Abstract
Context:
Insulinomas are the most common cause of endogenous hyperinsulinemic hypoglycemia in nondiabetic adult patients. They are usually benign, and curative surgery is the “gold standard” treatment if they can be localized. Malignant insulinomas are seen in less than 10% of patients, and their prognosis is poor. The glucagon like peptide-1 receptor (GLP-1R) is markedly up-regulated in insulinomas—especially benign lesions, which are difficult to localize with current imaging techniques.
Objective:
The aim of the study was to assess the possibility of the detection of primary and metastatic insulinoma by positron emission tomography (PET) using [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 ([68Ga]Exendin-4) in a patient with severe hypoglycemia.
Design and Setting:
Dynamic and static PET/computed tomography (CT) examination of a patient was performed using [68Ga]Exendin-4 at Uppsala University Hospital, Uppsala, Sweden.
Patients:
A patient presented with hypoglycemia requiring continuous iv glucose infusions. A pancreatic insulinoma was suspected, and an exploratory laparotomy was urgently performed. At surgery, a tumor in the pancreatic tail with an adjacent metastasis was found, and a distal pancreatic resection (plus splenectomy) and removal of lymph node were performed. Histopathology showed a World Health Organization classification grade II insulinoma. Postoperatively, hypoglycemia persisted, but a PET/CT examination using the neuroendocrine marker [11C]-5-hydroxy-L-tryptophan was negative.
Interventions:
The patient was administered [68Ga]Exendin-4 and was examined by dynamic PET over the liver and pancreas.
Results:
The stable GLP-1 analog Exendin-4 was labeled with 68Ga for PET imaging of GLP-1R-expressing tumors. The patient was examined by [68Ga]Exendin-4-PET/CT, which confirmed several small GLP-1R-positive lesions in the liver and a lymph node that could not be conclusively identified by other imaging techniques. The results obtained from the [68Ga]Exendin-4-PET/CT examination provided the basis for continued systemic treatment.
Conclusion:
The results of the [68Ga]Exendin-4-PET/CT examination governed the treatment strategy of this particular patient and demonstrated the potential of this technique for future management of patients with this rare but potentially fatal disease.
Insulinomas, with an incidence of 1–3 per million per year, are the most common cause of endogenous hyperinsulinemic hypoglycemia in nondiabetic adult patients. More than 90% are benign, curative surgery is the “gold standard” of treatment, and accurate localization of the tumors is of the utmost importance. This report presents the impact of diagnostic imaging using positron emission tomography (PET) targeting glucagon like peptide-1 receptor (GLP-1R) in an insulinoma patient with postoperative hypoglycemia.
Case Report
A female presented with an attack of unconsciousness due to hypoglycemia (plasma glucose [p-glucose], 2.2 mmol/L) requiring continuous iv glucose infusions (10–30%) in July 2012. During the previous year, the patient had changed eating habits and gained 40 kg in weight. The patient was examined for suspected primary pancreatic insulinoma with a fast that had to be stopped after 4 hours (insulin, 88 mE/L [normal range, <11]; C-peptide, 1.76 nmol/L [normal range, <1.5]; and p-glucose, 1.8 mmol/L [normal range, >4]), where mE/L is mEquivalents/L. Preoperative imaging with contrast-enhanced computed tomography (CT) and ultrasound (US) was negative, but endoscopic ultrasonography indicated a small lesion in the body of the pancreas. Because of clinical urgency, an exploratory laparotomy with intraoperative ultrasonography was performed in August 2012, and a 2-cm tumor in the tail of the pancreas and an adjacent lymph node metastasis were found. A distal pancreatic resection plus splenectomy was performed. Histopathology verified a locally invasive insulinoma (World Health Organization classification grade II), positive for cytokeratin, chromogranin, synaptophysin, and insulin, with some cells positive for calcitonin, and one lymph node metastasis with a cell proliferation rate (Ki-67) of 5–10%. No positive immunostainings for glucagon, pancreatic polypeptide, somatostatin, or gastrin were detected. Multiple endocrine neoplasia type 1 (MEN 1) was excluded by normal serum calcium, PTH, and prolactin.
Severe hypoglycemia persisted postoperatively, indicating remaining lesions. Ultrasonography indicated two small lesions (<1 cm) in the liver, but biopsies showed normal liver parenchyma. [11C]5-hydroxy-L-tryptophan ([11C]5-HTP)-PET/CT (1), which according to our experience is more sensitive than conventional imaging and somatostatin receptor imaging with [111In]-DTPA-octreotide (Octreoscan) in localizing insulinomas (2), was negative in both the pancreas and the liver. Based on our clinical experience of prompt improvement of hypoglycemia in insulinoma patients, treatment with everolimus at 10 mg/d (3) was initiated in September 2012, which reduced iv glucose requirements from 6 L/d to 4 L/d after 1 week. Streptozocin and 5-fluorouracil (4), first-line treatment in metastatic insulinomas, was subsequently started. Three weeks later, the patient was normoglycemic, and hormone levels had decreased. Systemic treatment had to be withdrawn in October 2012, due to an infection with septicemia and elevated creatinine at 280 μmol/L (normal range, <90). [18F]Fluorodeoxyglucose ([18F]FDG)-PET/CT (without contrast due to elevated creatinine) showed uptake in the operated area, confirming a postoperative infection. Two small lesions in the liver were seen with unspecific uptake of [18F]FDG. During the following weeks, hypoglycemia recurred (p-glucose, 2.8 mmol/L), hormone levels increased (insulin, 187 mE/L; C-peptide, 2.8 nmol/L; proinsulin, 918 pmol/L), and iv glucose infusions had to be reinitiated; renal function slowly improved but was still impaired. Because of the patient's serious general condition, an intra-arterial calcium stimulation test with venous sampling was considered to be contraindicated.
Because we had not been able to clearly visualize metastatic lesions, at this time surgery, radiofrequency ablation, and liver embolization were not an option. Somatostatin analogs were considered, but in our experience could worsen hypoglycemia. Diazoxide was contraindicated because of elevated creatinine and risk for fluid retention, in particular because the patient weighed 120 kg. Peptide receptor radionuclide therapy using [177Lu]-(DOTA0-D-Phe1-Tyr3)octreotate (DOTATATE) was not a therapeutic option due to the elevated creatinine. Pretherapeutic somatostatin receptor expression confirmation using either [111In]-DTPA-octreotide (Octreoscan)/single photon emission CT (SPECT) or [68Ga]-(DOTA0-D-Phe1-Tyr3)octreotide (DOTATOC)-PET/CTwas not considered. Additionally, the lesions were assumed to be too small in size to be detected by Octreoscan/[68Ga]-DOTATOC because they were not detected by [11C]5-HTP-PET/CT.
In a further attempt to localize the insulinoma lesions, the patient was examined with [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 ([68Ga]Exendin-4)-PET/CT targeting GLP-1R. Previously, Exendin-4 has been proposed as an imaging biomarker for native β-cells in the context of quantifying changes in β-cell mass in response to disease progression for both type 1 and type 2 diabetes, as well as for evaluation of cellular replacement therapy (5, 6). GLP-1R is overexpressed by up to five times in insulinoma compared to normal human β-cells. Even so, the presence of a background of receptor-specific uptake in the pancreas may potentially cause difficulties in the diagnosis of pancreatic insulinoma. For example, focal concentrations of normal pancreatic cells with high GLP-1R expression may present as a false-positive insulinoma. This is also characteristic for [11C]5-HTP and [18F]-L-dihydroxyphenylalanine ([18F]L-DOPA) because the decarboxylation uptake mechanism is present and highly utilized also in normal islet cells (7).
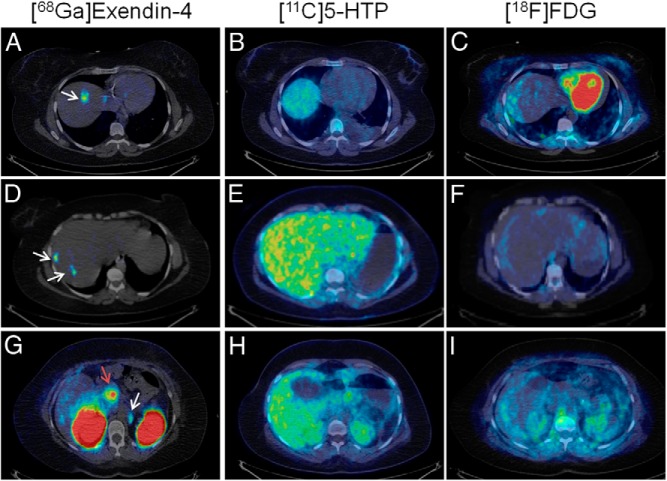
Whole-body [68Ga]Exendin-4-PET/CT examinations showed accumulation in multiple small nodes, presumably liver metastases, in both liver lobes (the largest, 1 cm) and a para-aortal lymph node (Figure 1, A, D, and G). Neither of the lesions had been conclusively detected by morphological imaging with CT and US or molecular imaging with [11C]5-HTP-PET/CT (Figure 1, B, E, and H) or [18F]FDG-PET/CT (Figure 1, C, F, and I). Native pancreas, containing a large number of cells positive for GLP-1R, exhibited marked uptake of [68Ga]Exendin-4 (Figure 1G). Maximum intensity projections of the abdomen showed the GLP-1R-positive lesions in liver and lymph nodes as well as native pancreas (Figure 2, A–C).
Figure 1.
[68Ga]Exendin-4 PET (left panels) confirmed several GLP-1R-positive lesions (white arrows) in the liver (A, D) and a para-aortal lymph node (G). No pancreatic or hepatic lesions could be conclusively detected by PET/CT using established tumor markers [11C]5-HTP (middle panels) and [18F]FDG (right panels). Each row shows the same transaxial abdominal sections for [68Ga]Exendin-4, [11C]5-HTP, and [18F]FDG, clearly demonstrating focal GLP-1R-positive lesions that could not be localized by established PET techniques. β-Cells in normal pancreas (red arrow) have significant expression of GLP-1R and can also be visualized by this technique (G). All images are normalized to a standardized uptake value of 1 with no background subtracted.
Figure 2.
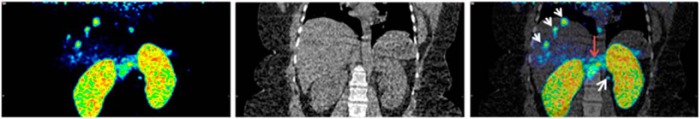
Three-dimensional maximum intensity projections of [68Ga]Exendin-4 PET (A), CT (B), and PET/CT fusion (C), which show the full tumor burden in liver and lymph nodes (white arrows) in addition to normal β-cells in pancreas (red arrow).
Because this was the first examination with this PET tracer, the uptake in the native pancreas is not known. However, because MEN 1 had been excluded in this patient, the high uptake in the pancreas most likely represents the presence of a large number of GLP-1R-expressing normal cells.
The results obtained from the [68Ga]Exendin-4-PET/CT examination provided the basis for continued systemic treatment. Because the patient had initially responded to everolimus and chemotherapy with normalization of glucose levels and reduction in hormone levels, both therapies were reinitiated when creatinine normalized in late November 2012. After two courses of chemotherapy, the patient was normoglycemic again with p-glucose levels at 6.9 mmol/L, insulin of 36 mE/L, C-peptide of 1.39 nmol/L, and proinsulin of 104 pmol/L. Unfortunately, in April 2013 she had to stop everolimus because of pneumonitis, a known side effect of this drug. Chemotherapy alone did not control hyperinsulinemia, and a CT with contrast of the abdomen in May 2013 showed progression in both liver lobes, with some lesions now measuring 1.5 cm. In June 2013, a bland embolization with embospheres of the right hepatic artery supplying most of the liver metastases was performed. A few weeks later, everolimus was reinitiated at a lower dose (10 mg three times a week and 5 mg four times a week); the patient then managed without glucose infusions, and a CT of the abdomen in August 2013 showed regression of some of the metastases. Recently, pneumonitis recurred, and everolimus was again withdrawn. Unfortunately, hormone levels are increasing, and a pretherapeutic diagnosis with Octreoscan or [68Ga]-DOTATATE for the determination of somatostatin receptor expression is scheduled. If positive, peptide receptor radionuclide therapy with [177Lu]-DOTATATE may be considered.
Methods
Radiochemistry
Good manufacturing practice-compliant production and quality control of the tracer were accomplished within 1 hour using the generator eluate fractionation method (8) with purification of the final product (9). 68Ga (T[1/2] = 68 min) was obtained from a 68Ge/68Ga generator system (1850 MBq; Eckert & Ziegler, Eurotope GmbH). The first fraction of 1.5 mL was discarded, and the next 1.0 mL containing over 70% of the total radioactivity was collected and buffered with 200 μL acetate buffer and 10 μL sodium hydroxide to provide a pH of 4.6 ± 0.4. To suppress radiolysis, 100 μL of ethanol was added. Then the mixture was transferred to a glass vial containing 10.5 nmol of good manufacturing practice grade DO3A-VS-Cys40-Exendin-4 (C S Bio Company, Inc) and incubated at 75°C for 15 minutes. The product was purified using C-8 (Sep-Pak Light C8 cartridge; Waters) reversed solid-phase extraction cartridge, eluted in 1.0 mL of 50% ethanol, formulated in sterile phosphate buffer (pH 7.4), and passed through a 0.22-μm filter into a sterile injection vial. A sample was taken for the determination of identity, radiochemical and chemical purity, pH, estimation of the peptide content, as well as control of sterility and endotoxins.
PET/CT examination protocol
The subject was positioned to include the liver in the center of the 15-cm axial field of view of a Discovery ST PET/CT scanner (GE Healthcare) by assistance of a low-dose CT scout view (140 kV, 10 mA). Attenuation correction was acquired by CT (140 kV, 10–80 mA) immediately before the PET examination. Diagnostic CT with contrast was not performed because of elevated creatinine.
The patient was then administered iv [68Ga]Exendin-4 (0.88 MBq/kg; 0.17 μg/kg; specific radioactivity, 56 MBq/nmol) and examined by dynamic PET over the liver and pancreas for 45 minutes. This was followed by a whole-body CT (for attenuation correction) and multibed whole-body PET examinations (4-min acquisition per bed position) 1.7 and 2 hours after tracer administration. Image acquisition was performed in three-dimensional mode and reconstructed using an iterative OSEM VUE Point algorithm (two iterations/21 subsets, in a 128 × 128 matrix). Reconstructed data were analyzed using Xeleris (GE Healthcare).
Diagnosis of Insulinoma: A Review of the Literature
Insulinomas are most often localized to the pancreas, but a small percentage (<2%) are extrapancreatic. Approximately 85% are solitary, 6–13% are multiple, and 4–6% are associated with MEN 1. Malignant insulinomas constitute < 10% of all insulinomas, and the prognosis of survival for patients with liver metastases has been estimated to be less than 2 years (10).
The biochemical diagnosis of insulinomas is established by measuring p-glucose, serum insulin, C-peptide, and proinsulin during a 12- to 72-hour fast and showing inappropriately high insulin/proinsulin levels for a glucose level below 2.5 mmol/L. Eighty percent of patients with insulinomas will present hypoglycemic symptoms and positive fast after 24 hours, 90% after 48 hours, and 100% after 72 hours (11). The possibility of the insulinoma being a part of MEN 1 should also be excluded, especially in young patients, in the presence of a family history, or if there are multiple tumors in the pancreas. If so, biochemical studies including serum PTH, ionized calcium, and prolactin should be performed.
Once the biochemical diagnosis of an insulinoma has been established, localization procedures should be undertaken. Preoperative imaging is critical to optimize surgical intervention. Due to the small size of the tumors (82% < 2 cm, 47% < 1 cm), they are often difficult to detect. US, CT, and magnetic resonance imaging are widely available but are only conclusive in < 50% of cases. Endoscopic US scans are positive in 70–95% of all cases. However, the technique often fails to detect tumors in the tail of the pancreas. Because insulinomas are highly vascularized, selective angiography can detect the lesion in about 60% of subjects, and if combined with venous sampling for insulin after intra-arterial calcium stimulation administration, the accuracy increases to about 60–80%. The procedure is, however, invasive and is accompanied by risks for complications (12).
Functional imaging with somatostatin receptor scintigraphy (SRS) and SPECT/CT is positive in < 50% of benign insulinomas because of low or absent expression of somatostatin receptor subtypes 2 and 5 that bind octreotide with high affinity. Malignant insulinomas, in contrast, may express these receptors and consequently show high uptake at SRS (predicting benefit from treatment with unlabeled or radiolabeled somatostatin analogs). Recently, several studies have demonstrated that PET with 68Ga-labeled somatostatin analogs, when combined with CT, has a higher sensitivity for smaller lesions than SRS or other modalities (13). Other PET tracers used for imaging of NET, available in some centers, are [11C]5-HTP and [18F]L-DOPA based on the amine-precursor uptake mechanisms of the tumors. PET using [11C]5-HTP has been shown to be a highly sensitive modality for neuroendocrine tumor (NET) imaging that surpasses that of SRS and CT. A comparison of [11C]5-HTP and [18F]L-DOPA showed that the former more accurately visualized pancreatic NET (14). Still, in a recent study, where [11C]5-HTP-PET imaging was correlated to surgical findings, it failed to detect two of six insulinomas, although it had higher sensitivity than other methods (2).
GLP-1R is expressed in human β-cells and highly overexpressed in insulinomas (15). The endogenous peptide GLP-1 has a very short biological half-life, and thus the metabolically more stable synthetic peptide analog, Exendin-4, has been developed and conjugated with a variety of chelator moieties for subsequent radiolabeling with 111In, 99Tc, or 68Ga. The resulting tracers have been evaluated preclinically for the targeting of insulinoma (16–18). Recently, [18F]-labeled Exendin-4 probes have also been developed (19, 20). [Lys40(Ahx-DTPA-111In)NH2]Exendin-4 has been used clinically for the detection of occult insulinoma by SPECT/CT (21) and also for preoperative localization of insulinoma (22). GLP-1R has been shown to have high sensitivity for localization of especially benign insulinoma (21). Recently, a prospective, open-label, multicenter phase II trial evaluated the insulinoma detection rate of [Lys40(Ahx-DTPA-111In)NH2]Exendin-4 SPECT/CT compared to CT or magnetic resonance imaging. In 25 patients who underwent surgery with histopathological verification, the sensitivities for the two methods were 97 and 47%, respectively (23). Malignant insulinomas have been described to have a lower density of GLP-1Rs (15, 24). Positron-emitting 68Ga (T[1/2] = 68 min; 89% β+) in combination with PET/CT potentially provides advantages to SPECT, such as improved sensitivity, higher resolution, faster acquisition time, and accurate quantification. This may enable detection of smaller lesions resulting in accurate staging and improved patient management. Moreover, the availability of 68Ga from generator systems and the possibility for labeling chemistry under radiopharmacy practice make [68Ga]Exendin-4 affordable at any clinical center.
Conclusion
This is the first clinical experience using [68Ga]Exendin-4-PET/CT for visualization of GLP-1R in insulinoma and in native pancreas. Unfortunately, we were not able to verify the lesions in the liver histopathologically by biopsies because they were too small (<1 cm), and representative tumor tissue could not be obtained. Even so, the results of the examination confirmed the presence of suspected liver and lymph node metastases, which could not be conclusively detected by other state-of-the-art imaging techniques. The presented results demonstrate the potential for this technique to become an important diagnostic imaging choice for the detection and staging of insulinomas.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council (65X-12219-15-6), the VINNOVA Foundation (2007-00069), the JDRF, ExoDiab, Barndiabetesfonden, and Tore Nilssons Foundation for Medical Research. O.K.'s position is supported by the National Institutes of Health (2U01AI065192-06). O.E.'s position is supported by ExoDiab.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Disclosure Summary: The authors declare that they have no conflicts of interests.
Footnotes
- CT
- computed tomography
- [18F]L-DOPA
- [18F]-L-dihydroxyphenylalanine
- DOTATATE
- (DOTA0-D-Phe1-Tyr3)octreotate
- DOTATOC
- (DOTA0-D-Phe1-Tyr3)octreotide
- [68Ga]Exendin-4
- [68Ga]Ga-DO3A-VS-Cys40-Exendin-4
- [18F]FDG
- [18F]fluorodeoxyglucose
- GLP-1R
- glucagon like peptide-1 receptor
- [11C]5-HTP
- [11C]5-hydroxy-L-tryptophan
- MEN 1
- multiple endocrine neoplasia type 1
- NET
- neuroendocrine tumor
- PET
- positron emission tomography
- p-glucose
- plasma glucose
- SPECT
- single photon emission CT
- SRS
- somatostatin receptor scintigraphy
- US
- ultrasound.
References
- 1. Orlefors H, Sundin A, Garske U, et al. Whole-body (11)C-5-hydroxytryptophan positron emission tomography as a universal imaging technique for neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and computed tomography. J Clin Endocrinol Metab. 2005;90:3392–3400 [DOI] [PubMed] [Google Scholar]
- 2. Orlefors H, Sundin A, Eriksson B, et al. PET-guided surgery—high correlation between positron emission tomography with 11C-5-hydroxytryptophane (5-HTP) and surgical findings in abdominal neuroendocrine tumours. Cancers. 2012;4:100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frilling A, Akerström G, Falconi M, et al. Neuroendocrine tumor disease: an evolving landscape. Endocr Relat Cancer. 2012;19:R163–R185 [DOI] [PubMed] [Google Scholar]
- 5. Selvaraju RK, Velikyan I, Johansson L, et al. In vivo imaging of the glucagonlike peptide 1 receptor in the pancreas with 68Ga-labeled DO3A-exendin-4. J Nucl Med. 2013;54:1458–1463 [DOI] [PubMed] [Google Scholar]
- 6. Brom M, Woliner-Van der Weg W, Joosten L, et al. Non-invasive quantification of the beta cell mass by SPECT with 111In-labelled exendin [published online February 1, 2014]. Diabetologia. doi:10.1007/s00125-014-3166-3 [DOI] [PubMed] [Google Scholar]
- 7. Eriksson O, Selvaraju RK, Johansson L, et al. Quantitative imaging of serotonergic biosynthesis and degradation in the endocrine pancreas [published online February 13, 2014]. J Nucl Med. doi:10.2967/jnumed.113.125187 [DOI] [PubMed] [Google Scholar]
- 8. Velikyan I, Beyer GJ, Långström B. Microwave-supported preparation of 68Ga bioconjugates with high specific radioactivity. Bioconjug Chem. 2004;15:554–560 [DOI] [PubMed] [Google Scholar]
- 9. Velikyan I, Xu H, Nair M, Hall H. Robust labeling and comparative preclinical characterization of DOTA-TOC and DOTA-TATE. Nucl Med Biol. 2012;39:628–639 [DOI] [PubMed] [Google Scholar]
- 10. Vanderveen K, Grant C. Insulinoma. Cancer Treat Res. 2010;153:235–252 [DOI] [PubMed] [Google Scholar]
- 11. Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19:783–798 [DOI] [PubMed] [Google Scholar]
- 12. Jensen RT, Cadiot G, Brandi ML, et al. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maecke HR, Hofmann M, Haberkorn U. (68)Ga-labeled peptides in tumor imaging. J Nucl Med 2005;46:172S–178S [PubMed] [Google Scholar]
- 14. Koopmans KP, Neels OC, Kema IP, et al. Improved staging of patients with carcinoid and islet cell tumors with 18F-dihydroxy-phenyl-alanine and 11C-5-hydroxy-tryptophan positron emission tomography. J Clin Oncol. 2008;26:1489–1495 [DOI] [PubMed] [Google Scholar]
- 15. Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007;48:736–743 [DOI] [PubMed] [Google Scholar]
- 16. Brom M, Oyen WJ, Joosten L, Gotthardt M, Boerman OC. 68Ga-labelled exendin-3, a new agent for the detection of insulinomas with PET. Eur J Nucl Med Mol Imaging. 2010;37:1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wild D, Béhé M, Wicki A, et al. [Lys40(Ahx-DTPA-111In)NH2]exendin-4, a very promising ligand for glucagon-like peptide-1 (GLP-1) receptor targeting. J Nucl Med. 2006;47:2025–2033 [PubMed] [Google Scholar]
- 18. Wild D, Wicki A, Mansi R, et al. Exendin-4-based radiopharmaceuticals for glucagonlike peptide-1 receptor PET/CT and SPECT/CT. J Nucl Med. 2010;51:1059–1067 [DOI] [PubMed] [Google Scholar]
- 19. Kiesewetter DO, Gao H, Ma Y, et al. 18F-radiolabeled analogs of exendin-4 for PET imaging of GLP-1 in insulinoma. Eur J Nucl Med Mol Imaging. 2012;39:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu H, Liang S, Liu S, Pan Y, Cheng D, Zhang Y. 18F-radiolabeled GLP-1 analog exendin-4 for PET/CT imaging of insulinoma in small animals. Nucl Med Commun. 2013;34:701–708 [DOI] [PubMed] [Google Scholar]
- 21. Wild D, Mäcke H, Christ E, Gloor B, Reubi JC. Glucagon-like peptide 1-receptor scans to localize occult insulinomas. N Engl J Med. 2008;359:766–768 [DOI] [PubMed] [Google Scholar]
- 22. Christ E, Wild D, Forrer F, et al. Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J Clin Endocrinol Metab. 2009;94:4398–4405 [DOI] [PubMed] [Google Scholar]
- 23. Christ E, Wild D, Ederer S, et al. Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: a prospective multicentre imaging study. Lancet Diabetes Endocrinol. 2013;1:115–122 [DOI] [PubMed] [Google Scholar]
- 24. Wild D, Christ E, Caplin ME, et al. Glucagon-like peptide-1 versus somatostatin receptor targeting reveals 2 distinct forms of malignant insulinomas. J Nucl Med. 2011;52:1073–1078 [DOI] [PubMed] [Google Scholar]