |
| Antiepileptic medications | Levetiracetam, oxcarbazepine | Topiramate | None |
| Bicarbonate therapy | Yes | Yes | Yes |
| Ambulatory | No | No | Yes |
| Vitamin D supplementation | Yes | Yes | Yes |
| Calcium supplementation | No | Yes (50 mg/kg/d) | Yes (35 mg/kg/d) |
| Serum calcium, mg/dL (ref, 8.8–10.1) | 12.3 | 13.9 | 13.2 |
| Urinary calcium:creatinine, mg/mg (ref, <0.2) | 0.32 | 0.29 | 0.2 |
| Serum phosphorus, mg/dL (ref, 3.8–6.5) | 5.1 | 4.8 | 6.1 |
| Alkaline phosphatase, U/L (ref, 145–320) | 66 | 108 | 107 |
| 25(OH)D, ng/mL (ref, 30–80) | 33.9 | 39.9 | 33.7 |
| 1,25(OH)2D, pg/mL (ref, 15–75) | 11 | <3 | |
| PTH, pg/mL (ref, 9–52) | <3.4 | 4.8 | <10 |
| Urinary N-telopeptide, nmol BCE/mmol Cr | ND | 214 | 152 |
| Serum C-telopeptide, pg/mL | ND | 479 | 126 |
| Serum PTHrP (pmol/L) (ref, 0–4) | <3.3 | <3 | <1.5 |
| Beta-hydroxybutyrate, mmol/L (ref, 0–0.9) | 1.9 | 3.9 | 5.8 |
| Albumin, g/dL (ref, 3.5–4.6) | 4 | 4.2 | 4.5 |
| pH | 7.44 | 7.38 | ND |
| Serum bicarbonate, mmol/L (ref, 20–26) | 28.6 | 30 | 26 |
Abbreviations: ND, not determined; BCE, bone collagen equivalents; PTHrP, parathyroid hormone related peptide.
Figure 1.
Serum calcium in the days before and after commencing calcitonin therapy. The reference range is 8.8–10.1 mg/dL (gray area).
Case 2
Case 2 was a 2-year-old male with a chromosome 2q24.3 deletion encompassing 14 genes, including SCN1A, SCN2A, and SCN9A. His epilepsy was refractory to triple therapy including levetiracetam, phenobarbitone, and topiramate, and he started a ketogenic diet at 19 months of age. His calcium was 9.6 mg/dL (ref, 8.8–10.1) before commencing this diet. While on this diet, he was also treated with sodium bicarbonate, calcium supplementation (50 mg/kg/d elemental calcium), and cholecalciferol. He presented with hypercalcemia 1 year after commencing the ketogenic diet. Over the preceding 6 months, he had become poorly tolerant of oral feeds and was having increased vomiting and reflux. His weight had fallen from 10.9 to 8.4 kg over the same period.
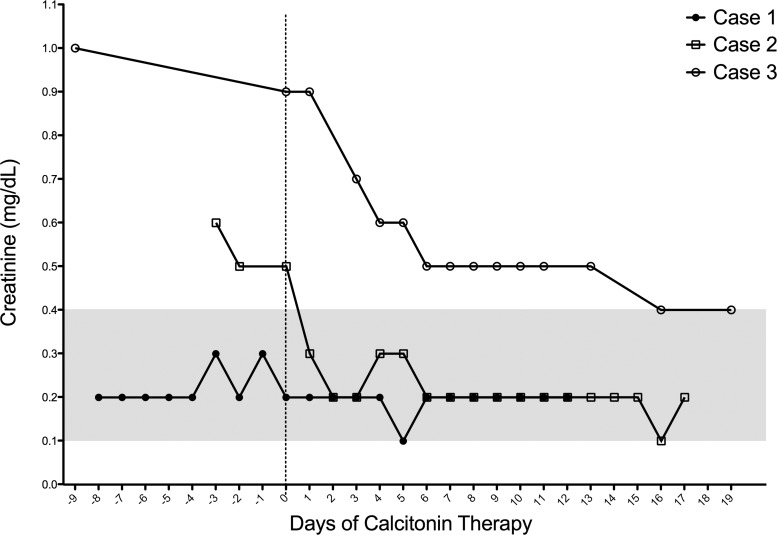
At presentation, his serum calcium was 13.9 mg/dL (ref, 8.8–10.1), serum intact PTH was 4.8 pg/mL (ref, 9–52), 1,25-dihydroxyvitamin D [1,25(OH)2D] was 11 pg/mL (ref, 15–75), and alkaline phosphatase was 108 U/L (ref, 145–320) (Table 1). His urinary calcium:creatinine ratio was 0.29 (ref, <0.2) (Table 1). His serum creatinine was elevated at 0.6 mg/dL (ref, 0.1–0.4), and SUN was 11 mg/dL (ref, 2–19). One year previously, his serum creatinine was 0.2 mg/dL, his SUN was 3 mg/dL, and his alkaline phosphatase was 332 U/L. He was treated with iv saline at 1.5 to 2 times maintenance, and he was commenced on sc calcitonin 4U/kg/d divided twice daily. This dose was increased to 6 U/kg/d divided three times daily to establish a response. His elevated calcium (Figure 1) and serum creatinine (Figure 2) had begun to normalize on hydration alone but improved further after introduction of calcitonin.
Figure 2.
Serum creatinine in the days before and after commencing calcitonin therapy. The reference range is 0.1–0.4 mg/dL (gray area).
Case 3
Case 3 presented at age 4 years. He had a background of developmental delay noted at 4 months of age. His fine motor and gross motor milestones were delayed, and at 2 years of age he had weak and clumsy fine motor skills with significant ataxia. He had his first seizure at 3 years of age and was initially treated with levetiracetam. His seizure frequency increased, and he was transitioned to divalproex sodium. He commenced the ketogenic diet at 4 years of age. At initiation of the ketogenic diet, his serum calcium was 9.9 mg/dL (ref, 8.8–10.1), creatinine was 0.3 mg/dL, SUN was 17 mg/dL, and alkaline phosphatase was 206 U/L (ref, 150–380).
He presented 8 months after starting the ketogenic diet with a 4-month history of poor oral intake, fatigue, constipation, and episodes of vomiting approximately twice weekly. There had been 1 kg of weight loss, from 19.1 to 18.1 kg, over the preceding 5 months, and his BMI at presentation was 16.2 kg/m2. His calcium was 13.2 mg/dL (ref, 8.8–10.1). He had low serum levels of intact PTH, 1,25(OH)2D, and alkaline phosphatase (Table 1). The urinary calcium:creatinine ratio was 0.2 (Table 1). Of note, his serum creatinine was 0.7 mg/dL (ref, 0.1–0.4), and SUN was 17 mg/dL (ref, 5–17).
He was treated with iv saline and established on sc calcitonin 3 U/kg/d divided twice daily. His serum levels of calcium (Figure 1) and creatinine (Figure 2) normalized on therapy. Calcitonin was successfully weaned over the following 4 months.
Discussion
The three patients described here show remarkable similarities in the clinical and biochemical features at the time hypercalcemia was identified and hence suggest a previously undescribed association between the ketogenic diet and hypercalcemia. All three patients had been on the ketogenic diet for 6 to 12 months, and all had similar biochemical features, specifically suppressed or low levels of PTH and 1,25(OH)2D, reduced alkaline phosphatase activity, and a normal to moderately elevated urinary calcium:creatinine ratio. Urinary N-telopeptide and serum C-telopeptide levels, which indicate osteoclastic activity, were low for age (Table 1).
It is conceivable that the ketogenic diet impairs osteoblastic activity and deposition of calcium into bone while having only a modest effect on bone resorption, thereby leading to a net movement of calcium out of the skeleton. This effect is likely compensated by increased urinary excretion of calcium in most patients. This hypothesis is consistent with previous reports that bone mass is reduced in patients on the ketogenic diet (13), and it is substantiated by the amelioration of hypercalcemia that we noted after treatment with calcitonin. Hence, we propose that the pathophysiology of hypercalcemia is based first on disproportionate bone resorption with inadequate disposition of the excess skeletal calcium, and second, an impairment in urinary excretion that abrogates a safety valve against developing hypercalcemia.
At present, the basis for the effect(s) of the ketogenic diet on bone and mineral metabolism is uncertain because the ketogenic diet results in a variety of metabolic consequences that can influence differentiation and/or function of osteoblasts and osteoclasts. These proposed mechanisms have recently been reviewed (5, 16), and they involve carbohydrate reduction, activation of ATP-sensitive potassium channels, inhibition of the mammalian target of rapamycin pathway, and enhanced availability of the endogenous ligand adenosine (17, 18). Adenosine, acting at G protein-coupled receptors expressed on both osteoblasts and osteoclasts (19, 20), is a particularly appealing candidate for the effects of the ketogenic diet on bone and mineral metabolism.
Adenosine is generated both intracellularly and extracellularly from the hydrolysis of adenine nucleotides. The half-life of extracellular adenosine is short as a result of deamination by adenosine deaminase (ADA) to inosine as well as cellular uptake by equilibrative nucleoside transporter 1. Intracellular adenosine is generated by cytoplasmic 5′-nucleotidases and is transported out of the cell by equilibrative nucleoside transporter 1 or metabolized either by ADA to inosine or by adenosine kinase to AMP. Recently, Masino et al (18) have shown that mice fed a ketogenic diet exhibit marked reductions in the expression of adenosine kinase, the major adenosine-metabolizing enzyme.
On a molecular level, extracellular adenosine can bind to a family of four adenosine receptors, termed A1, A2A, A2B, and A3. Active A1 receptors are critically important for osteoclast differentiation and function, whereas adenosine-dependent activation of other adenosine receptors appears to inhibit osteoclastogenesis (21). By contrast, both A1 and A2B receptors are highly expressed on osteoblast progenitors and osteoblasts, with activation of A1 receptors leading to induction of adipogenesis, whereas activation of A2B receptors inhibits adipogenesis and stimulates osteoblast differentiation (22). Given the variable expression of adenosine receptors on bone and stromal cells, it is difficult to predict the effect that increased extracellular adenosine, induced by the ketogenic diet, might have on bone metabolism.
Relevant insight may derive from studies of children with a deficiency of ADA, a genetic disorder that leads to elevated levels of extracellular adenosine. These children have a severe combined immunodeficiency and also exhibit marked skeletal abnormalities, which have been related to increased osteoclast bone resorption through activation of cytokine molecules (23) and an increased receptor activator of nuclear factor-κB ligand (RANKL):osteoprotegerin (OPG) ratio in the plasma (24). Using a mouse model of genetic deficiency of ADA (Ada−/−), Sauer et al (24) showed that Ada−/− mice have reduced trabecular bone volume and reduced bone turnover, with reduced RANKL but no change in OPG in the plasma. Overall, the Ada−/− mice appear to have decreased osteoblastogenesis but little change in osteoclastogenesis, features that are consistent with the bone markers present in our patients on the ketogenic diet and their therapeutic response to calcitonin. Taken in context, these observations suggest that adenosine exerts its principal effects on bone metabolism through inhibition of osteoblastogenesis.
Another potential adverse effect of the ketogenic diet on bone metabolism is acidosis. This is common in children on the ketogenic diet who receive inadequate treatment with citrate or bicarbonate and may contribute in several ways to the hypercalciuria and low bone mass present in this population. Acidosis decreases renal tubular calcium reabsorption and therefore increases urinary calcium excretion. The increased urine calcium excretion in acidosis is not associated with an increase in intestinal calcium absorption, but rather results from increased bone mineral resorption, possibly through activation of proton-sensing receptors on osteoclasts (25–27). Hence, both acidosis and ketosis per se produce significant perturbations in bone and mineral metabolism. Over time, these metabolic conditions may account for the progressive loss of BMD (13) and the hypercalciuria (14, 28) that are well-known features of the ketogenic diet. We propose that even a slight decrease in the ability of the kidney to excrete the excess extracellular calcium may precipitate the development of hypercalcemia because, contrary to expectations, renal excretion of calcium was not elevated in the patients we describe here.
Management of hypercalcemia in these children provides another challenge because the natural history is not known. It is often not an option to stop the ketogenic diet because this may be the only effective therapy for these patients' refractory epilepsy. In one of these patients, hypercalcemia resolved and there was no requirement for long-term treatment. As a result, the two other patients are currently being treated with calcitonin, in the expectation that this is a transient phenomenon. Bisphosphonates may have a role and can be considered if hypercalcemia persists or low bone mass becomes problematic.
Our study has several limitations. First, this analysis is retrospective, and we are unable to obtain more information regarding overall bone metabolism in these patients. Second, we are unable to describe the natural history of this phenomenon. Nevertheless, our observations suggest that chronic ketosis must be considered in the differential diagnosis of unexplained hypercalcemia.
This appears to be either an underappreciated or uncommon association, given that we have only seen three cases at our institution, where over 150 children are using this therapy. Future studies will be required to determine the true prevalence of hypercalcemia in this population. Monitoring of bone mineral metabolism is recommended by international consensus guidelines, where checking calcium at baseline and follow-up visits is advised (29). Hypercalcemia has not previously been described as a direct risk associated with the ketogenic diet, but we recommend that providers caring for children on this therapy be aware of this potential association.
Acknowledgments
C.P.H. is supported by a PhD grant from the National Children's Research Centre, Dublin, Ireland. This work was supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK079970) and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- ADA
- adenosine deaminase
- BMD
- bone mineral density
- BMI
- body mass index
- 1,25(OH)2D
- 1,25 dihydroxyvitamin D
- 25(OH)D
- 25-hydroxyvitamin D
- ref
- reference
- SUN
- serum urea nitrate.
References
- 1. Vestergaard P, Tigaran S, Rejnmark L, Tigaran C, Dam M, Mosekilde L. Fracture risk is increased in epilepsy. Acta Neurol Scand. 1999;99:269–275 [DOI] [PubMed] [Google Scholar]
- 2. Khanna S, Pillai KK, Vohora D. Insights into liaison between antiepileptic drugs and bone. Drug Discov Today. 2009;14:428–435 [DOI] [PubMed] [Google Scholar]
- 3. Levine MA. Assessing bone health in children and adolescents. Indian J Endocrinol Metab. 2012;16:S205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guelpa GM. La lutte contre l'épilepsie par la désintoxication et par la rééducation alimentaire. Revue de Therapie Medico-Chirurgicale. 1911;78:8–13 [Google Scholar]
- 5. Politi K, Shemer-Meiri L, Shuper A, Aharoni S. The ketogenic diet 2011: how it works. Epilepsy Res Treat. 2011;2011:963637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48:43–58 [DOI] [PubMed] [Google Scholar]
- 7. Wang HS, Lin KL. Ketogenic diet: an early option for epilepsy treatment, instead of a last choice only. Biomed J. 2013;36:16–17 [DOI] [PubMed] [Google Scholar]
- 8. Kossoff EH, Rho JM. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics. 2009;6:406–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang HC, Chung DE, Kim DW, Kim HD. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004;45:1116–1123 [DOI] [PubMed] [Google Scholar]
- 10. Keene DL. A systematic review of the use of the ketogenic diet in childhood epilepsy. Pediatr Neurol. 2006;35:1–5 [DOI] [PubMed] [Google Scholar]
- 11. McNally MA, Pyzik PL, Rubenstein JE, Hamdy RF, Kossoff EH. Empiric use of potassium citrate reduces kidney-stone incidence with the ketogenic diet. Pediatrics. 2009;124:e300–e304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hahn TJ, Halstead LR, DeVivo DC. Disordered mineral metabolism produced by ketogenic diet therapy. Calcif Tissue Int. 1979;28:17–22 [DOI] [PubMed] [Google Scholar]
- 13. Bergqvist AG, Schall JI, Stallings VA, Zemel BS. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr. 2008;88:1678–1684 [DOI] [PubMed] [Google Scholar]
- 14. Furth SL, Casey JC, Pyzik PL, et al. Risk factors for urolithiasis in children on the ketogenic diet. Pediatr Nephrol. 2000;15:125–128 [DOI] [PubMed] [Google Scholar]
- 15. Matos V, van Melle G, Boulat O, Markert M, Bachmann C, Guignard JP. Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr. 1997;131:252–257 [DOI] [PubMed] [Google Scholar]
- 16. Danial NN, Hartman AL, Stafstrom CE, Thio LL. How does the ketogenic diet work? Four potential mechanisms. J Child Neurol. 2013;28:1027–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greene RW. Adenosine: front and center in linking nutrition and metabolism to neuronal activity. J Clin Invest. 2011;121:2548–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masino SA, Li T, Theofilas P, et al. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J Clin Invest. 2011;121:2679–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ham J, Evans BA. An emerging role for adenosine and its receptors in bone homeostasis. Front Endocrinol (Lausanne). 2012;3:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mediero A, Cronstein BN. Adenosine and bone metabolism. Trends Endocrinol Metab. 2013;24:290–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mediero A, Kara FM, Wilder T, Cronstein BN. Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am J Pathol. 2012;180:775–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gharibi B, Abraham AA, Ham J, Evans BA. Contrasting effects of A1 and A2b adenosine receptors on adipogenesis. Int J Obes (Lond). 2012;36:397–406 [DOI] [PubMed] [Google Scholar]
- 23. Hong R. Associations of the skeletal and immune systems. Am J Med Genet. 1989;34:55–59 [DOI] [PubMed] [Google Scholar]
- 24. Sauer AV, Mrak E, Hernandez RJ, et al. ADA-deficient SCID is associated with a specific microenvironment and bone phenotype characterized by RANKL/OPG imbalance and osteoblast insufficiency. Blood. 2009;114:3216–3226 [DOI] [PubMed] [Google Scholar]
- 25. Brandao-Burch A, Utting JC, Orriss IR, Arnett TR. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int. 2005;77:167–174 [DOI] [PubMed] [Google Scholar]
- 26. Kato K, Matsushita M. Proton concentrations can be a major contributor to the modification of osteoclast and osteoblast differentiation, working independently of extracellular bicarbonate ions. J Bone Miner Metab. 2014;32:17–28 [DOI] [PubMed] [Google Scholar]
- 27. Pereverzev A, Komarova SV, Korcok J, et al. Extracellular acidification enhances osteoclast survival through an NFAT-independent, protein kinase C-dependent pathway. Bone. 2008;42:150–161 [DOI] [PubMed] [Google Scholar]
- 28. Sampath A, Kossoff EH, Furth SL, Pyzik PL, Vining EP. Kidney stones and the ketogenic diet: risk factors and prevention. J Child Neurol. 2007;22:375–378 [DOI] [PubMed] [Google Scholar]
- 29. Kossoff EH, Zupec-Kania BA, Amark PE, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50:304–317 [DOI] [PubMed] [Google Scholar]