Abstract
Context:
Factors common to multiple autoimmune diseases have been sought vigorously. Graves' disease (GD) and type 1 diabetes mellitus (T1DM) involve end-organ remodeling. Fibrocytes participate in inflammatory diseases and were recently shown to express thyroid-specific proteins such as the thyrotropin receptor and thyroglobulin.
Objective:
The objective of the study was to determine whether a broader repertoire of autoantigen expression, such as proteins associated with T1DM, can be ascribed to fibrocytes.
Design, Setting, and Participants:
Fibrocytes and fibroblasts were collected and analyzed from healthy individuals and those with autoimmune diseases in an academic clinical practice.
Main Outcome Measures:
Real-time PCR, Western blot analysis, gene promoter analysis, cell transfections, and flow cytometric cell sorting were performed.
Results:
Islet cell antigen ICA512 (IA-2) and islet cell autoantigen of 69 kDa (ICA69), two islet-specific proteins implicated in T1DM, are expressed by fibrocytes from healthy donors and those with T1DM, GD, and multiple sclerosis. Both transcripts are detected by PCR, the proteins are resolved on Western blots, and both gene promoters are active in fibrocytes. Levels of ICA69 are substantially higher than those of IA-2 in fibrocytes. ICA69 localizes to CD34+ GD orbital fibroblasts putatively derived from fibrocytes, whereas higher levels of IA-2 are found in CD34− fibroblasts.
Conclusions:
In addition to autoantigens implicated in thyroid autoimmunity, fibrocytes and derivative fibroblasts express multiple autoantigens associated with T1DM. This expression results from active gene promoters and abundant steady-state mRNA encoding ICA69 and IA-2. These latest findings demonstrate that fibrocytes express antigens relevant to multiple forms of endocrine autoimmunity. They suggest the potential for these cells playing a direct role in immune reactivity directed at the thyroid and pancreatic islets.
Autoimmune diseases, such as those exhibiting end-organ manifestations, can cluster in families exhibiting multiple forms of these diseases (1). Furthermore, epiphenomenal antibodies associated with one disease can frequently be detected in individuals diagnosed with another (2). Those patients with multiple autoantibodies are at increased risk for developing a second disease (3). Multiple circulating autoantibodies directed at pancreatic islet cell proteins, including islet cell autoantigen of 69 kDa (ICA69), islet tyrosine phosphatase-like protein/islet cell antigen ICA512 (IA-2), glutamate decarboxylase 65, zinc transporter, ZnT8, and insulin are associated with type 1 diabetes mellitus (T1DM) (4–12). These proteins are autoimmune targets, the expression of which had been believed to be limited to pancreatic islet cells. Subsequently, many tissue-specific proteins have been detected in multiple peripheral organs. Initially, epithelial cells in the thymus were shown to express self-antigens, providing an attractive mechanism for establishing central immune tolerance to these proteins through a process known as thymic education (13). More recent studies have disclosed autoantigen expression in extrathymic cells residing in the spleen and lymph nodes (14). These ground-breaking discoveries have now prompted a wider scope of inquiry into the broader expression of proteins previously thought to be tightly restricted.
CD34+ fibrocytes are monocyte lineage progenitor cells that traffic to areas of tissue injury and participate in tissue remodeling, wound healing, and fibrosis (15). They support immune function by presenting antigens, providing second signals to lymphocytes, and producing a wide array of cytokines in response to inflammatory cues (16–18). In experimental animals, fibrocytes are recruited to areas of injury, such as in models of lung fibrosis in which they serve as precursor cells (19). At least a component of this trafficking is mediated through the chemokine ligand 12/CXCR4 chemokine pathway (20). Fibrocytes display a characteristic array of surface markers, including CD45, CD11b, CD34, CXCR4, and collagen1 (21). They exhibit substantial plasticity and as cultured cells can terminally differentiate into adipocytes and myofibroblasts, depending on the molecular cues they receive from their environment (22).
Very recently we reported that circulating fibrocytes cultivated from the peripheral circulation become overabundant in Graves' disease (GD), and infiltrate the orbit and thyroid (23, 24). Unexpectedly, fibrocytes from healthy donors and those with GD were found to express two functional thyroid-specific proteins, namely the thyrotropin receptor (TSHR) (23) and thyroglobulin (Tg) (25). This expression derives from moderately abundant steady-state levels of their respective transcripts. These two proteins continue to represent leading candidate autoantigens involved in thyroid autoimmunity, including GD and Hashimoto's thyroiditis. Thus, fibrocytes might participate directly in the immunological reactions associated with these diseases.
We now report that in addition to TSHR and Tg, CD34+ fibrocytes surprisingly express IA-2 and ICA69, two islet cell antigens associated with T1DM. Their respective transcripts are detectable in fibrocytes cultivated from peripheral blood of healthy donors and those with T1DM, multiple sclerosis (MS), and GD. Expression of these antigens is the consequence of activity in fibrocytes of the respective gene promoters. Our identification of promiscuous islet-specific protein expression in fibrocytes suggests that these cells may play a role in multiple autoimmune endocrine diseases.
Materials and Methods
Materials
DMEM containing 4.5g/L D-glucose and L-glutamine was purchased from Life Technology (catalog number 11965–092). Fetal bovine serum (FBS) was from Life Technology (catalog number 16000–044). The gene promoter construct for IA-2 was generously provided by Dr A. L. Notkins (National Institutes of Health, Bethesda, Maryland) (26). Wild-type and mutant ICA69 gene promoter constructs were cloned as reported previously (27).
Fibroblast, fibrocyte, and NMB7 cell cultivation
Fibrocytes from a total of 18 healthy donors, 10 with GD, eight with T1DM, one with GD and T1DM, and one with MS were used in these studies. Five individuals with GD donated orbital fibroblasts, whereas two healthy individuals provided dermal fibroblasts and two donated orbital fibroblasts. Surgical waste from which orbital fibroblasts were cultivated was obtained during orbital decompressions for severe thyroid-associated ophthalmopathy (TAO) or from healthy tissues removed during cosmetic surgery. These activities have been approved by the Institutional Review Board of the University of Michigan Health System. Fibroblasts were allowed to proliferate as described previously (28), and monolayers were covered with DMEM containing 10% FBS, 2 mM glutamine, sodium pyruvate (110 mg/mL), penicillin/streptomycin (100 U/mL), and 4.5% glucose. They were maintained in a 37°C, humidified, 5% CO2 environment. Culture strains were used between the fifth and 12th passages, an interval during which cell phenotypes remain constant (28, 29). The medium was changed every 4 days.
Human fibrocytes were isolated from peripheral blood by Ficoll density centrifugation as described (23). Typically, 107 peripheral blood mononuclear cells were inoculated into each well of a six-well array and incubated in DMEM supplemented with 10% FBS, penicillin/streptomycin, and glutamine for 10–14 days before experimental manipulations. Greater than 90% were found to display the CD45+CD34+TSHR+CXCR4+ phenotype by flow cytometric analysis (21). Established NMB7, a brain-derived cell line (27), was cultured to near confluence in the same medium.
RNA isolation and quantitative RT-PCR
Confluent cultures were shifted to medium containing 1% FBS for 16 hours before treatment with IL-1β (10 ng/mL) or the other test agents indicated. Cellular RNA was extracted using the Aurum total RNA minikit (Bio-Rad Laboratories; catalog number 732–6820) according to the manufacturer's protocol. cDNA was generated by reverse transcription using oligo(deoxythymidine) and SuperScript III reverse transcriptase (Invitrogen Inc; catalog number 205311). Real-time PCR was performed using iQ SYBR Green Supermix (Bio-Rad Laboratories; catalog number 170–8882) in a reaction mixture also containing iTaq DNA polymerase, and deoxynucleotide triphosphates on a CFX96 real-time PCR system (Bio-Rad Laboratories). Each sample was analyzed in triplicate with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) used as the reference control. Primer sequences were as follows: GAPDH forward, 5′-TTGCCATCAATGACCCCTTCA-3′, reverse, 5′-CGCCCCACTTGATTTTGGA-3′; IA-2, Hs00160947_m1; ICA69, Hs00245256_m1*; IL-8 forward, 5-CTTCCTGAT-TTCTGCAGCT-3′ and reverse, 5′-CCACTCTCAATCACTCTCAG-3′; IL-6 forward, 5′-TGAGAAAGGAGACATGTAACAAGAGT-3′, and reverse, 5-TTGTTCCTCACTACTCTCAAATCTGT-3′; TNF-α forward, 5′-GTCTCCTACCAGACCAAG-3′ and reverse, 5′-CAAAGTAGACCTGCCCAGACTC-3′. Reactions were performed at 95°C for 5 minutes, 40 cycles at 95°C for 10 seconds, and 60°C for 30 seconds.
Western blot analysis
Fibrocytes were sonicated, taken up in extraction buffer (Invitrogen), and pelleted at 4°C cells. Protein was quantified with the BCA protein assay (Pierce Protein Research/Thermo Fisher), separated by 10% SDS-PAGE, and transferred to nitrocellulose filters. These were then incubated with primary polyclonal rabbit antibodies against ICA69 (6) or anti-PTP/IA-2 (Santa Cruz Biotechnology; catalog number 54749) (both 1:100). After washes these filters were then incubated with antirabbit IgG-horseradish peroxidase (Amersham) or antigoat IgG-horseradish peroxidase (Santa Cruz Biotechnology) (both 1:5000 dilution), respectively. Bands were detected by chemiluminescence using SuperSignal West Pico chemiluminescent substrate (Pierce/Fisher).
Gene promoter activity assays
Constructs containing 1031- and 746-bp fragments of the ICA69 and IA-2 gene promoters, respectively, were transiently transfected into fibrocytes using Lipofectamine according to a protocol provided by the supplier (Invitrogen; catalog number 18324–012) and as published previously (30). Twenty hours after transfection, cells were harvested and subjected to the dual-luciferase reporter assay (Promega). All transfection assays were performed in triplicate and repeated at least three times. Lumicount data were normalized to their Renilla transfection controls. Fibrocyte transfections used Amaxa nucleofection Technology (Amaxa, Koeln) using program U23 as described (25). Cells were then recultured for 48 hours before triplicate samples were subjected to the luciferase assay. Results were normalized to cellular protein content.
Cell sorting
Cell sorting was performed as previously described (31). Briefly, mixed (parental) TAO orbital fibroblasts in cultures were stained with fluorescein isothiocyanate-conjugated antihuman CD34 for 30 minutes at 4°C. Washed cells were sorted under sterile conditions with a FACSAria III (BD Biosciences). Sorted cells were recultured for 48 hours before harvest and RNA extraction.
Statistics
Significance was determined with a two-tailed Student's t test. Data are presented as the mean ± SD of independent replicates unless otherwise specified. Statistical significance was placed at a confidence level of P < .05.
Results
IA-2 and ICA69 protein are expressed in fibrocytes
We first interrogated fibrocytes from healthy donors for ICA69 and IA-2 protein. As Figure 1 demonstrates, ICA69 resolves as a doublet at 58 kDa on Western blot analysis in fibrocytes (Figure 1A), whereas IA-2 resolves as a single band of 108 kDa (Figure 1B), in fibrocytes from two different healthy individuals. Migration of both proteins resembles that of their respective recombinant controls. Thus, fibrocytes express not only antigens associated with thyroid autoimmunity (24) but also two proteins usually associated with pancreatic islet cells and neuroepithelium that have been implicated in T1DM (4, 6, 32–34). In contrast, glutamate decarboxylase 65 and insulin were undetectable (data not shown).
Figure 1.
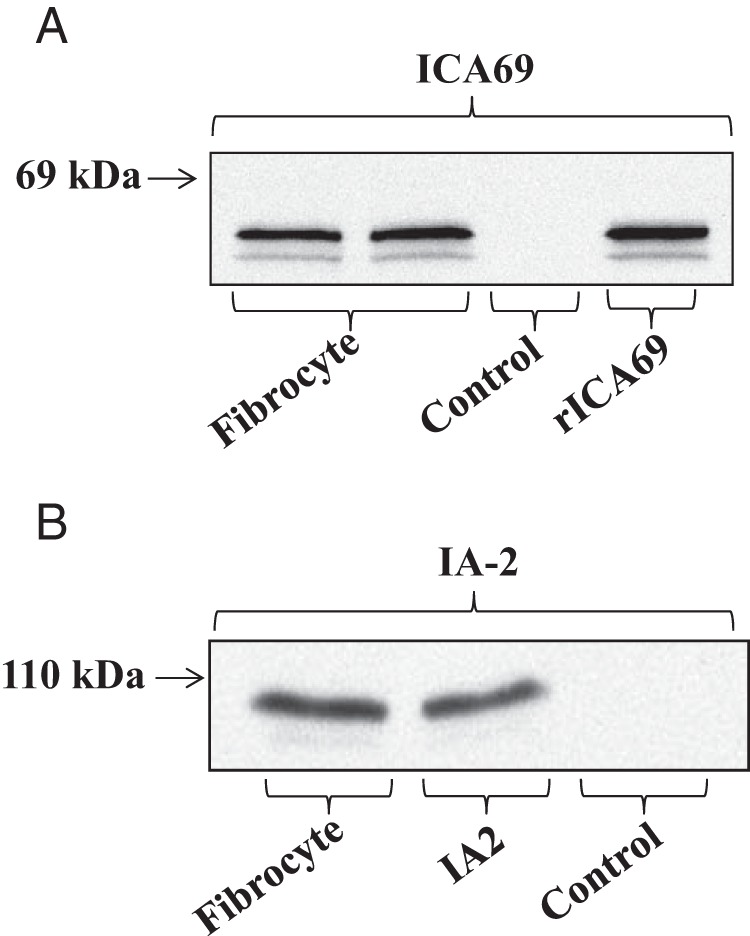
Fibrocytes express ICA69 and IA-2 protein. Cultured fibrocytes were lysed and proteins separated on 10% SDS-PAGE, transferred onto nitrocellulose filters, and detected using the primary and secondary antibodies described in Materials and Methods. Bands were detected by chemiluminescence with the SuperSignal West Pico chemiluminescent substrate (Pierce/Fisher). Mouse recombinant ICA69 and human full-length IA-2 were used as standards.
Fibrocytes express variable levels of IA-2 and ICA69 mRNAs
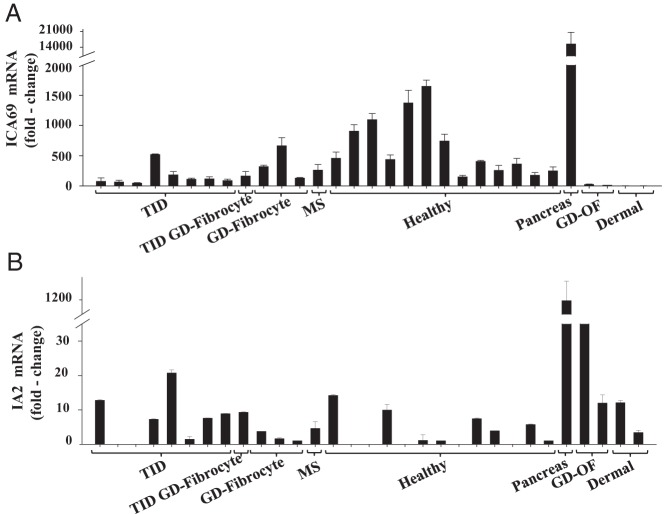
We next examined fibrocytes for their expression of the transcripts encoding these two pancreatic proteins by real-time PCR. Several strains of fibrocytes, each from a different donor, were examined for mRNAs encoding IA-2 and ICA69. As the data in Figure 2A indicate, ICA69 mRNA was detectable in all 26 fibrocyte strains tested but was undetectable in any of the fibroblasts, regardless of the health status of the donor or anatomic location from which they derived. Examination of fibrocytes from individuals with T1DM revealed that ICA69 mRNA was detected in all eight examples (Figure 2A). With regard to the expression of IA-2, levels of the transcript varied widely and the mRNA was detectable in six of the eight fibrocyte strains from individuals with T1DM (Figure 2B). Moreover, levels were considerably lower than those of ICA69. Surprisingly, it could be detected in two orbital fibroblast strains from donors with GD as well as dermal fibroblasts from two healthy donors. It should be noted that human pancreatic tissue expressed approximately 10-fold higher levels of these transcripts than did any of the isolated, cultured cells examined. Several strains of fibrocytes and fibroblasts were next analyzed for levels of Tg and TSHR mRNA. As Figure 3 demonstrates, all fibrocyte strains, regardless of the health status of the donor, exhibited at least a low-level expression of both transcripts. Thus, it would appear that fibrocytes express autoantigens associated with multiple endocrine diseases, regardless of the state of health of the donor. Aggregate data appear in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Figure 2.
ICA69 mRNA levels (A) and IA2 mRNA levels (B) in fibrocytes from individuals with type 1 diabetes (T1D Fibrocyte), with T1D and Graves' disease (T1D+GD), Graves' disease (GD Fibrocyte), MS (MS Fibrocyte), and healthy individuals (Healthy Fibrocyte). Also quantified were levels in pancreas, orbital fibroblasts from individuals with GD (GD-OF), and skin fibroblasts (Dermal). ICA69 mRNA and IA-2 mRNA were quantified by reversed transcribing RNA. cDNA was subjected to quantitative real time-PCR. Values were normalized to their respective GAPDH signals. Data are expressed as the mean ± SD of triplicate independent determinations.
Figure 3.
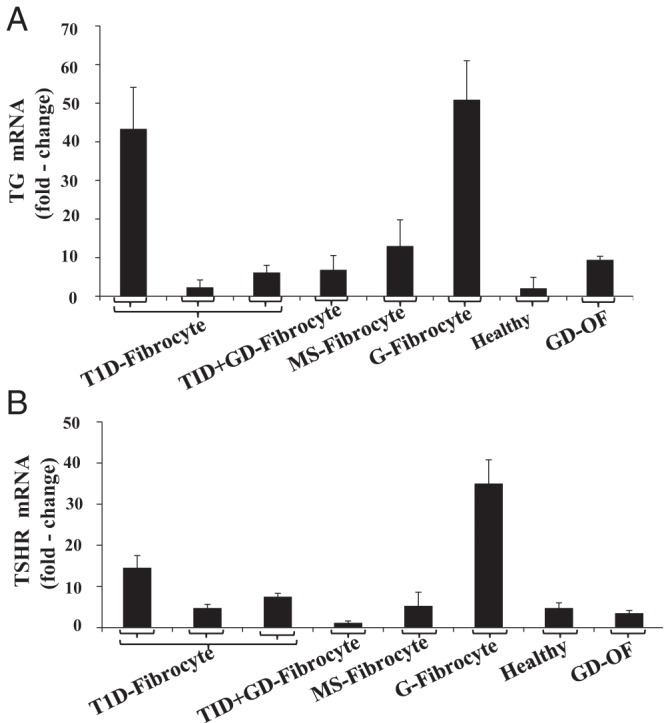
Tg mRNA (A) and TSHR mRNA (B) levels in fibrocytes and fibroblasts from healthy individuals and those with a variety of autoimmune diseases, as defined in the legend to Figure 2. The respective mRNAs were quantified by reversed transcribing RNA. cDNA was subjected to quantitative real-time-PCR. Values were normalized to their respective GAPDH signals. Data are expressed as the mean ± SD of triplicate independent determinations.
IA-2 and ICA69 gene promoters are active in fibrocytes
We next transfected fragments of the respective gene promoters that had been fused to luciferase reporters into fibrocytes and NMB7 cells. As the results in Figure 4 demonstrate, levels of the promoter for ICA69 is very active in NMB7 cells (Figure 4B) and are equivalent to those found in fibrocytes (Figure 4C) transfected in parallel. Introduction of a mutation using a site-directed technique within the cAMP response element-binding protein (CREB) site extending from −174 nt to −171 nt failed to alter the high activity levels seen with the wild-type fragment. In contrast, mutating the specificity protein-1 (Sp1)/glucocorticoid (GC) element from −196 nt to −193 nt substantially reduced activity in both NMB7 cells and fibrocytes when compared with the wild-type fragment. The activity is approximately 4-fold greater than that of the empty vector control. Thus, it would appear that although binding to the Sp1/GC site is critical to ICA69 expression in fibrocytes, the CREB site may be uninvolved in the promoter activity in these cells. IA-2 promoter activity can also be easily detected in fibrocytes as it is in NMB7 cells (Figure 4D).
Figure 4.
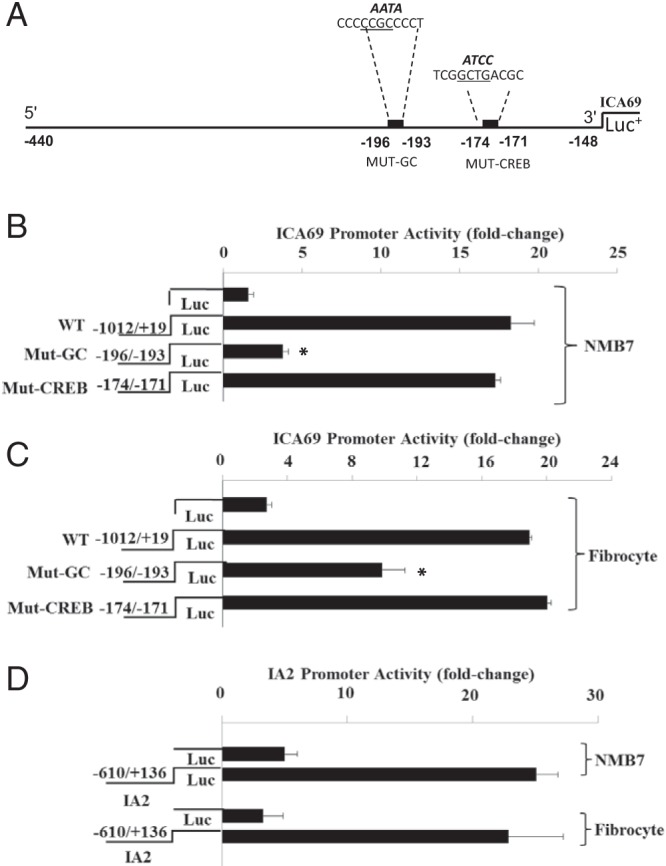
ICA69 and IA-2 gene promoter activities in NMB7 cells and fibrocytes. A, Sequence diagram of a fragment of human ICA69 gene promoter extending from −1012 to +19 nt. Mutations of the CREB (−174 to −171 nt) and Sp1/GC elements (−196 nt to −193 nt) are shown with the wt sequences below their emboldened, mutated counterparts. Activities of wild-type (WT) and mutated (Mut) ICA69 gene promoter/reporter constructs transfected into NMB7 cells (B) and fibrocytes (C) are shown. D, Activity of the IA-2 gene promoter/reporter construct transfected into NMB7 cells and fibrocytes. Fibrocyte cultures were transfected with plasmid containing empty reporter or one fused with the respective constructs, as described in Materials and Methods. Data are expressed as the mean ± SD. *, P < .05 vs wild type.
Divergence of ICA69 and IA-2 expression in subsets of TAO orbital fibroblasts
From the results thus far obtained, it appears that ICA69 expression is more frequently detected in fibrocytes, whereas IA-2 is detected more consistently in fibroblasts (Figures 2). TAO orbital fibroblasts comprise a mixture of CD34+ cells putatively deriving from fibrocytes and CD34− cells that represent native orbital fibroblasts (23, 25). Therefore, we interrogated these cells by subjecting them to sorting into pure subsets based on the display of CD34. As the flow plot contained in Figure 5A demonstrates, both CD34− and CD34+ fibroblasts are well represented in parental strains derived from a patient with severe TAO. Significantly higher levels of ICA69 expression localized to CD34+ fibroblasts (Figure 5B), whereas IA-2 localized to the CD34− subset (Figure 5C). Data from all experiments performed with sorted fibroblasts appear in Supplemental Table 2.
Figure 5.
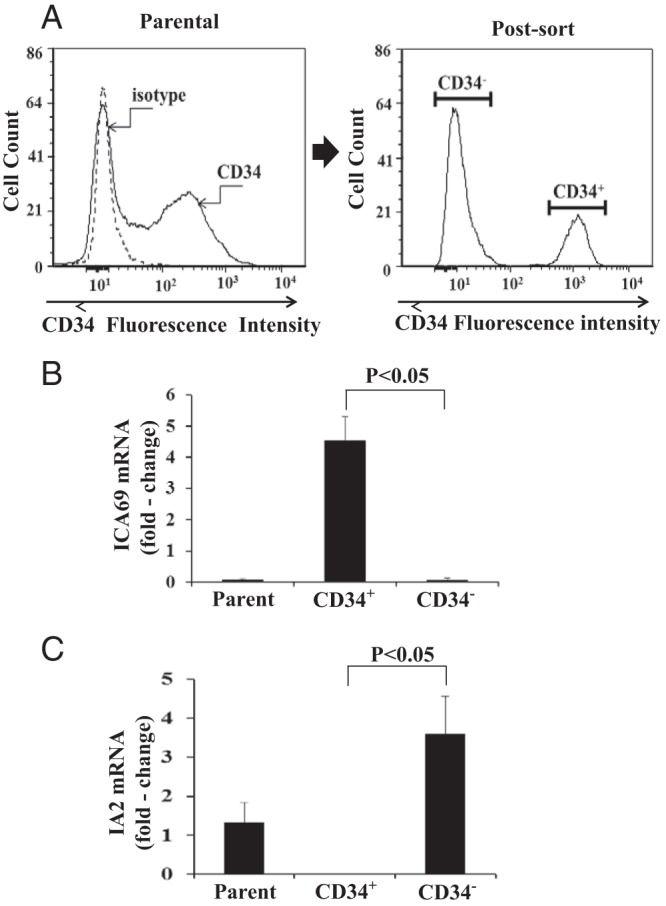
A, Left panel, Distribution of CD34+ and CD34− fibroblasts in a mixed (parental) orbital fibroblast culture from a patient with severe TAO, as determined by flow cytometry, demonstrates two distinct populations. Right panel, Cytometric sorting results in two pure populations. ICA69 (B) and IA-2 (C) mRNA levels in pure CD34+ and CD34− fibroblast subsets from the patient shown in panel A. Mixed (parental) strain of GD-OF containing CD34− and CD34+ cells was sorted into pure subsets by fluorescence-activated cell sorting. Cells were then cultured for 48 hours and RNA extracted and subjected to real-time PCR for the respective mRNA levels. Values were normalized to their respective GAPDH signals and expressed as the mean ± SD of three independent determinations. P < .05, CD34+ vs CD34− strain. Results shown are representative of four separate experiments.
Discussion
Antibodies directed at ICA69 and IA-2 are associated with T1DM (3–7, 32). The basis for implicating these antibodies in that disease derives from their frequent detection in newly diagnosed patients and their first-degree relatives (7, 35–37). In addition, autoreactive T cells to ICA69 can be detected in children with T1DM (38). From the current studies, ICA69 and IA-2 join TSHR and Tg as proteins that are strongly associated with human autoimmunity and are expressed promiscuously by human fibrocytes. In addition to fibrocytes, IA-2 mRNA can be detected more consistently, albeit at low levels, in fibroblasts. These observations are congruent with the earlier findings regarding TSHR in multiple fibroblast types and suggest a wider distribution of ICA69 and IA-2 than previously thought. The current studies demonstrate that these proteins are expressed by fibrocytes from many donors, regardless of whether they are healthy or manifest T1DM or other autoimmune diseases such as GD. Thus, although our results inform about their expression outside the pancreas, any role that they might play in T1DM or other forms of human autoimmunity by virtue of their expression pattern is speculative. Whether this wide distribution of expression plays any role in the generation of antibodies to either ICA69 or IA-2 remains uncertain.
Also emerging from these studies are the interesting patterns of IA-2 and ICA69 expression in TAO orbital fibroblasts after their sorting into pure CD34+ and CD34− subsets (Figure 5). The results suggest that despite its expression in fibrocytes, levels of IA-2 are extremely low in pure CD34+ fibroblasts, which appear to derive from those cells (25). It is possible that once fibrocytes infiltrate tissues, the expression of certain genes such as ICA69 can be revived once the cells are removed from the negative influences imposed by CD34− fibroblasts, whereas others such as IA-2 cannot. Although the molecular basis for these patterns of gene expression cannot be solved by the current studies, the results provide strong evidence for widespread expression of these two T1DM-related autoantigens. Furthermore, they indicate that the two proteins are present in divergent patterns with regard to cell expression.
Fibrocytes exhibit remarkable phenotypic plasticity, can efficiently present antigens, produce several cytokines, and generate collagen and vitronectin (20–25). Evidence for their potential involvement in autoimmunity is relatively recent but has become substantial. For instance, Galligan et al (39) found excessive phosphorylation of signaling intermediates in circulating fibrocytes isolated from donors with rheumatoid arthritis (RA). The abundance of circulating fibrocytes increases in asthma (40) in which these cells appear to serve as myofibroblast precursors within the lung (19). Bucala (41) has suggested participation of fibrocytes in the pathogenesis of nephrogenic systemic fibrosis. Our current findings raise the important possibility that fibrocytes express antigens implicated in the pathogenesis of multiple autoimmune endocrine diseases. The particularly striking and unanticipated nature of our results challenges the dogma surrounding patterns of tissue-specific expression of these proteins. To date, fibrocytes have been found to express TSHR and Tg as well as ICA69 and IA-2. At issue is what role these proteins might play in the normal function of fibrocytes and therefore prompts the question of their involvement in host defense and tissue remodeling. With regard to TSHR, we now know that TSH induces several important cytokines, including IL-1, IL-6, IL-8, TNF-α, and monocyte chemotactic protein-1 (42). Tg expressed by fibrocytes appears to be functional in that it becomes iodinated in situ (24). However, unlike the thyroid, Tg localizes within the fibrocyte and does not get exported. Its intracellular biology has yet to be determined. IA-2 localizes to dense core insulin-secretory granules found within β cells. In addition to the pancreas, it can also be detected in brain and adrenal medulla (15, 16, 34, 43–45). IA-2 promotes β-cell proliferation, regulates insulin exocytosis, and interacts with insulin-tethering proteins that form complexes with the cytoskeleton (17, 18).
The implications surrounding promiscuous expression of IA-2 and ICA69 to the broken immune tolerance toward these proteins found in T1DM are considerable but as yet only speculative. The role of IA-2 in the pathogenesis of T1DM appears to be complex and was initially linked to immunoreactivity of the protein's intracellular domain (6). Morran et al (37) demonstrated the potential pathogenic importance of antigenic determinants located within the extracellular domain of IA-2. Importantly, humoral reactivity directed against the region stretching from amino acids 26–577 may be particularly useful in predicting progression to T1DM. Like IA-2, immune reactivity to ICA69 also appears to carry predictive value in individuals with T1DM. In addition to their presence in nearly half of individuals with prediabetes (7), antibodies directed against ICA69 have been detected in patients with Sjögren's syndrome and RA (46). Presence of these antibodies seems to carry less specificity than those against IA2 for T1DM. Anti-ICA69 antibodies could be detected in similar proportions of individuals with RA as those with T1DM (47). Because fibrocytes present antigen through major histocompatibility complex class II (18), it is possible that they participate in the generation of T cell autoreactive responses against self-antigens. Further scrutiny into their seemingly complex interactions with other components of the immune system is warranted, including whether immune reactivity against Tg and TSHR expressed by fibrocytes might play a role in thyroid autoimmunity.
Another aspect emerging from these studies concerns whether fibrocytes might participate in the tissue remodeling observed in T1DM. For instance, renal failure represents an all-too-frequent consequence of longstanding diabetes mellitus (48). Kidney fibrosis, the consequence of longstanding and progressive disease, results in the loss of normal tissue architecture and diminished renal function (49). It has been proposed that kidney inflammation in diabetes mellitus leads to fibrosis and that fibrocytes might play important roles in this process (48). Furthermore, targeting fibrocytes infiltrating the kidney might represent an effective strategy for altering the course of disease to a more favorable conclusion. Although not yet investigated, the remodeling of the pancreas in T1DM, which leads to the loss of β-cell function could also involve infiltrating fibrocytes and thus must be investigated.
The current studies disclose that fibrocytes, regardless of whether they come from healthy donors or those with autoimmune disease, express several autoantigens. Our findings raise the possibility that these proteins may play physiological roles outside the thyroid and pancreas.
Acknowledgments
We thank Dr A. Notkins (National Institutes of Health, Bethesda, Maryland) for providing the human IA-2 promoter.
The authors have no proprietary or commercial interest in any material discussed in this article.
This work was supported in part by National Institutes of Health Grants EY008976, EY011708, DK063121, DK053456, DK56200 and DK073724; Core Center for Vision grant EY007003 from the National Eye Institute; an unrestricted grant from Research to Prevent Blindness; the Bell Charitable Foundation; and the Clinical and Translational Science Program Award UL1RR024986.
Disclosure Summary: All authors have nothing to declare.
Footnotes
- CREB
- cAMP response element-binding protein
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GC
- glucocorticoid
- GD
- Graves' disease
- IA-2
- islet cell antigen ICA512
- ICA69
- islet cell autoantigen of 69 kDa
- MS
- multiple sclerosis
- RA
- rheumatoid arthritis
- Sp1
- specificity protein-1
- TAO
- thyroid-associated ophthalmopathy
- T1DM
- type 1 diabetes mellitus
- Tg
- thyroglobulin
- TSHR
- thyrotropin receptor.
References
- 1. Cardenas-Roldan J, Rojas-Villarraga A, Anaya JM. How do autoimmune diseases cluster in families? A systemic review and meta-analysis. BMC Med. 2013;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sellner J, Kalluri SR, Cepok S, Hemmer B, Berthele A. Thyroid antibodies in aquaporin 4 antibody positive central nervous system autoimmunity and multiple sclerosis. Clin Endocrinol (Oxf). 2011;75:271–272 [DOI] [PubMed] [Google Scholar]
- 3. Pietropaolo M, Peakman M, Pietropaolo SL, et al. Combined analysis of GAD65 and ICA512(IA-2) autoantibodies in organ and non-organ-specific autoimmune diseases confers high specificity for insulin-dependent diabetes mellitus. J Autoimmun. 1998;11:1–10 [DOI] [PubMed] [Google Scholar]
- 4. Trajkovski M, Mziaut H, Schubert S, Kalaidzdis Y, Altkruger A, Solimena M. Regulation of insulin granule turnover in pancreatic β-cells by cleaved ICA512. J Biol Chem. 2008;48:33719–33729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karges W, Hammond-McKibben D, Gaedigk R, Shibuya N, Cheung R, Dosch HM. Loss of self-tolerance to ICA69 in nonobese diabetic mice. Diabetes. 1997;10:1548–1556 [DOI] [PubMed] [Google Scholar]
- 6. Kawasaki E, Yu L, Gianni R, et al. Evaluation of islet cell antigen (ICA) 512/IA-2 autoantibody radioassays using overlapping ICA512/IA-2 constructs. J Clin Endocrinol Metab. 1997;2:375–380 [DOI] [PubMed] [Google Scholar]
- 7. Pietropaolo M, Castaño L, Babu S, et al. Islet cell autoantigen 69 kD (ICA69). Molecular cloning and characterization of a novel diabetes-associated autoantigen. J Clin Invest. 1993;1:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pietropaolo M, Towns R, Eisenbarth GS. Humoral autoimmunity in type 1 diabetes: prediction, significance, and detection of distinct disease subtypes. Cold Spring Harb Perspect Med. 2012;10:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baekkeskov S, Aanstoot H, Christgau S, et al. Identification of the 64K autoantigen in insulin dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–156 [DOI] [PubMed] [Google Scholar]
- 10. Palmer JP, Asplin CM, Clemons P, et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222:1337–1339 [DOI] [PubMed] [Google Scholar]
- 11. Lan MS, Lu J, Goto Y, Notkins AL. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994;13:505–514 [DOI] [PubMed] [Google Scholar]
- 12. Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (SIc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abramson J, Giruad M, Benoist C, Mathis D. Aire's partners in the molecular control of immunological tolerance. Cell. 2010;140:123–135 [DOI] [PubMed] [Google Scholar]
- 14. Gardner JM, DeVoss JJ, Friedman RS, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81 [PMC free article] [PubMed] [Google Scholar]
- 16. Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562 [DOI] [PubMed] [Google Scholar]
- 17. Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425 [PubMed] [Google Scholar]
- 18. Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-processing cell capable of priming naïve T cells in situ. Proc Natl Acad Sci USA. 1997;94:6307–6312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389 [DOI] [PubMed] [Google Scholar]
- 20. Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piling D, Fan T, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-β and peroxisome proliferator-activated receptor γ. J Biol Chem. 2007;282:22910–22920 [DOI] [PubMed] [Google Scholar]
- 23. Douglas RS, Afifyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy, J Clin Endocrinol Metab. 2010;95:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith TJ, Padovani-Claudio DA, Raychaudhuri N, et al. Fibroblasts expressing the thyrotropin receptor overarch thyroid and orbit in Graves' disease. J Clin Endocrinol Metab. 2011;96:3827–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernando R, Atkins S, Raychaudhuri N, et al. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci USA. 2012;109:7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie J, Zhang B, Lan MS, Notkins AL. Genomic structure of promoter sequence of the insulin-dependent diabetes mellitus autoantigen, IA-2 (PTPRN). Genomics. 1998;54:338–343 [DOI] [PubMed] [Google Scholar]
- 27. Friday RP, Pietropaolo SL, Profozich J, Trucco M, Pietropaolo M. Alternative core promoters regulate tissue-specific transcription from the autoimmune diabetes-related ICA1 (ICA69) gene locus. J Biol Chem. 2003;278:853–863 [DOI] [PubMed] [Google Scholar]
- 28. Smith TJ, Koumas L, Gagnon A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endo Metab. 2002;87:385–392 [DOI] [PubMed] [Google Scholar]
- 29. Smith TJ, Sempowski GD, Wang HS, Del Vecchio PJ, Lippe SD, Phipps RP. Evidence for cellular heterogeneity in primary cultures of human orbital fibroblasts. J Clin Endocrinol Metab. 1995;80:2620–2625 [DOI] [PubMed] [Google Scholar]
- 30. Tsui S, Fernando R, Chen B, Smith TJ. Divergent Sp1 protein levels may underlie differential expression of UDP glucose dehydrogenase by fibroblasts: role in susceptibility to orbital Graves' disease. J Biol Chem. 2011;286:24487–24499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li B, Smith TJ. Divergent expression of IL-1 receptor antagonists in CD34+ fibrocytes and orbital fibroblasts in thyroid-associated ophthalmopathy: contribution of fibrocytes to orbital inflammation. J Clin Endocrinol Metab. 2013;98:2783–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mziaut H, Trajkovski M, Kersting S, et al. Synergy of glucose and growth hormone signaling in islet cells through ICA512 and STAT5. Nat Cell Biol. 2006;8:435–445 [DOI] [PubMed] [Google Scholar]
- 33. Harashima S, Clark A, Christie MR, Notkins AL. The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. Proc Natl Acad Sci USA. 2005;102:8704–8709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solimena M, Dirkx R, Jr, Hermel JM, et al. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J. 1996;15:2102–2114 [PMC free article] [PubMed] [Google Scholar]
- 35. Martin S, Kardorf J, Schulte B, et al. Autoantibodies to the islet antigen ICA69 occur in IDDM and in rheumatoid arthritis. Diabetologia. 1995;38:351–355 [DOI] [PubMed] [Google Scholar]
- 36. Song A, Winer S, Tsui H, et al. Deviation of islet autoreactivity to cryptic epitopes protects NOD mice from diabetes. Eur J Immunol. 2003;33:546–555 [DOI] [PubMed] [Google Scholar]
- 37. Morran MP, Casu A, Arena VC, et al. Humoral autoimmunity against the extracellular domain of the neuroendocrine autoantigen IA-2 heightens the risk of type I diabetes. Endocrinology. 2010;151:2528–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen C, Bergerot I, Elliott JF, et al. Evidence that a peptide spanning the B-C junction of proinsulin is an early auto-antigen epitope in the pathogenesis of type I diabetes. J Immunol. 2001;167:4926–4935 [DOI] [PubMed] [Google Scholar]
- 39. Galligan CL, Siminovitch KA, Keystone EC, Bykerk V, Perez OD, Fish EN. Fibrocyte activation in rheumatoid arthritis. Rheum. 2010;49:640–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang CH, Huang CD, Lin HC, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Repir Crit Care Med. 2008;178:583–591 [DOI] [PubMed] [Google Scholar]
- 41. Bucala R. Circulating fibrocytes: Cellular basis for NSF. J Am Coll Radiol. 2008;5:36–39 [DOI] [PubMed] [Google Scholar]
- 42. Gillespie EF, Papageorgiou K, Fernando R, et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol Metab. 2012;5:E740–E746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakajima K, Wu G, Sakudo A, Onadera T, Takeyama N. Distinct subcellular localization of three isoforms of insulinoma-associated protein 2β in neuroendocrine tissues. Life Sci. 2011;88:798–802 [DOI] [PubMed] [Google Scholar]
- 44. Spitzenberger F, Pietropaolo S, Verkade P, et al. Islet cell autoantigen of 69 kDa is an arfaptin-related protein associated with the Golgi complex of insulinoma INS-1 cells. J Biol Chem. 2003;278:26166–26173 [DOI] [PubMed] [Google Scholar]
- 45. Pilon M, Peng X-R, Spence AM, Plasterk RHA, Dosch HM. The diabetes autoantigen ICA69 and its Caernorhabditis elegans homologue, ric-19, are conserved regulators of neuroendocrine secretion. Mol Biol Cell. 11:3277–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kanasaki K, Taduri G, Koya D. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol. 2013;4:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kerokoski P, Ilonen J, Gaedigk R, et al. Production of the islet cell antigen ICA69 (p69) with baculovirus expression system: analysis with a solid-phase time-resolved fluorescence method of sera from patients with IDDM and rheumatoid arthritis. Autoimmunity. 1999;29:281–289 [DOI] [PubMed] [Google Scholar]
- 48. Lin WR, Inatomi O, Lee CY, et al. Bone marrow-derived cells contribute to cerulean-induced pancreatic fibrosis in the mouse. Int J Exp Pathol. 2012;93:130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gordon TP, Cavill D, Neufing P, Zhang YJ, Pietropaolo M. ICA69 autoantibodies in primary Sjögren's syndrome. Lupus. 2004;13:483–484 [DOI] [PubMed] [Google Scholar]